Abstract
Patient: Female, 47
Final Diagnosis: Antiphospholipid antibody syndrome
Symptoms: Liver failure • pneumonia • renal failure
Medication: —
Clinical Procedure: Live donor liver transplantation
Specialty: Transplantology
Objective:
Rare co-existance of disease or pathology
Background:
Acute-on-chronic liver failure was first defined within the last 10 years as acute decompensation of chronic liver disease accompanied by multiorgan failure and poor outcome. Budd-Chiari syndrome is a rare and potentially deadly hepatic condition. To the best of our knowledge, this is the first case report of a live liver donor recipient with antiphospholipid antibody syndrome.
Case Report:
A 47-year-old woman from Sudan with acute-on-chronic liver failure and subacute Budd-Chiari syndrome triggered by active pneumonia was evacuated to Amman, Jordan. In Amman, she was transferred to our hospital for liver transplant evaluation.
She presented with progressive liver failure, acute kidney failure, acute respiratory failure, and encephalopathy stage IV. Multidisciplinary therapy was initiated with IV anti-infective drugs and optimizing mechanical ventilation.
Clinically, we stopped her progressive deterioration after 48 h and she improved slightly in our ICU. Accelerated work-up for donors and recipient was completed and her daughter was selected as a medically appropriate donor despite the fact that she was found to have heterozygote factor V Leiden mutation and antiphospholipid antibody syndrome, similar to her mother.
A lifesaving live-donor liver transplantation was carried out after 72 h. Donor and recipient were discharged in good condition with normal liver function and both were discharged on anticoagulant Rivaroxaban 20 mg.
Conclusions:
We present the first case of a patient with acute-on-chronic liver failure with subacute Budd-Chiari syndrome, which was triggered by bacterial pneumonia and was successfully treated by live-donor liver transplantation from a donor with antiphospholipid antibody syndrome.
MeSH Keywords: Budd-Chiari Syndrome, Liver Failure, Acute, Liver Transplantation, Pneumonia, Bacterial
Background
Budd-Chiari syndrome (BCS) is an uncommon and potentially deadly hepatic disorder caused by obstruction of hepatic venous outflow; retrohepatic vena cava is occasionally involved [1]. The prevalence of BCS is unclear but has been estimated globally as 1/100 000 [2], with a higher prevalence in Asia and Africa [3]. BCS is more common in females and is more likely to occur in the third or fourth decade of life [4].
Antiphospholipid antibody syndrome (APS), primary myeloproliferative disorders (MPD), and factor V Leiden mutation (FVLM) are the most common etiological underlying disorders of BCS [4,5].
Acute-on-chronic liver failure (ACLF) is a newly described syndrome of acute decompensation of chronic liver disease accompanied by multiorgan failure and poor outcome. The Asian Pacific Association published the first consensus statement regarding ACLF for the Study of the Liver (APASL) in 2009 [6]. The classification of ACLF in different stages is carried out according to the number of failed organs. Thus, ACLF is graded into 3 stages: ACLF-1=single renal or non-renal organ failure if accompanied by renal and/or cerebral impairment; ACLF-2=2 organ failures; and ACLF-3=3–6 organ malfunctions [7].
ACLF is not rare and can affect up to 40% of patients admitted to the ICU for acute cirrhosis deterioration. The most common triggering factors in ACLF are bacterial infections and alcoholism [8].
The pathophysiology of ACLF is unclear, but the occurrence of an extreme inflammatory reaction seems to be responsible [9].
While there are no generally recognized listing criteria for patients with ACLF grade 3, these patients are frequently not allowed to participate in a fair liver allocation.
Liver allocation for patients with acute-on-chronic liver failure grade 3 with 3 or more organ failures from deceased donors is still debatable because of the anticipated inferior post-transplant outcome and the organ shortage. Patients presenting with active pneumonia and ACLF are usually not considered for liver transplantation as it is uncertain if these patients would recover from pneumonia while under immunosuppression [10–13].
In 2011, Goralczyk et al. proposed that patients with ACLF grade 3 generated by active pneumonia improved under goal-directed therapy and then underwent liver transplantation shortly thereafter, only to recover quickly from infection. They concluded that liver transplantation in ACLF 3 patients with pneumonia is a potentially curable treatment that should not be unwisely denied [14].
A recent study by Artu et al. showed significantly different outcomes of liver transplantation in patients with acute-on-chronic liver failure grade 3 compared with a non-transplanted patient group. The study shows that liver transplantation can be safe in patients with ACLF grade 3 if the patient’s situation matches the objectives of the “transplantation window”. Patients were transplanted after prompt treatment in the ICU and a rapid decision by the Interdisciplinary Transplant Board, if they somehow responded to medical therapy and stabilized [15].
