Abstract
Essentials.
The incidence of venous thrombosis has remained stable over the past decade.
Risk factors and diagnostic and prophylaxis strategies are determinants of the overall incidence.
Given current trends of the determinants, we are making progress in reducing the burden.
More progress can be made by implementing validated risk assessment models.
Venous thrombosis is a major contributor to the global disease burden. In this review we aim to answer two important questions: (1) are we making progress in reducing this disease burden and (2) how can we further improve? To answer these questions, we first evaluated the disease burden, that is, the incidence of first venous thrombosis over the past decade(s) and discuss its most important determinants. We found that the incidence of first venous thrombosis remained relatively unchanged, despite an increase in risk factor prevalence and a rise in identification of subsegmental pulmonary emboli due to enhanced image quality and utilization. This is, however, balanced by improved thromboprophylaxis strategies, resulting in an overall unchanged venous thrombosis incidence. We can further improve by developing, validating, and implementing risk assessment strategies, allowing us to identify persons at high or low risk in whom thromboprophylaxis can be provided or withheld, respectively.
Keywords: epidemiology, incidence, risk assessment, risk factors, venous thrombosis
1. INTRODUCTION
Venous thrombosis is the collective term for deep vein thrombosis and pulmonary embolism, indicating the presence of a blood clot obstructing flow in the deep veins or pulmonary arteries, respectively. At an estimated incidence rate of 1‐2 per 1000 persons every year, it is the third most common cardiovascular disease and is associated with substantial short‐ and long‐term morbidity and mortality.1, 2, 3, 4 Short‐term consequences of venous thrombosis include the absolute need for anticoagulant therapy, which is inevitably associated with an increased bleeding risk5; the estimated case‐fatality rate is approximately 6% after 30 days.2 Long‐term consequences concern the risk of disease recurrence, as approximately 20‐25% of all patients have a recurrence within 5 years.6 Other long‐term consequences include the risk of developing post‐thrombotic syndrome (PTS, in 20‐50% of patients with a deep vein thrombosis)7 and the rare but potentially life threatening condition chronic thromboembolic pulmonary hypertension (CTEPH) that occurs in approximately 0.6% of patients with pulmonary embolism.8 Venous thrombosis is a leading cause of disability‐adjusted life years (DALYs) lost9 and associated with substantial healthcare costs.10, 11 In the past decades, several changes in the prevention, diagnostic strategies, and management of venous thrombosis have been achieved. For instance, guidelines now suggest extended or lifelong duration of anticoagulant therapy in men with a first unprovoked event, while previously, a limited treatment duration was recommended for these patients.12 Pulmonary embolism is now almost always diagnosed with computed tomography (CT) angiography or ventilation perfusion scan instead of (the gold standard) pulmonary angiography,13 and for several risk situations, such as surgery, near‐universal thromboprophylaxis strategies have been implemented.14, 15, 16 In addition, the prevalence of risk factors for venous thrombosis in populations is continuously changing. There is an epidemic of obesity in the aging Westernized population in which, for instance, 32.5% of the adult population in the Netherlands was overweight (body mass index [BMI] ≥ 25 kg/m2) in 1995 which increased to 41.7% in 2010,17 while the use of hormone replacement therapy in women over 50 has declined after it was shown in the early 2000s that such therapy increases both the risk of arterial cardiovascular disease as well as venous thrombosis.18, 19 Therefore, it is important to evaluate the burden of venous thrombosis over time, to assess whether we are making progress in the prevention of this disease and to find leads as to how we can reduce the venous thrombotic disease burden even further. In this article, by reviewing the available evidence including state of the art research presented at the International Society on Thrombosis and Haemostasis (ISTH) Congress 2017, we aim to answer two questions: (1) are we making progress in decreasing the burden of venous thrombosis in the general population, and (2) how can we proceed further in preventing venous thrombosis disease in the community?
2. VENOUS THROMBOSIS INCIDENCE OVER TIME
One of the ways to evaluate disease burden is by studying its incidence (number of persons who get the disease in a population at risk, per size of the population and a given time period). However, the total burden of the disease goes beyond first events, as it also includes post‐thrombotic syndrome, anticoagulant treatment, mortality, recurrent events and health‐care associated costs. To estimate the total burden, we will therefore consider the first event as a “marker” of the total burden of thrombotic disease that follows after a first event. For this, we need data on trends on venous thrombosis incidence from populations‐based studies.
In a United States population‐based study by Heit and colleagues, the average age‐ and sex‐adjusted incidence of a first venous thrombosis in the Olmsted County population was 10.2 (95% CI 10.2‐10.3) per 10 000 person‐years which did not change over the course of 30 years (1981‐2010 based on 3293 events, Figure 1A).3 In contrast, in another population‐based study from the United States, Huang and colleagues showed that the age‐ and sex‐adjusted incidence of a first event increased in the period 1985‐2009 from 7.3 (95% CI 6.4‐8.2) per 10 000 person‐years to 13.3 (95% CI 12.2‐14.3), based on 3887 events (Figure 1B). The authors observed that the rise was mainly due to an increase in the incidence of pulmonary embolism.20 Alotaibi and colleagues assessed the incidence of venous thrombosis over calendar‐time in Alberta, Canada (4 million individuals).21 Between 2004 and 2012 there were 31 656 incident acute venous thrombosis events, with an overall age‐ and sex‐standardized incidence rate of 13.8 (95% CI 13.7‐14.0) per 10 000 person‐years. This incidence rate did not change during the study period.21 In the Norwegian Tromsø study, in which 26 855 persons aged 25‐97 were followed from 1994/1995 throughout 2012, the incidence of a first symptomatic venous thrombosis (based on 693 events) increased from 15.8 (95% CI 11.6‐19.9) per 10 000 persons years in 1996 and 1997 to 20.1 (95% CI 16.0‐24.3) in 2010 and 2011.22
Figure 1.
