Abstract
Objectives
To examine 2-year safety, efficacy and radiographic outcomes of sarilumab in adults with RA and inadequate response to MTX (MTX-IR).
Methods
In the randomized, placebo-controlled MOBILITY trial, MTX-IR patients received subcutaneous sarilumab (150 or 200 mg) or placebo every 2 weeks (q2w) plus MTX for up to 1 year. Upon study completion, patients could enrol in the open-label, long-term extension study (EXTEND, NCT011046652), in which all patients received sarilumab 200 mg q2w plus MTX. Dose reduction to 150 mg q2w was allowed for abnormal laboratory findings and per investigator’s discretion.
Results
Of 1197 patients participating in MOBILITY, 901 entered EXTEND. Over the 2-year period, treatment-emergent adverse events (TEAEs) and serious AEs occurred at rates of 279.6 events per 100 patient-years and 16.6 events per 100 patient-years, respectively. The most common TEAEs were neutropenia, injection site erythema, increased alanine aminotransferase and upper respiratory tract infections. After 1 year in the open-label, long-term extension, disease activity reached similar levels regardless of initial treatment. Modified total Sharp scores at year 1 were maintained through year 2. Best radiographic outcomes were observed in patients initially randomized to sarilumab 200 mg q2w. After dose reduction, 89.4% of patients continued the study through 2 years.
Conclusion
Sarilumab safety through year 2 was consistent with IL-6 receptor blockade. Clinical response was similar irrespective of initial treatment, and radiographic progression stabilized. Patients initiated on sarilumab 200 mg q2w had the best radiographic outcomes. Dose reduction allowed most patients to continue with the study.
Keywords: sarilumab, rheumatoid arthritis, IL-6, radiographic outcomes, disease activity, physical function
Rheumatology key messages
The 2-year, safety/efficacy profile of sarilumab is consistent with prior findings and IL-6R inhibition.
Sarilumab dose reductions successfully managed laboratory abnormalities while retaining treatment efficacy.
Patients initiated on sarilumab 200 mg q2w had best radiographic and physical function outcomes.
Introduction
RA is a chronic, progressive, autoimmune disease characterized by joint inflammation, pain, morning stiffness and progressive joint destruction [1]. Long-term treatment is required to reduce joint damage progression and conserve health-related quality of life and work productivity [1]. Therapy for RA includes conventional synthetic and biologic DMARDs (csDMARDs and bDMARDs) [2]. Current EULAR treatment recommendations [3] state that MTX should be part of the first-line therapy for RA, while ACR guidelines [4] for RA in DMARD-naïve patients recommend csDMARDs as first-line therapy, with MTX as the preferred initial therapy.
An important mediator of immune-stimulatory processes in RA is IL-6, a proinflammatory cytokine [5]. Blockade of IL-6 signalling is an effective therapeutic approach for the treatment of RA [6]. Sarilumab is a human immunoglobulin G1 (IgG1) mAb that binds specifically to both soluble and membrane-bound IL-6 receptors (sIL-6Rα and mIL-6Rα), and has been shown to inhibit IL-6-mediated signalling through these receptors [7–9]. In the double-blind, placebo-controlled, 52-week MOBILITY study in adult patients with active, moderate-to-severe RA and inadequate response to MTX, s.c. sarilumab administered at doses of 150 or 200 mg every 2 weeks (q2w) plus weekly MTX significantly reduced disease activity, improved physical function and inhibited radiographic progression compared with placebo plus MTX [10]. Over the 52-week study, the most common treatment-emergent adverse events (TEAEs) were infections, injection site reactions, increased transaminases and neutropenia. Neutropenia was not associated with an increased risk of infections or serious infections [10].
Because RA is a chronic disease that requires long-term therapy, this open-label extension (OLE) study was designed to assess long-term safety and efficacy. The current analysis was conducted in patients who completed MOBILITY to examine the safety and durability of response to sarilumab over 2 years and to assess the effect of dose reduction from 200 mg to 150 mg to manage decreased absolute neutrophil count (ANC), decreased platelet count or increased liver enzymes during the OLE.
Methods
Study design
The 52-week, phase III MOBILITY study (NCT01061736) assessed the safety and efficacy of sarilumab (150 or 200 mg q2w s.c.) plus MTX vs placebo plus MTX in patients with active, moderate-to-severe RA and inadequate response to MTX [10]. Starting at week 16, patients who did not achieve ⩾20% improvement from baseline in swollen joint count or tender joint count at two consecutive assessments could receive rescue therapy with open-label sarilumab 200 mg q2w. Long-term outcomes of treatment with sarilumab were examined in an OLE study in patients previously randomized in sarilumab clinical studies (NCT01146652; planned study dates: June 2010 to December 2020). This 2-year analysis focuses exclusively on those patients from MOBILITY who enrolled into the extension study (EXTEND).
