Abstract
Fluctuations in gonadal hormones over the course of the menstrual cycle are known to cause functional brain changes and are thought to modulate changes in the balance of cortical excitation and inhibition. Animal research has shown this occurs primarily via the major metabolite of progesterone, allopregnanolone, and its action as a positive allosteric modulator of the GABAA receptor. Our study used EEG to record gamma oscillations induced in the visual cortex using stationary and moving gratings. Recordings took place during twenty females’ mid‐luteal phase when progesterone and estradiol are highest, and early follicular phase when progesterone and estradiol are lowest. Significantly higher (∼5 Hz) gamma frequency was recorded during the luteal compared to the follicular phase for both stimuli types. Using dynamic causal modeling, these changes were linked to stronger self‐inhibition of superficial pyramidal cells in the luteal compared to the follicular phase. In addition, the connection from inhibitory interneurons to deep pyramidal cells was found to be stronger in the follicular compared to the luteal phase. These findings show that complex functional changes in synaptic microcircuitry occur across the menstrual cycle and that menstrual cycle phase should be taken into consideration when including female participants in research into gamma‐band oscillations.
Keywords: allopregnanolone, electroencephalography, GABA, menstrual cycle, visual gamma oscillations
1. INTRODUCTION
In healthy women, fluctuations in gonadal hormones lead to functional changes in the brain over the course of each menstrual cycle. The interaction of these hormones with the balance of cortical excitation and inhibition has formed the basis of research attempting to characterize changes across both the healthy menstrual cycle and menstrual cycle linked disorders, such as catamenial epilepsy and premenstrual dysphoric disorder (PMDD) (Bäckström et al., 2014; Reddy, 2004). As the primary mediator of cortical inhibition, the γ‐aminobutyric acid (GABA) system and aberrant GABAergic inhibition has been implicated in these menstrual cycle related disorders (Bäckström et al., 2003). Over the course of the menstrual cycle, changes in the GABA system are modulated primarily by the major metabolite of progesterone, allopregnanolone. Allopregnanolone, similar to benzodiazepines, produces this change via its action as a potent positive allosteric modulator of the GABAA receptor. This mechanism of action, in combination with the readiness with which progesterone and its metabolites cross the blood brain barrier (around 83% for progesterone (Pardridge & Mietus, 1979) has led to research into allopregnanolone's use as a central nervous system therapeutic agent (Limmroth, Lee, & Moskowitz, 1996; Pardridge & Mietus, 1979; Zhu, Wang, Bäckström, & Wahlström, 2001). By binding to the neurosteroidal site on the GABAA receptor allopregnanolone potentiates the effect of GABA, leading to an overall increase in inhibition of neuronal excitability (Birzniece et al., 2006; Majewska, Harrison, Schwartz, Barker, & Paul, 1986). The effect of allopregnanolone on the balance of cortical excitation and inhibition is beginning to become apparent in animal research (Smith, Shen, Gong, & Zhou, 2007). However, in humans, there are limitations in measuring functional changes in vivo, specifically in finding valid noninvasive techniques, which has meant a major gap in understanding remains.
Studies using magnetic resonance spectroscopy (MRS) have found variations in levels of GABA both across the healthy menstrual cycle and in atypical populations. The first of these studies reported finding reduced GABA levels across the menstrual cycle in PMDD compared to healthy controls (Epperson et al., 2002). Furthermore, Epperson et al. (2002) found that GABA levels overall were increased in the luteal phase compared to the follicular phase in healthy controls. The opposite was found for participants with PMDD. Similar trends were found in a study that compared smokers with healthy controls (Epperson et al., 2005) indicating this effect was not specific to either group. However, GABA concentration, as measured with MRS, only provides a bulk concentration measurement and it is unclear how these measures directly index functional synaptic inhibition (Stagg, Bachtiar, & Johansen‐Berg, 2011). That said, these studies (Epperson et al., 2002, 2005) do give evidence for significant functional changes in the human female GABA system over the course of the menstrual cycle.
A number of studies have also attempted to elucidate functional changes in the GABA system that occur over the menstrual cycle. Neurosteroids have been linked to changes in tonic inhibition as well as changes in receptor density (Lovick, Griffiths, Dunn, & Martin, 2005; Maguire, Stell, Rafizadeh, & Mody, 2005). Furthermore, direct evidence of allopregnanolone influencing changes in GABAA receptor expression and sensitivity has been found in animals (Lovick et al., 2005; Maguire et al., 2005; Türkmen, Bäckström, Wahlström, Andreen, & Johansson, 2011). In humans, recent research using administered allopregnanolone indicated reduced sensitivity in the luteal phase compared to the follicular phase by showing reduced sedation in the luteal phase, measured using saccadic eye movement (SEM) (Timby et al., 2016). However, earlier research, also measuring SEMs, found no change in healthy participants using midazolam (a GABA enhancing drug) and administered pregnanolone (Sundstrom et al., 1998; Sundstrom, Nyberg, & Bäckström, 1997).
Neural oscillations recorded using EEG represent another noninvasive method of studying humans and have been related to GABAergic inhibition. In particular beta oscillations have been linked to changes in GABAergic inhibition (Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000). Increased beta power has been shown with a number of GABA enhancing drugs such as benzodiazepines (Hall, Barnes, Furlong, Seri, & Hillebrand, 2010; Jensen et al., 2005); interestingly a relative decrease in beta power in has also been found in the luteal phase (Feshchenko, Veselis, & Reinsel, 1997; Solís‐Ortiz, Ramos, Arce, Guevara, & Corsi‐Cabrera, 1994; van Lier, Drinkenburg, van Eeten, & Coenen, 2004). Alpha oscillations have a well‐known relationship with GABAergic inhibition (Jensen & Mazaheri, 2010). However, the mechanisms by which alpha is modulated by changes in GABA are less well established as the modulations that occur are not always predictable (Jensen & Mazaheri, 2010; Lozano‐Soldevilla, ter Huurne, Cools, & Jensen, 2014) and though they have been correlated with changes over the menstrual cycle, the results have been conflicting (Bazanova, Kondratenko, Kuzminova, Muravlyova, & Petrova, 2014; Brötzner, Klimesch, Doppelmayr, Zauner, & Kerschbaum, 2014; Solís‐Ortiz et al., 1994).
Visually induced gamma oscillations are an attractive approach for understanding changes in cortical inhibition. Animal in vivo and in vitro studies have established that both power and frequency of gamma oscillations are intrinsically linked to changes in GABAergic inhibitory processes (Bartos, Vida, & Jonas, 2007; Gonzalez‐Burgos & Lewis, 2008). Buzsaki and Wang (2012) illustrate how changes in synchronicity of gamma oscillations can be mediated by inhibitory GABAergic interneurons exerting their control on large populations of pyramidal cell firing. Via this model, increases in GABAergic inhibition leads to synchronization of the previously stochastic firing of pyramidal cells. Electrophysiological recording demonstrates a decrease in frequency and an increase in gamma amplitude in response to increases in GABAergic inhibition (Buzsaki & Wang, 2012; Gonzalez‐Burgos & Lewis, 2008). This is a useful model for both MEG and EEG recordings of gamma oscillations, as both methods are particularly sensitive to changes in local field potentials associated with cortical pyramidal cells (Buzsáki, Anastassiou, & Koch, 2012).