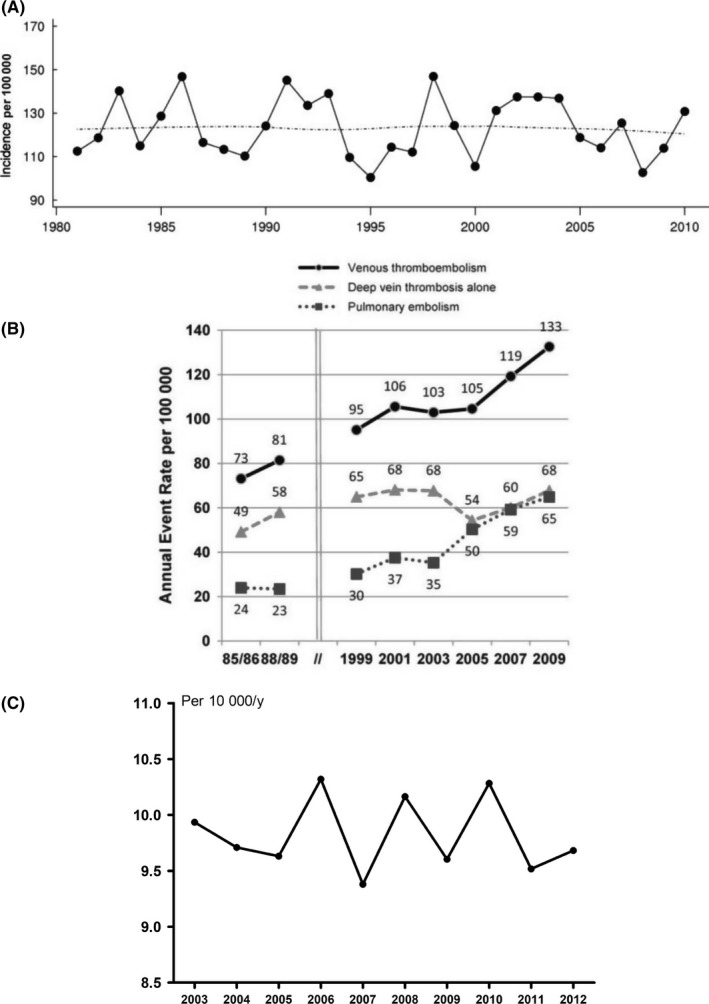
Absolute risk of first venous thrombosis over the course of time in three independent studies. Age‐adjusted incidence rates of first venous thrombosis: A, among Olmsted County, Minnesota residents, 1981‐2010 based on 3293 events as published by Heit and colleagues,3 printed with permission from Schattauer publishers; B, among residents of Worcester, Massachusetts, 1985‐2009 based on 3887 events, printed with permission of Elsevier as published by Huang and colleagues20; and C, over the course of 10 years in three large anticoagulation clinics in the Netherlands, adapted from Scheres et al.,24 based on 14 253 events
To investigate the incidence of first venous thrombosis events over calendar time in the Netherlands, we conducted a dynamic population‐based study between 2003 and 2012, as was previously described by Kort et al.23, 24 Since only patients treated with vitamin K antagonists are included in the study, patients using long term low‐molecular‐weight heparins for venous thrombosis treatment (such as patients with a malignancy or pregnant patients) are underrepresented. Patients who died as a result of or shortly after the event are therefore neither in the study. The results of this study were presented at the ISTH congress of 2017, as depicted in Figure 1C (adapted from Scheres et al.24), from which it appears that, based on these 14 253 events, the overall age‐adjusted incidence rate of first venous thrombosis did not change over the course of 10 years.
Taken the results from these five studies together, it can be concluded that the incidence of (diagnosed) first venous thrombosis remained relatively unchanged over the past decades. This would suggest that we are not making progress in preventing venous thrombotic disease in the general population. However, such an interpretation might be too superficial and we first need to examine the determinants of these incidences more closely.
3. DETERMINANTS OF VENOUS THROMBOSIS INCIDENCE
The three main determinants that can influence the incidence of venous thrombosis in a general population are: (1) the prevalence of risk factors for venous thrombosis, (2) the number and method of diagnostic procedures, and (3) the implementation of thrombophrophylactic strategies.
3.1. Prevalence of risk factors
The incidence of any disease is dependent on the prevalence of risk factors in the population at risk for the disease. Obviously, when more individuals have (multiple) risk factors, the more likely it becomes that these individuals will actually get the disease.
