Abstract
Purpose: Cryotherapy has been employed to reduce postoperative inflammation for enhancement of the recovery of total knee arthroplasty (TKA). However, the clinical advantages in functional recovery after TKA remain controversial. This study was conducted to clarify the postoperative alterations in deep temperature around the knee and to evaluate the association between the temperature changes and functional recovery in the early phase after TKA. Methods: Postoperative changes in deep temperature around the knee were evaluated with the probe that can measure subcutaneous tissue temperature at the depth of 1 cm in 28 patients with medial knee osteoarthritis undergoing unilateral TKA through medial parapatellar approach. The same rehabilitation protocol was provided without cryotherapy. Outcome assessment included knee range of motion (ROM) and 10-meter fast speed walking test. Results: The operated knee showed a greater increase in deep temperature at postoperative days 1 and 2, followed by a gradual decrease by day 14 when the temperature was still higher than the baseline. When deep temperature change around the operated knee was calculated by subtracting the preoperative temperature from the highest postoperative one, significant association was found between deep temperature change and knee ROM recovery at day 14. The operated knees with more than 2°C increase in postoperative deep temperature resulted in poor ROM recovery. There was no association of deep temperature change with 10-meter fast speed walking test improvement at day 14 or ROM recovery at 1-year follow-up. Conclusions: This study has provided the first data on deep temperature alterations around the knee after TKA. More than 2°C increase in postoperative deep temperature could result in poor ROM recovery after TKA. The results may support establishment of adequate procedures of cryotherapy for early gain in knee motion after TKA.
Keywords: Total knee arthroplasty, Deep temperature, Cryotherapy, Rehabilitation, Knee motion
Inflammation commonly appears in the perioperative tissues after total knee arthroplasty (TKA). The severity of inflammation may determine the compliance with the prescribed postoperative physical therapy of mobilization and regaining range of motion (ROM) of the knee. ROM has been recognized as an important outcome measure of TKA and is a major component of joint-specific scoring systems1).
Local heat in association with inflammation potentially increases the perioperative tissue temperature after TKA. However, time course of deep tissue temperature changes around the joint after TKA have not been reported in the literature. Cryotherapy in combination with compression has been employed to mitigate postoperative inflammation for enhancement of the recovery of TKA. Local application of cold penetrates to a depth of 4 cm below the skin2). Indeed, the cold reduces intraarticular temperature after arthroscopy when applied over the joint. The mean difference in the intraarticular temperature is 6°C between the knees with and without cryotherapy for one hour after arthroscopy3). Thus, cryotherapy could cause beneficial effects on functional recovery after TKA as a result of the intraarticular temperature decrease. However, the clinical outcomes on ROM have been equivocal. Some studies have shown a benefit4-7), whereas others have demonstrated no difference8-11). One of the reasons for these controversial results may be different procedures of cryotherapy used in different studies. In order to design an appropriate postoperative cryotherapy, it is necessary to elucidate deep tissue temperature changes around the joint after TKA.
Our goals for this study were to clarify the postoperative alterations in deep temperature around the knee and to evaluate the association between the temperature changes and functional recovery in the early phase after TKA.
Methods
1. Patients
Fifty consecutive patients from the preoperative waiting list were scheduled for unilateral primary TKA over a period of six months from April 2015. The exclusion criteria included previous surgery on the evaluated knee, previous arthroplasties of other joints, rheumatoid arthritis, and decreased mobility due to non-joint-related factors (e.g., Parkinson disease). Twenty patients were excluded based on these criteria. Two patients were lost to 1-year followup because of their remote relocation. The remaining 28 patients with medial knee osteoarthritis undergoing unilateral TKA were enrolled to evaluate postoperative changes in deep temperature around the knee. All patients received the same standardized postoperative care with the same medical rehabilitation protocol without cryotherapy. Physical therapy including mobilization started on the first postoperative day. Informed consent was obtained from each patient in strict accordance with the Declaration of Helsinki. All procedures in this study were approved by the ethics committee of Kobe City Medical Center General Hospital (the acknowledgment number: zn170903). No infection was seen in the knees with TKA during the entire postoperative course.