There is promising translation of this cellular model to noninvasive human studies via induction of gamma oscillations in the visual cortex. Visual gamma oscillations appear to be a highly robust biological trait marker (Muthukumaraswamy, Singh, Swettenham, & Jones, 2010; Tan, Gross, & Uhlhaas, 2016) that varies across age (Gaetz, Roberts, Singh, & Muthukumaraswamy, 2012; Orekhova et al., 2015), and is strongly genetically determined (van Pelt, Boomsma, & Fries, 2012). A number of pharmaco‐MEG studies have been able to show that they are consistently and predictably modulated by changes in GABAergic inhibition (Muthukumaraswamy, 2014). Drugs that increase GABAergic inhibition via positive allosteric modulation including alcohol, and the benzodiazepine lorazepam have been shown to lead to decreases in gamma frequency and increases in gamma power (Campbell, Sumner, Singh, & Muthukumaraswamy, 2014; Lozano‐Soldevilla et al., 2014). Similarly, tiagabine, a GABA reuptake inhibitor used in the treatment of epilepsy has been shown to decrease the frequency but not modify the amplitude of gamma oscillations (Magazzini et al., 2016). Furthermore, recent research has linked gamma oscillations in the human primary visual cortex (V1) to changes in GABAA receptor density (Kujala et al., 2015).
Recently, dynamic causal modeling of steady‐state responses (DCM‐SSR) has been used to explain spectral data at the level of network changes within prescribed generative neural mass models. DCM allows inferences to be made about underlying microcircuitry of a cortical response acting as a “mathematical microscope” (Moran, Pinotsis, & Friston, 2013). Of particular interest in analyses of visually induced gamma oscillations is the ability to make inferences about intrinsic connectivity between populations of superficial pyramidal cells and inhibitory interneurons, potentially reflecting the neural dynamics underlying the generation of gamma oscillations (Buzsaki & Wang, 2012). Recently Shaw et al. (2017) developed a customized canonical microcircuit model that better reflects the synaptic physiology of V1 than the generic mass models used in previous DCM work, in order to study visually induced gamma oscillations which arise from V1. Using their model of V1, Shaw et al. (2017) were able to show significant modulation of parameter strength when visually induced gamma activity was modified by tiagabine. Furthermore, they were able to differentiate between laminar specific events including localizing the generation of gamma oscillations to primarily superficial layers through pyramidal‐interneuron loops. The study was also able to show model sensitivity to the effect of tiagabine on the underlying time‐constant of GABAergic inhibition. As such, this approach provides a potentially attractive method for understanding the changes in the GABA system across the menstrual cycle.
The aim of this study was to quantify visual gamma oscillation parameters over the course of the menstrual cycle in healthy women. Furthermore, using the DCM approach developed by Shaw et al. (2017), we sought to make inferences about functional changes in the GABA system that may contribute to any changes found. The study used EEG to record visually induced gamma oscillations in the early follicular phase compared to the mid luteal phase, when hormones are at their lowest compared to their highest levels.
2. METHODS
2.1. Participants and study design
Twenty females aged 21–23 years volunteered to participate in the study and completed both study days. Participants were required to have no history of neurological or psychiatric disorder including premenstrual dysphoric disorder or any self‐reported major menstrual cycle related changes in mood. They were also required to not be taking any ongoing prescription medications or use hormonal forms of contraception. This study was approved by the University of Auckland Human Participants Ethics Committee. Participants provided informed written consent prior to participation.
The study used a two‐session crossover design. One session took place during the follicular phase when estradiol and progesterone are, on average, at their lowest point in the cycle while the other session took place in the mid‐ luteal phase when estradiol and progesterone are, on average, at their peak in the menstrual cycle (Bäckström et al., 2003; Genazzani et al., 1998). The order of sessions was counterbalanced. All study sessions began between 2 and 4 pm to control for diurnal variations in neurosteroid levels (Tiihonen Möller, Bäckström, Söndergaard, Kushnir, & Bergquist, 2016).
Participants were tracked for 3 menstrual cycles leading up to their first study date. This allowed the best estimate of cycle length to be made. Based on the average menstrual cycle length of 28 days, the follicular phase was defined as between days 1 and 5 of the menstrual cycle; the luteal phase, was defined as between 20 and 25 of the menstrual cycle. Several participants had considerably longer or shorter cycles than 28 days. For these participants, the luteal study date was adjusted accordingly. For this reason, blood samples were taken for confirmation of correct cycle timing.
2.2. Blood samples
For participants with an average cycle length, blood samples were taken on the study day to test for progesterone and estradiol levels in plasma. Participants with longer or shorter cycles came in for an additional sample the day before to confirm they were in the right phase. Cycle timing was determined using the guidelines provided by local laboratory guidelines (Lab Plus NZ, May 2016), who outline that plasma concentration of estradiol is typically between 50–850 pmol/L in the follicular phase, and 160–770+ pmol/L during the luteal phase. Progesterone is typically between 0 and 6 nmol/L in the follicular phase, and 6 and 80 nmol/L during the luteal phase. Due to the overlap in healthy estradiol levels in either phase, finding greater levels of estradiol was only used to support the decision that a participant within the luteal phase, progesterone levels >6 nmol/L were used as the confirmation of correct timing. Blood samples were collected using BD vacutainer® PST™ II tubes. Quantification of estradiol and progesterone concentration in plasma was carried out by LabPlus, Auckland Hospital, by electrochemiluminescence immunoassay. Assays were performed according to Roche Oestradiol III (2016) and Progesterone III (2015) assay guidelines using a Roche Cobas 8000 analyzer (e602 module).
2.3. EEG acquisition and paradigm
Continuous EEG was recorded using 64 channel Acticap Ag/AgCl active shielded electrodes and Brain Products MRPlus amplifiers. Data were recorded in Brain Vision Recorder (Brain Products GmbH, Germany) with a 1,000 Hz sampling rate, and 0.1 μV resolution. FCz was used as an online reference, AFz as ground. Electrode impedance below 10 kΩ was achieved prior to recording.
Stimuli were displayed on an ASUS VG248QE computer monitor with a screen resolution of 1,920 × 1,080 and 144 Hz refresh rate. TTL pulses generated through the parallel port of the display computer provided synchronization of stimulus events with EEG acquisition. All stimuli were generated by MATLAB (The Mathworks, Inc., Natick, MA) using the Psychophysics Toolbox (Brainard, 1997; Kleiner et al., 2007; Pelli, 1997).
The stimuli for this task was a black and white annular grating with a spatial frequency of 3 cycles per degree, subtending 16° of visual angle (Figure 1a). The grating was presented at 90% contrast, in the centre of the screen, on a grey background. A red dot provided a central fixation point. Participants were seated 90 cm from the screen. There were two conditions in this experiment (Figure 1a). In condition one, the stimuli moved inwardly at a rate of 1.33° of visual angle per second. In condition two, the stimuli were stationary. Each condition was presented 3 times each in a block of 84 presentations, providing a total of 252 trials per condition. In both conditions, the stimulus was on for between 1 and 1.1 s (pseudorandomly jittered). Participants were instructed to press the spacebar as soon as the stimulus disappeared from the screen. If the response was too slow the paradigm paused while participants were prompted to “press space to continue.” Following a participant's response, there was then a 1 s intertrial interval. The order of blocks was counterbalanced. Each block was separated by a forced 20 s break. Two participants only received the moving grating condition, leaving 16 participants for the static gratings analyses and 18 for the moving grating analyses.
Figure 1.