After completion of the randomized trial, all patients became eligible for enrolment in the OLE, where they received sarilumab 200 mg q2w plus MTX. Patients could continue receiving the MTX background therapy that they received in the original study, but the dose could be reduced or discontinued, or a patient could be switched to an alternative csDMARD for safety or tolerability reasons. Per protocol, investigators could reduce the dose of sarilumab to 150 mg q2w for ANC ⩾0.5 to <1.0 Giga/L, platelet count ⩾50 to <100 Giga/L or alanine aminotransferase (ALT) 3–5× upper limit of normal or per investigator judgement. Sarilumab was permanently discontinued in cases of opportunistic infections, hypersensitivity or anaphylactic reactions, severe neurological disease, significant laboratory abnormalities, pregnancy, use of other biologics or any adverse event (AE) the investigator deemed would jeopardize patient safety.
The protocols for the randomized phase and OLE were approved by the appropriate ethics committees/institutional review boards, and each patient gave written informed consent. The study was conducted in compliance with institutional review board regulations, the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Patient population and study assessments
Patients were excluded from the OLE if they had AEs or other abnormalities that would affect participation, or if they had any AE leading to permanent study drug discontinuation. As some patients were switching from placebo, safety parameters, including haematological and liver function tests, were assessed q2w up to week 12 and then every 12 weeks; AEs were recorded at each study visit. Safety was captured through week 52 for this analysis. Efficacy was assessed every 4 weeks until week 12 to capture onset of effect of sarilumab in patients who initially received placebo; subsequently, assessments were performed every 12 weeks. Efficacy was captured through week 48 for this analysis. Progression of structural joint disease was monitored using radiographs of hands and feet at entry, at the end of year 1 and at 2 years (year 1 in the OLE).
Study objectives
The primary objective of the OLE was assessment of safety. Parameters assessed included the incidence of TEAEs, serious TEAEs (SAEs), AEs of special interest and changes in specific laboratory tests. AEs were coded using the Medical Dictionary for Regulatory Activities, whereas AEs of special interests were identified using pre-specified criteria. Major adverse cardiovascular (CV) events (MACE) were defined as CV death, myocardial infarction, stroke, hospitalization for unstable angina or hospitalization for transient ischaemic attack. Possible MACE were evaluated by an independent CV adjudication committee. Efficacy measurements included ACR disease activity measures (ACR20/50/70 responses), DAS28-CRP, Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), HAQ-Disability Index (HAQ-DI) and ACR/EULAR Boolean-based remission. Radiographic progression was assessed using the modified Sharp–van der Heijde scoring (SHS) system. Radiographs of patients that were available at baseline, in the first year and in the second year were read centrally by two readers independently, who were blinded to treatment assignment, chronological order of the radiographs and patients’ clinical status.
Statistical analysis
The safety population included all patients who received at least one dose of sarilumab. Response rates were computed using three different denominators: patients who were randomized into the double-blind period [intention-to-treat (ITT) population], patients who enrolled in the OLE and patients who completed 1 year in the OLE (completers, defined as patients who completed efficacy assessments at 48 weeks and those who completed safety assessments at 52 weeks). Analysis of clinical endpoints was based on all available data as observed. Continuous clinical endpoints including DAS28-CRP, CDAI and SDAI were set to missing after treatment discontinuation but not after rescue with open-label sarilumab. In calculations of disease remission rates or ACR response rates, patients were considered non-responders for all time points beyond treatment discontinuation or rescue during the double-blind period (non-responder imputation). Data collected during the OLE were summarized according to the original randomized treatment; no statistical comparisons were made on these data. Linear extrapolation of radiographic data was applied if a radiograph was performed before the scheduled 1- or 2-year assessment.
Results
Patient disposition
In MOBILITY, 1197 adult patients with active, moderate-to-severe RA and an inadequate response to MTX were initially randomized (1:1:1) to receive placebo (n = 398), sarilumab 150 mg (n = 400) or sarilumab 200 mg (n = 399) q2w s.c. added to weekly MTX (ITT population). A total of 901 of these patients enrolled in the extension study and received sarilumab 200 mg q2w (supplementary Fig. S1, available at Rheumatology online) [10]. At baseline, demographic and disease characteristics were similar across the ITT population and across those patients entering the extension study (Table 1).
Table 1.
