Abstract
Macroencapsulation devices provide the dual possibility of immunoprotecting transplanted cells while also being retrievable, the latter bearing importance for safety in future trials with stem cell–derived cells. However, macroencapsulation entails a problem with oxygen supply to the encapsulated cells. The βAir device solves this with an incorporated refillable oxygen tank. This phase 1 study evaluated the safety and efficacy of implanting the βAir device containing allogeneic human pancreatic islets into patients with type 1 diabetes. Four patients were transplanted with 1‐2 βAir devices, each containing 155 000‐180 000 islet equivalents (ie, 1800‐4600 islet equivalents per kg body weight), and monitored for 3‐6 months, followed by the recovery of devices. Implantation of the βAir device was safe and successfully prevented immunization and rejection of the transplanted tissue. However, although beta cells survived in the device, only minute levels of circulating C‐peptide were observed with no impact on metabolic control. Fibrotic tissue with immune cells was formed in capsule surroundings. Recovered devices displayed a blunted glucose‐stimulated insulin response, and amyloid formation in the endocrine tissue. We conclude that the βAir device is safe and can support survival of allogeneic islets for several months, although the function of the transplanted cells was limited (Clinicaltrials.gov: NCT02064309).
Keywords: cellular biology, clinical research/practice, diabetes: type 1, encapsulation, endocrinology/diabetology, islet transplantation, islets of Langerhans, translational research/science
Short abstract
An investigator‐initiated phase 1 study of the safety and efficacy of implanting the macroencapsulation device βAir with an incorporated refillable oxygen tank containing allogeneic human pancreatic islets into 4 patients with type 1 diabetes shows that the device is safe and can support survival of allogeneic islets for several months, though the function of the transplanted cells is limited.
Abbreviations
- ANA
antinuclear antibody
- ANOVA
Analysis of variance
- CGM
continuous glucose monitoring
- CRP
C‐reactive protein
- DBD
donor after brain death
- DTSQ
Diabetes Treatment Satisfaction Questionnaire
- GAD
glutamic acid decarboxylase
- GMP
Good manufacturing practice
- GSIS
glucose‐stimulated insulin secretion
- GPAIS
glucose‐potentiated arginine stimulation
- HbA1c
Hemoglobin A1c
- HIV
Human immunodeficiency virus
- HRQoL
health‐related quality of Life
- IA‐2
insulinoma associated antigen 2
- IAPP
islet amyloid polypeptide
- KRBH
Krebs Ringer Bicarbonate HEPES buffer
- MMTT
Mixed Meal Tolerance Test
- PET
positron emission tomography
- [11C]‐5‐HTP
[11C]‐5‐hydroxytryptophan
1. INTRODUCTION
Pancreatic islet transplantation is at present an established procedure with well‐documented efficacy for the treatment of patients with type 1 diabetes complicated with unstable glycemic control and hypoglycemia unawareness.1 Yet, in most cases, the side effects with the currently available systemic unspecific immunosuppressive drugs shift the “risk‐benefit” analysis in favor of not performing transplantation. Therefore, focus is now intensified to foster development of novel means to control the immune system to enable large‐scale clinical application.
A valid strategy to prevent rejection and recurrence of autoimmunity is encapsulation of the insulin‐producing cells. Encapsulation introduces a physical barrier that prevents access of immune cells to the transplanted cells, but also precludes revascularization. Macroencapsulation devices hold the possibility for graft recovery, which is advantageous from a safety point of view, in particular in trials with stem cell–derived cells. However, such a system also aggravates problems with nutrient supply to the transplanted tissue, with hypoxia‐related functional impairment and cell death in the clustered highly metabolically active islet tissue.2, 3 To solve this matter, the βAir device with an incorporated refillable oxygen tank was developed and successfully tested in small and large animal models.4, 5, 6 The present clinical phase 1 study was conducted to evaluate the safety of implanting the βAir device7 containing isolated allogeneic human islets in patients with type 1 diabetes. The device was implanted subcutaneously in subjects with well‐controlled and uncomplicated type 1 diabetes.
2. MATERIALS AND METHODS
2.1. Patient selection
The study was approved by the Uppsala County ethics board and the Swedish Medical Products Agency. The reported investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000. All participants were provided oral and written information and signed a written consent form prior to inclusion in the study. Patient inclusion criteria were the following: type 1 diabetes duration >5 years, age >18 years, intensive diabetes self‐management defined as ≥3 blood glucose measurements daily and ≥3 insulin injections daily or insulin‐pump therapy. Patient exclusion criteria were the following: fulfillment of clinical criteria for regular therapeutic pancreas or islet transplantation, any previous or scheduled organ transplantation, treatment with any immunosuppressive drug, random C‐peptide >0.003 nmol/L; HbA1c >10% (>85.8 mmol/mol); BMI >30 kg/m2; insulin requirements >1U/kg/24 hours; use of drugs other than insulin to treat diabetes; pregnancy or planned pregnancy; active infections including hepatitis B and C, HIV, and tuberculosis; known drug abuse; any coagulopathy or anticoagulant therapy; severe coexisting cardiac disease; renal failure (GFR <60 mL/min). The aim was to include equal numbers of male and female patients.
