Abstract
Objectives
Minimally important changes (MICs) for SPondyloArthritis Research Consortium of Canada (SPARCC) MRI scores are ⩾2.5 for SI joint and ⩾5 for spine. This post hoc analysis assessed achievement of MIC in SPARCC scores in biologic-naïve patients with AS treated with tofacitinib or placebo, and correlation with clinical responses.
Methods
Adult AS patients in a 12-week phase 2 study (n = 207) were randomized 1: 1: 1: 1 to tofacitinib 2, 5 or 10 mg twice daily (BID) or placebo. MIC in SPARCC SI joint and spine scores were assessed for patients with available MRI data (N = 164; 79%). Clinical endpoints at week 12, including Assessment of SpondyloArthritis international Society 20% improvement (ASAS20), were compared between patients achieving/not achieving MIC.
Results
A greater proportion of patients achieved MIC with tofacitinib 2, 5 and 10 mg BID vs placebo for SI joint (28.6, 38.6, 29.6 vs 11.8%) and spine scores (29.3, 36.4, 40.9 vs 11.8%). Generally, a greater proportion of patients treated with tofacitinib 2, 5 and 10 mg BID or placebo, respectively, who achieved MIC for SI joint and spine scores achieved ASAS20 (SI joint: 75.0, 88.2, 69.2, 75.0%; spine: 91.7, 85.7, 72.2, 75.0%) vs patients who did not achieve MIC (SI joint: 51.7, 84.0, 58.1, 48.3%; spine: 46.4, 85.7, 53.8, 48.3%). Numerically greater responses were seen in those patients achieving vs not achieving MIC across a range of other efficacy assessments.
Conclusion
Approximately one-third of tofacitinib-treated AS patients experienced clinically meaningful reductions in spinal MRI inflammation at week 12. Patients achieving MIC for MRI inflammation had greater clinical response.
Keywords: ankylosing spondylitis, inflammation, magnetic resonance imaging, minimally important change, sacroiliac joints, spine, SPondyloArthritis Research Consortium of Canada, tofacitinib
Rheumatology key messages
Approximately one-third tofacitinib-treated AS patients experienced clinically meaningful spinal MRI inflammation reductions at week 12.
Almost three times more tofacitinib-treated AS patients achieved the minimally important change in MRI inflammation versus placebo.
Greater clinical response was observed in AS patients achieving the minimally important change for MRI inflammation.
Introduction
AS is a chronic, immune-mediated systemic inflammatory disease of the axial skeleton [1]. MRI can be used to assess inflammation in the SI joint and spine in patients with AS based on SPondyloArthritis Research Consortium of Canada (SPARCC) scores [2, 3]. Unlike many clinical assessments for AS, which rely on subjective measures of disease symptoms, MRI scoring provides an objective measure of inflammation and disease activity. Although MRI assessments have been shown to be highly discriminatory between treatments in studies of TNF inhibitors (TNFi) vs placebo [4–6], the correlation between improvements in MRI and clinical outcomes is less well-defined [4, 7–10]. Minimally important change (MIC) can be a helpful concept to understand the number of patients showing meaningful change and can be applied to both clinical and imaging parameters so that associations in treatment responses can be evaluated more readily by clinicians.
The MICs for SPARCC SI joint and spine scores in patients with AS have previously been established using data from a randomized, controlled study of adalimumab vs placebo using an anchor-based approach, with a global evaluation of change (no change vs change in overall MRI) based on expert radiologist opinion as the external anchor [11]. Receiver-operating characteristic curves were used to determine the MIC as ⩾2.5-point change for SI joint scores, and ⩾five-point change for spine scores.
Tofacitinib is an oral Janus kinase (JAK) inhibitor. It preferentially inhibits signaling via JAK3 and/or JAK1 with functional selectivity over JAK2 [12]. As such, tofacitinib affects signaling via IL-17, IL-21 and IL-23, thereby modulating immune responses and reducing or preventing inflammation [13]. The efficacy and safety of tofacitinib have been investigated in a phase 2 study in biologic-naïve patients with active AS [14]. This study demonstrated that tofacitinib 5 and 10 mg twice daily (BID) provided greater efficacy vs placebo in reducing signs and symptoms of active AS over 12 weeks based on clinical assessments. MRI assessments showed significantly greater improvements from baseline in SPARCC SI joint and spine scores at week 12 with tofacitinib compared with placebo [14].
The aim of the present analysis was to assess the proportion of patients achieving MIC in SPARCC SI joint and spine scores with tofacitinib vs placebo in patients with AS who participated in the first phase 2 study of tofacitinib for AS, and to determine whether achievement of MRI MIC corresponded with clinical improvements.
Methods
Study design and patients
This was a post hoc analysis of data from a 16-week (12-week treatment; 4-week washout/off-treatment follow-up period), phase 2, multicentre, randomized, double-blind, placebo-controlled, dose-ranging study (clinicaltrials.gov: NCT01786668). Patient inclusion and exclusion criteria have been described previously [14]. Briefly, patients were aged ⩾18 years, fulfilled the modified New York criteria for AS [15] confirmed by centralized reading of sacroiliac radiographs, had active disease based on BASDAI score ⩾4 and total back pain score ⩾4, and had an inadequate response or intolerance to NSAID drug therapy. Patients who were receiving current treatment, or who had received prior treatment with biologic DMARDs were excluded. Patients were permitted to continue current treatment with MTX, SSZ and stable oral corticosteroids (⩽10 mg/day prednisone or equivalent). There were no eligibility criteria relating to MRI scores.