Summary of patient demographic features and disease activity at study entry, by original randomization group
| MOBILITY | EXTEND (OLE) | |||||
|---|---|---|---|---|---|---|
| Sarilumab | Sarilumab | |||||
| Placebo + MTX (n=398) | 150 mg q2w + MTX (n=400) | 200 mg q2w + MTX (n=399) | Placebo + MTX (n=307) | 150 mg q2w + MTX (n=300) | 200 mg q2w + MTX (n=294) | |
| Female, n (%) | 321 (81) | 319 (80) | 337 (84) | 246 (80) | 241 (80) | 246 (84) |
| Age, mean (s.d.), years | 50.9 (11.2) | 50.1 (11.9) | 50.8 (11.8) | 50.8 (10.7) | 50.3 (11.8) | 50.2 (11.6) |
| Prior biologic DMARD use, n (%) | 86 (22) | 87 (22) | 84 (21) | 71 (23) | 75 (25) | 64 (22) |
| Duration of RA, mean (range), years | 9 (0–44) | 10 (0–45) | 9 (0–34) | 9 (0–44) | 10 (0–45) | 9 (0–34) |
| Seropositive for RF, n (%) | 336 (84) | 345 (87)a | 328 (83)a | 260 (85) | 261 (88)b | 250 (85)b |
| Seropositive for anti-CCP autoantibody, n (%) | 340 (85) | 359 (90)c | 337 (85)c | 264 (86) | 273 (91) | 255 (87)d |
| Tender joint count (0–68), mean (s.d.) | 26.8 (13.7) | 27.2 (14.1) | 26.5 (14.5) | 26.8 (13.6) | 27.4 (14.4) | 26.9 (14.4) |
| Swollen joint count (0–66), mean (s.d.) | 16.7 (9.3) | 16.6 (9.0) | 16.8 (9.7) | 17.1 (9.4) | 16.7 (9.2) | 17.0 (9.5) |
| CRP, mean (s.d.), mg/l | 20.5 (23.0) | 22.5 (23.1) | 22.2 (23.8) | 20.1 (22.1) | 22.8 (24.0) | 21.5 (20.6) |
| DAS28-CRP, mean (s.d.) | 5.9 (0.9) | 6.0 (0.9) | 6.0 (0.9) | 5.9 (0.9) | 6.0 (0.9) | 6.0 (0.9) |
| HAQ-DI, mean (s.d.) | 1.6 (0.7) | 1.6 (0.6) | 1.7 (0.6) | 1.6 (0.7) | 1.6 (0.6) | 1.7 (0.6) |
For RF, n=396 for sarilumab 150 mg and n=397 for sarilumab 200 mg.
For RF, n=298 for sarilumab 150 mg and n=293 for sarilumab 200 mg.
For anti-CCP, n=398 for sarilumab 150 mg and n=397 for sarilumab 200 mg.
n=293. q2w: every 2 weeks.
The OLE population included 77.1% (307/398) of the patients originally randomized to placebo, 75.0% (300/400) of the patients originally randomized to sarilumab 150 mg q2w and 73.7% (294/399) of the patients originally randomized to sarilumab 200 mg q2w. Of the 901 patients who enrolled in the OLE, 776 participants completed year 2 of the study. During the extension study, discontinuation rates were similar across original treatment groups: year 2 completers were 87.0% (267/307) of those patients originally randomized to placebo, 85.0% (255/300) of those originally randomized to sarilumab 150 mg q2w and 86.4% (254/294) of those originally randomized to sarilumab 200 mg q2w (supplementary Fig. S2, available at Rheumatology online).
Safety
During 2 years of treatment with any dose of sarilumab, TEAEs occurred at a rate of 279.6 events per 100 patient-years (PY), and SAEs occurred at a rate of 16.6 events per 100 PY (Table 2). The most frequently reported TEAEs (occurring in ⩾5% of patients) were neutropenia (20.6 events/100 PY), injection site erythema (20.2 events/100 PY), increased ALT (9.6 events/100 PY) and upper respiratory tract infections (9.6 events/100 PY) (Table 2). Treatment discontinuation rates during the OLE were similar among original randomization groups (13.0–15.0% of patients) and occurred because of AEs in 7.8–9.7% of patients (supplementary Table S1, available at Rheumatology online). Serious and opportunistic infections occurred at rates of 4.6 and 1.0 events per 100 PY, respectively (supplementary Table S2, available at Rheumatology online).
Table 2.
TEAEs over entire 2-year period
| Any initial dose (PY = 1810.9), nE (nE/100 PY) | |
|---|---|
| TEAEs | 5064 (279.6) |
| SAEs | 301 (16.6) |
| Deaths | 7 (0.4) |
| Discontinuations due to TEAEs | 266 (14.7) |
| Most frequently reported TEAEs (≥5% in any dose group) by MedDRA Preferred Term | |
| Neutropenia | 373 (20.6) |
| Injection site erythema | 365 (20.2) |
| Upper respiratory tract infection | 174 (9.6) |
| ALT increased | 173 (9.6) |
| Accidental overdosea | 141 (7.8) |
| Urinary tract infection | 119 (6.6) |
| Nasopharyngitis | 107 (5.9) |
| Bronchitis | 99 (5.5) |
| Leucopenia | 97 (5.4) |
| Diarrhoea | 74 (4.1) |
| Hypertension | 71 (3.9) |
| RA | 61 (3.4) |
nE (nE/100 PY) = number of events and number of events per 100 PY. PY for a treatment group is the total duration on sarilumab of the treatment group. aProtocol defined as the administration of ≥2 doses of study drug during an interval <11 days. ALT: alanine aminotransferase; MedDRA: Medical Dictionary for Regulatory Activities; PY: patient-years; SAE: serious treatment–emergent adverse event; TEAE: treatment-emergent adverse event.