2.2. Study design
The study was an open‐label, investigator‐driven clinical phase 1 trial primarily assessing the safety and secondarily the efficacy of allogeneic transplantation of macroencapsulated human islets within the bioartificial pancreas βAir (BetaO2 Technologies Ltd, Israel). The study was designed to remove the devices 180 days posttransplantation, with a safety follow‐up for an additional 6 months. After inclusion, the insulin therapy was intensified during a run‐in period in order to optimize metabolic control. During the trial, all patients recorded daily blood glucose levels and insulin doses in a diary. Clinical visits were performed at least every other week posttransplantation until device removal. The study design and all inclusion and exclusion criteria can be found on clinicaltrials.gov (NCT02064309).
2.3. Immunological monitoring
Before transplantation, all patients were characterized regarding blood group, HLA, HLA antibodies, glutamic acid decarboxylase (GAD), and insulinoma‐associated antigen 2 (IA‐2) antibodies, and for antinuclear antibodies (ANAs) according to hospital routines. All parameters except blood group and HLA type were repeatedly evaluated during the follow‐up.
2.4. Islet isolation and transplantation of the βAir device
Human pancreatic islets were isolated from pancreases procured donation after brain death (DBD) donors in GMP facilities according to clinical routine.8 Descriptive data for each islet donor are provided in Table S1. The criteria for islet preparations were the following: islet viability >80%, islet purity >80%; for functional tests of islets prior to transplantation see Table S1 and Figure S1. The islets, 155 000‐180 000 islet equivalents, were mixed with alginate and loaded in 12 separate alginate slabs into a single βAir device. Surgery was performed under general anesthesia. A midline skin incision was made and a subcutaneous pocket to fit the device was created by blunt dissection. On the opposite side, 2 smaller incisions were made to implant the 2 ports used for oxygen refueling. The tubes that connected the device and the ports were tunneled subcutaneously. Bleeding was stopped by diathermia, and the subcutaneous pocket was observed for 5‐10 minutes to exclude bleeding prior to insertion of the device. Once the device was inserted, the tubes were connected and both ports were tested by manual refueling of oxygen using a syringe connected to the oxygen refueling device. The wounds were thereafter sutured. Three of the four patients received one βAir device, whereas patient 3 received 2 devices. Oxygen refilling was carried out on a daily basis by the patients themselves using a refilling device. The βAir capsule is designed to contain enough oxygen for the implanted islets for at least 30 hours.6
2.5. Safety tests
All patients were admitted to the hospital on the day before surgery. The patients were examined clinically, and blood samples were obtained to again verify that all inclusion criteria and none of the exclusion criteria were fulfilled. An ultrasonography was performed 3 hours after surgery to exclude bleeding at the site of device implantation. The patients were hospitalized for 3‐5 days. Clinical parameters, blood glucose levels, insulin needs, status of wound, and blood samples were evaluated daily. Blood glucose concentrations were measured ≥7 times daily to adjust insulin doses. Follow‐up visits were performed on postoperative days 7 and 10, and thereafter once weekly during the first month and then every other week up until device removal. MRI scans were performed one, 3, and 6 months posttransplantation to exclude fibrosis or inflammation surrounding the device and oxygen refueling ports. At device removal, biopsy samples were harvested from the tissue surrounding the device and evaluated by immunohistochemistry.
2.6. Clinical efficacy tests
HbA1c levels and plasma C‐peptide concentrations were analyzed at the central laboratory of Uppsala University Hospital. Self‐assessed blood glucose levels and insulin requirements were monitored daily in a patient diary. A blinded continuous glucose monitoring (CGM; iPro2, Medtronic, Solna, Sweden) was performed during the run‐in period, immediately posttransplantation, 3 months posttransplantation, before device removal, and 6 months after device removal. A Mixed Meal Tolerance Test (MMTT; Resource Protein, 6 mL/kg [maximum 360 mL],Novartis) was performed 3 months posttransplantation, and in one case also 6 months posttransplantation, to evaluate the secretory capacity of the transplanted islets. Prior to the MMTT, the patient was admitted to the hospital and blood glucose was maintained between 4.5 and 8 mmol/L overnight by an intravenous insulin infusion (Actrapid, Novo Nordisk Scandinavia AB, Malmö, Sweden). Plasma C‐peptide and blood glucose concentrations were determined at 0, 15, 30, 60, 90, and 120 minutes during the MMTT.
All patients filled out a Diabetes Treatment Satisfaction Questionnaire (DTSQ) (Table S1)9 and RAND‐36,10 a health‐related quality of life (HRQoL) questionnaire, prior to transplantation, and this was repeated 3 months posttransplantation, prior to explantation, and 6 months after explantation.
A positron emission tomography (PET) with radioactive labeled water ([15O]H2O) and [11C]‐5‐hydroxytryptophan ([11C]‐5‐HTP) was performed as described previously11 in 2 of the patients before explantation of the devices to evaluate the in vivo kinetics over the immune barrier of the device.
2.7. Ex vivo efficacy tests
After explantation, the whole device was evaluated with a static glucose‐stimulated insulin secretion (GSIS) test followed by a glucose‐potentiated arginine stimulation (GPAIS) test.
The oxygenated devices were placed in glass beakers containing 100 mL of Krebs Ringer bicarbonate HEPES buffer (KRBH) with addition of 0.2% (wt/vol) albumin in an incubator maintained at a temperature of 37°C and a CO2 concentration of 5%. Initially, the device was incubated for 30 minutes in 2.8 mmol/L glucose (Fresenius Kabi, Uppsala, Sweden) as a washing step. The device was then incubated for 45 minutes in each of the following glucose concentrations: 2.8 mmol/L, 16.7 mmol/L, and finally 16.7 mmol/L glucose with the addition of 10 mmol/L arginine (Sigma, Stockholm, Sweden). Medium samples were collected after 0, 5, 10, 15, 30, and 45 minutes at each incubation step.