Eligible patients were randomized 1: 1: 1: 1 to receive tofacitinib 2 mg, tofacitinib 5 mg, tofacitinib 10 mg or placebo BID. Patients received study treatment from baseline to week 12, followed by a 4-week off-treatment follow-up period. The study was conducted in accordance with applicable legal and regulatory requirements, and the general principles set forth in the International Ethical Guidelines for Biomedical Research Involving Human Subjects, International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent. Institutional review boards or independent ethics committees at each investigational centre approved the study. This post hoc analysis did not require additional approval; the international classification of diseases for the study covered the use of data outside of the primary objectives of the study, permitting the re-analysis of study results at a later date for future research.
MRI assessments of the SI joint and spine
The first MRI assessment for each patient was performed after screening and at least 7 days prior to the baseline visit and the first dose of study medication. A second MRI assessment was performed within 7 days prior to the week 12 visit, while patients were still receiving study medication. For patients who withdrew at, or after week 6 of the study, an MRI was obtained within 10 days of the last dose of study medication; patients who withdrew from the study prior to week 6 did not have a second MRI assessment.
The MRI exam for the spine and SI joint consisted of a T1-weighted spin echo series and a short T1 inversion recovery series. SPARCC scores were based on the short T1 inversion recovery sequence with the T1-weighted scans used for anatomical reference. SPARCC SI joint scores were based on the measurement of six consecutive slices, with each slice scored 0–12 for oedema, intensity and depth to give a total score of 0–72, with a higher score indicating greater inflammation [3]. Spine scores were based on the measurement of three consecutive sagittal slices per discovertebral unit, each scored 0–6 for oedema, intensity and depth to give a total score of 0–18, with a higher score indicating greater inflammation [2]. The sum of the six most severely affected discovertebral units was used for the SPARCC spine score, to give an overall range of 0–108 [2].
MRI scoring was conducted by two central readers, who assessed the scans independently and were blinded to time sequence and treatment. Discrepancies between the two readers were adjudicated by a third trained reader, blinded to the results of the two initial readers and visit. Discrepancies included: cases read by one reader and set as unreadable by the other reader; changes in SPARCC scores in different directions that differed by ⩾6 points; and changes in SPARCC scores in the same direction that differed by ⩾14 points. For cases requiring adjudication, the average score of the closest two of the three values was used as the final result for this study. The reliability of SPARCC scoring between readers was assessed for the total study population using intra-class correlation coefficients (ICC). The ICC for all SI joint scores was 0.73 and for spine scores was 0.90. The ICC for the total change scores between time points was 0.69 for SI joint scores and 0.61 for spine scores.
The number of patients achieving MIC in SPARCC SI joint and spine scores was determined based on the previously defined cut-offs of ⩾2.5-point change for SI joint scores and ⩾5-point change for spine scores [11]. The number of patients achieving MRI remission, defined as SI joint score <2 [16] and spine score <3, was also assessed.
Clinical assessments
Clinical assessments at week 12 included the proportions of patients achieving Assessment of SpondyloArthritis international Society 20% improvement (ASAS20), ASAS 40% improvement (ASAS40), AS DAS major improvement (ASDAS MI; change ⩾2.0 from baseline), ASDAS clinically important improvement (ASDAS CII; change ⩾1.1 from baseline), ASDAS inactive disease (ASDAS ID; <1.3) and ASDAS moderate disease activity (⩾1.3 to <2.1), and mean changes from baseline in ASDAS, BASDAI, BASFI and total back pain (numeric rating scale; 0 [no pain] –10 [most severe pain]).
Statistical analyses
Observed data are presented for patients who received tofacitinib 2 mg BID, tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo. Concordance between patients achieving MIC in SPARCC SI joint and spine scores and week 12 clinical response was summarized by treatment group. Pooled data for the tofacitinib 5 and 10 mg BID doses are also presented to provide a larger sample size for conducting meaningful statistical analyses. The two higher tofacitinib doses were chosen for this pooled analysis as these were deemed to be the most clinically relevant based on the primary and secondary study outcomes [14]. The association between achieving the MIC for SPARCC scores and clinical response at week 12 for the pooled tofacitinib 5 and 10 mg BID group was analysed using Fisher’s exact tests for binary outcomes and two-sample t-tests for continuous outcomes.
Results
Patients
Of the 207 patients who participated in the phase 2 study, 164 patients had MRI data at baseline and follow-up (week 12 or study withdrawal) and were included in this analysis. Follow-up MRI data were not available for 43 patients (16 patients had no baseline MRI; data for five patients were not available due to technical issues; two patients withdrew prior to MRI completion; two patients had a visit deviation for MRI; no reason for missing MRI was provided for 18 patients). For the patients included in this analysis, baseline demographics and characteristics were generally similar across treatment groups (Table 1) and were consistent with those of the full study population [14].
Table 1.