2. Deep tissue temperature measurement
In addition to body temperature measurement and hematological analyses including C-reactive protein (CRP) as routine examinations, deep tissue temperature was measured non-invasively with the thermometer on the basis of zero-heat-flow method12) (Core Temp CM-210, Terumo Corporation, Tokyo, Japan) before physical therapy at the identical time of day pre- and postoperatively in the same room where the temperature was kept at 25°C. This monitoring system has already been approved as a medical device in Japan and used clinically for deep temperature measurement throughout the perioperative process. The probe can measure the tissue temperature at the depth of 1 cm subcutaneous. In the previous study thermocouple probes have been placed intraarticularly into the medial gutter and the suprapatellar pouch under arthroscopy to measure the intraarticular temperature3). While skin incision for TKA hampered probe placement in the anterior aspect of the knee for measurement of deep temperature in reference to the suprapatellar pouch, we employed the medial aspect for measurement of deep temperature corresponding to the medial gutter. Preliminary setup according to the manufacture's instruction confirmed that the probe was securely positioned at the point 6 cm medial to the superior patellar pole with the knee fully extended and could provide consistent data on deep temperature in three separate measurements.
3. Surgical technique
All knees were approached through a midline anterior incision with a medial parapatellar arthrotomy. TKA was performed using Bi-Surface 5 total knee system (KYOCERA Medical Corporation, Osaka, Japan) with a tourniquet applied for 75 minutes in average (60-90 minutes). Before skin closure, hemostasis was controlled using diathermy. Wound drainage was used postoperatively. Postoperative pain control was conducted with the same regimen. After completing the surgery, epidural analgesia with fentanyl was administered in all patients by postoperative day 2. Thereafter, exacerbation of pain was treated with celecoxib 400 mg/day by postoperative day 14 at discharge.
4. Assessment of outcome
Patients were evaluated pre- and post-operatively by physical therapists experienced in the conduction of functional assessments. Knee flexion and extension were measured at discharge (day 14) and 1-year followup with the use of a hand-held goniometer. Postoperative recovery of ROM was evaluated by subtracting the baseline data at admission from the data at discharge. When ROM was greater or equal at discharge or 1-year followup than that at admission, postoperative recovery of ROM was considered as gain. In contrast, when ROM was smaller at discharge or 1-year followup, the ROM recovery was considered as loss. Performance-based function was assessed using a 10-meter fast speed walking test (10MWT) by measuring the time required to walk 10 meters as quickly and safely as possible, with or without assistive devices, on a level surface. Postoperative effects on 10MWT were evaluated by subtracting the baseline data at admission from those at postoperative day 14 at the time of discharge.
5. Statistical analyses
Data were presented as mean ± SD. After Shapiro-Wilk test of normality, data were compared by t test and Mann-Whitney test for parametric and nonparametric tests, respectively. Fisher's exact test was used to compare ROM recovery conditions on the basis of postoperative deep tissue temperature change. Odds ratios and respective 95% confidence intervals were presented. Significance was set at P<0.05. Statistical analyses were conducted in SPSS for Windows, Version 24.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Of 28 TKA patients, there were 20 females and 8 males. For full patient demographics, see Table 1.
Table 1.