The devices were then dismantled, and the same stimulation tests were performed on 2 alginate slabs, one from each side of the device, free floating in petri dishes containing 10 mL of media following the same protocol as for the whole device. The means of insulin release were calculated from experiments of slabs for each patient.
2.8. Immunohistochemistry
Remaining alginate slabs were either fixed in formalin and paraffin‐embedded or frozen for immunohistochemical evaluation. Sections of the slabs were stained for insulin (guinea pig polyclonal; dilution 1:400; Fitzgerald, Acton, MA), detected by MACH 3 Rabbit HRP‐Polymer Detection (Biocare Medical, Concord, CA) and visualized by 3,3′‐diaminobenzidine.
Alginate‐embedded formalin‐fixed slabs and islets from the pancreas donors were stained with Congo red according to Puchtler and Sweat,12 and the presence of amyloid was determined in polarized light.13
Biopsies from the tissue surrounding the device were obtained at explantation and fixed in formalin. Sections (5 μm) were stained for CD31 (mouse monoclonal, clone JC70A), CD4 (mouse monoclonal, clone 4B12), CD8 (mouse monoclonal, clone C8/144B), CD3 (rabbit polyclonal, code A0452), CD20cy (mouse monoclonal, clone L26), CD45 (mouse monoclonal, clone 2B11 + PD7/26), and CD68 (mouse monoclonal, clone KP1). Antibodies were purchased from Agilent Technologies (Kista, Sweden).
2.9. Statistical analysis
GraphPad Prism version 6.07 (GraphPad Software, La Jolla, CA) was used for statistical analysis. A one‐way analysis of variance (ANOVA) based on repeated measurements with Dunnett's post hoc test was applied for longitudinal follow‐up data using the parameters sampled on the day of transplantation as a control. For values of HbA1c, C‐peptide, and insulin doses, postoperative weeks 0‐12 and the 2 measurements following explantation were used for analysis, since these were the time points for which data were recorded for all 4 patients. In the individual plots all recorded data are given. For the CGM, DTSQ, and RAND‐36 data, Friedman's nonparametric repeated measurement test was applied with Dunn's post hoc test. For the CGM data, the pretransplantation data were used as a control, whereas for DTSQ and RAND‐36 data, multiple comparisons were applied. P < .05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of the patients
The patients included in the study were all well‐educated regarding self‐management of their diabetes and were highly motivated to participate in the study; for their characteristics see Table 1.
Table 1.
Descriptive patient data collected prior to transplantation
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Gender | Female | Male | Male | Female |
| Age (years) | 53 | 47 | 59 | 44 |
| Weight (kg) | 72 | 99 | 79 | 72 |
| Height (cm) | 174 | 189 | 179 | 172 |
| BMI (kg/m2) | 23.8 | 27.7 | 24.7 | 24.3 |
| Diabetes duration (years) | 41 | 30 | 30 | 39 |
| Retinopathy | Discrete | Discrete | Discrete | Discrete |
| Treatment regimen | Injections | Pump | Pump | Injections |
| HbA1c before run‐in (mmol/mol, DCCT %) | 73 (8.8) | 68 (8.4) | 66 (8.2) | 63 (7.9) |
| HbA1c after run‐in (mmol/mol, DCCT %) | 62 (7.8) | 71 (8.6) | 62 (7.8) | 63 (7.9) |
| Total insulin doses (U/24 h) | 36 | 54 | 46 | 30 |
| Insulin doses (U/24 h/kg) | 0.5 | 0.56 | 0.58 | 0.42 |
| Exercise | 3 times weekly | 2‐3 times weekly | 2‐3 times weekly | 2‐3 times weekly |
| Hypoglycemia unawareness | No | No | No | No |
| Other drugs | Levothyroxine | Statins | Levothyroxine | ‐ |
| Total number of islets (IEQ) transplanted and islet purity (%) |
155 000 96% |
180 000 81% |
180 000, 180 000 86%, 89% |
180 000 92% |
| Number of islets transplanted (IEQ/kg BW) | 2150 | 1800 | 4600 | 2500 |
Apart from discrete retinopathy none of the patients had any diabetes‐related long‐term complications. IEQ, islet equivalents.
3.2. Safety evaluation
A mild inflammation surrounding the surgical wound was observed in all patients during the first days after transplantation. In patient 1, the surgical wound was red and heated on day 4 posttransplantation, and as a precaution 7 days of treatment with clindamycin (300 mg twice daily) was administered. The bacterial culture from the wound was negative. In the same patient, a second surgery was performed 7 days after transplantation due to a rotation of the oxygen ports, which made it impossible to refuel oxygen. At this surgery, the ports were restored and fixed to the surrounding tissue. Because of this, there was a delay between the oxygen refilling of 32 hours, which is just above the maximal time acceptable, but no related peak in C‐peptide concentration suggestive of hypoxia‐related beta‐cell death was observed. Thereafter, none of the patients reported any missed oxygen refilling or problems refilling oxygen through the ports. C‐reactive protein (CRP) levels peaked at day 3 posttransplantation, range 19‐48 mg/L. After the first 10 days after transplantation, there were no signs of wound inflammation or infection either clinically or in blood samples in any of the patients. There were neither signs of HLA immunization nor changes in autoantibody frequency (GAD, IA2, ANA) posttransplantation. It is noteworthy that in patient 2, the islet donor blood group was incompatible with that of the recipient; despite this there were no signs of increasing ABO antibody titers. The patients carrying the devices generally had few complaints, but reported some stress related to remembering and performing oxygen refilling.