Baseline demographics and disease characteristics for patients with MRI data at baseline and follow-upa
| Patient characteristic | Tofacitinib 2 mg BID, | Tofacitinib 5 mg BID, | Tofacitinib 10 mg BID, | Placebo |
|---|---|---|---|---|
| n = 42 | n = 44 | n = 44 | n = 34 | |
| Gender, male, n (%) | 27 (64.3) | 32 (72.7) | 31 (70.5) | 24 (70.6) |
| Age, mean (s.d.), years | 41.1 (11.9) | 41.2 (10.3) | 39.5 (11.0) | 41.5 (13.4) |
| Race, white, n (%) | 30 (71.4) | 35 (79.5) | 35 (79.5) | 28 (82.4) |
| HLA-B27+, n (%) | 37 (88.1) | 38 (86.4) | 42 (95.5) | 28 (82.4) |
| BMI, mean (s.d.), kg/m2 | 25.6 (4.9) | 26.2 (5.0) | 25.5 (3.8) | 27.2 (6.3) |
| Disease duration since diagnosis, median, years | 4.4 | 3.5 | 1.9 | 3.2 |
| Concomitant csDMARDs, n (%) | 20 (47.6) | 14 (31.8) | 13 (29.5) | 10 (29.4) |
| hsCRP ≥ULN 0.287 mg/dl, n (%) | 30 (71.4) | 35 (79.5) | 32 (72.7) | 24 (70.6) |
| hsCRP ≥ULN 0.5 mg/dl, n (%) | 26 (61.9) | 30 (68.2) | 28 (63.6) | 18 (52.9) |
| ASDAS, mean (s.d.) | 3.6 (0.8) | 3.7 (0.9) | 3.7 (0.8) | 3.7 (0.8) |
| BASDAI, mean (s.d.) | 6.5 (1.3) | 6.4 (1.7) | 6.6 (1.4) | 6.7 (1.6) |
| BASFI, mean (s.d.) | 5.4 (1.8) | 5.8 (2.3) | 5.6 (2.5) | 5.5 (2.5) |
| SPARCC SI joint score, mean (s.d.) | 13.3 (15.4) | 12.7 (15.4) | 11.4 (15.0) | 8.0 (13.5) |
| SPARCC spine score, mean (s.d.) | 14.9 (15.7) | 19.2 (18.7) | 17.3 (21.2) | 16.1 (18.5) |
Week 12 or study withdrawal. BID: twice daily; csDMARD: conventional synthetic DMARD; hsCRP: high-sensitivity CRP; SPARCC: SPondyloArthritis Research Consortium of Canada; ULN: upper limit of normal.
Patients achieving MIC in SPARCC SI joint and spine scores
Changes from baseline in SPARCC SI joint and spine scores at week 12 have previously been reported for this study [14]. At week 12, mean reductions from baseline in SI joint scores were significantly greater with tofacitinib 5 and 10 mg compared with placebo, and mean reductions from baseline in spine scores were significantly greater with all tofacitinib doses compared with placebo.
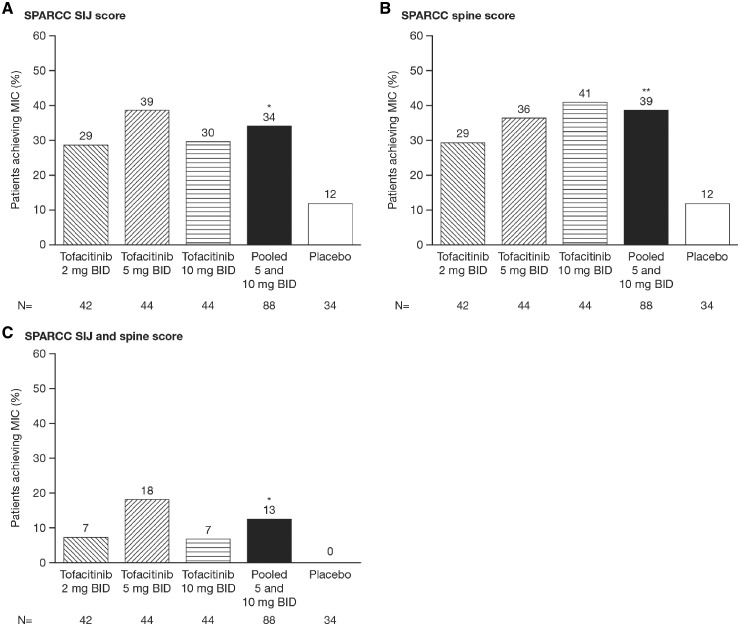
In the present analysis of patients with MRI data at baseline and follow-up, after 12 weeks of treatment, greater proportions of patients achieved reduction in MRI inflammation according to the MIC in SPARCC SI joint scores with tofacitinib 2 mg BID (28.6%), 5 mg BID (38.6%) and 10 mg BID (29.5%) compared with placebo (11.8%) (Fig. 1A). Similarly, greater proportions of patients achieved reduction in MRI inflammation according to the MIC in SPARCC spine scores with tofacitinib 2 mg BID (29.3%), 5 mg BID (36.4%) and 10 mg BID (40.9%) compared with placebo (11.8%) (Fig. 1B). No placebo-treated patients achieved the MIC for both SPARCC SI joint and spine scores, compared with 7.3% of patients treated with tofacitinib 2 mg BID, 18.2% with 5 mg BID and 6.8% with 10 mg BID (Fig. 1C). Statistical analysis for the pooled tofacitinib 5 and 10 mg BID group showed significantly greater proportions of patients achieving MIC in SPARCC SI joint (34.1%), spine (38.6%) and both SI joint and spine (12.5%) scores compared with placebo (11.8, 11.8 and 0%, respectively; all P < 0.05).
Fig. 1.
Proportion of patients achieving the MIC at week 12
(A) SPARCC SI joint score, (B) SPARCC spine score and (C) both SPARCC SI joint and spine score. *P < 0.05; **P < 0.01 vs placebo. BID: twice daily; MIC: minimally important change; n: number of patients in treatment group; SPARCC: SPondyloArthritis Research Consortium of Canada.