Among patients receiving any dose of sarilumab, there was one patient with lower gastrointestinal (GI) perforation among two patients with diverticulitis (GI perforation rate of 0.1 events/100 PY).
A total of 14 malignancies occurred in 13 patients (1.1%; 0.8 events/100 PY) and included basal cell carcinoma (2; 0.2%), breast cancer (1; 0.1%), ductal adenocarcinoma of pancreas (1; 0.1%), invasive lobular breast carcinoma (1; 0.1%), malignant melanoma (3; 0.3%), metastatic breast cancer (1; 0.1%), metastatic bronchial carcinoma (1; 0.1%), neoplasm of the appendix (1; 0.1%), papillary thyroid cancer (1; 0.1%), small intestine carcinoma (1; 0.1%) and squamous cell carcinoma of the skin (1; 0.1%).
The frequency of MACE in patients receiving sarilumab was 0.4 events/100 PY and included stroke (0.2 events/100 PY), CV death (0.1 events/100 PY) and myocardial infarction (0.1 events/100 PY; supplementary Table S2, available at Rheumatology online). Seven deaths occurred within the first year as previously reported [10], with no additional deaths reported during the first year of the extension study.
Laboratory abnormalities
In patients receiving any dose of sarilumab, elevated ALT levels (>3× upper limit of normal) occurred in 135 (11.9%) patients over the 2-year period (supplementary Table S3, available at Rheumatology online). No cases of Hy’s law were observed. ANC ⩽1.0 Giga/L occurred in 118 (10.4%) patients (supplementary Table S3, available at Rheumatology online). Decrease in ANC was not associated with increased risk of infection (supplementary Table S4, available at Rheumatology online).
Efficacy
Clinical efficacy
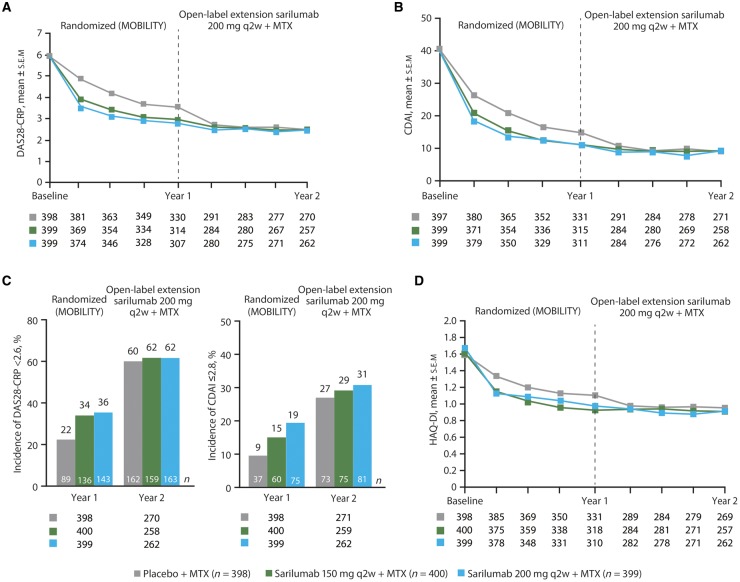
In the completer population, after switch to active treatment in the OLE, patients initially randomized to placebo exhibited similar clinical outcomes to those initially randomized to sarilumab 150 and 200 mg q2w (Fig. 1 and Table 3). In completers, disease activity [mean (s.e.m.)], assessed by DAS28-CRP, reached the same level regardless of initial treatment [2.5 (0.1) for patients initially randomized to placebo or sarilumab 150 mg and 2.4 (0.1) for patients initially randomized to sarilumab 200 mg; Fig. 1A]. A similar pattern was observed for completers with CDAI [mean (s.e.m.): 8.9 (0.5), 8.7 (0.6) and 8.6 (0.6) for patients initially randomized to placebo, sarilumab 150 mg and sarilumab 200 mg, respectively; Fig. 1B]. Percentages of patients achieving DAS28-CRP <2.6 and CDAI ⩽2.8 were similar for patients initially randomized to placebo, sarilumab 150 mg q2w and sarilumab 200 mg q2w when data were analysed for completers (Fig. 1C) or for the ITT population (supplementary Table S5, available at Rheumatology online). Additional assessments of clinical efficacy, including SDAI, incidence of SDAI remission (SDAI ⩽3.3), Boolean-based ACR/EULAR remission (Table 3) and proportion of ACR20/50/70 responses, revealed similar improvements across completers regardless of treatment received during the double-blind period.