The surgical wounds healed as expected in all transplanted patients, and a minor fibrotic resistance could be palpated surrounding the device and oxygen ports. MRI performed one, 3, and 6 months after transplantation showed only initial mild signs of inflammation and a limited degree of fibrosis in the surrounding tissue at later examinations. When the devices were explanted 3‐6 months posttransplantation, a nonadhesive thin fibrotic tissue was found surrounding the device. Implantation of 2 devices in one of the patients was not associated with any additional complications.
Morphological examination of tissue surrounding the device revealed a substantial foreign body reaction in 3 of 4 patients. Visual examination of the sections revealed an intense accumulation of CD45+ cells in the area close to the encapsulation device. A majority of these cells were CD68+ macrophages (Figure 1A). Accumulation of these cells was uniform on a single device, but varied to some extent between devices (Figure 1B). Similarly, accumulation of CD3+ T cells (Figure 1C‐D) varied between devices. A majority of the CD3+ cells were CD8+, and only small numbers of CD4+ cells could be found. CD20+ B cells were not frequently found in the inflammation close to the surface of the capsule (Figure 1E); however, these cells accumulated together with CD3+ CD8+ T cells around small blood vessels in the surrounding subcutaneous tissue (Figure 1E‐F). Only few CD31+ capillaries were found close to the surface of the device and in the area with inflammation; capillaries were in ordinary frequencies in the surrounding subcutaneous tissue (Figure 1H).
Figure 1.
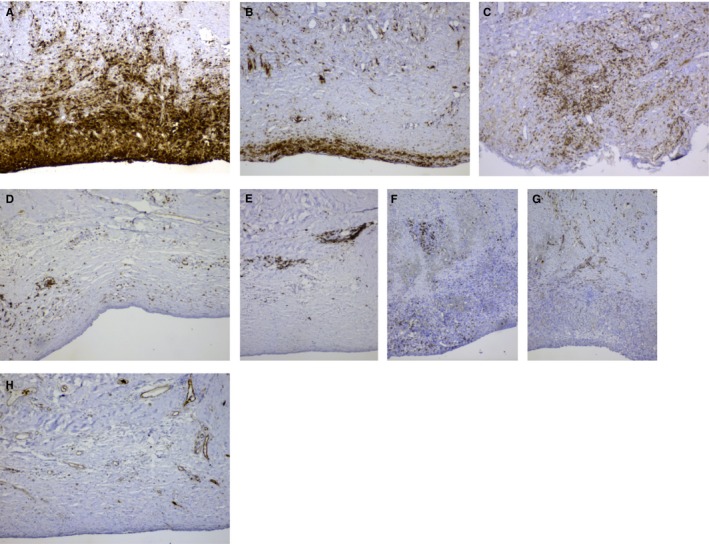
Accumulation of CD68+ macrophages (A and B) and CD3+ T cells (C and D) varied between devices. CD20+ B cells and CD3+ CD8+ T cells accumulated around small blood vessels in the surrounding subcutaneous tissue (E and F). Only few CD31+ capillaries were found close to the surface of the device and in the area with inflammation; however, capillaries were frequently observed in the surrounding subcutaneous tissue (G and H). Original magnification × 100
3.3. Efficacy evaluation
C‐peptide was detectable in blood plasma in all patients when measured 1 day posttransplantation (range 0.028‐0.093 nmol/L) and remained detectable up until 2‐4 weeks posttransplantation (Figure 2A). Only patient 1 reached a peak C‐peptide of 0.06 nmol/L during the MMTT at 3 months after transplantation, whereas plasma C‐peptide was not detectable in the remaining 3 patients (<0.003 nmol/L). Six months after transplantation, the C‐peptide concentration was below the detection limit during the MMTT, also in patient 1. Due to no measurable graft function, βAir devices were, according to protocol, recovered already 4‐5 months post‐transplantation in patients 2‐4.
Figure 2.