Relationship between MIC for SPARCC scores and clinical assessments
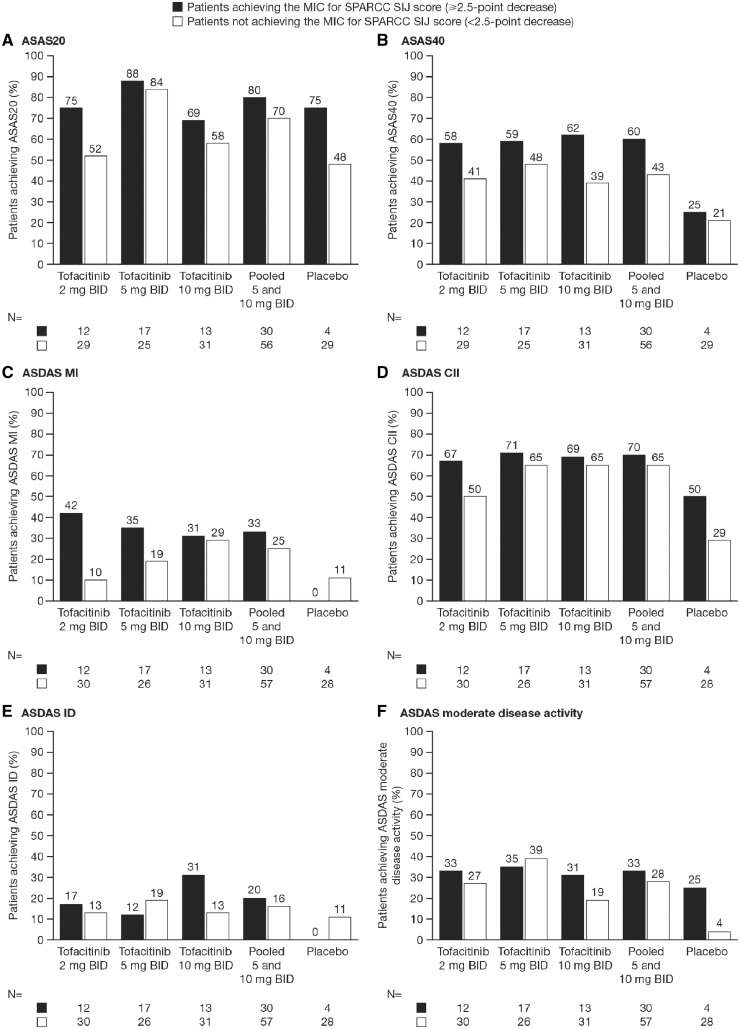
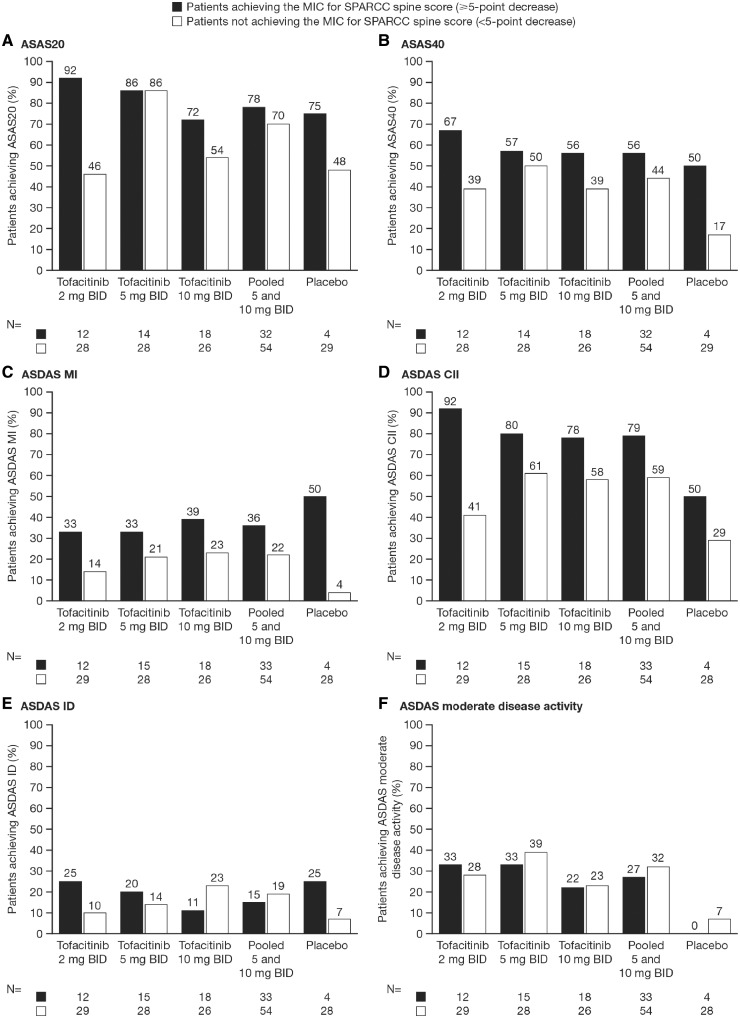
Across all treatment groups, this descriptive analysis showed that a greater proportion of patients who achieved the MIC in SPARCC SI joint or spine scores also achieved ASAS20 and ASAS40 responses compared with those patients who did not achieve the MIC in SPARCC SI jointor spine scores (Figs 2 and 3, respectively). A greater proportion of patients treated with tofacitinib who achieved the MIC in SPARCC SI joint or spine scores also achieved ASDAS MI and ASDAS CII compared with those patients who did not achieve MIC. However, the results were less clear for ASDAS ID and ASDAS moderate disease activity; clinical response was greater in patients achieving MIC in SI joint and spine scores in some, but not all, tofacitinib dose groups (Figs 2 and 3). In general, numerically more patients in the pooled 5 and 10 mg BID group who achieved MIC in SI joint or spine also achieved clinical responses; however, these differences did not reach statistical significance for any clinical outcome. The results for the placebo group varied more widely, which is likely to be due to the limited number of patients (n = 4) who achieved MIC for SI joint or spine scores, leading to high variability in this group.