For venous thrombosis, risk factors can roughly be classified into two main categories, ie, acquired and genetically determined.25 Acquired risk factors can be either transient or persistent. Transient risk factors are only present for a certain time period, such as, for example, long‐haul flights or pregnancy and the postpartum period. Examples of persistent acquired risk factors are inflammatory bowel disease or overweight and obesity. Genetically determined risk factors are non‐modifiable and always present such as male sex, mutations leading to thrombophilia or body height.25
In Table 1, risk factors for first venous thrombosis that we discuss in this review are shown. In addition, we have indicated whether we anticipate an increase or decrease in the prevalence of these risk factors in Western countries in coming years. As an example, trends in the prevalence of these risk factors over calendar time in the Netherlands are shown in Figure 2, based on data provided by Statistics Netherlands,17, 26, 27, 28, 29, 30 Royal Schiphol Group,31 and the Netherlands Comprehensive Cancer Organisation.32 In the near future, the prevalence of most of these risk factors is likely to increase further. As shown in Figure 2, the number of total hospitalizations and persons with an active malignancy has increased over the years (1.4‐1.5 fold, respectively, when comparing the year 2010 with 1995). A similar pattern was described by Torre and colleagues in 2015, who observed an increase in global cancer prevalence.33 In 1995, 13.7% of the Dutch population was aged >65, which increased to 16.0% in 2010. This is in concordance with the global aging of the population, as reported by the Global Aging Institute.34 The population was more often overweight/obese in 2010 than in 1995, in line with reports from the World Health Organization (WHO), describing a global increase of overweight and obese populations.35 Air travel from the main airport terminal in the Netherlands (Schiphol, Amsterdam) has nearly doubled from 1995 to 2010, a similar trend can be observed from data on worldwide air travel from the World Data Bank.36 Both the total number of surgeries as well as high‐risk venous thrombosis surgeries, such as total knee and hip replacements, increased from 1995 to 2010 in the Netherlands, where the number of knee replacements more than doubled over this timeframe. An increase in the global surgery volume was also reported by the WHO.37 In contrast, oral or injectable contraceptive use among women <50 years declined slightly and the number of live birth pregnancies was constant over time. However, the number of live birth pregnancies in women aged 40 years or older increased two‐fold from 1995 to 2010, who carry a higher risk for venous thrombosis than pregnant younger mothers.38, 39, 40 In a 2015 report by the United Nations, a slight global decrease in the number of pregancies is projected globally in the coming decades, where especially in Western countries, the maternal age at chilbirth is increasing.41 In addition, at the ISTH congress of 2017, O'Shaughnessy et al. reported on venous thrombosis risk factors in 16 218 women who delivered at the Rotunda Hospital in Dublin between September 2014 and December 2016. The majority, i.e, 82% of women, had at least one venous thrombosis risk factor (on top of pregancy) and over half of the women had two or more risk factors. Of all 16 218 women, 5380 (33.2%) were 35 years or older.42
Table 1.
Venous thrombosis risk factors and anticipated change of prevalence the coming years
| Risk factor | Estimated relative risk (compared to the general population)* | Anticipated prevalence increasing or decreasing | References for change in prevalence |
|---|---|---|---|
| Provoked, Transient | |||
| General, orthopedic surgery and hospitalization | 5‐50 | ↑ | 26, 27, 37 |
| Long‐haul (air) travel | 2.5‐3 | ↑ | 31, 36 |
| Infections | 1‐3 | ↑ | 27 |
| Pregnancy and postpartum period | 3‐5 | ↓/(↑ older maternal age) | 28, 41 |
| Oral contraceptive use | 4‐7 | ↓ | 29 |
| Hormone replacement therapy | 2‐5 | ↓ | 73 |
| Provoked, Persistent | |||
| Overweight and obesity | 2‐3 | ↑ | 17, 35 |
| Active malignancy | 7‐20 | ↑ | 32, 33 |
| Chronic (inflammatory) diseasesa | 1‐10 | ↑ | 74, 75 |
| Unprovoked | |||
| Increasing age | 1‐∞ | ↑ (older population) | 30, 34 |
| Body height | 1.5‐4 | =/↑ (global increase) | 17, 76 |
| Male sex | 2 | = | 30 |
| Genetic risk factorsb | 1‐20 | = | 77, 78 |
↑ increase. ↓; decrease. =; no change. *List of risk factors and relative risk adapted from Lijfering et al.79
Chronic kidney diseases, Human immunodeficiency virus, hyperthyroid disease, inflammatory bowel disease, systemic lupus erythematosus, amongst others.
Factor V Leiden mutation, Prothrombin G20210A mutation, genetic deficiencies of protein S, protein C, or antithrombin, non–O blood group amongst others.
Figure 2.
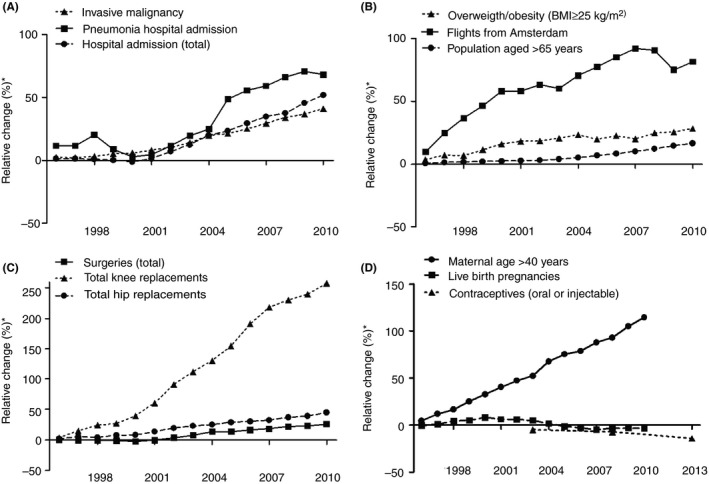
Trends in the prevalence of several risk factors for venous thrombosis, 1995‐2010. *Reference is 1995 except for contraceptives (oral or injectable) where the reference year is 1998. A denotes, major medical conditions; B, demographics and lifestyle; C, surgery (all) and orthopedic surgery; D, female‐specific risk factors. Adapted from statistics Netherlands,17, 26, 27, 28, 29, 30 Royal Schiphol Group,31 and Netherlands Comprehensive Cancer Organisation.32 Precise numbers are available in Table S1
Overall, the prevalence of these risk factors is increasing and many of these risk factors are currently unavoidable.