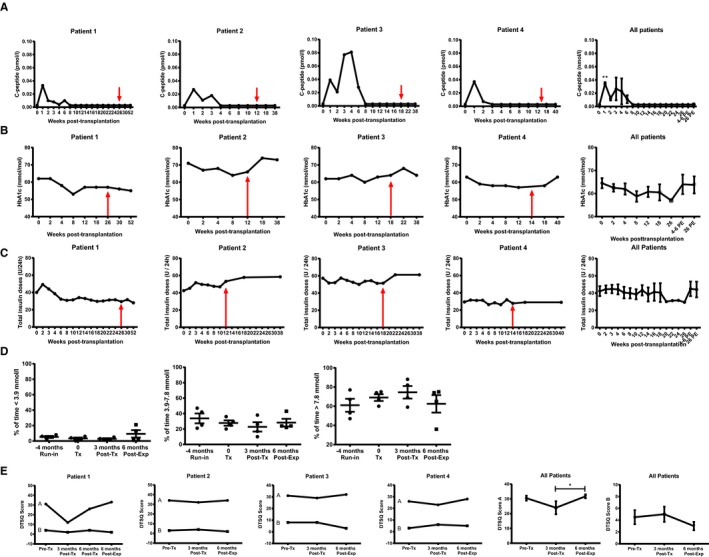
Clinical follow‐up data posttransplantation. (A) Fasting plasma C‐peptide concentrations were increased after transplantation and measurable for up to 8 weeks posttransplantation. HbA1c levels (B) and insulin requirements (C) did not change posttransplantation. In A‐C, data are first provided for each individual patient from the day of transplantation up until explantation of the device (indicated by red arrow), and for an additional 6 months. In the graphs to the right in A‐C, means ± standard error of the mean (SEM) for all the 4 patients are provided; the follow‐up visits are labeled 4‐6 PE and 26 PE, meaning 4‐6 and 26 weeks postexplantation, respectively. (D) Data from continuous glucose monitoring (CGM) prior to transplantation, posttransplantation, and 6‐months postexplantation of the device. Data are presented as means ± SEM for all patients with individual values given. Data are expressed as the percentage of time spent in target range of glucose (3.9‐7.8 mmol/L), above target (>7.8 mmol/L), and below target (<3.9 mmol/L). No change in glucose variability was observed posttransplantation. (E) Data from the Diabetes Treatment Satisfaction Questionnaire (DTSQ) were first plotted for each patient with both the positive (a) and negative responses (b) provided in the same graph. In the graph to the right, means ± SEM, separated for DTSQ a and b, for all the 4 patients are provided. *Denotes P < .05 when compared to prior to explantation of the device
Three of 4 patients improved their HbA1c posttransplantation, but this was not statistically significant (P = .21, Figure 2B). Insulin needs posttransplantation were not reduced (P = .33, Figure 2C) and there was no change in glucose variability after transplantation based on blinded CGM data (P = .24, P = .22, and P = .49, respectively, Figure 2D).
There was no change in the patient‐reported DTSQ post‐transplantation when compared to the run‐in period, but there was an increased satisfaction after removal of the device (P = .009, Figure 2E). There was no change in HRQoL for any of the domains in RAND‐36 (Figure S2).
Patients 1 and 3 underwent PET/CT examinations prior to explantation of the devices. The device/devices could be visualized easily in the CT scan. However, no signs of activity of [15O]H2O or [11C]‐5‐HTP after injection, within the devices, was observed.
The ex vivo evaluation of the intact devices revealed overall very low insulin secretion response to glucose and only an additional insulin secretory response to GPAIS test from one of the devices (patient 2; Figure 3A). This device overall showed the best response to both the GSIS and GPAIS tests. Insulin release during the GSIS increased slowly even from the devices in which a stimulatory response was observed (Figure 3B). The islets slabs recovered from the devices were found to be responsive to glucose ex vivo from 2 of 4 patients, and to GPAIS in 3 of 4 (Figure 3C). The best dynamic response to glucose was again obtained in slabs from the device removed from patient 2 (Figure 3D). It is noteworthy that the amount of insulin released from the devices was similar to that of individual islet slabs, even though the devices contained 12 times more islets (Figure 3A,C).
Figure 3.
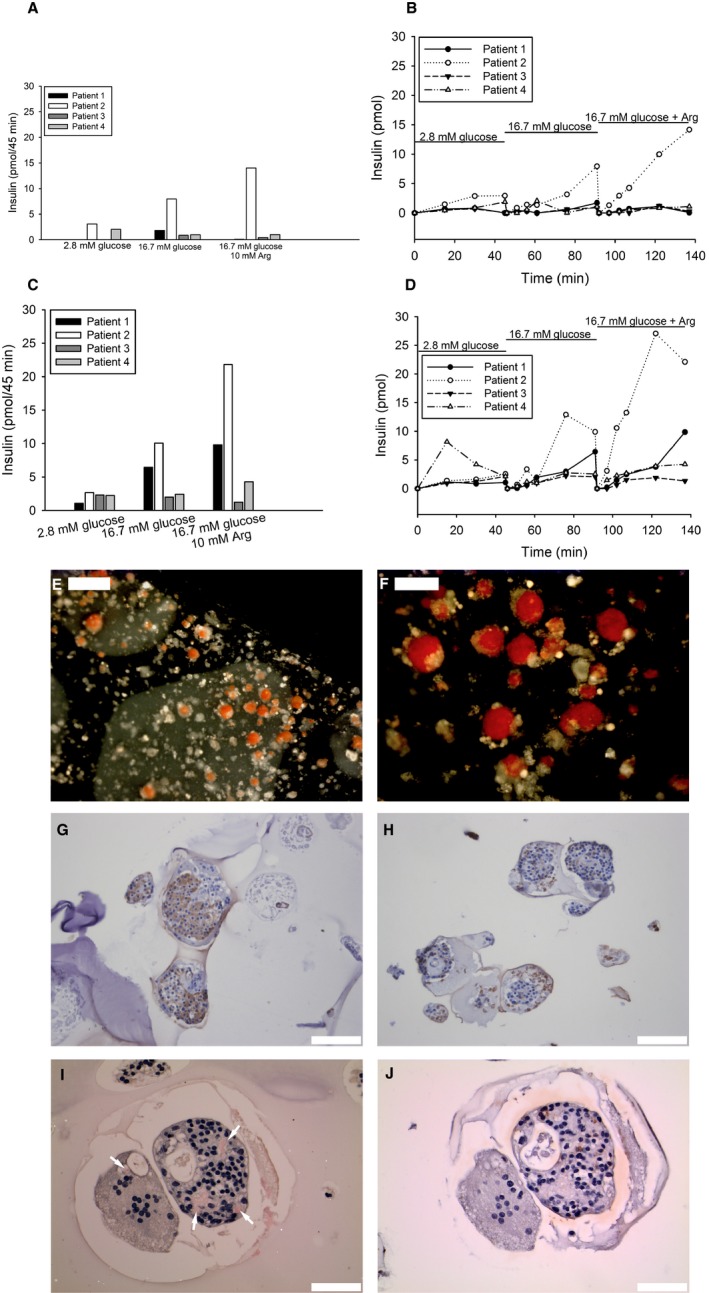
Recovered device (A‐B) and device containing slabs (C‐D) were evaluated ex vivo with respect to insulin secretion upon glucose and arginine stimulation. Insulin release was measured in 4 patients for 45 minutes at each condition (A). The dynamic insulin secretion was observed up to 135 minutes after continuous stimulation of device with low (2.8 mmol/L), high (16.7 mmol/L), and finally high glucose supplemented arginine solution (B). Insulin release was measured in slabs, recovered from the device, when stimulated with low, respectively, high glucose (2.8 vs 16.7 mmol/L) with or without arginine for 45 min (C). One slab from each patient was monitored for dynamic insulin secretion when stimulated with different concentrations of glucose (2.8 vs 16.7 mmol/L) with or without arginine (D). Separate slabs were stained with dithizone (red), and islets could easily be detected (E, scale bar 500 μm) and (F, scale bar 200 μm). Islet‐containing slabs were also further processed for immunohistochemistry and stained for insulin (brown). Insulin‐positive cells could be found in slabs from all patients (G), but also areas with fragmented islets and cellular debris were observed (H, scale bar 100 μm). Sections stained with Congo red identified amyloid‐containing islets (I, scale bar 50 μm; amyloid [red] indicated by arrows), and insulin‐positive cells (brown) were verified in consecutive sections (J, scale bar 50 μm). The left islet in (I), where a single deposit occurs, has a markedly reduced number of nucleated cells. The islet to the right contains multiple inclusions occupying almost 20% of the islet area, and the amyloid is surrounded by nucleated cells. The result suggests that amyloid formation proceeded for a long time, which requires functioning beta cells. Sections were counterstained with hematoxylin