Demographic data of 28 patients at admission and discharge
| admission | discharge | 1-year followup | |
|---|---|---|---|
| Values are expressed as mean±SD. *P<0.05 vs the data at admission by paired t test. The data on the operated knee motion are shown. BMI: body mass index, ROM: range of motion, 10MWT: 10-meter fast speed walking test. | |||
| age (years) | 76.6±6.9 | ||
| sex (female/male) | 20/8 | ||
| BMI (kg/m2) | 25.2±3.3 | ||
| knee flexion (°) | 119±16 | 108±14* | 119±8.0 |
| knee extension (°) | –12.5±6.2 | –10.3±5.7 | –1.6±3.1* |
| total ROM (°) | 106±19 | 97.3±17.5* | 117±9.7* |
| 10MWT (sec) | 9.5±2.5 | 13.1±9.8* | |
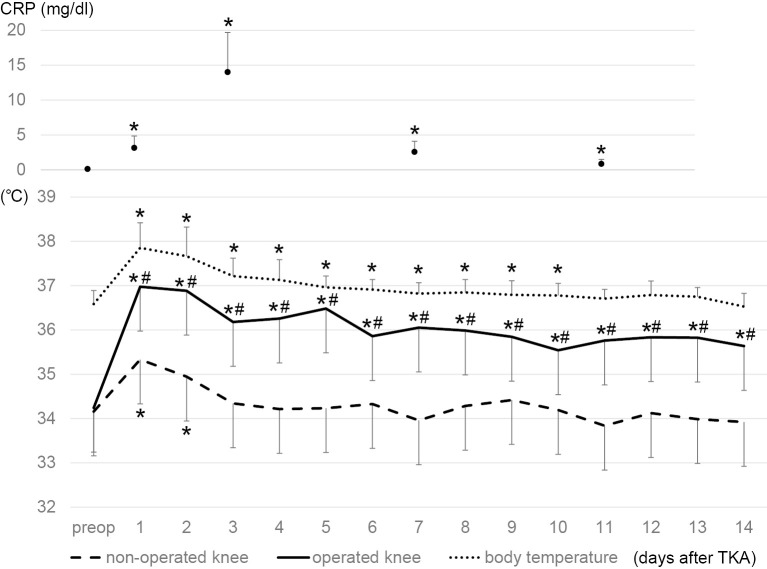
1. Time course of deep tissue temperature after TKA (Figure 1)
Figure 1.
Time courses of deep tissue temperature around the knee joints, body temperature, and C-reactive protein (CRP). Deep tissue temperature was measured with the probe that can measure the tissue temperature at the depth of 1 cm subcutaneous. Values are mean ± SD. *P<0.05 compared with the baseline data at admission (preop) by paired t test. #p < 0.05 compared with the temperature in the non-operated knee at the same postoperative day by paired t test.
There was no significant difference in preoperative deep tissue temperature at admission between the operated and non-operated control knees. After TKA, deep temperature around the control knee increased at day 1 and thereafter decreased and reached the preoperative levels by day 3. Compared with the control knee, the joint with TKA showed a greater increase in deep temperature at days 1 and 2, followed by a gradual decrease by day 14 at discharge when the temperature was still higher than the baseline at admission. Deep temperature around the joint with TKA was significantly higher at each postoperative day through the entire time course than the preoperative temperature around the same knee and the temperature around the control knee at the same postoperative day (Figure 1).
2. Association between deep temperature change and knee ROM recovery at discharge
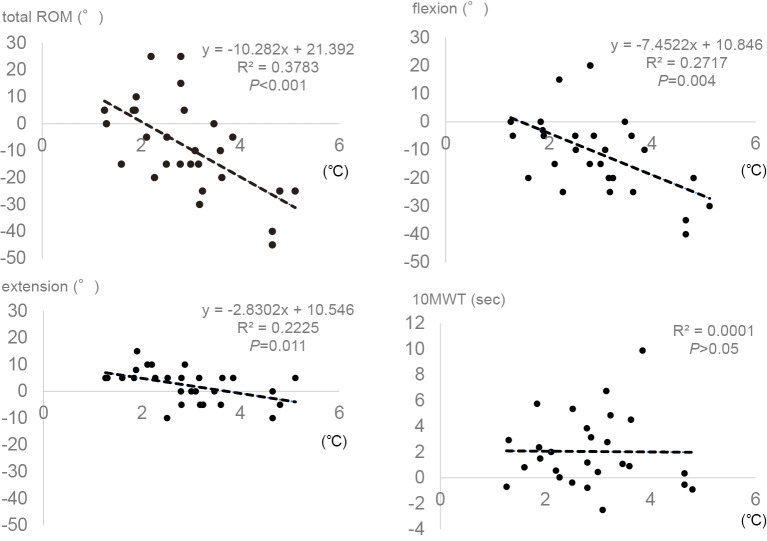
We calculated the deep temperature change around the operated knee by subtracting the baseline preoperative temperature at admission from the highest postoperative one. The scatter plots (Figure 2) demonstrated a significant association between the deep temperature change and each recovery of flexion, extension, and total ROM at day 14, although no improvement was found in comparison of knee motion between admission and discharge among all the 28 patients (Table 1). From the regression equation obtained (Figure 2), total ROM recovery was calculated to be almost zero when postoperative deep temperature change was 2°C. Thus, postoperative deep temperature change of 2°C could divide total ROM recovery into gain and loss. Of the 28 TKA patients, 6 and 22 patients showed less than and more than 2°C increases in deep tissue temperature, respectively. When demographic data at admission were compared between the two groups classified on the basis of the deep temperature change, there was no significant difference between the groups. In addition, no difference in the body temperature and CRP at days 1 and 3, respectively, was found between