3.2. Diagnostic procedures
Second, the incidence of venous thrombosis is dependent on the number of events identified. An increase in the number of imaging tests will result in an increased number of encountered (either symptomatic, asymptomatic or misclassified) events. As was previously described by Wiener and colleagues, there has been a rise in the use of CT pulmonary angiograms with increasing availability of CT, as it also allows finding other causes for pulmonary embolism–like symptoms. At the same time, the image quality is continuously improving, resulting in finding more smaller (subsegmental) pulmonary emboli and hence, an increase in incidence. In contrast, the age‐adjusted, in‐hospital case‐fatality rate of pulmonary embolism decreased from 12.1% in 1998 to 7.8% in 2006. This suggests that at least some of these pulmonary emboli are self limiting or misclassified events.43, 44 A similar observation was reported by Dentali and colleagues, in a study on admission for pulmonary embolism in a region with approximately 13 million inhabitants in Italy, where the incidence of pulmonary embolism increased between 2002 and 2012 from 4.0 to 6.2 per 10 000 persons per year in women and from 3.5 to 4.6 in men. The case‐fatality rate decreased over this time frame (from 15.6% to 10.2% in women and from 17.6% to 10.2% in men).45 In the Netherlands, we also observed an increase in pulmonary embolism diagnoses over calendar time, but a decrease in incidence of deep vein thrombosis (Figure 3), resulting in an overall unchanged incidence rate of first venous thrombosis. A similar observation was reported previously by Huang and colleagues, in their study among residents from Worcester in the United States from 2001 the incidence of (distal or proximal) first deep vein thrombosis decreased and the incidence first pulmonary embolism increased (Figure 1B).20 In parallel with this observation, there was an increase in noninvasive testing between 1985 and 2009. Notably, after 2005 the incidence of first (distal or proximal) deep vein thrombosis also seemed to increase again.20 A comparable finding was reported based on data from the United States Healtcare Cost and Utilization Nationwide Inpatient Sample, in which the hospitalization rates for pulmonary embolism increased from 1996 to 2014. The hospitalization rates for deep vein thrombosis initially increased up to 2012 and decreased afterwards.46 These observations also are in line with a report from the Norwegian Tromsø study that showed that the incidence of pulmonary embolism increased from 4.5 (95% CI 2.3‐6.7) per 10 000 person‐years in 1996 and 1997 to 11.3 (95% CI 8.2‐14.4) in 2010 and 2011, where the incidence of isolated deep vein thrombosis decreased during this timeframe, from 11.2 (95% CI 7.7‐14.6) per 10 000 person years in 1996 and 1997 to 8.8 (95% CI 6.1‐11.5) in 2010 and 2011.22 It is currently uncertain whether patients with subsegmental pulmonary emboli (either symtomatic or asymptomatic and outside of special patients groups) benefit from anticoagulant treatment. For this purpose, an international cohort management study currently investigates the safety of withholding anticoagulants in these patients (NCT01455818).
Figure 3.
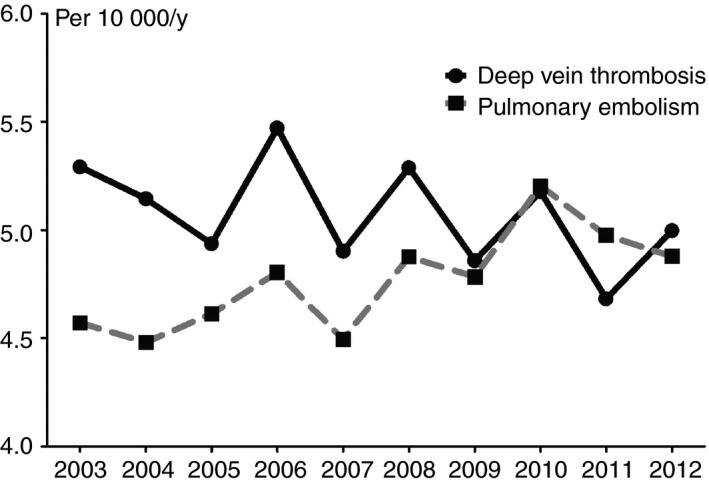
Absolute risk of first deep vein thrombosis and pulmonary embolism over the course of time in three large anticoagulation clinics in the Netherlands. Incidence of first pulmonary embolism and deep vein thrombosis over the course of 10 years in three large anticoagulation clinics in the Netherlands24
Although incidence data over time are limited, the increase in use of whole‐leg ultrasound as opposed to the use of only proximal ultrasound has likely led to an increase in identification of distal deep vein thromboses. Although these can present with troublesome symptoms, distal deep vein thromboses are less burdensome than proximal deep vein thrombosis as they are associated with a lower recurrence and mortality rate.47, 48 Whether patients (outside of special patient groups) with symptomatic distal deep vein thromboses benefit from anticoagulation therapy is currently debated.49, 50 Consequently, in centers in which patients with distal deep vein thrombosis are treated with anticoagulants, whole‐leg ultrasound is often used, whereas in centers where distal deep vein thrombosis is typically not treated, only proximal ultrasound is used. For this reason, distal deep vein thromboses are often not or only partly included in incidence studies. In contrast, there is strong evidence and global consensus for several decades that proximal deep vein thrombosis necessitates treatment with anticoagulant therapy. Therfore, the incidence of proximal deep vein thrombosis over the past years will not have been, or only slighty, affected by changes in diagnostic and treatment strategies.
In summary, the overall incidence of first venous thrombosis is dependent on the number of imaging tests performed. In the past few years there has been an increase in imaging quality and the number of diagnostic tests performed which has especially resulted in an increased number of (subsegmental) pulmonary emboli diagnoses. In contrast, the incidence of proximal deep vein thrombosis has decreased.