Liver transplantation for Budd-Chiari syndrome with ACLF is still a challenging surgical issue and is very rare. LDLT can provide grafts appropriate within the so-called “transplantation window”.
Case Report
A 47-year-old woman from Sudan, who presented with rapidly progressive liver failure, acute kidney failure, acute respiratory failure, and encephalopathy stage IV, was managed conservatively with intravenous fluids, IV antibiotics, and mechanical ventilation, but was progressing rapidly to severe organ deterioration.
She was evacuated by rescue airplane to a hospital nearby and progressed even more. After our consultation, she was transferred to our hospital for further evaluation of possible LDLT. Further information regarding liver function of the patient prior to deterioration was not available.
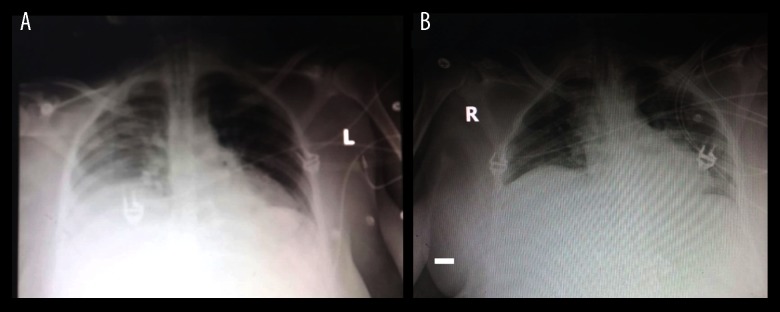
She received 3 anti-infective drugs in adjusted renal dose because of her acute renal failure: Imipenem/Cilastatin, Colistin, and ECALTA, according to microbiological findings in her blood and sputum showing Acinetobacter baumannii, Candida albicans, and Enterococcus faecalis. Subsequently, she improved clinically under this goal-directed therapy at our ICU. Oxygenation (PaO2/FIO2) improved and FIO2 needs on ventilator decreased from 100% to 50% within 3 days (Figure 1A, 1B).
Figure 1.
(A) Chest X-ray immediately after admission at our ICU showed right-sided pneumonia. (B) Chest X-ray improvement days after goal-directed therapy before LDLT.
Urine output increased markedly and serum creatinine dropped from 3.7 to 2.7 mg/dl. White blood cell count dropped from 37×109 to 18.0×109 (Table 1). In contrast, total bilirubin increased to 48 mg/dl and the hepatic encephalopathy did not improve, even without sedation for 10 days. A brain CT scan ruled out brain injuries such as hemorrhage, and chest and abdomen angio CT scanning was performed for precise assessment of the BCS severity, including possible vena cava involvement and cava replacement. The retrohepatic vena cava appeared severally narrowed and the main hepatic veins were occluded. The CT scan revealed a huge amount of ascites with bilateral pleural effusion.
Table 1.
Laboratory results on admission, just before the transplant, and at last follow-up visit.
| One week before LDLT | One day before LDLT | Last follow-up visit after LDLT | |
|---|---|---|---|
| AST (10/l) | 115 | 180 | 29 |
| ALT (10/l) | 100 | 80 | 15 |
| Total bilirubin (mg/dl) | 46 | 48 | 0.8 |
| Creatinine (mg/dl) | 3,7 | 2,7 | 0.6 |
| Leukocytes (109/l) | 37 | 18 | 6.9 |
| FI02 needs | 100% | 50% | No need |
Expedited work-up was completed and the psychiatric team and ethics committee approved liver donation. The liver transplant board accepted the indication for liver transplant and approved the living donor liver transplantation (LDLT).
The donor was selected as appropriate despite the fact that she was found to have an antiphospholipid antibody syndrome (APS) and heterozygote Factor V Leiden mutation, exactly matching her mother’s findings. The antiphospholipid antibodies were elevated in both of them.
Donor total liver volume was 1500 cm3 and future remnant liver volume was 700 cm3. The recipient weight was 100 Kg before her deterioration; the estimated graft-to-recipient weight ratio (GRWR) was at least 0.8%. Surgery was then carried out after 72 h. The donated graft had 1 right hepatic vein, 1 right artery, 1 right bile duct, and 1 right PV-Branch.
Intraoperatively, the inferior vena cava appeared narrowed due to liver compression, but after removing the liver, the cava lumen sufficiently expanded. In spite of this, we conducted posterior cavoplasty for enlargement of the hepatic venous outflow tract of the right lobe according to a technique published previously [16].