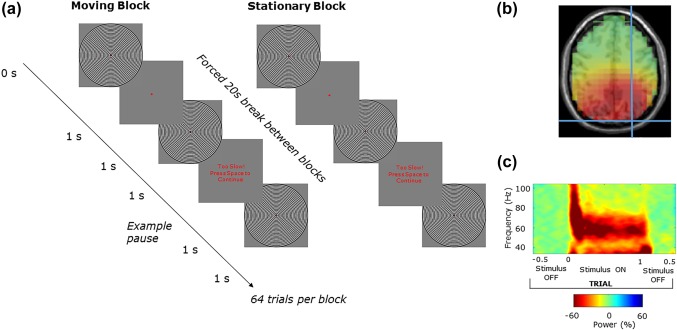
(a) An annular grating was used to induce visual gamma oscillations. For each trial the stimulus was on the screen for 1 s, and off for ∼1 s. The task was a block design with 84 trials per block, the stimuli was either moving or stationary for each of the 6 blocks. Participants were required to press the spacebar when the stimuli disappeared from the screen and were prompted if their response was too slow. Following source localization, a virtual sensor of the peak gamma intensity was chosen. (b) A single participants peak gamma at MNI coordinate 27 −90 36. (c) The time–frequency spectrogram (obtained using Hilbert transformation of bandpass filtered data) of gamma activity at this virtual sensor in relation to trial timing, exemplifying induced visual gamma activity and how it is time‐locked to the grating stimulus (in this case moving) [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. Data analysis
Data were first epoched into trials −0.5 s prestimulus and 1.5 s poststimulus onset. The data were then baselined using the 500 ms prestimulus time window. Semi‐automated artifact rejection was completed using the Fieldtrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011) as well as manually inspecting each trial for muscle and electrical interference. ICA was run on the remaining data and artifact components were visually identified and removed (mean number of removed components was 3.2 out of a possible maximum of 64). The average reference was then computed. A linearly constrained minimum variance (LCMV) beamformer (Van Veen, Van Drongelen, Yuchtman, & Suzuki, 1997) was applied with an 8 mm resolution and a broad 30–90 Hz bandpass filter using the template headmodel provided with fieldtrip. The result was projected into the Montreal Neurological Institute (MNI) coordinate system. The coordinate contributing the peak gamma power intensity was selected and used to construct a virtual sensor (Figure 1b,c).
2.5. Quality control procedure for reliable peak gamma frequency estimation
To estimate the peak frequency of gamma, a bootstrapping method was used which is outlined in more detail, and compared with other peak frequency estimation approaches, in Magazzini et al. (2016). The method produces a metric of the peak frequency reliability as a form of quality control (QC) (Magazzini et al., 2016) and was employed in the current study to address the relatively lower signal‐to noise ratio in EEG visual gamma data compared to MEG (Muthukumaraswamy & Singh, 2013) and to provide an objective measure of data reliability. Using this method, a spectral analysis was first completed that used a smoothed periodogram Fourier method (Bloomfield, 2004). For each trial, the time series is demeaned and tapered with a Hanning window. The raw periodogram was then computed for the baseline (−500 ms) and sustained gamma (250–750 ms) time periods, and smoothed with a Gaussian kernel. The single‐trial spectra were averaged across trials for the baseline and stimulus separately. From this, the amplitude spectrum was calculated as percent change from baseline. Bootstrapping was performed with 10,000 iterations on the spectral data with a frequency‐window of 35–90 Hz. Trials were resampled with replacement. Peak frequency was calculated as the greatest increase from baseline of the average of the resampled single‐trial data. The QC procedure provided a metric of reliability by calculating the frequency width required to accommodate 50% of the bootstrapped frequencies around the distribution mode. In this study a threshold of ±1 Hz was applied, meaning that if more than 50% of the iterations fell outside 1 Hz either side of the mode, the dataset was rejected. Note that the standard deviation of the bootstrap estimates serves as an estimator of the standard error. Moving gratings peak frequency estimation QC passed all 18 data sets (Figure 4), indicating reliable gamma peaks could be estimated for all participants. Peak frequency estimation QC for the static grating task passed 9/16 data sets indicating, as expected (Muthukumaraswamy & Singh, 2013), that the static grating produces a lower signal‐to‐noise ratio than moving gratings (Figure 6). To complement the bootstrapped individual response spectra (Figures 4 and 6), the original nonbootstrapped individual response spectra (showing stimulus induced gamma compared baseline) can be found in Supporting Information, Figure S1 for moving gratings and Figure S2 for static gratings.
Figure 4.
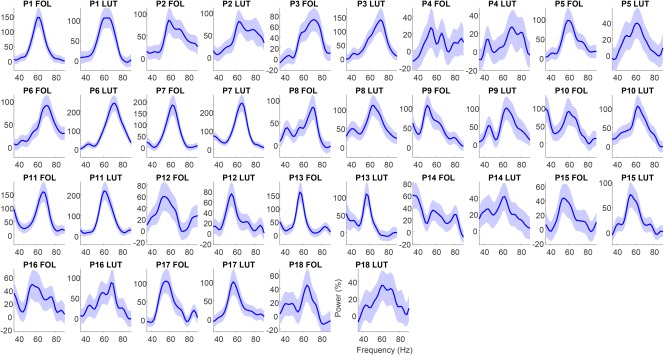
Amplitude as a function of frequency for the moving grating stimulus type for each participant's follicular and luteal sessions. In these QC graphs, blue indicates spectra which were automatically classified as good and those colored red (nil) were classified as bad. Graphs show relative change spectra (% change units) with shaded 95% confidence intervals [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
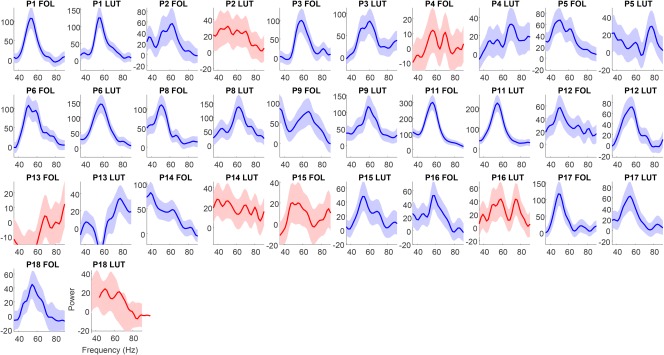
Amplitude as a function of frequency for the static grating stimulus type for each participant's follicular and luteal sessions. In these QC graphs, blue indicates spectra which were automatically classified as good and those colored red (P2 LUT, P4 FOL, P13 FOL, P14 LUT, P15 FOL, P16 LUT, and P18 LUT) were classified as bad. Graphs show relative change spectra (% change units) with shaded 95% confidence intervals. Note P7 and P10 are missing as for these participants only the moving grating stimulus type was collected [Color figure can be viewed at http://wileyonlinelibrary.com]
2.6. Dynamic causal modeling
Dynamic causal modeling for steady‐state responses (DCM‐SSR) was conducted using the methods of Shaw et al. (2017) which utilised a variation on the canonical microcircuit neural model (CMC). DCM uses a generative model comprising the neural‐mass model as well as an observation model, which when using EEG or MEG is typically a lead‐field weighting (Moran et al., 2009; Shaw et al., 2017). The neural mass model for the CMC comprises 4 types of interacting cell populations. For these the variation on the neural model employed by Shaw et al. (2017) includes six types of parameter, including time‐constants (T), local (G), and extrinsic (A) synaptic connectivity strengths, exogenous input (C) strength, delay (D), and presynaptic firing (S). Of particular interest is the modulation of intrinsic or local connectivity (parameter G) but also population time‐constants (parameter T) (Figure 2), these and an overall gain parameter (L) are allowed to vary all other parameter types outlined above are fixed in the model.