3.3. Prophylactic strategies
Third, the incidence of venous thrombosis is influenced by the usage of tromboprophylactic strategies. Increasing the use of thromboprophylaxis will decrease the number of venous thrombosis events. Prophylactic strategies can be implemented for high‐risk situations, such as hospitalization, medical illness, orthopedic surgery, trauma or pregnancy. As it is challenging, if not currently impossible, to use prophylaxis to prevent unprovoked trombotic events (since there is no preceding high‐risk situation that can be identified or anticipated on in these individuals), the efficacy of prophylactic strategies can only be inferred from assesing the incidence of provoked events over calender time because if profylactic strategies are succesful, this should be reflected by a decline in provoked events. This is indeed the case, as was shown by Heit and colleagues, who reported a decline in the incidence of provoked first venous thrombotic events from 1981‐2010.3 In addition, the implementation of a nationwide program for venous thrombosis prevention in England has resulted in a risk assesment in over 95% of patients admitted to an acute National Health Service hospital.51 In a subsequent study by Roberts and colleagues, it was shown that succesful risk assesment was also associated with a reduction in hospital‐associated venous thrombosis events in England; risk ratio 0.88 (95% CI 0.74‐0.98).51, 52
In brief, thromboprophylaxic strategies seem efficacious in reducing the incidence of venous thrombosis, when correctly and rigourously applied.
4. ARE WE MAKING PROGRESS?
To answer the question whether we are making progress one needs to combine knowledge on the epidemiological patterns of the venous thrombosis burden with knowledge on its determinants. This way, we can observe progress in three ways (summarized in Table 2). First, despite an increase in the prevalence of most risk factors, the incidence remained relatively unchanged over the past years: this means we must have made progress, otherwise the incidence would have increased in concordance with the rise in prevalence of risk factors. Second, over the years the incidence of pulmonary embolism has increased, due to the finding of more subsegmental pulmonary emboli.43, 44, 45 In addition, the incidence of proximal deep venous thrombosis, which has not been subject to changes in diagnostic management, seems to decline. As the overall incidence remains unchanged, a portion of deep venous thrombosis and pulmonary emboli must have been prevented, and “replaced” by less burdensome (subsegmental) or misclassified pulmonary emboli. Hence, there is progress. Third, over the years several thromboprophylaxis strategies have been implemented that resulted in a reduction of the number of hospital associated thromboses.51, 52 Taken together, although the incidence of venous thrombosis did not go down, we are making progress. There is, nevertheless, room for advancement, which we will discuss below.
Table 2.
Summary of the determinants of venous thrombosis incidence, the direction of their effect and current indicators that we are making progress
| Determinant | Increase results in ↓/↑ incidence | Decrease results in ↓/↑ incidence | Current indicator that we are making progress |
|---|---|---|---|
| Prevalence of risk factors | ↑ | ↓ | Despite an increase in risk factor prevalence, venous thrombosis incidence remained unchanged |
| Number and quality of diagnostic tests | ↑ | ↓ | Despite a rise in identification of subsegmental pulmonary emboli, venous thrombosis incidence remained unchanged |
| Usage of thromboprophylaxis strategies | ↓ | ↑ | More and better thromboprophylaxis strategies balance the rise in risk factors and number of diagnostic tests, resulting in an overall unchanged venous thrombosis incidence |
↑; increases the venous thrombosis incidence. ↓; decreases the venous thrombosis incidence.
5. HOW CAN WE FURTHER IMPROVE?
5.1. Prevention of first venous thrombosis
Knowledge on incidence of venous thrombosis and its determinants is useful for two purposes: (1) better understanding of the pathophysiology of venous thrombosis, and (2) prevention of venous thrombosis. However, for the latter, we are restricted to prevention of provoked events, as individuals at risk for unprovoked thrombosis are hard to identify. For individuals at risk of provoked events, the risk can be reduced whilst the provoking risk factor is in play. For example, patients undergoing major (orthopedic) surgery receive prophylaxis for a certain period after the surgery. Such prophylactic strategies can be implemented in two ways, either in all patients during a high‐risk situation or limited to patients with actual (estimated) high risk of an event during the high‐risk situation.
5.2. General prophylaxis in high‐risk situations
This first possible strategy, which is currently applied, is to provide thromboprophylaxis to all patients during a high‐risk situation. This is frequently done when the duration of the risk situation is relatively short and thromboprophylaxis is easily administered, for example in immobilized patients admitted to the hospital or in patients recovering from major surgery.15, 16, 53 This strategy is more challenging and impractical when the high‐risk situation is of longer duration, for example during pregnancy in women with inherited antithrombin deficiency,54 or in patients with an active malignancy.55 Moreover, the longer patients receive prophylaxis, the higher the chances of a bleeding event. In addition, we have to keep in mind that the absolute risk of an event can still be relatively low, generally a few percent. Hence, there are many patients in high‐risk situations who will not develop a venous thrombosis, possibly not even without prophylaxis. To complicate matters even further, there will be patients who will develop thrombosis, despite taking prophylaxis. As an example, in a Cochrane review on thromboprophylaxis after major orthopedic surgery, 2% of patients developed venous thrombosis despite thromboprophylaxis with low‐molecular‐weight heparin.56 In addition, in the POT‐KAST and POT‐CAST trials low‐dosed thromboprophylaxis was compared with no thromboprophylaxis for preventing symptomatic venous thrombosis in patients who underwent knee arthroscopy for the duration of eight days and for the full period of immobilization patients with casting of the low leg, respectively. There was no difference in the incidence of the primary outcome, ie, symptomatic venous thrombosis, between both arms in these trials. In the treatments arms of these trials, thromboprophylaxis did not prevent thrombosis in 0.7% (POT‐KAST) and 1.4% (POT‐CAST) of the patients, respectively.57 In line with this, the aforementioned study on the risk assessment program after achieving coverage of >90% of hospitalized patients being risk assessed in England resulted in fact in only a small risk reduction, risk ratio 0.88 (95% CI 0.79‐0.98), of hospital associated thrombosis.52 In another study, investigators set out to assess the effect of achieving nearly universal thromboprophylaxis use at the Mayo Clinic hospital (Rochester, MN, USA) on incidence of in hospital venous thrombosis (defined as events which occurred in the hospital or within 92 days after any hospital discharge) over the years 2005‐2010.14 The authors concluded that, as the incidence remained unchanged, despite an increase in the rate of thromboprophylaxis to ~90% near the midpoint of the study period, the thromboprophylaxis had been insufficient.14
Overall, general (low‐dosed) thromboprophylaxis strategies do not seem sufficient for all high‐risk patients. An obvious explanation for this observation is that venous thrombosis is a multicausal disease, implying that several risk factors need to be present to reach a certain “thrombosis threshold” and the disease to occur.58 The number of risk factors present in an individual, however, is likely be different among individuals. Furthermore, the risk in an individual is also dependent on the strength of the risk factors present. As illustrations, in women using oral contraceptives, the activated protein C (APC) resistant effects may increase the risk of a venous thrombosis synergistically when a prothrombotic mutation such as Factor V Leiden is also present.59 In a different example, the increased risk of venous thrombosis is stronger in tall and short persons than persons of average size during long haul flights, likely because they are more prone to obstructed blood flow due to the unnatural sitting circumstances.60 In summary, venous thrombosis can result from different combinations of risk factors, where some combinations result in stronger effects than others and can vary among patient characteristics. This can explain why some patients develop thrombosis despite anticoagulant treatment, while many others never develop it, even in the absence of treatment. This concept suggests the need for an individualized approach. Combining and integrating knowledge on the presence of predisposing factors in individuals in statistical models, should ideally lead to the possibility to determine the size of the risk of venous thrombosis in a particular patient in a particular situation.