Fig. 1.
Clinical efficacy for patients completing 1 year of open-label extension, by original randomization
(A) Mean DAS28-CRP scores. (B) Mean CDAI scores. (C) Proportion of patients achieving DAS28-CRP <2.6 and CDAI remission (CDAI ≤2.8) in the ITT (year 1) and completer (year 2) populations. (D) Mean HAQ-DI scores. Data are for patients who completed the 52-week MOBILITY study and 1 year of the open-label extension (completer population). Patients were tabulated according to their original randomization group in MOBILITY. Numbers below the x-axes represent patients for whom data were available at a particular time point. CDAI: Clinical Disease Activity Index; ITT: intention-to-treat; q2w: every 2 weeks.
Table 3.
Clinical data for patients completing 1 year of open-label extension (completers)
| Placebo + MTX → sarilumab 200 mg q2w + MTX | Sarilumab 150 mg q2w + MTX → sarilumab 200 mg q2w + MTX | Sarilumab 200 mg q2w + MTX | |
|---|---|---|---|
| DAS28-CRP, mean (s.e.m.)a | |||
| Baseline | 5.9 (0.0) | 6.0 (0.0) | 6.0 (0.0) |
| Year 1 | 3.6 (0.1) | 2.9 (0.1) | 2.8 (0.1) |
| Year 2 | 2.5 (0.1) | 2.5 (0.1) | 2.4 (0.1) |
| Incidence of DAS28-CRP <2.6, yes, n/N (%)a | |||
| Year 2 | 162/270 (60.0) | 159/258 (61.6) | 163/262 (62.2) |
| CDAI, mean (s.e.m.)a | |||
| Baseline | 40.6 (0.6) | 40.5 (0.6) | 40.4 (0.6) |
| Year 1 | 14.7 (0.6) | 11.1 (0.6) | 11.0 (0.6) |
| Year 2 | 8.9 (0.5) | 8.7 (0.6) | 8.6 (0.6) |
| Incidence of CDAI remission (CDAI ≤2.8), yes, n/N (%)a | |||
| Year 2 | 73/271 (26.9) | 75/259 (29.0) | 81/262 (30.9) |
| SDAI, mean (s.e.m.) | |||
| Baseline | 42.7 (0.6) | 42.6 (0.7) | 42.7 (0.6) |
| Year 1 | 16.0 (0.7) | 11.7 (0.6) | 11.3 (0.6) |
| Year 2 | 9.2 (0.6) | 9.0 (0.6) | 8.8 (0.6) |
| SDAI remission (SDAI ≤3.3), yes, n/N (%) | |||
| Year 2 | 81/270 (30.0) | 78/258 (30.2) | 85/262 (32.4) |
| Incidence of Boolean-based ACR/EULAR remission, yes, n/N (%) | |||
| Year 2 | 47/272 (17.3) | 52/259 (20.1) | 60/262 (22.9) |
| HAQ-DI score, mean (s.e.m.) | |||
| Baseline | 1.6 (0.03) | 1.6 (0.03) | 1.7 (0.03) |
| Year 1 | 1.1 (0.04) | 0.9 (0.04) | 1.0 (0.04) |
| Year 2 | 1.0 (0.04) | 0.9 (0.04) | 0.9 (0.04) |
Patients were tabulated according to their randomized treatment in MOBILITY. aClinical endpoints were set to missing after early treatment discontinuation but not after rescue with open-label sarilumab. CDAI: Clinical Disease Activity Index; q2w: every 2 weeks; SDAI: Simplified Disease Activity Index.
Improvement in physical function, assessed by HAQ-DI, was similar in completers regardless of initial randomized treatment group [mean (s.e.m.): 0.96 (0.04), 0.91 (0.04) and 0.92 (0.04) for patients initially randomized to placebo, sarilumab 150 mg and sarilumab 200 mg, respectively], although the greatest improvement was seen in patients randomized to sarilumab 200 mg q2w during the double-blind period [mean (s.e.m.) change from baseline in HAQ-DI: –0.66 (0.04), –0.68 (0.04) and –0.77 (0.04) for patients initially randomized to placebo, sarilumab 150 mg and sarilumab 200 mg, respectively; Fig. 1D].