Figure 2.
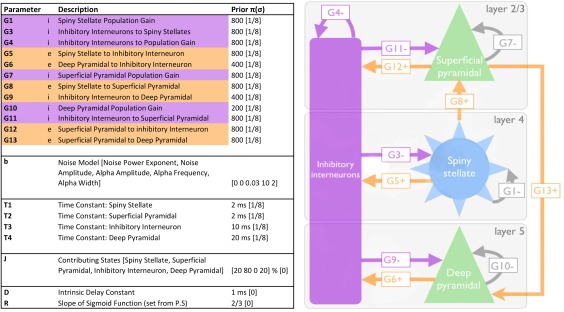
Adapted from Shaw et al. (2017). Depicts and describes the canonical microcircuit (CMC) used in the DCM procedure. Left: Three‐layer model, excitatory connections are shown in orange, and inhibitory (i.e., GABAergic) connections in purple. Grey arrows represent self‐inhibition within each of the excitatory cell populations. Right: Descriptions of parameters that define the model, including their prior values and their precisions (sigma) [Color figure can be viewed at http://wileyonlinelibrary.com]
Under this model, it was possible to characterize the local synaptic connectivity between inhibitory interneurons and excitatory pyramidal, and stellate cells (Figure 2). Two nonreciprocal excitatory connections exist, one between L4 stellate to L2/3 pyramidal cells, the second between L2 pyramidal to L5/6 pyramidal cells. Each population also has an inhibitory, self‐modulatory gain connection modeled by G1, G4, G7, and G10. Reciprocal L2/3 pyramidal to interneuron connection are modeled by the G11 and G12 parameters that correspond physiologically to the generators of gamma rhythm, according to the PING model (Bartos et al., 2007; Tiesinga & Sejnowski, 2009; Whittington et al., 2000). Although this model may be overly simplified in terms of the true cytoarchitecture, mean‐field models balance biological complexity against computational estimability. Indeed, the model has been shown to be sufficiently detailed enough to recapitulate variations in local connectivity induced by subtle pharmacological manipulation (Shaw et al., 2017).
Initial starting values for each of the model parameters were derived by first fitting the CMC model to the mean spectral density across participants and conditions. These values form the priors that are used in the individual DCM‐SSR fits for each of the individual datasets. G1, G3, G10, and G13 were fixed as in the study by Shaw et al. (2017) where these parameters were found to have little to no effect on the fitted spectral density. Similarly, we also fixed T1 as it was found to have a profound effect on model stability (Shaw et al., 2017). The output provided values of individual parameter strengths for each condition.
3. RESULTS
3.1. Blood sampling and cycle timing confirmation
All participants were confirmed to have progesterone and estradiol levels in the range consistent with the follicular and luteal phases for each study session day (Figure 3).
Figure 3.
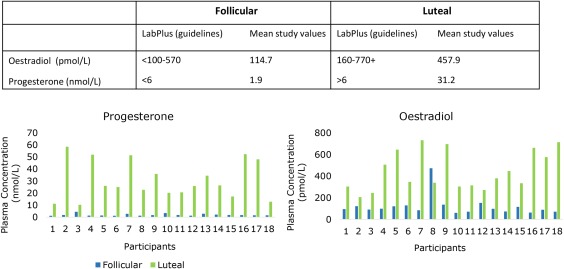
Plasma levels of progesterone and estradiol taken at the EEG recording to confirm cycle timing. The data indicate that 100% of participants were in the correct phase at the time of testing [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Peak frequency not amplitude is modulated over the menstrual cycle
Moving gratings peak frequency estimation QC passed all 18 data sets meaning all participants were included in the subsequent analyses (Figure 4). All Wilcoxon‐signed rank tests were subjected to false discovery rate (FDR) correction for multiple comparisons (Benjamini & Hochberg, 1995). A Wilcoxon‐signed rank test showed that participants had significantly higher peak mean gamma frequency in the luteal phase (M = 63.42 Hz, SD = 5.30 Hz) compared to the follicular phase (M = 59.86 Hz, SD = 7.19 Hz; Z = −2.42, p = .032 FDR) (3.56 Hz difference) (Figure 5). The corresponding peak amplitudes were also subjected to a Wilcoxon‐signed rank test. However, there was no significant difference in percent signal change between peak amplitude for the luteal (M = 111.01%, SD = 66.51 Hz) and follicular phases (M = 95.72%, SD = 44.70%; Z = −0.21, p = .836 FDR) (Figure 5).
Figure 5.
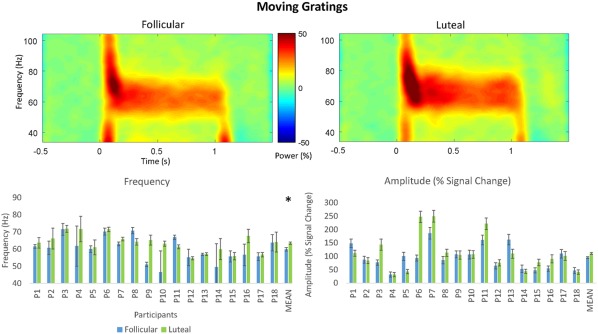
Top: Grand‐averaged time–frequency spectrogram for the follicular and luteal phases for the static grating stimulus type. Intensity of color warmth indicates changes in power (%) from the baseline. Bottom: Individual participant frequency and amplitude results. Study means show significantly higher peak mean frequency in the luteal compared to follicular phase. No significant differences were found for amplitude. Error bars show standard error [Color figure can be viewed at http://wileyonlinelibrary.com]
Peak frequency estimation QC for the static grating task passed 9/16 data sets to be included in subsequent analyses. As mention above, this is expected (Muthukumaraswamy & Singh, 2013), as the static grating produces a lower signal‐to‐noise ratio than moving gratings (Figure 6). Despite the low numbers, consistent with the finding for moving gratings, a Wilcoxon‐signed rank test also showed that participants had significantly higher peak mean gamma frequency in the luteal phase (M = 58.16 Hz, SD = 3.95 Hz) compared to the follicular phase (M = 52.41 Hz, SD = 3.00 Hz; Z = −2.43, p = .032 FDR) (5.75 Hz difference) (Figure 7). There was also no significant difference in percent signal change of peak mean amplitude for the luteal (M = 114.49%, SD = 57.12%) compared to the follicular phase (M = 120.84%, SD = 71.19%; Z = −0.42, p = .836 FDR) (Figure 7).
Figure 7.