5.3. Risk prediction tailored prophylaxis
To further improve the current situation and continue to reduce the number of venous thrombosis evens, risk assessment models (also known as prediction models or RAMs) seem promising. These models ideally allow identification of individuals at high or low venous thrombosis risk during a specific risk situation.61 In recent years, several models have been developed for different patient populations such as patients with cancer62 on which a novel model was also presented at the ISTH 2017 congress by Pabinger and colleagues,63 major trauma,64 lower extremity cast‐immobilization,65 medical inpatients,66 or postpartum women,67 among others. In order to optimize the safe utilization of these models, they first need to be externally validated (ie, tested in a dataset different from the set in which the models were built). Models may seem to perform well in the dataset in which they were built, but performance is often limited in external validation studies (even when the target population for the model is similar).68 Next, models can be improved by studying the potential added value (so called incremental value studies) of a new predictor which was not yet used in the initial version of the model.69 A potential pitfall in the era of risk assessment models is when all efforts are aimed towards the development of new separate models instead of focusing on the validation and improvement of currently available models. In a systematic review on available risk assessment models for cardiovascular disease in the general population it was shown that there is an excess of separate models (n = 363).70 Moreover, external validation, incremental value studies or studies comparing the performance of models were often not performed.70 Although this systematic review excluded venous thrombosis models and a systematic overview of venous thrombosis models is lacking, the same challenges apply. As an example, at the ISTH congress of 2017, Blondon and colleagues presented the results of an external validation and comparison to the Geneva RAM of the Improve RAM for thromboprophylaxis for acutely ill medical patients.71 Finally, to actually achieve a reduction in the venous thrombosis burden by preventing events by using these models, efforts towards the implementation and prospective evaluation (eg, impact studies) of well performing available models are necessary.72 In brief, we can further proceed in reducing the burden of venous thrombosis by the development, validation, and implementation of well‐performing risk assessment models that will allow identification of patients who require (duration and dose tailored) thromboprophylaxis. As general (low‐dose) thromboprophylaxis does not seem effective in all high‐risk patients, trials are needed to determine the optimal dose and duration of thromboprophylaxis in these patients.
6. CONCLUSION
To conclude, we are definitely making progress in reducing the venous thrombosis burden. This progress can be deduced from a relatively unchanged incidence rate over the past decade(s), despite an increasing prevalence of risk factors and identification of subsegmental emboli. This progress can be attributed to better and more frequent implementation of thromboprophylaxis strategies which have resulted in a reduced number of events in patients during high‐risk situations. We can likely proceed further by developing, validating, and implementing risk assessment strategies, in which persons at high‐risk during risk situations can be identified and prescribed adequate thromboprophylaxis, whereas in patients at low risk prophylaxis can be safely withheld.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
Supporting information
ACKNOWLEDGMENTS
We are grateful to Helga Vermaas, Nynke Wiersma, and Felix van der Meer from the anticoagulation clinics from The Hague, Utrecht, and Leiden, respectively, for providing the data which were reviewed in this article.
Scheres LJJ, Lijfering WM, Cannegieter SC. Current and future burden of venous thrombosis: Not simply predictable. Res Pract Thromb Haemost. 2018;2:199–208. 10.1002/rth2.12101
Funding Information
L.J.J. Scheres is a PhD candidate supported by the Netherlands Heart Foundation, CREW project (2013T083).
Contributor Information
Luuk J. J. Scheres, https://twitter.com/ScheresLuuk.
Suzanne C. Cannegieter, Email: s.c.cannegieter@lumc.nl, https://twitter.com/s_cannegieter.