Fig. 2.
Relationship between patients achieving MIC for SPARCC SI joint score and clinical responses at week 12
Clinical responses measured by (A) ASAS20, (B) ASAS40, (C) ASDAS MI, (D) ASDAS CII, (E) ASDAS ID and (F) ASDAS moderate disease activity. Statistical analysis for the pooled 5 and 10 mg BID group using Fisher’s exact test showed no significant association between MIC and any clinical response SI joint ≥MIC (2.5): patients achieving the MIC for SPARCC SI joint score (≥2.5-point decrease) SI joint <MIC (2.5): patients not achieving the MIC for SPARCC SI joint score (<2.5-point decrease). ASAS: Assessment of SpondyloArthritis international Society; BID: twice daily; CII: clinically important improvement; ID: inactive disease; MI: major improvement; MIC: minimally important change; n: number of patients achieving/not achieving MIC; SPARCC: SPondyloArthritis Research Consortium of Canada.
Fig. 3.
Relationship between patients achieving MIC for SPARCC spine score and clinical responses at week 12
Clinical responses measured by (A) ASAS20, (B) ASAS40, (C) ASDAS MI, (D) ASDAS CII, (E) ASDAS ID and (F) ASDAS moderate disease activity. Statistical analysis for the pooled 5 and 10 mg BID group using Fisher’s exact test showed no significant association between MIC and any clinical response ASAS. ASAS: Assessment of SpondyloArthritis international Society; BID: twice daily; CII: clinically important improvement; ID: inactive disease; MI: major improvement; MIC: minimally important change; n: number of patients achieving/not achieving MIC; SPARCC: SPondyloArthritis Research Consortium of Canada.
Mean improvements from baseline in ASDAS, BASDAI, BASFI and total back pain were generally numerically greater across all treatment groups in patients who achieved the MIC in SPARCC SI joint and spine scores compared with those who did not achieve the MIC (Table 2). Statistical analysis for the pooled tofacitinib 5 and 10 mg BID dose group showed a significant difference in change from baseline in BASDAI between patients achieving the MIC in SI joint score and those not achieving the MIC; numerical differences were observed for ASDAS, BASFI and total back pain score, but did not reach statistical significance.
Table 2.
Mean clinical end point changes from baseline in patients achieving/not achieving MIC at week 12
| Change in | Tofacitinib | Tofacitinib | Tofacitinib | Pooled tofacitinib | Placebo |
|---|---|---|---|---|---|
| disease outcome | 2 mg BID | 5 mg BID | 10 mg BID | 5 and 10 mg BID | |
| ΔASDAS, mean (s.d.) | |||||
| SI joint ≥MIC (2.5) | −1.7 (1.2) | −1.6 (0.8) | −1.6 (1.2) | −1.6 (1.0) | −1.0 (0.7) |
| n | 12 | 17 | 13 | 30 | 4 |
| SI joint <MIC (2.5) | −1.1 (0.9) | −1.4 (0.8) | −1.4 (0.7) | −1.4 (0.8) | −0.7 (0.9) |
| n | 30 | 26 | 31 | 57 | 28 |
| Spine ≥MIC (5) | −1.9 (0.8) | −1.6 (0.8) | −1.8 (0.7) | −1.7 (0.8) | −1.3 (1.8) |
| n | 12 | 15 | 18 | 33 | 4 |
| Spine <MIC (5) | −1.1 (1.0) | −1.4 (0.9) | −1.3 (0.9) | −1.4 (0.9) | −0.6 (0.7) |
| n | 29 | 2.8 | 26 | 54 | 28 |
| ΔBASDAI, mean ( s.d.) | |||||
| SI joint ≥MIC (2.5) | −4.0 (2.6) | −3.3 (1.8) | −3.8 (2.0) | −3.5 (1.9) | −2.5 (1.7) |
| n | 12 | 17 | 13 | 30* | 4 |
| SI joint <MIC (2.5) | −2.5 (2.1) | −2.8 (1.6) | −2.6 (1.7) | −2.7 (1.6) | −1.8 (2.1) |
| n | 30 | 26 | 31 | 57 | 29 |
| Spine ≥MIC (5) | −4.2 (1.9) | −3.2 (2.1) | −2.7 (1.9) | −3.0 (2.0) | −3.8 (3.0) |
| n | 12 | 15 | 18 | 33 | 4 |
| Spine <MIC (5) | −2.4 (2.3) | −2.9 (1.5) | −3.1 (1.8) | −3.0 (1.6) | −1.6 (1.8) |
| n | 29 | 28 | 26 | 54 | 29 |
| ΔBASFI, mean ( s.d.) | |||||
| SI joint ≥MIC (2.5) | −2.8 (2.1) | −2.9 (1.8) | −2.4 (2.4) | −2.7 (2.0) | −0.8 (0.9) |
| n | 12 | 17 | 13 | 30 | 4 |
| SI joint <MIC (2.5) | −1.7 (1.9) | −2.4 (1.9) | −2.4 (2.0) | −2.4 (1.9) | −1.4 (1.8) |
| n | 30 | 26 | 31 | 57 | 29 |
| Spine ≥MIC (5) | −3.0 (1.9) | −3.0 (2.2) | −2.8 (2.0) | −2.9 (2.1) | −3.0 (2.7) |
| n | 12 | 15 | 18 | 33 | 4 |