Postoperatively, donor and recipient immediately received heparin IV targeting therapeutic partial thromboplastin time (60 s). Doppler ultrasound examination for donor and recipient was routinely performed daily during the first postoperative week. With regard to the portal vein and hepatic artery, the Doppler ultrasound showed normal postoperative findings.
In the post-transplant course of both recipient and donor, the International Normalized Ratio (INR) level was the following:
Recipient INR was 2.5 on (post-op day) POD1, 2 on POD3, 1.6 on POD6, and 1.2 on POD10. Donor INR on POD1 was 2.2, 1.9 on POD3, 1.4 on POD6, and 1 on POD 10 (Figure 2).
Figure 2.
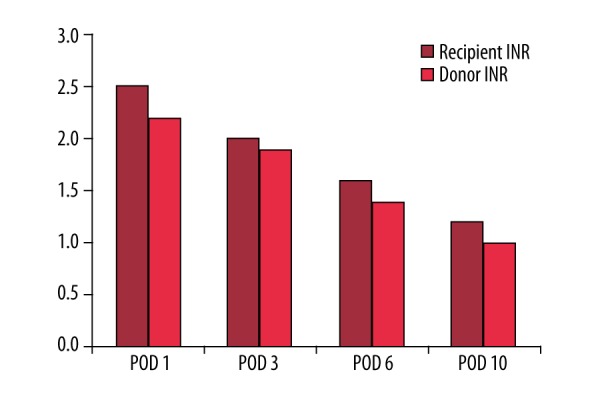
Improvement of recipient’s and donor’s INR after LDLT.
Postoperatively, the anti-infective therapy with Imipenem/Cilastatin. Colistin and ECALTA were continued for 10 days and decreased over 1 week and was always adjusted to the improving renal function.
Donor ambulation and oral feeding started at postoperative day 1. The abdominal drain was removed on postoperative day 3. The recipient was discharged 10 days after surgery in good condition and with normal liver function.
The pathology report revealed Budd-Chiari syndrome with severe acute centrolobular hepatocyte necrosis and thrombosed major portal vein branches intrahepatically. Cirrhosis was not seen.
After the LDLT, the recipient was kept intubated for 3 days and continued on the same antibiotics/antifungal therapy for an additional 10 days. Tacrolimus was delayed for 5 days to allow fast kidney recovery.
She remained 7 days in the ICU and was discharged after 23 days in good condition with normal renal and respiratory function. Total bilirubin decreased to 1.8 mg/dl and liver enzymes returned to normal levels. Both donor and recipient were discharged on Rivaroxaban 20 mg for at least 6 months (Xarelto®; Bayer AG, Germany).
Discussion
Budd-Chiari syndrome is a rare syndrome with an incidence of 0.1 to 10 per million people a year, presenting blocked venous outflow from the liver, mostly at the level of hepatic veins and inferior vena cava [1,17]. The leading etiological factors are hypercoagulable disorders and myeloproliferative diseases.
Without treatment, 90% of patients would die within 3 years, mostly due to complications of liver cirrhosis. BCS is usually classified according to etiology (primary or secondary) and clinical course (acute, chronic, acute, or chronic lesion) [2,3,18].
BCS is recognized by establishing blockade of the venous out-flow and imaging variations of the liver and the portal venous system. Laboratory tests are essential to complete work-up and are crucial in identifying coagulation and hematological disorders that can be identified in up to 75% of patients with BCS [19].
In general, there are many factors related to an inferior prognosis in patients receiving treatment: refractory ascites, old diagnosis, and liver failure at the time of presentation.
Liver transplant cures not only liver disease, but also protein S, C, or AT3 deficiency.
The antiphospholipid antibody syndrome is an autoimmune disorder presenting a broad variety of clinical descriptions, but mainly is recognized as thrombosis-associated adverse events. APS is associated with the existence of antiphospholipid antibodies, incorporating the supposed lupus anticoagulant [20].
The 1-year survival rate in non-transplanted patients with ACLF-3 is about 20% and BCS alone has a 90% 3-year mortality rate in the chronic phase. Recommendations suggest liver transplantation as a last option for salvage treatment if medical and endovascular treatment failed to restore patency of veins [9].
Acute-on-chronic liver failure is a newly described syndrome of acute decompensation of chronic liver disease, accompanied by multiorgan failure and poor outcome. ACLF is not uncommon and can affect up to 40% of patients admitted to the ICU for acute cirrhosis deterioration. The most common triggering factors in ACLF are bacterial infections and alcoholism.