REFERENCES
- 1. Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real‐world population: the Q‐VTE Study Cohort. Am J Med. 2013;126:832.e13–e21. [DOI] [PubMed] [Google Scholar]
- 2. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5:692–9. [DOI] [PubMed] [Google Scholar]
- 3. Heit JA, Ashrani A, Crusan DJ, McBane RD, Petterson TM, Bailey KR. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost. 2017;117:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delluc A, Tromeur C, Le Ven F, et al. Current incidence of venous thromboembolism and comparison with 1998: a community‐based study in Western France. Thromb Haemost. 2016;116:967–74. [DOI] [PubMed] [Google Scholar]
- 5. van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–75. [DOI] [PubMed] [Google Scholar]
- 6. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. 2010;376:2032–9. [DOI] [PubMed] [Google Scholar]
- 7. Rabinovich A, Kahn SR. The postthrombotic syndrome: current evidence and future challenges. J Thromb Haemost. 2017;15:230–41. [DOI] [PubMed] [Google Scholar]
- 8. Ende‐Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49:pii: 1601792. [DOI] [PubMed] [Google Scholar]
- 9. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–71. [DOI] [PubMed] [Google Scholar]
- 10. Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union‐28: an annualised cost‐of‐illness model for venous thromboembolism. Thromb Haemost. 2016;115:800–8. [DOI] [PubMed] [Google Scholar]
- 11. Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital‐acquired and preventable costs utilising long‐term attack rates. Thromb Haemost. 2012;108:291–302. [DOI] [PubMed] [Google Scholar]
- 12. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 13. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. [DOI] [PubMed] [Google Scholar]
- 14. Heit JA, Crusan DJ, Ashrani AA, Petterson TM, Bailey KR. Effect of a near‐universal hospitalization‐based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood. 2017;130:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e227S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falck‐Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e278S–325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Statistics Netherlands . Statline: height and weight, 1995‐2010. [updated 2017 November 1]. Available from http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=81565NED&D1=0-4&D2=a&D3=0-1,5&D4=0&D5=14-29&HDR=T&STB=G1,G2,G3,G4&VW=T.
- 18. Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. the Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–96. [DOI] [PubMed] [Google Scholar]
- 19. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. [DOI] [PubMed] [Google Scholar]
- 20. Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985‐2009). Am J Med. 2014;127:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: the AB‐VTE population‐based study. Am J Med. 2016;129:879.e19–e25. [DOI] [PubMed] [Google Scholar]
- 22. Arshad N, Isaksen T, Hansen JB, Braekkan SK. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur J Epidemiol. 2017;32:299–305. [DOI] [PubMed] [Google Scholar]
- 23. Kort D, van Rein N, van der Meer FJM, et al. Relationship between neighbourhood socioeconomic status and venous thromboembolism: results from a population‐based study. J Thromb Haemost. 2017;15:2352–60. [DOI] [PubMed] [Google Scholar]
- 24. Scheres LJJ, Kort D, van Rein N, et al. Abstract: Sex‐ specific incidence rates of deep vein thrombosis and pulmonary embolism in The Netherlands. Res Pract Thromb Haemost. 2017;1:39–40. [Google Scholar]
- 25. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14:1480–3. [DOI] [PubMed] [Google Scholar]
- 26. Statistics Netherlands . Statline: clinical surgeries, 1995‐2010[updated 2017 November 1]. Available from http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=80386NED&D1=0-3&D2=0&D3=0&D4=0,19,22&D5;=a&HDR=T&STB=G4,G1,G2,G3&VW=T.
- 27. Statistics Netherlands . Statline: hospital admissions, 1995‐2010 [updated 2017 November 1]. Available from http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=71540ned&D1=a&D2=0&D3=l&D4=a&D5=0,8,45,91&D6=l&D7=0-15&HDR=T,G5,G1,G2,G6&STB=G4,G3&VW=T.
- 28. Statistics Netherlands . Statline: births, 1995‐2010. [updated 2017 November 1]. Available from http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=37422ned&D1=0,4-5,7,9,11,13,17,26,35,40-41,48&D2=45-60&HDR=G1&STB=T&VW=T.
- 29. Statistics Netherlands . Statline: birth control, 1993‐2013. [updated 2017 November 1]. Available from http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=37459&D1=0-9&D2=0&D3=a&HDR=T&STB=G1,G2&VW=T.
- 30. Statistics Netherlands . Statline: sex and age, 1995‐2010. [updated 2017 November 1]. Available from http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=70233ned
- 31. Royal Schiphol Group . Traffic and transport figures. [updated 2017 November 1]. Available from https://www.schiphol.nl/nl/schiphol-group/pagina/verkeer-en-vervoer-cijfers/
- 32. Netherlands Comprehensive Cancer Organisation . Dutch cancer figures [updated 2017 November 1]. Available from http://www.cijfersoverkanker.nl/selecties/dataset_1/img59fb188268062.