Islets could be visualized within the alginate slabs by dithizone staining (Figure 3E‐F). Immunohistochemistry revealed many intact islets rich in beta cells found in all alginate slabs (Figure 3G). However, there were also significant numbers of damaged islets, for example, fragmented islets and islets with central necrosis, as well as apparently morphologically intact islets with only a few insulin positive cells (Figure 3H). Amyloid was not detected in any islets in the pancreas from the organ donors. However, after explantation, amyloid was detected in islets from all patients, but the amount and the number of affected islets varied. Particularly, islets recovered from patient 3 showed substantial amyloid deposits (Figure 3I), whereas in other cases amyloid was located mainly intracellularly.
4. DISCUSSION
The present study was initially planned to enroll 8 subjects. After inclusion and transplantation of 4 patients that demonstrated good safety profile, the study was stopped because of limited metabolic usefulness, with only minute and transient levels of circulating C‐peptide levels. In line with the protocol, 3 or 4 patients ended the study prematurely and had their devices explanted due to nonsignificant graft function.
No subject developed donor‐specific HLA antibodies despite the absence of systemic immunosuppression. Likewise, no islet infiltration of immune cells was observed, and remaining beta cells were regularly found in the encapsulated grafts. The results presented support the notion that the βAir device is safe and successfully prevents immunization and rejection of allogeneic islets in humans with type 1 diabetes even in the presence of circulating islet autoantibodies.
The avascularity of the encapsulated cells means that they must rely on oxygen and nutrient delivery solely by diffusion for their survival and function. The βAir device partly solves the problem with oxygen delivery, but requires daily refilling of the oxygen chamber through one of the 2 subcutaneous Port‐A‐Cath ports.7 Other than for the initial technical problems with rotation of the ports encountered in patient 2, there were no reports of missed or failed oxygen refillings during the course of study, which indicated that this strategy is feasible. However, the frequent demands of refilling and need to carry the equipment while traveling obviously caused some stress for the patients, as judged from their feedback and diabetes treatment satisfaction questionnaires. Nevertheless, although morphologically intact islets were regularly found after retrieval of the βAir device, also islets with central necrotic areas and lost integrity were frequently observed. An inherent problem in islet transplantation is the continuous synthesis and secretion of islet amyloid polypeptide (IAPP). If this molecule is not accurately transported from the islets, for example, due to impaired vascularization, amyloid deposits will form that impair islet function.14, 15 In the present study, no amyloid could be found within the islets preimplantation; however, after retrieval of the βAir device intra‐ and extracellular islet deposits of amyloid were regularly observed. Amyloid formation has previously been reported to occur in clinical islet transplantation to the liver.14, 16 Impaired diffusion kinetics of IAPP over the immune barrier might have aggravated amyloid formation when compared to that observed after transplantation of nonencapsulated islets.15
Two subjects in the present study (patients 1 and 3) underwent PET imaging, and no activity within the device after injection of [15O]H2O or [11C]5‐HTP could be detected during a PET imaging period of 10 and 60 minutes, respectively. [11C]5‐HTP is of similar molecular weight as glucose (180 Da). The absence of [15O]H2O and [11C]5‐HTP activity is likely due to no tracer transport across the device membrane. The result suggests a substantial delay in diffusion of water, 5‐HTP, and therefore likely also glucose into the device. Diffusion of larger molecules, for example, insulin (5800 Da) and IAPP (3900 Da) secreted from the encapsulated islets, would be expected to be even more delayed. These observations are in agreement with findings reported previously in a patient in whom the βAir device with human islets was implanted in the pre‐peritoneal space, a site considered to be superior when compared with subcutaneous implantation.7 Even so, the kinetics of glucose‐stimulated insulin release during an intravenous glucose tolerance test showed a significant delay in secreted C‐peptide over a 4‐hour period also at the pre‐peritoneal space.7 Given the physiological differences between different tissue compartments, especially regarding vascularity, the most advantageous site for transplantation of macroencapsulation devices remains to be determined. Although a nonsignificant decrease in HbA1c posttransplantation was observed in 3 patients, this was not reflected in lowered doses of administrated insulin or a better outcome of function measured as C‐peptide production in vivo. However, because C‐peptide released from the device is introduced into the subcutaneous space, and not directly into the circulation, local degradation in the tissue could have contributed to the low or nonrecordable C‐peptide concentrations.17 Of interest, the ex vivo glucose responsiveness with insulin from the device removed from patient 2 was better than that from the others, which indicates that the islets within this device benefited from the reduced in vivo foreign body reaction.