Radiographic outcomes
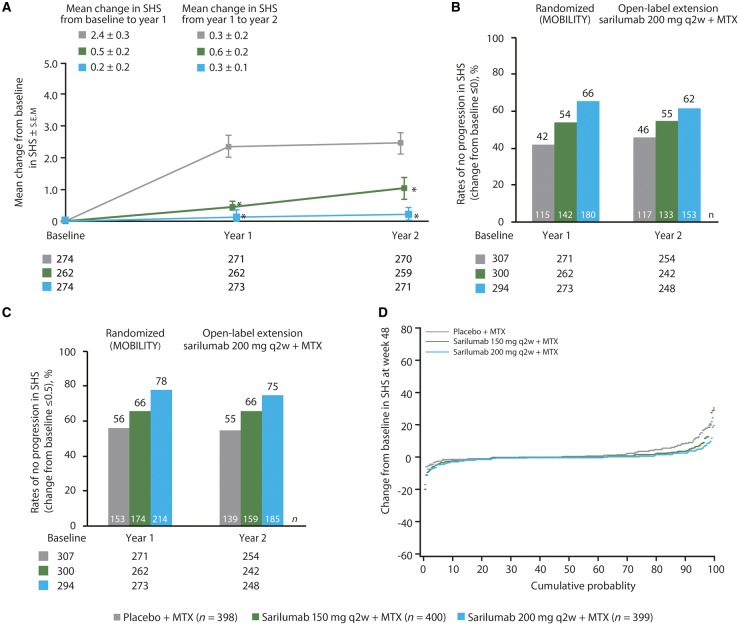
Two-year radiographic data were available for 800 patients. At year 2, missing or post-rescue data were imputed by a linear extrapolation approach for 56 patients (supplementary Fig. S3, available at Rheumatology online). After 2 years of follow-up, completers initiated on sarilumab 200 mg q2w had the most favourable radiographic outcomes (Fig. 2A). Completers initially randomized to the placebo group showed markedly reduced progression of mean SHS once moved to active treatment [increase of 0.27 (0.16) between the end of the randomized study and 1 year in the OLE]. In completers already receiving active treatment, SHS remained stable between the end of the randomized study and 1 year in the OLE [increase of 0.56 (0.22) and 0.25 (0.12) in the initial sarilumab 150 and 200 mg q2w groups, respectively; Fig. 2A and Table 4). Of the patients initially randomized to sarilumab 200 mg q2w plus MTX in the double-blind period, 62% had no radiographic progression after 1 year in the OLE (i.e. change from baseline upon enrolment into the randomized study in SHS of ⩽0 to end of year 1 of OLE) compared with 55% of patients initially randomized to sarilumab 150 mg q2w plus MTX and 46% of patients initially randomized to placebo plus MTX (Fig. 2B). A similar pattern was observed when the cut-off used to define no radiographic progression was change from baseline in SHS ⩽0.5 (Fig. 2C). Overall, patients initially randomized to sarilumab 200 mg q2w plus MTX in the double-blind period had a smaller probability of radiographic progression at the end of year 2 than patients initially randomized to sarilumab 150 mg q2w plus MTX or placebo plus MTX (Fig. 2D).
Fig. 2.
Radiographic progression in patients, by original randomization
(A) Mean change from baseline in SHS (≤0). *Nominal P<0.01 vs placebo plus MTX. (B) Percentage of patients with no progression (change from baseline ≤0) of structural damage at year 2 for the OLE population. (C) Percentage of patients with no progression (change from baseline ≤0.5) of structural damage at year 2 for the OLE population. (D) Cumulative probability distribution plot for change in SHS at the end of year 2. OLE: open-label extension; q2w: every 2 weeks; SHS: modified Sharp–van der Heijde score.
Table 4.
Radiographic data at baseline, years 1 and 2
| Placebo + MTX → sarilumab 200 mg q2w + MTX | Sarilumab 150 mg q2w + MTX → sarilumab 200 mg q2w + MTX | Sarilumab 200 mg q2w + MTX | |
|---|---|---|---|
| Randomized population | 398 | 400 | 399 |
| Completers with X-ray/OLE population, n/N (%)a | 270/307 (87.9) | 259/300 (86.3) | 271/294 (92.2) |
| SHS score, mean (s.e.m.) | |||
| Baseline | 45.8 (3.8) | 49.2 (3.6) | 43.2 (3.4) |
| Year 1 | 48.4 (3.9) | 49.6 (3.6) | 43.1 (3.4) |
| Year 2 | 48.3 (3.9) | 50.5 (3.7) | 43.3 (3.4) |
| Erosion score, mean (s.e.m.) | |||
| Baseline | 22.4 (2.0) | 22.2 (1.8) | 19.7 (1.7) |
| Year 1 | 23.6 (2.0) | 22.5 (1.8) | 19.6 (1.7) |
| Year 2 | 23.6 (2.0) | 22.9 (1.8) | 19.7 (1.7) |
| Joint space narrowing score, mean (s.e.m.) | |||
| Baseline | 23.4 (1.9) | 26.9 (2.0) | 23.5 (1.8) |
| Year 1 | 24.8 (2.0) | 27.2 (2.0) | 23.5 (1.8) |
| Year 2 | 24.7 (2.0) | 27.6 (2.0) | 23.6 (1.8) |
| SHS change from baseline, mean (s.e.m.) | |||
| Δ Baseline – year 1 | 2.4 (0.3) | 0.5 (0.2) | 0.2 (0.2) |
| Δ Baseline – year 2 | 2.5 (0.3) | 1.1 (0.3) | 0.2 (0.2) |
| Δ Year 1 – year 2 | 0.3 (0.2) | 0.6 (0.2) | 0.3 (0.1) |
Patients were tabulated according to their randomized treatment in MOBILITY. OLE: open-label extension; q2w: every 2 weeks; SHS: modified Sharp–van der Heijde score.