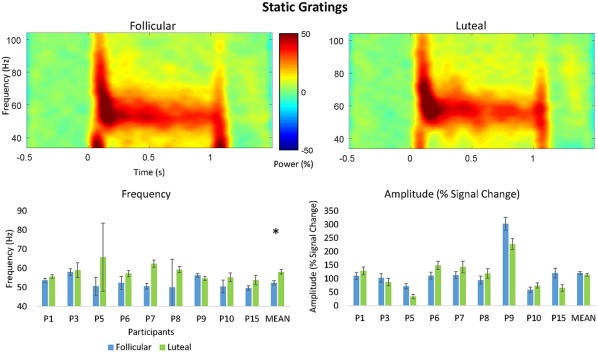
Top: Grand‐averaged time–frequency spectrogram for the follicular and luteal phases for the moving grating stimulus type. Intensity of color warmth indicates changes in power (%) from the baseline. Bottom: Individual participant frequency and amplitude results. Study means show significantly higher peak mean frequency in the luteal compared to follicular phase. No significant differences were found for amplitude. Error bars show standard error calculated as the standard deviation of the bootstrapped distribution [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Hormone levels are not correlated with frequency or amplitude
Spearman's correlations were conducted to check for potential correlations between change in amplitude between the luteal and follicular phase, change in frequency, and estradiol or progesterone (Figure 9). For moving gratings, no significant correlation was found between progesterone (M = 28.66 nmol/L, SD = 15.76 nmol/L) and frequency (M = 3.57 Hz, SD = 6.38 Hz; r s = .388, p = .111) or percent signal change of peak mean amplitude (M = 15.29%, SD = 49.94%; r s = −.120, p = .636). Similarly, no significant correlation was found between estradiol (M = 326.61 pmol/L, SD = 217.28 pmol/L) and frequency (r s = .442, p = .066) or percent signal change of peak mean amplitude (r s = −0.178, p = .481). Similarly, for static gratings, no significant correlation was found between progesterone (M = 25.56 nmol/L, SD = 15.02 nmol/L) and frequency (M = 5.76 Hz, SD = 5.40 Hz; r s = .452, p = .222) or percent signal change of peak mean amplitude (M = −6.34%, SD = 41.04%; r s = .218, p = .574). Simialrly, no significant correlation was found between estradiol (M = 354.22 pmol/L, SD = 198.79 pmol/L) and frequency (r s = 0.617, p = .077) percent signal change of peak mean amplitude (r s = .383, p = .308).
Figure 9.
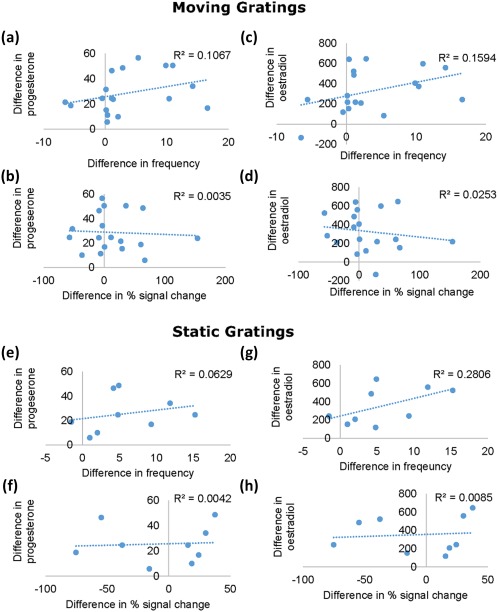
Scatter plots of the difference between the follicular and luteal phase in gamma frequency, and percent signal change correlated with the difference in plasma concentrations of estradiol and progesterone. Upper panel: Graphs of the moving gratings condition show the correlation between plasma concentrations of progesterone and frequency (a), and progesterone and percent signal change (b). Estradiol and frequency (c), and estradiol and percent signal change (d). Lower panel: Scatter plots for the static gratings condition show the correlation between plasma concentrations of progesterone and frequency (e), and progesterone and percent signal change (f). Estradiol and frequency (g), and estradiol and percent signal change (h). There were no significant effects of plasma concentration of ether hormone on frequency or percent signal change [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DYNAMIC CAUSAL MODELING
4.1. Cycle and mean parameter strength
A repeated measures ANOVA was run to explore the effects of menstrual cycle phase (follicular versus luteal), and grating (moving versus static) on the G (local connection) and T (time constant) parameters (Figure 8). There was no significant phase by grating‐type interaction (F (1,10) = 0.38, p = .866). However, because the primary purpose of this analysis is to explore the effect of menstrual cycle phase on the parameters, the main effects are interpreted. A significant main effect of menstrual cycle phase was found (F (1,10) = 4465.82, p = .012). Two parameters showed this main effect of phase. G7 (F (1,10) = 6.77, p = .026), and G9 (F (1,10) = 7.10, p = .024). Comparison of estimated marginal means shows that G7 is stronger in the luteal phase (M = 1.33, SE = 0.056) than the follicular phase (M = 1.18, SE = 0.044). G9 is stronger in the follicular (M = 2.11, SE = 0.028) than the luteal phase (M = 2.03, SE = 0.029). No significant main effect of grating was found (F (1,10) = 34.88, p = .131).
Figure 8.
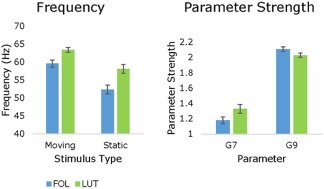
Left: Peak mean frequencies from the QC estimation show significantly greater frequency in the luteal compared to follicular phase for both moving (+3.56 Hz) and static gratings (+5.75 Hz). Right: These are shown side‐by‐side with the significant difference in estimated marginal means of the parameter strengths for G7 and G9 in the follicular compared to luteal phase. G7 is significantly stronger in the luteal phase and G9 is significantly stronger in the follicular phase. Error bars show standard error calculated as the standard deviation of the bootstrapped distribution [Color figure can be viewed at http://wileyonlinelibrary.com]
5. DISCUSSION
Using EEG to record visual gamma oscillations during the luteal and follicular phases of the menstrual cycle, this study found significantly higher peak gamma frequency in the luteal phase compared to the follicular phase. This result shows that endogenous modulation of gamma oscillations can be reliably measured using EEG. Evidence of this ability is important as the majority of existing work with gamma oscillations has been completed using MEG. This is largely due to the relatively poorer signal‐to noise ratio of EEG and historical lack of advanced signal processing techniques to overcome this. However, EEG is both cheaper and more widely available both at academic institutions and hospitals than MEG, an important factor for future applications of this research.
Making any detailed inferences about specific neurobiological causes for our findings are complicated and limited by the observational nature of the study design. However, the lack of any correlation between peripheral measures of progesterone or estradiol and peak gamma amplitude or frequency do not support the argument that absolute neurosteroid concentrations are the key contributors to the effects of menstrual cycle found in this study, as peripheral concentrations of progesterone and estradiol have been found to be well correlated with concentrations in the brain (Bixo, Bäckström, Winblad, & Andersson, 1995; Wang, Seippel, Purdy, & Bäckström, 1996). Although we did not directly measure allopregnanolone levels, these are correlated with progesterone, particularly in the luteal phase (Wang et al., 1996). Instead, because gamma oscillations in V1 have been correlated with GABAA receptor properties such as density in human visual cortex (Kujala et al., 2015), this suggests that receptor dynamics may be more important.
In this study, we only found a change in gamma frequency and not amplitude. This has been found in one other study on the effects of tiagabine on visual gamma oscillations (Magazzini et al., 2016). In our case, this may have been due to EEG signal to noise ratio being generally lower than that of MEG (Muthukumaraswamy & Singh, 2013). However, this is unlikely as the reliability of the peak frequency estimation was as good as, or at least comparable to, the findings of Magazzini et al. (2016), indicating high signal‐to‐noise ratio. It has also been proposed that the neural mechanisms behind amplitude and frequency of gamma can be differentially modulated, with frequency more linked to the time‐constant of inhibitory processes (Magazzini et al., 2016). To support this, when the data for the tiagabine data as presented by Magazzini et al. (2016) was subjected to DCM‐SSR by Shaw et al. (2017), it was found that individual variability in the time constant of inhibitory interneurons was found to be significantly modulated by gamma frequency but not amplitude. Furthermore, a contribution analysis was completed in Shaw et al. (2017) to determine the key parameters contributing to gamma frequency. This was found to be parameter G7; the self‐inhibition of superficial pyramidal cells. By contrast, gamma amplitude was found to be primarily determined by G11; the strength of the inhibitory interneuron to superficial pyramidal cell connection. However, it is also important to consider that, even using MEG, within‐subject test–retest reliability of visual gamma amplitude effects measured in sensor space are lower than that for frequency (Tan et al., 2016). Therefore, replication of this study will be a key to confirming whether indeed there is no effect of menstrual cycle on gamma amplitude.