the groups (Table 2). In contrast to one of the 6 patients with less than 2°C increase, 17 of the 22 patients with more than 2°C increase in postoperative deep temperature showed total ROM recovery loss at day 14. Thus, more than 2°C increase in postoperative deep temperature significantly correlated with the incidence of total ROM recovery loss in the TKA patients at day 14 (Table 3). When ROM were compared between admission and discharge, the TKA group with more than 2°C increase in postoperative deep temperature resulted in a significant decrease in total ROM and flexion at day 14. The group with less than 2°C increase showed a significant increase in extension at day 14 (Table 4).
Figure 2.
Linear regression analyses of postoperative deep temperature change with knee motion recovery and postoperative effects on 10-meter fast speed walking test (10MWT). Deep temperature change around the operated knee was calculated by subtracting the baseline temperature at admission from the highest postoperative one. Postoperative recovery of range of motion (ROM) in the operated knee and postoperative effects on 10MWT were evaluated by subtracting the baseline data at admission from the data at discharge.
Table 2.
Comparison of demographic data between the two groups classified on the basis of postoperative deep temperature change
| deep temperature change | <2℃ | >2℃ | P |
|---|---|---|---|
| Values are expressed as mean±SD. After deep temperature change was calculated by subtracting the baseline temperature at admission from the highest postoperative one, 28 TKA patients were classified into two groups, <2℃ (n=6) and >2℃ (n=22). The data on the operated knee motion are shown. After Shapiro-Wilk test of normality, data were compared by t test (*) and Mann-Whitney test (**) for parametric and nonparametric tests, respectively. BMI: body mass index, ROM: range of motion, CRP: C-reactive protein. | |||
| sex (female/male) | 4/2 | 16/6 | |
| age (years) | 79.8±6.5 | 75.6±7.1 | 0.206* |
| BMI (kg/m2) | 25.1±1.6 | 25.2±3.6 | 0.984* |
| flexion at admission (°) | 113±12 | 120±16 | 0.234** |
| extension at admission (°) | –13.3±6.1 | –12.3±6.3 | 0.716* |
| total ROM at admission (°) | 100±16 | 108±20 | 0.502* |
| body temperature at day 1 (℃) | 37.8±0.4 | 37.9±0.6 | 0.815* |
| CRP at day 3 (mg/dl) | 14.0±5.1 | 14.0±5.9 | 0.993* |
Table 3.
Comparison between deep temperature change and knee motion recovery conditions
| total ROM recovery | deep temperature change | P* | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| <2℃ | >2℃ | |||||
| After deep tissue temperature change was calculated by subtracting the baseline temperature at admission from the highest postoperative one, 28 TKA patients were classified into two groups, <2℃ (n=6) and >2℃ (n=22). When total range of motion (ROM) in the joint with total knee arthroplasty was greater or equal at discharge than that at admission, total ROM recovery was considered as gain. In contrast, when total ROM was smaller at discharge, total ROM recovery was considered as loss. *Comparison by Fisher’s exact test. | ||||||
| discharge | gain | 5 | 5 | 0.013 | 17.0 | 1.59-181 |
| loss | 1 | 17 | ||||
| 1-year followup | gain | 6 | 16 | 0.198 | ||
| loss | 0 | 6 | ||||
Table 4.