Acute-on-chronic liver failure is the deadliest complication of liver cirrhosis; it can affect inpatients as well as outpatients with cirrhosis [21]. Severe acute alcoholic hepatitis (AAH) can also lead to the same clinical picture as ACLF, with a 1-year mortality rate over 70%. Bacterial and fungal infections are very common in ACLF and are linked to severe systemic inflammation and high mortality. Most patients with ACLF rapidly develop infections [22,23].
Liver allocation from deceased donors is still debatable for patients with acute-on-chronic liver failure grade 3 and with 3 organ failures or more, because of the anticipated inferior post-transplant outcome and organ shortage. Patients presenting with active pneumonia and ACLF are usually not considered for liver transplantation, since it is uncertain if these patients would recover from pneumonia while under immunosuppression. While there are no generally recognized listing criteria for patients with ACLF grade 3 and these patients are frequently not allowed to participate in a fair and potentially lifesaving liver allocation.
Selva et al. published a 2017 retrospective study with patients who fulfilled the criteria of ACLF and underwent liver transplantation with a 100% 1-year survival rate [24].
Recently, further studies with similar patient groups have shown that patients with ACLF who subsequently underwent liver transplantation had a 100% 1-year survival rate. This promising result suggests that high-urgency allocation of liver transplantation should be considered for ACLF patients [25,26].
Artru et al. found that liver transplant significantly affects the survival of patients with ACLF-3, with a 1-year survival similar to that of patients with a lower grade of ACLF. Furthermore, they showed a significant outcome of liver transplantation in patients with acute-on-chronic liver failure grade 3 compared with a non-transplanted patients group.
They recommended a fast administrative process due to the short supposed “transplantation window”, proposing that patients with ACLF-3 must be promptly referred to a specific liver ICU. They concluded that liver transplantation increases the survival of patients with ACLF [15].
In 2011, Goralczyk et al. published an important small observational study, which demonstrated excellent survival rates in patients with ACLF grade 3 triggered by active pneumonia, who improved under the so-called “goal-directed therapy” and then underwent liver transplantation shortly thereafter. They concluded that liver transplantation in ACLF 3 is an essential treatment that should not be unwisely denied [14].
Based on the 2011 publication by Goralczyk et al., we proceeded with transplantation after making sure that the goal-directed therapy showed a clear improvement of the recipient’s sepsis. If this obvious improvement of the patient’s clinical condition had not taken place, we would never have proceeded with transplantation. Soon after ensuring that sepsis was controlled by our goal-directed therapy, liver transplantation was carried out.
Regarding the donor, we focussed on reducing/preventing the risk of thromboembolic events. Thus, an extensive evaluation was performed, which revealed acceptable risk. In addition, we started immediate postoperative heparinization intravenously, which was replaced by Xarelto after a few days and was continued for approximately 6 months. Thereby, the risk was minimized by an aggressive preventative therapy.
In patients with ACLF-3, we support very short selection processes in order to reduce the risks of complications and further deterioration of multiple organs, which cause potentially preventable deaths, and reducing the probabilities of the patients being appropriately transplanted.
LDLT is capable of providing ACLF-3 patients with “just in time” live-saving grafts within the proposed transplantation window. Living donors with antiphospholipid antibody syndrome should be treated with extreme caution because they are likely to develop thromboembolic events. Indeed, some transplant centers regard such donor candidates as contraindicated.
We suggest that donors with antiphospholipid antibody syndrome without clinical manifestation should not be excluded from further evaluation. If extended evaluation and liver biopsy do not show any abnormal results and potential donors are in good health, they may be considered for donation. We do not recommend a general change in donor selection strategy, but rather suggest decision-making on a case-by-case basis, especially under life-threating conditions as seen with our recipient. For the safety of donors, anticoagulation after live donation for the period of 3–6 months appears to be essential.
Conclusions
In summary, we report the first case of a patient with acute-on-chronic liver failure with subacute BCS, triggered by bacterial pneumonia, who was successfully treated by LDLT from a donor with antiphospholipid antibody syndrome. In case of +APS donors, live donor liver transplantation is safe and feasible for donor and recipient and, above all, is life-saving for the recipient.
Abbreviations:
- BCS
Budd-Chiari syndrome;
- APS
antiphospholipid antibody syndrome;
- MPD
primary myeloproliferative disorders;
- FVLM
factor V leiden mutation;
- ACLF
acute-on-chronic liver failure;
- LDLT
living donor liver transplantation;
- GRWR
graft-to-recipient weight ratio
Footnotes
Conflict of interests
None.