A delayed or blunted glucose‐stimulated insulin release from the encapsulated islets was observed when compared to the rapid responsiveness of the islets prior to their transplantation. In fact, the insulin secretion response of the whole devices, containing 12 alginate slabs with >100 000 islets, was similar in magnitude to that of one slab with islets, which suggests severely delayed kinetics of insulin release from the intact device. Notably, total insulin release from the intact device was in the range of what is expected from only 100 islets prior to encapsulation, cf. Figure S1. Diffusion of substances in and out of the device is concentration dependent, but also critically depends on the surface‐to‐volume ratio as well as the diffusion capacity over the immune barrier. The βAir device would tentatively benefit from increasing its surface‐to‐volume ratio; however, with the limitations that the device will still have to keep dimensions reasonable for clinical application. Notably, a membrane with pore sizes as large as 0.45 μm seems able to control islet allograft rejection in rodent models,5, 18, 19 as well as in this and a previous clinical trial,20 ie, preventing cell‐to‐cell contact between donor and recipient cells seems sufficient to prevent HLA immunization and allograft rejection. Implantation of the islets in an alginate gel potentially also impairs diffusion. Islets even in small alginate capsules (≈ 350 μm in diameter) demonstrated a severely blunted response to high glucose and theophylline, whereas islets encapsulated in larger capsules (≈650 μm in diameter) failed to respond.21 Notably, using the same in vitro conditions, the authors showed that the response to glucose by nonencapsulated islets was 7‐fold greater when compared to islets in small capsules. Alginate may also function as a “Ca2+ trap,” since divalent cations cross‐link the alginate molecules. Therefore, aims might be to minimize also the volume of alginate in any encapsulation device. The permeability of the alginate to cytokines and antibodies was not determined postexplantation.
The remarkable results presented in small animals using various encapsulation strategies have been difficult to transfer to large animal models and in clinical trials.22, 23, 24 The problems encountered could be attributed to (1) the significant contribution of glucose effectiveness for glucose metabolism in small animals,25 (2) limited islet survival due to diffusion problems of oxygen and nutrients occurring when the device has been upscaled from harboring a few hundred islets to encompass several hundred thousands of islets, and (3) an uncontrolled foreign body reaction resulting in excessive formation of scar tissue.26, 27
Nevertheless, this first‐in‐human trial shows that the βAir device can prevent rejection and maintain viable transplanted beta cells for a period of several months. Although the function of transplanted cells was limited, the procedure was safe. Therefore, the approach described can be applied to guide not only further development of encapsulation devices, but also initial clinical trials with insulin‐producing cells derived from stem cells, evaluating the safety of such cells in humans over a period of months. This would provide important knowledge on how such cells behave in humans with respect to differentiation and potential teratoma development, prior to optimization of implantation procedure for a later clinical trial aiming for efficacy.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Avi Rotem, Baruch Zimermann, Helena Grinberg, Tali Goldman, Uriel Barkai, and Yuval Avni are all employed by Beta‐O2 Technologies Ltd and receive options in the company in addition to salary. Håkan Ahlström is one of the founders of Antaros Medical AB and Olof Eriksson is employed by Antaros Medical AB. This company has not been involved in the presented study. The remaining authors have no conflicts of interest to disclose.