| Spine <MIC (5) | −1.7 (1.9) | −2.4 (1.6) | −2.1 (2.1) | −2.2 (2.1) | −1.1 (1.5) |
| n | 29 | 28 | 26 | 54 | 29 |
| ΔTotal back pain, mean ( s.d.) | |||||
| SI joint ≥MIC (2.5) | −4.0 (2.8) | −3.8 (2.5) | −3.4 (3.3) | −3.6 (2.8) | −2.0 (1.8) |
| n | 12 | 17 | 13 | 30 | 4 |
| SI joint <MIC (2.5) | −2.6 (2.6) | −3.1 (1.9) | −2.6 (2.2) | −2.8 (2.1) | −1.8 (2.4) |
| N | 29 | 25 | 31 | 56 | 29 |
| Spine ≥MIC (5) | −4.6 (2.0) | −3.6 (2.1) | −3.5 (2.3) | −3.5 (2.1) | −4.3 (3.1) |
| n | 12 | 14 | 18 | 32 | 4 |
| Spine <MIC (5) | −2.4 (2.7) | −3.3 (2.3) | −2.4 (2.7) | −2.9 (2.5) | −1.5 (2.0) |
| n | 28 | 28 | 26 | 54 | 29 |
P < 0.05 vs patients not achieving the MIC using two-sample t test SI joint ≥MIC (2.5): patients achieving the MIC for SPARCC SI joint score (≥2.5-point decrease); SI joint <MIC (2.5): patients not achieving the MIC for SPARCC SI joint score (<2.5-point decrease) Spine ≥MIC (5): patients achieving the MIC for SPARCC spine score (≥5-point decrease); Spine <MIC (5): patients not achieving the MIC for SPARCC spine score (<5-point decrease). Δ: change from baseline; ASDAS: Ankylosing Spondylitis Disease Activity Score; BID: twice daily; MIC: minimally important change; SI joint: sacroiliac joint; SPARCC: SPondyloArthritis Research Consortium of Canada.
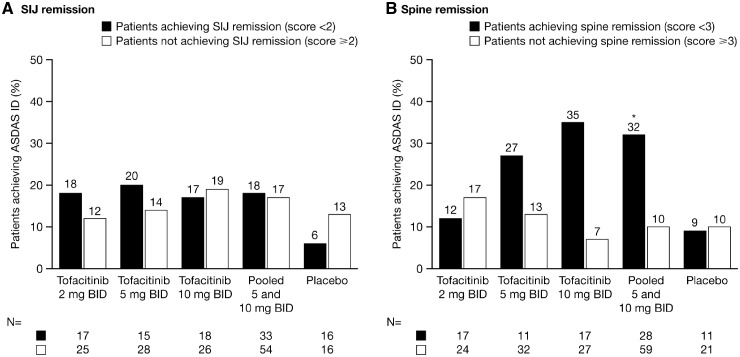
SI joint MRI remission, defined as a SPARCC score <2, was achieved by 40.5% of patients receiving tofacitinib 2 mg BID, 36.4% receiving 5 mg BID, 40.9% receiving 10 mg BID and 52.9% receiving placebo. Spine MRI remission, defined as a SPARCC score <3, was achieved by 40.5, 25.0, 38.6 and 32.4% of patients, respectively. There was no clear association between patients who achieved SI joint remission and those who achieved ASDAS ID (Fig. 4). A greater proportion of patients receiving tofacitinib 5 or 10 mg BID who achieved spine remission also achieved ASDAS ID; this association was statistically significant for the pooled tofacitinib 5 and 10 mg BID dose group (Fig. 4). Both SI joint and spine remission were achieved by nine (21.4%) patients who received tofacitinib 2 mg BID, three (6.8%) who received 5 mg BID, five (11.4%) who received 10 mg BID and six (17.6%) who received placebo. Among patients treated with tofacitinib 2, 5 and 10 mg BID, a greater proportion who achieved both SI joint and spine remission also achieved ASDAS ID (22.2, 33.3 and 20.0%, respectively) compared with those patients who did not achieve remission (12.1, 15.0 and 17.9%, respectively), however, conclusions are limited by the small numbers of patients who achieved both remission criteria.
Fig. 4.
Relationship between SPARCC SI joint and spine remission in patients achieving ASDAS ID at week 12
(A) SPARCC SI joint remission and (B) SPARCC spine remission. *P < 0.05 vs not achieving remission using Fisher’s exact test for any endpoint in the pooled 5 and 10 mg BID group. BID: twice daily; ID: inactive disease; MIC: minimally important change; n: number of patients achieving/not achieving MIC; SPARCC: SPondyloArthritis Research Consortium of Canada.
Discussion
MRI assessments provide an objective measure of inflammation and disease activity in patients with AS, and the SPARCC scoring system has been widely used and validated in AS [2, 3]. A 12-week phase 2 study of tofacitinib for the treatment of AS previously demonstrated significantly greater improvements from baseline in SPARCC SI joint and spine scores with tofacitinib 5 and 10 mg BID compared with placebo [14]. This post hoc analysis of MRI and clinical data from the phase 2 study showed that a greater proportion of tofacitinib-treated patients achieved the MIC in both SPARCC SI joint and spine scores compared with placebo. Clinically relevant reduction in spinal and SI joint inflammation was observed in ∼30% of patients treated with tofacitinib compared with 12% of placebo-treated patients.