Comparison of knee motion recovery on the basis of postoperative deep temperature change
| <2℃ | >2℃ | |||||
|---|---|---|---|---|---|---|
| admission | discharge | 1-year | admission | discharge | 1-year | |
| Values are mean±SD. After deep tissue temperature change was calculated by subtracting the baseline temperature at admission from the highest postoperative one, 28 TKA patients were classified into two groups, <2℃ (n=6) and >2℃ (n=22). The data on the operated knee motion are shown. *P<0.05 vs the data at admission in the same group by paired t test. | ||||||
| flexion | 113±12 | 108±12 | 122±8.8* | 120±16 | 107±15* | 118±7.8 |
| extension | –13.3±6.1 | –6.2±4.3* | –0.8±2.0* | –12.3±6.3 | –11.4±5.6 | –1.8±3.3* |
| total ROM | 100±16 | 102±15 | 121±9.7* | 108±19 | 96±18* | 116±9.7* |
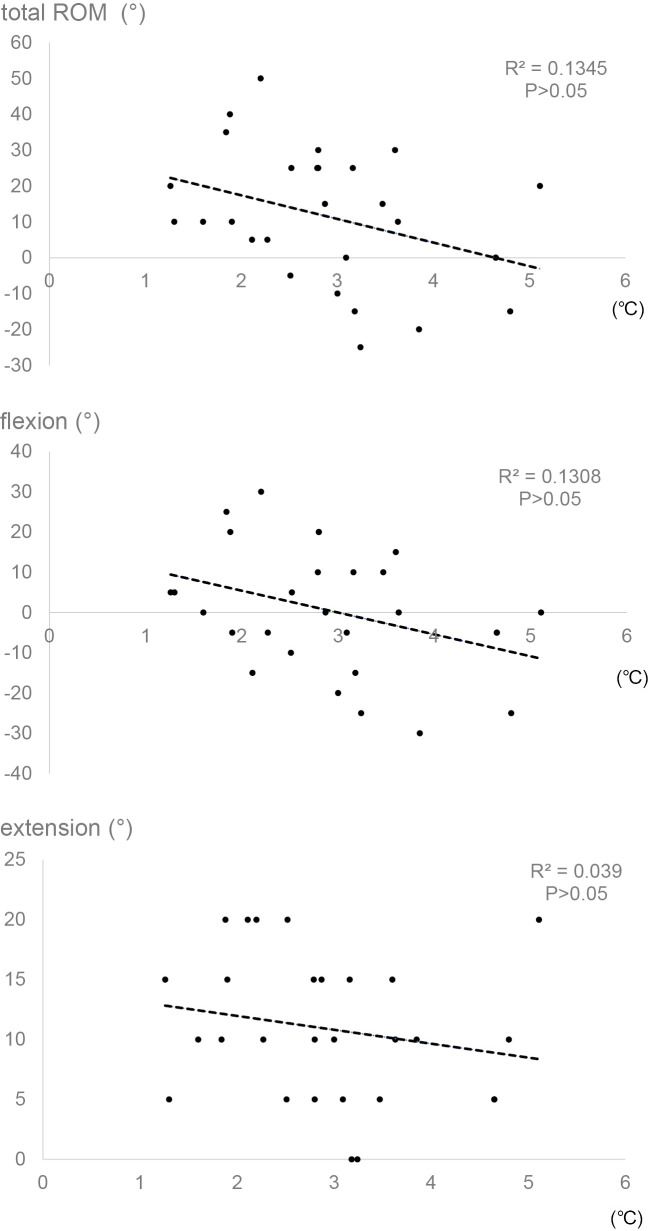
3. Association between deep temperature change and knee ROM recovery at 1-year followup
At 1-year followup, a significant increase in extension and total ROM was found in comparison of knee motion between admission and discharge among all the 28 patients (Table 1). However, there was no association between the deep temperature change and each recovery of flexion, extension, and total ROM (Figure 3). Every patient with less than 2°C increase in postoperative deep temperature showed total ROM recovery gain, while 16 of the 22 patients with more than 2°C increase exhibited total ROM recovery gain at 1-year followup (Table 3). Whereas the TKA group with less than 2°C increase in postoperative deep temperature resulted in a significant gain in flexion, extension, and total ROM, the group with more than 2°C increase showed a significant increase in extension and total ROM at the followup (Table 4).