Supporting information
ACKNOWLEDGMENTS
POC, DE, and OK designed the trial. POC, DE, and AS were clinically responsible for the patients in the study. AS performed the surgical procedures. DE, JO, OK, BZ, AR, and HG conducted the ex vivo experiments. POC, DE, GTW, HA, LC, OE, JO, and OK researched the data. AR, BZ, HG, TG, UB, and YA developed the βAir device. POC, DE, and OK wrote the manuscript and all other authors critically reviewed the manuscript. The research nurses Violeta Armijo Del Valle, Maria Svenaeus Lundgren Rebecka Hilmius, and Karin Kjellström (Uppsala University Hospital), as well as Sofie Ingvast and Magnus Ståhle, are gratefully acknowledged for their assistance and great efforts in this trial. This study was supported by the Juvenile Diabetes Research Foundation, the Swedish Medical Research Council (VR K2015‐54X‐12219‐19‐4, K2013‐55X‐15043, K2016‐GTW, 2016‐01040 and 921‐2014‐7054), the Diabetes Wellness Foundation, the Novo Nordisk Foundation, the Swedish Diabetes Association, The Ernfors Family Fund, HumEn HEALTH‐F4‐2013‐602889, 646075‐ELASTISLET, Helmsley Charitable Trust and Barndiabetesfonden. Human isolated islets were provided by The Nordic Network for Clinical Islet Transplantation, supported by the Swedish national strategic research initiative EXODIAB (Excellence of Diabetes Research in Sweden).
Carlsson P‐O, Espes D, Sedigh A, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18:1735–1744. 10.1111/ajt.14642
Per‐Ola Carlsson and Daniel Espes contributed equally in this study.
REFERENCES
- 1. Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colton CK. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev. 2014;67–68:93‐110. [DOI] [PubMed] [Google Scholar]
- 3. Lau J, Henriksnas J, Svensson J, Carlsson PO. Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant. 2009;14(6):688‐693. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig B, Rotem A, Schmid J, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci USA. 2012;109(13):5022‐5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barkai U, Weir GC, Colton CK, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22(8):1463‐1476. [DOI] [PubMed] [Google Scholar]
- 6. Neufeld T, Ludwig B, Barkai U, et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE. 2013;8(8):e70150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ludwig B, Reichel A, Steffen A, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA. 2013;110(47):19054‐19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goto M, Eich TM, Felldin M, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78(9):1367‐1375. [DOI] [PubMed] [Google Scholar]
- 9. Bradley C, Lewis KS. Measures of psychological well‐being and treatment satisfaction developed from the responses of people with tablet‐treated diabetes. Diabet Med. 1990;7(5):445‐451. [DOI] [PubMed] [Google Scholar]
- 10. Hays RD, Sherbourne CD, Mazel RM. The RAND 36‐item health survey 1.0. Health Econ. 1993;2(3):217‐227. [DOI] [PubMed] [Google Scholar]
- 11. Eriksson O, Espes D, Selvaraju RK, et al. Positron emission tomography ligand [11C]5‐hydroxy‐tryptophan can be used as a surrogate marker for the human endocrine pancreas. Diabetes. 2014;63(10):3428‐3437. [DOI] [PubMed] [Google Scholar]
- 12. Puchtler H, Sweat F. Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem. 1965;13(8):693‐694. [DOI] [PubMed] [Google Scholar]
- 13. Westermark GT, Johnson KH, Westermark P. Staining methods for identification of amyloid in tissue. Methods Enzymol. 1999;309:3‐25. [DOI] [PubMed] [Google Scholar]
- 14. Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359(9):977‐979. [DOI] [PubMed] [Google Scholar]
- 15. Bohman S, Westermark GT. Extensive amyloid formation in transplanted microencapsulated mouse and human islets. Amyloid. 2012;19(2):87‐93. [DOI] [PubMed] [Google Scholar]
- 16. Westermark GT, Davalli AM, Secchi A, et al. Further evidence for amyloid deposition in clinical pancreatic islet grafts. Transplantation. 2012;93(2):219‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekberg K, Brismar T, Johansson BL, et al. C‐Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care. 2007;30(1):71‐76. [DOI] [PubMed] [Google Scholar]
- 18. Brauker J, Martinson LA, Young SK, Johnson RC. Local inflammatory response around diffusion chambers containing xenografts. Nonspecific destruction of tissues and decreased local vascularization. Transplantation. 1996;61(12):1671‐1677. [DOI] [PubMed] [Google Scholar]
- 19. Sorenby AK, Wu GS, Zhu S, Wernerson AM, Sumitran‐Holgersson S, Tibell AB. Macroencapsulation protects against sensitization after allogeneic islet transplantation in rats. Transplantation. 2006;82(3):393‐397. [DOI] [PubMed] [Google Scholar]
- 20. Tibell A, Rafael E, Wennberg L, et al. Survival of macroencapsulated allogeneic parathyroid tissue one year after transplantation in nonimmunosuppressed humans. Cell Transplant. 2001;10(7):591‐599. [PubMed] [Google Scholar]
- 21. Chicheportiche D, Reach G. In vitro kinetics of insulin release by microencapsulated rat islets: effect of the size of the microcapsules. Diabetologia. 1988;31(1):54‐57. [DOI] [PubMed] [Google Scholar]
- 22. Calafiore R, Basta G, Luca G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137‐138. [DOI] [PubMed] [Google Scholar]
- 23. Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14(2):157‐161. [DOI] [PubMed] [Google Scholar]
- 24. Tuch BE, Keogh GW, Williams LJ, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887‐1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korsgren E, Korsgren O. Glucose effectiveness: the mouse trap in the development of novel ss‐cell replacement therapies. Transplantation. 2016;100(1):111‐115. [DOI] [PubMed] [Google Scholar]
- 26. De Vos P, Van Straaten JF, Nieuwenhuizen AG, et al. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes. 1999;48(7):1381‐1388. [DOI] [PubMed] [Google Scholar]
- 27. Leu FJ, Chen CF, Chiang WE, et al. Microencapsulated pancreatic islets: a pathologic study. J Formos Med Assoc. 1992;91(9):849‐858. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