In general, a numerically greater proportion of patients who achieved MIC in SPARCC SI joint and spine scores also achieved clinical response, based on ASAS and ASDAS assessments, compared with those patients who did not achieve MIC. Similarly, achievement of MIC in SPARCC SI joint and spine scores was associated with numerically greater improvements from baseline in ASDAS, BASDAI, BASFI and total back pain score across all treatment groups. While responses were greater in patients who achieved the MIC for SI joint and spine inflammation, these results did not reach statistical significance, and differences between groups were relatively small for some endpoints. Achievement of MRI remission appeared to be associated with clinical remission for spine scores. However, results were less conclusive for SI joint scores. A higher rate of MRI remission was observed in the placebo group compared with tofacitinib groups for SI joint scores, which is likely due to the lower baseline SI joint score for the placebo group (8.0) vs the tofacitinib groups (11.4–13.3). However, the difference in the overall proportion of patients achieving remission is unlikely to have influenced the association between MRI remission and clinical response. Findings for all endpoints were limited by the small numbers of patients in each individual treatment group, which resulted in a wide variability in responses.
Previous analyses of the association between objective MRI assessments and clinical measures have provided mixed results [4, 7–10, 17, 18], and several studies have reported a more consistent association between MRI assessment and markers of inflammation, such as CRP and serum CTX-1, compared with clinical assessments [8, 17, 18]. However, to date, only one study has evaluated the association between clinical measures and attainment of the MIC for MRI assessments. Maksymowych et al. [11] compared the proportion of patients achieving clinical response between those achieving or not achieving MIC for SPARCC scores following treatment with adalimumab or placebo, and reported a significant association in patients receiving placebo, but not in those receiving adalimumab.
The MICs for SPARCC scores provide valid measurements to assess the minimum detectable change in spinal and SI joint inflammation based on MRI assessments. However, the results presented here and previously [11] show limited correlation between MIC in MRI inflammation and clinical responses in established AS. This suggests that the MIC for SPARCC scores may not be suitable for use as a minimum clinically important difference, as reduction in inflammation does not appear to be directly linked to improvements in clinical assessments and patient outcomes. Understanding of the cause of symptoms in patients with AS is still limited, and it is possible that treatments for AS that provide improvement in clinical assessments and patient outcomes may have an impact on some symptoms of AS, such as mechanisms of pain, that are not related to the inflammation observed by MRI.
Structural progression could not be meaningfully assessed in this study owing to the short 12-week duration. Therefore, comparisons between MRI inflammation and structural progression were not possible. However, a recent study indicated that TNFi therapy can reduce structural damage progression in the spine in patients with AS, which is mediated by the effect of TNF inhibition on decreasing inflammation and disease activity [19]. Indeed, near-complete inhibition of spinal damage progression was observed in patients with inactive disease status, indicating that in order to stop structural damage progression, remission, rather than a reduction in clinical disease activity/inflammation, may be required [19]. Further evidence has shown that a reduction in inflammation of the SI joint (defined by SPARCC MRI score) with TNFi therapy is independently associated with a reduction in erosion (defined by SPARCC MRI SI joint structural score) over 2 years [20]. In addition, reduction in inflammation of the SI joint was observed as early as 12 weeks in a randomized placebo-controlled trial of etanercept in non-radiographic axial spondyloarthritis, and after 48 weeks an association was observed between decreases in SPARCC SI joint inflammation and reductions in erosion score [21].
A study by Weiß et al. [10] in patients with AS treated with etanercept or adalimumab showed a significant correlation between changes in BASDAI and SI joint score in patients with disease duration of <4 years, but a poor correlation in patients with longer disease duration. This suggests that in patients with longer disease duration, inflammation may not be the direct cause of AS symptoms, while inflammation may play a more direct role in patients with shorter disease duration. This is supported by findings from the Etanercept (ETN) Against a Placebo for Etanercept on a Background NSAIDs in the Treatment of Early SpA Patients Who do Not Have X-ray Structural Changes (EMBARK) study, which did show a correlation between clinical response and SPARCC SI joint scores in patients with early AS after 48 weeks of etanercept treatment [22].
This analysis is limited by the relatively short (12-week) study duration, and effects of tofacitinib on MRI inflammation and clinical responses in AS may differ over a longer treatment duration. For example, in the EMBARK study of etanercept for the treatment of AS, while limited correlations were observed between clinical response and SPARCC SI joint scores after 12 weeks of treatment, the strength of these correlations increased after 48 weeks [22]. In addition, it is possible that clinical responses over 12 weeks may be affected by the relatively high placebo response (40.1% for ASAS20 response) seen in the present study [14]. A further limitation was that the small number of patients included in this phase 2 study precluded statistical analysis for individual treatment groups, and numerical differences reported here should be interpreted with caution. Even after pooling data for the 5 and 10 mg BID doses, the sample size was still relatively small, particularly when split further into those patients achieving or not achieving the MIC. Furthermore, not all patients had MRI data recorded. Although some MRI data were missing due to technical issues, there were a number of patients who refused the follow-up MRI. These patients are likely to be those with greater disease severity, who may have greater pain or disability.