Figure 3.
Linear regression analyses between postoperative deep temperature change and knee motion recovery at 1-year followup. Deep temperature change around the operated knee was calculated by subtracting the baseline temperature at admission from the highest postoperative one. Postoperative recovery of ROM in the operated knee was evaluated by subtracting the baseline data at admission from the data at 1-year followup.
4. No association of deep temperature change with 10MWT
Significant delay was found in 10MWT at the time of discharge compared with admission (Table 1). The scatter plots showed marginal association between the deep temperature change and the postoperative effects on 10MWT at day 14 (Figure 2).
Discussion
This study has demonstrated the first data on natural time course of deep tissue temperature around the joint with TKA. Surgical intervention-induced inflammation resulted in a significant increase in deep temperature in the operated knee. In the previous studies using a flexible catheter probe inserted invasively into the knee joint cavity with various arthritic diseases, intraarticular and skin surface baseline temperatures are 35-36°C and 31-33°C in average, respectively13). Similar studies in the healthy knee joint using the same method have shown that intraarticular and skin surface baseline temperatures are 32-33°C and 27-29°C in average, respectively14). Skin surface temperature is lower than the intraarticular temperature, probably because skin can cool and rewarm at a faster rate15). In contrast to skin surface temperature, the baseline deep temperature of 34.2°C in average in the present series of osteoarthritic knee joints is considered to be compatible with the intraarticular temperature. Therefore, the deep tissue temperature that can be non-invasively measured on the basis of zero-heat-flow method12) may be a surrogate marker for evaluating the intraarticular temperature in the knee.
This study has clearly shown association between deep temperature change and knee ROM recovery in the early phase after TKA, which may support a role of cryotherapy in early ROM gain after TKA. Cryotherapy after TKA has been employed as standard care in some facilities yet infrequently used in others16,17). There is evidence that cryotherapy in combination with compression for 3 days after TKA improves ROM during postoperative 7-21 days6). In addition, a meta-analysis evaluating the efficacy of cryotherapy within 48 hours after TKA has revealed that cryotherapy has benefits for early ROM recovery at discharge18). However, no studies have measured the intraarticular or deep tissue temperature before and after cryotherapy, or have compared alterations in the temperatures between the knees with and without cryotherapy. Because the operated knees with more than 2°C increase in deep temperature may result in poor recovery of total ROM at discharge, it is likely that cryotherapy is applied to keep the deep temperature change within 2°C for the first and second days after TKA. Our observations of postoperative alterations in deep tissue temperature around the knee joint with TKA during the time period of 14 days indicate a basis for further investigation on the establishment of appropriate cryotherapy after TKA. What causes different postoperative increases in deep tissue temperature around the knee among patients remains to be elucidated.
Limitations of our study include relatively small number of cases studied. At three months after TKA, one study has shown that cryotherapy provides a favorable effect on knee flexion19) whereas the other has demonstrated no effect by cryotherapy5). We found no association of deep tissue temperature change with ROM recovery at one year after TKA. From the results of the meta-analysis18) and the present study, cryotherapy could improve knee motion in the early postoperative phase. Early gain in knee motion by cryotherapy may speed up the time for mobilization because restricted knee ROM potentially impedes early medical rehabilitation. Future prospective studies with more patients should be conducted to address this issue. Another limitation is different thickness of the soft tissue surrounding the knee joint in different patients. Because thermal conductivity is different between adipose tissue and muscle, deep tissue temperature at the depth of 1 cm could differently reflect the intraarticular temperature among patients. However, the patients enrolled in the present study exhibited no difference in BMI between the operated knees with less than and more than 2°C increase in deep temperature.