Dose reduction
Dose reduction from sarilumab 200 mg q2w to 150 mg q2w occurred in 14.4% of patients (n=123). Consistent with the protocol recommendations, the most common reasons for dose reduction were decreased ANC (9.1%; n=78) and increased ALT levels (3.6%; n=31) (supplementary Table S6, available at Rheumatology online). Dose reduction for a decrease in platelet counts (0.7%; n=6) or for other AEs (0.8%; n=7) occurred infrequently (supplementary Table S6, available at Rheumatology online). Reducing the dose of sarilumab from 200 mg q2w to 150 mg q2w allowed most of these patients (n=110; 89.4%) to continue the study through 2 years.
Laboratory abnormalities began to show improvement within 1 month of dose reduction and continued through 6 months after dose reduction (supplementary Table S7, available at Rheumatology online). Among those patients who had a dose reduction because of decreased ANC, the proportion of patients with ANC ⩾0.5 to <1.0 Giga/L decreased from 55.1% before dose reduction to 14.3 and 6.8% at 1 and 6 months after dose reduction, respectively. Efficacy of sarilumab was maintained as assessed by improvements in DAS28-CRP (–3.15 mean change from baseline before and –3.31 at 24 weeks after dose reduction), CDAI (–29.21 mean change from baseline before and –31.34 at 24 weeks after dose reduction) and HAQ-DI scores (–0.64 mean change from baseline before and –0.68 at 24 weeks after dose reduction).
Discussion
Upon completion of the randomized, placebo-controlled trial, patients could enrol in the long-term extension study in which all patients received sarilumab 200 mg q2w plus MTX. As RA is a chronic disease requiring long-term treatment, the primary objective of the OLE was to examine the long-term safety of sarilumab. In this analysis of treatment with sarilumab for 2 years, the safety profile was consistent with the anticipated effects of IL-6R inhibition, and no new safety concerns were identified. Discontinuation rates were similar across original randomization groups. The most common non-laboratory TEAEs were injection site erythema, upper respiratory tract infection and urinary tract infection. Malignancies, MACE and lower GI perforations were infrequent. The most common laboratory abnormalities were neutropenia and elevations in ALT.
These 2-year observations are consistent with the sarilumab safety profile observed in other sarilumab clinical trials of shorter duration [10–12] and are consistent with previously published studies of other IL-6R inhibitors [13], including the long-term safety for tocilizumab when administered intravenously [14] or subcutaneously [15]. The 2-year attrition was comparable to that observed at year 2 in a long-term extension study for tocilizumab [14]. In addition, decreased ANC was not associated with increased rates of infections, which is in alignment with observations from previous studies with sarilumab [10, 12] and an analysis of pooled data from phases III to IV studies of tocilizumab [16].
Extension studies have shown that the clinical benefits achieved with active therapy with biologic and targeted synthetic DMARDs can be sustained in the long term [14, 15, 17, 18]. Durable clinical responses were maintained with sarilumab 200 mg q2w, even with dose reduction to 150 mg q2w to manage changes in laboratory parameters. Disease activity and radiographic progression were reduced with sarilumab, after initiation in the randomized study or after switch to sarilumab 200 mg q2w in the OLE. Across the initial randomization groups, DAS28-CRP and CDAI scores reached the same levels, and percentages of patients achieving CDAI remission and DAS28-CRP <2.6 were similar.
One of the most important treatment goals for RA is inhibition of joint damage progression and conservation of health-related quality of life [1]. Active treatment with sarilumab reduced structural progression of joint damage in all patient groups, but the best radiographic outcomes were seen in patients initially randomized to sarilumab 200 mg q2w plus MTX during the double-blind period. Improvements in radiographic outcomes were greater in patients initially randomized to sarilumab 200 mg q2w than in patients who increased their dose from 150 to 200 mg q2w. A prior post hoc analysis of MOBILITY indicated that reduction in radiographic progression at 1 year was generally associated with overall greater improvement in physical function [19]. In this analysis, sarilumab improved physical function in all patient groups, as assessed by HAQ-DI. These data reinforce the importance of early interventions for RA.