The pharmaco‐MEG literature provides a number of examples showing that increasing GABAergic inhibition via GABA enhancing drugs leads to a decrease in gamma frequency (Campbell et al., 2014; Lozano‐Soldevilla et al., 2014; Magazzini et al., 2016). From this, it could be speculated that our finding of relatively higher frequency in the luteal compared to the follicular phase found in this study is interpretable as reflecting greater GABAergic inhibition in the follicular phase relative to the luteal phase. This might seem counterintuitive as during the luteal phase endogenous allopregnanolone is higher, and based on this one might then expect lower frequency gamma in the luteal phase. However, if mechanisms for allopregnanolone tolerance like those described in Lovick et al. (2005) in animals, become active due to sustained exposure to allopregnanolone in the luteal phase, this could create the paradoxical effect that we have observed. Consistent with this idea, Timby et al. (2016) found in healthy human females decreased sensitivity to administered allopregnanolone in the luteal phase compared to the follicular phase and suggested that this may be due to tolerance to allopregnanolone over the menstrual cycle. Furthermore, based on animal literature, the primary mediator of apparent changes in GABAergic inhibition over the menstrual cycle is related to upregulated expression of GABAA receptors containing α4 and δ subunits (Smith et al., 2007), and a potential decrease in sensitivity may represent a break down or modulation of α4 containing receptors found (Lovick et al., 2005). If the mechanism for reported changes in GABAergic inhibition are related to α4 receptors, this may also be accounting for the conflict between the findings with administered benzodiazepines and allopregnanolone (Sundstrom et al., 1997; Timby et al., 2016).
While it is important to reconcile our findings with the existing pharmaco‐MEG literature, the argument of tolerance relies on the assumption that while targeting the mid‐luteal phase, the majority of tests took place during the later stages of the mid‐luteal phase when tolerance begins to take effect (Türkmen et al., 2011). This is because in the early half of the mid‐luteal phase this argument becomes difficult to reconcile with rodent findings of increased potentiation of GABAergic inhibition during late dioestrus (comparable to the early‐ to mid‐luteal phase of human females) compared to during estrus, demonstrated by measuring the peak amplitude of inhibitory post synaptic currents (Maguire et al., 2005). However, one could potentially test hypotheses around tolerance in an advancement of this study using administered allopregnanolone similar to Timby et al. (2016). Even more informative would be to focus on more specific subsections of the luteal phase (e.g., early‐mid and late‐mid halves of the mid‐luteal phase).
In the current DCM analyses, we found several microcircuit parameters that were modulated by menstrual cycle phase; parameters G7 and G9. As explained above, G7 represents superficial pyramidal self‐inhibition and was increased in the luteal phase. Whereas G9 represents inhibitory interneuron connections to deep pyramidal cells and was increased in the follicular phase. The previous contribution analysis run by Shaw et al. (2017) determined four parameters with the greatest contribution to beta and gamma peak amplitude and frequency by testing a parameter's sensitivity to variation in each respective spectral feature, two of which are relevant here. Parameter G7 (self‐inhibition of superficial pyramidal cells) was the predominant determinant of peak gamma frequency where increasing G7 leads to corresponding increases in gamma frequency. This is entirely consistent with the current results. From a neurobiological perspective, G7 as a self‐inhibition parameter is a “lumped” parameter that could subsume a variety of potential gain‐control mechanisms, including neuromodulation, receptor cycling or receptor desensitization, any of which could be at play across the endogenous menstrual cycle. Furthermore, any of these mechanisms may feasibly be modulated by GABAergic changes. As has already been mentioned, in response to increases in allopregnanolone, the primary mediator of apparent changes in inhibition is upregulated expression of GABAA receptors containing α4 and δ subunits (Smith et al., 2007). δ‐GABAA receptors are extrasynaptic mediators of tonic inhibition and have been related to gain‐control mechanisms (Semyanov, Walker, Kullmann, & Silver, 2004; Stell, Brickley, Tang, Farrant, & Mody, 2003). They are also at particularly high density in layer 2/3 (Drasbek & Jensen, 2006), where G7 is modeled.
With respect to G9 representing inhibitory interneuron to deep pyramidal cells, Shaw et al. (2017) found this parameter was positively correlated with beta rather than gamma amplitude. G9 more directly suggests modification of GABAergic interneuron system, although specifically in its laminar interaction with the deep pyramidal cells. Interestingly, and in contrast to the above, this modification indicates that there is greater inhibition in the follicular phase than luteal phase. This is proposed to be in keeping with one of the unique actions of allopregnanolone at endogenous levels. Below certain levels allopregnanolone can produce a paradoxical effect and depress inhibition via polarity‐dependent action on Cl− influx and efflux (Bäckström et al., 2011; S. Smith et al., 2007). However, δ‐GABAA receptors are more sensitive to lower levels of allopregnanolone, and in the healthy menstrual cycle, still produce an overall increase in GABAergic inhibition when allopregnanolone levels increase endogenously (Belelli et al., 2009; Lovick et al., 2005; Maguire et al., 2005; Smith et al., 2007). Research has shown that there is reduced expression of δ‐GABAA receptors in layer 5 (Drasbek & Jensen, 2006) where parameter G9 is modeled. Rather, tonic inhibition is modulated by α5‐GABA receptors which allopregananolone has a much lower affinity for Peng et al. (2009) but can also produce a paradoxical effect (Burgard, Tietz, Neelands, & Macdonald, 1996; Smith et al., 2007). The reduced sensitivity to allopregnanolone within layer 5 due to the absence of δ‐GABAA receptors may be leading to the opposing difference in inhibition found in the luteal compared to the follicular phase.
Taken together, these modifications suggest a change in the laminar functioning of the (visual) cortex across the menstrual cycle with relatively less superficial pyramidal cell activity in the luteal phase and relatively more GABAergic inhibition of deep‐level activity in the follicular phase. In invasive animal recordings, gamma rhythm activity predominates in the superficial layers whereas in deeper layers beta oscillations are more dominant (Maier, Adams, Aura, & Leopold, 2010; Xing, Yeh, Burns, & Shapley, 2012). From a theoretical perspective, this may suggest alterations in hierarchical prediction coding mechanisms where it has been suggested that superficial cells encode ascending prediction errors while predictions are encoded by deep pyramidal cells and then transmitted to lower levels of the cortical hierarchy (Bastos et al., 2012; Friston, Bastos, Pinotsis, & Litvak, 2015). On the basis of the present data alone, any argument we could make about prediction coding mechanisms across the menstrual cycle would be speculative, but we note that in the same experimental cohort, we recorded not only resting‐state EEG but mismatch negativity (MMN) data. The MMN in particular specifically allows measures of hierarchical predictive coding to be made—albeit typically in the auditory system (Garrido, Kilner, Kiebel, et al., 2009; Garrido, Kilner, Stephan, & Friston, 2009). As such, although it is beyond the scope of this work, it is possible that in the near future we may be able to explicitly test these speculations regarding predictive coding.