In summary, in this post hoc analysis of data from a phase 2 study in patients with active AS, ∼30% of patients treated with tofacitinib for 12 weeks experienced clinically meaningful reductions in axial MRI inflammation, and almost three times more patients who received tofacitinib 2, 5 or 10 mg BID achieved MIC in SPARCC SI joint and spine scores compared with placebo. There was a trend for greater clinical responses among tofacitinib-treated patients who achieved MIC for MRI inflammation compared with those not achieving MIC across all doses, although findings were limited by the small patient numbers in each group and differences between groups were small for some endpoints. Therefore, further assessments are needed in larger study populations and over a longer time-course to confirm if the MIC for SPARCC assessments is related to clinical outcomes and therefore can be viewed as a true minimum clinically important difference in patients with AS in general, and those treated with tofacitinib specifically.
Acknowledgements
The authors would like to thank the study patients and investigators. This study was sponsored by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Alice MacLachlan, PhD, at Complete Medical Communications, Glasgow, UK, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–4).
Funding: This work was supported by Pfizer Inc.
Disclosure statement: W.P.M. has received research grants from AbbVie, Pfizer Inc, Sanofi and UCB, has acted as a consultant for AbbVie, Eli Lilly, Janssen, Merck, Novartis, Pfizer Inc, Sanofi and UCB and is Chief Medical Officer of Canadian Research Education (CaRE) Arthritis Ltd. D.vd.H. is the Director of Imaging Rheumatology BV and has acted as a consultant for AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer Inc, Regeneron, Roche, Sanofi, Takeda and UCB. X.B. has received research grants from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, MSD, Novartis, Pfizer Inc, Roche and UCB. A.D. has received research grants from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer Inc and UCB and has been an advisory board member for Eli Lilly, Janssen, Novartis, Pfizer Inc and UCB. S.P.S., D.L., D.F., T.H. and K.S.K. are employees and stockholders of Pfizer Inc.
References
- 1. Braun J, Sieper J.. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- 2. Maksymowych WP, Inman RD, Salonen D. et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. [DOI] [PubMed] [Google Scholar]
- 3. Maksymowych WP, Inman RD, Salonen D. et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703–9. [DOI] [PubMed] [Google Scholar]
- 4. Lambert RG, Salonen D, Rahman P. et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2007;56:4005–14. [DOI] [PubMed] [Google Scholar]
- 5. Rudwaleit M, Baraliakos X, Listing J. et al. Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept. Ann Rheum Dis 2005;64:1305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun J, Landewé R, Hermann KG. et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum 2006;54:1646–52. [DOI] [PubMed] [Google Scholar]
- 7. Rudwaleit M, Schwarzlose S, Hilgert ES. et al. MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis 2008;67:1276–81. [DOI] [PubMed] [Google Scholar]
- 8. Bredella MA, Steinbach LS, Morgan S, Ward M, Davis JC.. MRI of the sacroiliac joints in patients with moderate to severe ankylosing spondylitis. Am J Roentgenol 2006;187:1420–6. [DOI] [PubMed] [Google Scholar]
- 9. Goh L, Suresh P, Gafoor A, Hughes P, Hickling P.. Disease activity in longstanding ankylosing spondylitis: a correlation of clinical and magnetic resonance imaging findings. Clin Rheumatol 2008;27:449–55. [DOI] [PubMed] [Google Scholar]
- 10. Weiß A, Song IH, Haibel H, Listing J, Sieper J.. Good correlation between changes in objective and subjective signs of inflammation in patients with short- but not long duration of axial spondyloarthritis treated with tumor necrosis factor-blockers. Arthritis Res Ther 2014;16:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maksymowych WP, Lambert RG, Brown LS, Pangan AL.. Defining the minimally important change for the Spondyloarthritis Research Consortium of Canada spine and sacroiliac joint magnetic resonance imaging indices for ankylosing spondylitis. J Rheumatol 2012;39:1666–74. [DOI] [PubMed] [Google Scholar]
- 12. Meyer DM, Jesson MI, Li X. et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm 2010;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghoreschi K, Jesson MI, Li X. et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690, 550). J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Heijde D, Deodhar A, Wei JC. et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017;76:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 16. van den Berg R, de Hooge M, Bakker PA. et al. Metric properties of the SPARCC score of the sacroiliac joints - data from baseline, 3-month, and 12-month followup in the SPACE cohort. J Rheumatol 2015;42:1186–93. [DOI] [PubMed] [Google Scholar]
- 17. Machado P, Landewé RB, Braun J. et al. MRI inflammation and its relation with measures of clinical disease activity and different treatment responses in patients with ankylosing spondylitis treated with a tumour necrosis factor inhibitor. Ann Rheum Dis 2012;71:2002–5. [DOI] [PubMed] [Google Scholar]
- 18. Kang KY, Jung JY, Hong YS, Ju JH, Park SH.. Positive correlation between inflammation on sacroiliac joint MRI and serum C-terminal telopeptide of type-I collagen in ankylosing spondylitis but not in non-radiographic axial spondyloarthritis. Clin Exp Rheumatol 2017;35:415–22. [PubMed] [Google Scholar]
- 19. Molnar C, Scherer A, Baraliakos X. et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018;77:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pedersen SJ, Wichuk S, Chiowchanwisawakit P, Lambert RG, Maksymowych WP.. Tumor necrosis factor inhibitor therapy but not standard therapy is associated with resolution of erosion in the sacroiliac joints of patients with axial spondyloarthritis. Arthritis Res Ther 2014;16:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maksymowych WP, Wichuk S, Dougados M. et al. Modification of structural lesions on MRI of the sacroiliac joints by etanercept in the EMBARK trial: a 12-week randomised placebo-controlled trial in patients with non-radiographic axial spondyloarthritis. Ann Rheum Dis 2018;77:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maksymowych WP, Dougados M, van der Heijde D. et al. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis 2016;75:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]