Conclusion
This study is the first to demonstrate alterations in deep tissue temperature around the knee after TKA. Significant association was found between deep temperature change and postoperative ROM recovery. TKA patients with more than 2°C increase in postoperative deep temperature could result in poor ROM recovery in the early phase. The present results may provide the basic data to establish adequate procedures of cryotherapy after TKA for early gain in knee motion.
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- 1. Insall JN, Dorr LD, et al.: Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989; 248: 13-14. [PubMed] [Google Scholar]
- 2. Lowdon BJ and Moore RJ: Determinants and nature of intramuscular temperature changes during cold therapy. Am J Phys Med. 1975; 54: 223-233. [PubMed] [Google Scholar]
- 3. Martin SS, Spindler KP, et al.: Does cryotherapy affect intraarticular temperature after knee arthroscopy? Clin Orthop Relat Res. 2002; 400: 184-189. [DOI] [PubMed] [Google Scholar]
- 4. Levy AS and Marmar E: The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993; 297: 174-178. [PubMed] [Google Scholar]
- 5. Webb JM, Williams D, et al.: The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998; 21: 59-61. [DOI] [PubMed] [Google Scholar]
- 6. Kullenberg B, Ylipää S, et al.: Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006; 21: 1175-1179. [DOI] [PubMed] [Google Scholar]
- 7. Morsi E: Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002; 17: 718-722. [DOI] [PubMed] [Google Scholar]
- 8. Gibbons CE, Solan MC, et al.: Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001; 25: 250-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Healy WL, Seidman J, et al.: Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994; 299: 143-146. [PubMed] [Google Scholar]
- 10. Scarcella JB and Cohn BT: The effect of cold therapy on the postoperative course of total hip and knee arthroplasty patients. Am J Orthop (Belle Mead NJ). 1995; 24: 847-852. [PubMed] [Google Scholar]
- 11. Smith J, Stevens J, et al.: A randomized, controlled trial comparing compression bandaging and cold therapy in postoperative total knee replacement surgery. Orthop Nurs. 2002; 21: 61-66. [DOI] [PubMed] [Google Scholar]
- 12. Fox RH and Solman AJ: A new technique for monitoring the deep body temperature in man from the intact skin surface. J Physiol. 1971; 212: 8-10. [PubMed] [Google Scholar]
- 13. Oosterveld FG and Rasker JJ: Effects of local heat and cold treatment on surface and articular temperature of arthritic knees. Arthritis Rheum. 1994; 37: 1578-1582. [DOI] [PubMed] [Google Scholar]
- 14. Oosterveld FG, Rasker JJ, et al.: The effect of local heat and cold therapy on the intraarticular and skin surface temperature of the knee. Arthritis Rheum. 1992; 35: 146-151. [DOI] [PubMed] [Google Scholar]
- 15. Merrick MA, Knight KL, et al.: The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993; 28: 236-245. [PMC free article] [PubMed] [Google Scholar]
- 16. Barry S, Wallace L, et al.: Cryotherapy after total knee replacement: a survey of current practice. Physiother Res Int. 2003; 8: 111-120. [DOI] [PubMed] [Google Scholar]
- 17. Naylor J, Harmer A, et al.: Status of physiotherapy rehabilitation after total knee replacement in Australia. Physiother Res Int. 2006; 11: 35-47. [DOI] [PubMed] [Google Scholar]
- 18. Addie S, Naylor JM, et al.: Cryotherapy after total knee arthroplasty a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2010; 25: 709-715. [DOI] [PubMed] [Google Scholar]
- 19. Walker RH, Morris BA, et al.: Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation, and continuous cooling pad following total knee arthroplasty. J Arthroplasty. 1991; 6: 151-156. [DOI] [PubMed] [Google Scholar]