References:
- 1.Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50(1):195–203. doi: 10.1016/j.jhep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Valla DC. The diagnosis and management of the Budd-Chiari syndrome: Consensus and controversies. Hepatology. 2003;38(4):793–803. doi: 10.1053/jhep.2003.50415. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZG, Jones RS. Budd-Chiari syndrome. Curr Probl Surg. 1996;33(2):83–211. doi: 10.1016/s0011-3840(96)80001-3. [DOI] [PubMed] [Google Scholar]
- 4.Valla DC. Hepatic vein thrombosis (Budd-Chiari syndrome) Semin Liver Dis. 2002;22(1):5–14. doi: 10.1055/s-2002-23202. [DOI] [PubMed] [Google Scholar]
- 5.Janssen HL, Garcia-Pagan JC, Elias E, et al. Budd-Chiari syndrome: A review by an expert panel. J Hepatol. 2003;38(3):364–71. doi: 10.1016/s0168-8278(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 6.Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol. 2012;57(6):1336–48. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo V, Jalan R. Acute-on-chronic liver failure: Definition, diagnosis, and clinical characteristics. Semin Liver Dis. 2016;36(2):109–16. doi: 10.1055/s-0036-1583202. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–82. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arroyo V, Moreau R, Jalan R, Ginès P, EASL-CLIF Consortium CANONIC Study Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62(1 Suppl.):S131–43. doi: 10.1016/j.jhep.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Bahirwani R, Shaked O, Bewtra M, et al. Acute-on-chronic liver failure before liver transplantation: Impact on posttransplant outcomes. Transplantation. 2011;92:952–57. doi: 10.1097/TP.0b013e31822e6eda. [DOI] [PubMed] [Google Scholar]
- 11.Duan BW, Lu SC, Wang ML, et al. Liver transplantation in acute-on-chronic liver failure patients with high model for end-stage liver disease (MELD) scores: A single center experience of 100 consecutive cases. J Surg Res. 2013;183:936–43. doi: 10.1016/j.jss.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Finkenstedt A, Nachbaur K, Zoller H, et al. Acute-on-chronic liver failure: Excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879–86. doi: 10.1002/lt.23678. [DOI] [PubMed] [Google Scholar]
- 13.Zheng MH, Shi KQ, Fan YC, et al. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol. 2011;9(4):351–56.e3. doi: 10.1016/j.cgh.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Goralczyk AD, Abu-Ajaj W, Tsui TY, et al. Liver transplantation in patients with liver cirrhosis and active pneumonia: an observational study. Transpl Int. 2011;24(11):1068–74. doi: 10.1111/j.1432-2277.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- 15.Artru F, Louvet A, Ruiz I, et al. J Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. Hepatol. 2017;67(4):708–15. doi: 10.1016/j.jhep.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 16/.Goralczyk AD, Obed A, Beham A, et al. Posterior cavoplasty: A new approach to avoid venous outflow obstruction and symptoms for small-for-size syndrome in right lobe living donor liver transplantation. Langenbecks Arch Surg. 2011;396(3):389–95. doi: 10.1007/s00423-010-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa G, Font J, García-Pagan JC, et al. Budd-Chiari syndrome secondary to antiphospholipid syndrome: Clinical and immunologic characteristics of 43 patients. Medicine. 2001;80(6):345–54. doi: 10.1097/00005792-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 18.DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49(5):1729–64. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajesh S, Mukund A, Arora A. Imaging diagnosis of splanchnic venous thrombosis. Gastroenterol Res Pract. 2015;2015:101029. doi: 10.1155/2015/101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denninger MH, Chaït Y, Casadevall N, et al. Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology. 2000;31:587–91. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- 21.Piano S, Tonon M, Vettore E, et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017;67(6):1177–84. doi: 10.1016/j.jhep.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Testino G, Leone S. Acute alcoholic hepatitis: A literature review and proposal of treatment. Minerva Med. 2017 doi: 10.23736/S0026-4806.17.05431-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut. :2017. doi: 10.1136/gutjnl-2017-314240. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Selva Rajoo A, Lim SG, Phyo WW, et al. Acute-on-chronic liver failure in a multi-ethnic Asian city: A comparison of patients identified by Asia-Pacific Association for the Study of the Liver and European Association for the Study of the Liver definitions. World J Hepatol. 2017;9(28):1133–40. doi: 10.4254/wjh.v9.i28.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkenstedt A, Nachbaur K, Zoller H, et al. Acute-on-chronic liver failure: Excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879–86. doi: 10.1002/lt.23678. [DOI] [PubMed] [Google Scholar]
- 26.Putignano A, Gustot T. New concepts in acute-on-chronic liver failure: Implications for liver transplantation. Liver Transpl. 2017;23:234–43. doi: 10.1002/lt.24654. [DOI] [PubMed] [Google Scholar]