- 33. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 34. Global Aging Institute . About global aging. 2017. [updated 2017 November 1]. Available from http://www.globalaginginstitute.org/about-gai/about-global-aging.html
- 35. World Health Organization . Global Health Observatory data: overweight and obesity. 2016. [updated 2017 November 1]. Avaialble from http://www.who.int/gho/ncd/risk_factors/overweight/en/
- 36. The World Data Bank . Air transport, passengers carried. [updated 2017 November 1]. Avaialble from https://data.worldbank.org/indicator/IS.AIR.PSGR
- 37. Weiser TG, Haynes AB, Molina G, et al. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ. 2016;94:201–209f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108:56–60. [DOI] [PubMed] [Google Scholar]
- 39. Jacobsen AF, Skjeldestad FE, Sandset PM. Ante‐ and postnatal risk factors of venous thrombosis: a hospital‐based case‐control study. J Thromb Haemost. 2008;6:905–12. [DOI] [PubMed] [Google Scholar]
- 40. Sultan AA, Tata LJ, West J, et al. Risk factors for first venous thromboembolism around pregnancy: a population‐based cohort study from the United Kingdom. Blood. 2013;121:3953–61. [DOI] [PubMed] [Google Scholar]
- 41. United Nations , Department of Economic and Social Affairs, Division P. World fertility patterns 2015. [Accessed 2017 November 1] Available from http://www.un.org/en/development/desa/population/publications/pdf/fertility/world-fertility-patterns-2015.pdf
- 42. O'Shaughnessy F, Donnelly J, Cooley S, Bennett K, Ní Áinle F, Cleary B. Abstract: VTE Risk Factors in an Irish Urban Obstetric Population. Res Pract Thromb Haemost. 2017;1:196–7. [Google Scholar]
- 43. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;71:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Time trends and case fatality rate of in‐hospital treated pulmonary embolism during 11 years of observation in Northwestern Italy. Thromb Haemost. 2016;115:399–405. [DOI] [PubMed] [Google Scholar]
- 46. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- 47. Galanaud JP, Sevestre MA, Genty C, et al. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost. 2014;12:436–43. [DOI] [PubMed] [Google Scholar]
- 48. Barco S, Corti M, Trinchero A, et al. Survival and recurrent venous thromboembolism in patients with first proximal or isolated distal deep vein thrombosis and no pulmonary embolism. J Thromb Haemost. 2017;15:1436–42. [DOI] [PubMed] [Google Scholar]
- 49. Garry J, Duke A, Labropoulos N. Systematic review of the complications following isolated calf deep vein thrombosis. Br J Surg. 2016;103:789–96. [DOI] [PubMed] [Google Scholar]
- 50. Righini M, Galanaud JP, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double‐blind, placebo‐controlled trial. Lancet Haematol. 2016;3:e556–62. [DOI] [PubMed] [Google Scholar]
- 51. Roberts LN, Durkin M, Arya R. Annotation: developing a national programme for VTE prevention. Br J Haematol. 2017;178:162–70. [DOI] [PubMed] [Google Scholar]
- 52. Roberts LN, Porter G, Barker RD, et al. Comprehensive VTE prevention program incorporating mandatory risk assessment reduces the incidence of hospital‐associated thrombosis. Chest. 2013;144:1276–81. [DOI] [PubMed] [Google Scholar]
- 53. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e195S–226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Croles FN, Nasserinejad K, Duvekot JJ, Kruip MJ, Meijer K, Leebeek FW. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and bayesian meta‐analysis. BMJ. 2017;359:j4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- 56. Forster R, Stewart M. Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair. Cochrane Database Syst Rev 2016;3:CD004179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Adrichem RA, Nemeth B, Algra A, et al. Thromboprophylaxis after knee arthroscopy and lower‐leg casting. N Engl J Med. 2017;376:515–25. [DOI] [PubMed] [Google Scholar]
- 58. Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–73. [DOI] [PubMed] [Google Scholar]
- 59. Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–7. [DOI] [PubMed] [Google Scholar]
- 60. Cannegieter SC, Doggen CJ, van Houwelingen HC, Rosendaal FR. Travel‐related venous thrombosis: results from a large population‐based case control study (MEGA study). PLoS Med. 2006;3:e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. [DOI] [PubMed] [Google Scholar]
- 62. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pabinger I, van Es N, Heinze G, et al. Abstract: development and external validation of a risk assessment model for cancer‐associated venous thromboembolism. Res Pract Thromb Haemost. 2017;1:203–4. [Google Scholar]
- 64. Ho KM, Rao S, Rittenhouse KJ, Rogers FB. Use of the Trauma Embolic Scoring System (TESS) to predict symptomatic deep vein thrombosis and fatal and non‐fatal pulmonary embolism in severely injured patients. Anaesth Intensive Care. 2014;42:709–14. [DOI] [PubMed] [Google Scholar]
- 65. Nemeth B, van Adrichem RA, van Hylckama Vlieg A, et al. venous thrombosis risk after cast immobilization of the lower extremity: derivation and validation of a clinical prediction score, L‐TRiP(cast), in three population‐based case‐control studies. PLoS Med. 2015;12:e1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450–7. [DOI] [PubMed] [Google Scholar]
- 67. Sultan AA, West J, Grainge MJ, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ. 2016;355:i6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Steyerberg EW, Pencina MJ, Lingsma HF, Kattan MW, Vickers AJ, Van Calster B. Assessing the incremental value of diagnostic and prognostic markers: a review and illustration. Eur J Clin Invest. 2012;42:216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blondon M, Spirk D, Kucher N, et al. Abstract: external validation and comparison of the improve risk assessment model with the Geneva Risk Assessment Model in the ESTIMATE Cohort. Res Pract Thromb Haemost. 2017;1:180. [Google Scholar]
- 72. Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. [DOI] [PubMed] [Google Scholar]
- 73. Foundation for Pharmaceutical Statistics . 3,5% vrouwen tussen 45‐60 jaar krijgt hormoontherapie. 2014. [updated 2017 November 1] Available from https://www.sfk.nl/publicaties/PW/2014/3-5-vrouwen-tussen-45-60-jaar-krijgt-hormoontherapie.
- 74. van Oostrom SH, Gijsen R, Stirbu I, et al. Time trends in prevalence of chronic diseases and multimorbidity not only due to aging: data from general practices and health surveys. PLoS ONE. 2016;11:e0160264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. World Health Organization . Global status report on noncommunicable diseases. Geneva: WHO; 2014. [DOI] [PubMed] [Google Scholar]
- 76. Roser M. Human height 2017. [updated 1 November 2017]. Available from https://www.OurWorldInData.org.
- 77. Hille ET, Westendorp RG, Vandenbroucke JP, Rosendaal FR. Mortality and causes of death in families with the factor V Leiden mutation (resistance to activated protein C). Blood. 1997;89:1963–7. [PubMed] [Google Scholar]
- 78. van Mens TE, Levi M, Middeldorp S. Evolution of factor V Leiden. Thromb Haemost. 2013;110:23–30. [DOI] [PubMed] [Google Scholar]
- 79. Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis ‐ current understanding from an epidemiological point of view. Br J Haematol. 2010;149:824–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