The interpretation above focuses heavily on the role of progesterone, over estradiol, when considering the impact of receptor dynamics. While estradiol does have an influence on the balance of excitation and inhibition, studies have typically found that it is progesterone and its inhibitory metabolites that are the primary driver of changes observed in the mid‐luteal phase, particularly when compared to the early follicular phase (Epperson et al., 2002, 2005; Lovick et al., 2005; Maguire et al., 2005). While administering oestradiol has been found to recover reduced gamma power in ovariectomized rats (Schroeder et al., 2017), to our knowledge, there is no literature isolating the effect of estradiol on gamma oscillations during natural fluctuations, such as during the menstrual cycle. There was also no effect of the menstrual cycle on gamma power found in the present study.
Furthermore, using paired‐pulse transcranial magnetic stimulation (TMS) and motor evoked potentials as an indicator of cortical inhibition, studies have found greater inhibition in the luteal compared to the follicular phase, in contrast, during ovulation there is an increase in excitation (Smith, Adams, Schmidt, Rubinow, & Wassermann, 2002; Smith et al., 1999). Estradiol is known to have a depressive effect on GABAergic inhibitory input (Wójtowicz & Mozrzymas, 2010), and facilitate increased excitation by enhancing glutamatergic transmission (Yokomaku et al., 2003). This suggests that while the excitatory effects of estradiol are dominant during ovulation, it is the inhibitory effects of progesterone that have the greatest influence during the luteal phase. Unfortunately, TMS based metrics of inhibition have struggled to consistently show an effect of menstrual cycle meaning there remains a requirement for alternate methods of measuring changes (Hattemer et al., 2007; Zoghi, Vaseghi, Bastani, Jaberzadeh, & Galea, 2015). Estradiol has been shown to be less influential on overall GABA levels, and hence inhibition, than the effect of progesterone and its metabolites in humans (Epperson et al., 2002, 2005). Furthermore, it is progesterone and its metabolites that are more frequently implicated in menstrual cycle related disorders such as PMDD (Bäckström et al., 2014; Barth, Villringer, & Sacher, 2015; Epperson et al., 2002; Girdler, Straneva, Light, Pedersen, & Morrow, 2001; Reddy, 2004).
As well as providing a valuable tool for measuring functional changes in the brain related to changes in balance of excitation and inhibition, one of the most important impacts of our research is that it shows that endogenous changes across the menstrual cycle significantly affect the EEG signal during visual gamma tasks. Our study also makes clear that consideration of menstrual timing in women is important if they are to be included in either healthy control or patient studies. Where hormones are not accounted for as a potential source of variation, in clinical (Rowland et al., 2013), pharmacological (Campbell et al., 2014), and genetic research (van Pelt et al., 2012), true effects may in fact be underestimated. Also of particular relevance is repeated‐measures research where recordings may take place at different phases of the menstrual cycle. One way to overcome this would be to ensure participants are all studied at the same phase of their menstrual cycle. The follicular phase is by far the most straightforward to verify as its initiation is signaled by the onset of menstrual bleeding. Alternatively, females on oral contraception, while taking active hormone pills will be at a predictable and constant stage in their cycle. Any other form of hormonal contraception or therapy should also have its specific impact on the menstrual cycle and gonadal hormones taken into account. For example, modern contraceptive implants, such as Implanon, can lead to increased irregularity of menstrual bleeding, so in these cases, follicular phase would be again easier to estimate (Mansour, Korver, Marintcheva‐Petrova, & Fraser, 2008).
5.1. Strengths, limitations, and future directions
A key strength of this study was the rigorous cycle tracking and confirmation of cycle timing. Participants were tracked for three full cycles prior to their first study date. Participants with any irregular cycle lengths, despite often self‐reporting regular cycles, came in the day before a potential study date for a blood sample. The study date was rescheduled if they were not in their luteal phase. This happened on a small number of occasions. Within the average menstrual cycle there is very common, and healthy, variance in intraindividual cycle length and regularity (Fehring, Schneider, & Raviele, 2006). This leads us to the conclusion that in studies without blood or saliva quantification of progesterone, it cannot be unequivocally stated that testing took place during the luteal phase. Even counting back from menstrual onset after the study, though successful in some cases, will not pick up on anovulatory cycles. Anovulatory cycles have no surge in hormones during the luteal phase and can affect up to 38% of women aged 20–24 for at least 1/3 cycles (Metcalf & Mackenzie, 1980). In addition, as already mentioned, all study sessions began between 2 and 4 pm to control for diurnal variations in neurosteroid levels (Tiihonen Möller et al., 2016).
One of the limitations of this study was the apparent tradeoff between moving and static gratings. Moving gratings produced the cleanest data according to the QC thresholding. This leads to the greatest number of useable datasets to take forward through data processing. It has been reported previously that visually induced gamma oscillation can hit a frequency ceiling at around 70 Hz and that moving gratings tend to induce gamma at a frequency closer to 70 Hz than static (Swettenham, Muthukumaraswamy, & Singh, 2009). This was referred to as a potential saturation of the visually induced gamma effect (Swettenham et al., 2009). The relatively smaller mean change in gamma frequency between cycle phases for moving (3.56 Hz) compared to static gratings (5.75 Hz) may be some indication of this occurring in our data. A potential criticism of our data analysis may come from the nature of the QC approach to analyzing visual gamma, where data that do not contain robust gamma gets rejected. This means that individuals that do not produce or record robust gamma get rejected alongside poor‐quality datasets. This raises questions around whether results are representative of the general population, an important point also echoed by Magazzini et al. (2016). However, all of our participants passed QC for at least the moving stimulus, so this effect appears to be representative for the majority of our participants and therefore potentially the larger population of at least young women.
It is a common criticism of MEG and especially EEG measurements of high frequency oscillations that they can be highly contaminated by electromyography (EMG) artifact (Whitham et al., 2007). There are several features of our data that argue strongly against the likelihood that the occipital gamma band increases observed reflect simply EMG changes across the menstrual cycle. We note that we have followed most of the best recommendations by Muthukumaraswamy (2013) to avoid muscle artifacts. This included the use of ICA as well as beamforming for source localization which, as explained by (Muthukumaraswamy, 2013), allows for the positive identification of each individuals peak gamma source as being attributable to visual cortex generation. Typically systematic modifications of neck muscle will localize to a bilateral source distribution (as well as showing broadband frequency changes). Our results show typical peak frequencies for induced occipital gamma between 40 and 70 Hz and a typical bandwidth (Figures 5 and 7, as well as individual spectra in Figures 4 and 6). Furthermore, because our results are computed relative to a prestimulus baseline, this would imply that any EMG contamination must be occurring in a boxcar similar to the stimulus frequency. This seems unlikely for muscle movements. While this could conceivably occur for onset microsaccades (Yuval‐Greenberg, Tomer, Keren, Nelken, & Deouell, 2008), our main analysis takes in the sustained period of induced gamma activity beyond the time period where early onset saccades might be present. Furthermore, onset saccades typically have much broader frequency content than we observe here.
Attributing our findings to EMG activity also implies EMG activity has relatively higher frequency during the luteal than the follicular phase. To the best of our knowledge, there is no evidence to support that this effect of the menstrual cycle occurs. The research around menstrual cycle effects on muscle contractions in general is highly inconclusive, however, where positive results are found, they indicate that metrics of muscle activity including EMG, contraction strength, and the like are strongest during ovulation, no difference is found between the early follicular and mid‐luteal phases when tested (Drake, 2001; Drake, Evetovich, Eschbach, & Webster, 2003; Jonge, Boot, Thom, Ruell, & Thompson, 2001; Kossioni & Karkazis, 1993; Phillips, Sanderson, Birch, Bruce, & Woledge, 1996; Sarwar, Niclos, & Rutherford, 1996). Furthermore, when investigating EMG contamination, the most commonly cited evidence is an increase in gamma power (Whitham et al., 2007), which was not found in this study.