Laboratory abnormalities (e.g. decreased ANC and increased ALT levels) were successfully managed by reducing the dose of sarilumab from 200 mg q2w to 150 mg q2w, allowing most of these patients to remain on study drug while maintaining efficacy. Clinical and functional efficacy over 2 years was similar between patients who experienced dose reductions and those who did not.
A limitation of OLE studies is that they include patients who perform well and exclude those who discontinue for any reason, including lack or loss of efficacy or AEs. As such, evaluating long-term efficacy using continuous variables (including SHS) can be influenced by progressively smaller numbers of patients remaining on study, and a non-responder imputation method may not adequately address this concern. Follow-up was relatively short at the time of this analysis. However, this OLE study is ongoing, and future analyses will provide further insights into durability of response to sarilumab, including 5-year X-ray data.
In conclusion, 2-year treatment of active, moderate-to-severe RA with sarilumab, along with dose reduction in the event of laboratory abnormalities, resulted in durable efficacy outcomes and a safety profile consistent with previous reports and IL-6R blockade.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contribution of all study investigators, and Deborah Dukovic and Shyamalie Jayawardena, Sanofi Genzyme, for additional statistical support. Editorial assistance was provided under the direction of the authors by Dusica Curanovic, PhD, Todd Parker, PhD and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Funding: This study was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Disclosure statement: M.C.G. has received research grants and/or consulting fees from Roche, Sanofi, GlaxoSmithKline, R-Pharm and Bird Rock Bio. J.vA. was an employee of Regeneron Pharmaceuticals, Inc., at the time of analysis and may hold stock and/or stock options in the company. C.F. and H.vH. are employees of Sanofi Genzyme and may hold stock and/or stock options in the company. N.M.H.G., J.P. and E.K.M. are employees of Regeneron Pharmaceuticals, Inc., and may hold stock and/or stock options in the company. T.W.J.H. has received consulting fees from Sanofi. D.vdH. has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Roche, Sanofi, Takeda and UCB, and is the director of Imaging Rheumatology. The other author has declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Scott DL, Wolfe F, Huizinga TW.. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- 2. Upchurch KS, Kay J.. Evolution of treatment for rheumatoid arthritis. Rheumatology 2012;51(Suppl 6):vi28–36. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewe R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 4. Singh JA, Saag KG, Bridges SL. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 5. Srirangan S, Choy EH.. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis 2010;2:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genentech, Inc. Actemra [package insert]. South San Francisco, CA, USA, 2016.
- 7. Rafique A, Martin J, Blome M. et al. Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human IL-6 receptor (IL-6r) alpha. Ann Rheum Dis 2013;72(Suppl 3):A797. [Google Scholar]
- 8. Wang L-H, Xue Y, Liu X. et al. FRI0020 Preclinical development of sarilumab, the first fully human monoclonal antibody (MAB) against IL-6r alpha: utilization and value of double humanized animal model. Ann Rheum Dis 2013;72(Suppl 3):A375. [Google Scholar]
- 9. Zhang L, Luan B, Adler A. et al. Sarilumab (REGN88), a fully-human anti-IL6R antibody, inhibits tumor growth in preclinical models, as a single agent and in combination with the VEGF blocker aflibercept. Cancer Res 2012;72(8 Suppl):2723. [Google Scholar]
- 10. Genovese MC, Fleischmann R, Kivitz AJ. et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 11. Emery P, Rondon J, Garg A. et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with RA. Arthritis Rheumatol 2015;67(Suppl 10): http://acrabstracts.org/abstract/safety-and-tolerability-of-subcutaneous-sarilumab-compared-to-intravenous-tocilizumab-in-patients-with-ra/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleischmann R, van Adelsberg J, Lin Y. et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol 2017;69:277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolen JS, Beaulieu A, Rubbert-Roth A. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 14. Nishimoto N, Miyasaka N, Yamamoto K. et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009;68:1580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kivitz A, Wallace T, Olech E. et al. Long-term safety and efficacy of subcutaneously administered tocilizumab for adult rheumatoid arthritis: a multicenter phase 3b long-term extension study. Rheumatol Ther 2016;3:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moots RJ, Sebba A, Rigby W. et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology 2017;56:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinblatt ME, Keystone EC, Furst DE. et al. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended study. Ann Rheum Dis 2006;65:753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wollenhaupt J, Silverfield J, Lee EB. et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 19. Genovese MC, van Hoogstraten H, Kampman W, Jayawardena S, Huizinga TWJ.. Association between clinical and radiographic responses, and physical function in a phase 3 study of sarilumab plus methotrexate in patients with active, moderate-to-severe rheumatoid arthritis. Ann Rheum Dis 2017;76(Suppl 2):576–7.27489225 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.