To explore the apparent changes in the GABA system further in the future, it would be useful to record induced gamma oscillations at the very beginning of the luteal phase as well as the end to compare changes over time. This may help disentangle and explain some of the contradictory findings in the human literature as well as provide a dimension of long‐term changes that are not provided in studies of the far shorter menstrual cycle of rodents (4 days). In addition, to exclude a potential interaction of estradiol, a recording during ovulation when progesterone levels are low and estradiol high may provide important insight, especially because, as explained earlier, estradiol has an opposing depressive effect on GABAergic inhibitory input (Wójtowicz & Mozrzymas, 2010), as well as an excitatory effect via its effects on glutamatergic transmission (Yokomaku et al., 2003). However, in terms of the contribution of such an approach, the peak during ovulation is far shorter than that of the luteal phase and may not capture the longer term dynamics, instead offering an entirely unique piece of information. As such, it would be difficult, if not impossible, to quantify the contribution of progesterone and estradiol independently in studies of purely endogenous changes in the mid‐late luteal phase. Further potential avenues for future research include investigating to what extent our findings contribute to understanding GABA‐related disorders such as schizophrenia and epilepsy (Gonzalez‐Burgos & Lewis, 2008; Reddy, 2004; Rowland et al., 2013). One particular possibility to consider is the effect of changing sensitivity GABAergic drugs when administered daily at a uniformed dose over the course of the menstrual cycle (as is typical).
In conclusion, this study provides evidence for menstrual‐cycle‐related changes in visual gamma oscillations. Increased gamma frequency was found in the luteal compared to the follicular phase, thus demonstrating endogenous synaptic modification of excitation–inhibition occurring across the menstrual cycle. Our findings also indicate that there are complex functional changes within the cortical microcircuitry across the menstrual cycle and exemplify the potential value of DCM in elucidating the mechanisms behind these changes, in addition to analyses of spectral data features.
AUTHOR CONTRIBUTIONS
Conceptualization: RLS and SDM; methodology: RLS, SDM, ADS, and KDS; investigation: RLS, SDM, and RLM; formal analysis: RLS; writing—original draft: RLS and SDM; writing—review and editing: RLS, SDM, ADS, KDS, RLM, and FS; funding acquisition: SDM; supervision: SDM and FS.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
RLS is supported by Auckland Medical Research Foundation Doctoral Scholarship. SDM is supported by a Rutherford Discovery Fellowship administered by the Royal Society of New Zealand. AS is supported by a Wellcome Trust Strategic Award (104943/Z/14/Z). KDS is supported by the UK MEG Partnership Grant (MRC/EPSRC, MR/K005464/1), CUBRIC, and the School of Psychology at Cardiff University. The work was supported by a Faculty Research Development Fund, University of Auckland grant.
Sumner RL, McMillan RL, Shaw AD, Singh KD, Sundram F, Muthukumaraswamy SD. Peak visual gamma frequency is modified across the healthy menstrual cycle. Hum Brain Mapp. 2018;39:3187–3202. 10.1002/hbm.24069
Funding information Auckland Medical Research Foundation Doctoral Scholarship; Royal Society of New Zealand; Wellcome Trust Strategic Award, Grant/Award Number: 104943/Z/14/Z; UK MEG Partnership Grant (MRC/EPSRC), Grant/Award Number: MR/K005464/1; CUBRIC; School of Psychology at Cardiff University; Faculty Research Development Fund, University of Auckland
The authors have no conflicts of interest to declare.
REFERENCES
- Bäckström, T. , Andersson, A. , Andreé, L. , Birzniece, V. , Bixo, M. , Björn, I. , … Zingmark, E. (2003). Pathogenesis in menstrual cycle‐linked CNS disorders. Annals of the New York Academy of Sciences, 1007, 42–53. [DOI] [PubMed] [Google Scholar]
- Bäckström, T. , Bixo, M. , Johansson, M. , Nyberg, S. , Ossewaarde, L. , Ragagnin, G. , … van Wingen, G. (2014). Allopregnanolone and mood disorders. Progress in Neurobiology, 113, 88–94. 10.1016/j.pneurobio.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Bäckström, T. , Haage, D. , Löfgren, M. , Johansson, I. M. , Strömberg, J. , Nyberg, S. , … Bengtsson, S. K. (2011). Paradoxical effects of GABA‐A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience, 191(Supplement C), 46–54. [DOI] [PubMed] [Google Scholar]
- Barth, C. , Villringer, A. , & Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience, 9(37). 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos, M. , Vida, I. , & Jonas, P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience, 8(1), 45–56. 10.1038/Nrn2044 [DOI] [PubMed] [Google Scholar]
- Bastos, A. M. , Usrey, W. M. , Adams, R. A. , Mangun, G. R. , Fries, P. , & Friston, K. J. (2012). Canonical microcircuits for predictive coding. Neuron, 76(4), 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazanova, O. M. , Kondratenko, A. V. , Kuzminova, O. I. , Muravlyova, K. B. , & Petrova, S. E. (2014). EEG alpha indices depending on the menstrual cycle phase and salivary progesterone level. Human Physiology, 40(2), 140–148. 10.1134/S0362119714020030 [DOI] [PubMed] [Google Scholar]
- Belelli, D. , Harrison, N. L. , Maguire, J. , Macdonald, R. L. , Walker, M. C. , & Cope, D. W. (2009). Extrasynaptic GABA(A) receptors: Form, pharmacology and function. Journal of Neuroscience, 29(41), 12757–12763. 10.1523/jneurosci.3340-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Birzniece, V. , Bäckström, T. , Johansson, I.‐M. , Lindblad, C. , Lundgren, P. , Löfgren, M. , … Zhu, D. (2006). Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Research Reviews, 51(2), 212–239. [DOI] [PubMed] [Google Scholar]
- Bixo, M. , Bäckström, T. R. , Winblad, B. , & Andersson, A. (1995). Estradiol and testosterone in specific regions of the human female brain in different endocrine states. Journal of Steroid Biochemistry and Molecular Biology, 55(3–4), 297–303. [DOI] [PubMed] [Google Scholar]
- Bloomfield, P. (2004). Fourier analysis of time series: An introduction: John Wiley & Sons. [Google Scholar]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Brötzner, C. P. , Klimesch, W. , Doppelmayr, M. , Zauner, A. , & Kerschbaum, H. H. (2014). Resting state alpha frequency is associated with menstrual cycle phase, estradiol and use of oral contraceptives. Brain Research, 1577(100), 36–44. 10.1016/j.brainres.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard, E. C. , Tietz, E. I. , Neelands, T. R. , & Macdonald, R. L. (1996). Properties of recombinant gamma‐aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Molecular Pharmacology, 50(1), 119–127. [PubMed] [Google Scholar]
- Buzsáki, G. , Anastassiou, C. A. , & Koch, C. (2012). The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience, 13(6), 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki, G. , & Wang, X. J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35, 203–225. 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, A. E. , Sumner, P. , Singh, K. D. , & Muthukumaraswamy, S. D. (2014). Acute effects of alcohol on stimulus‐induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacology, 39(9), 2104–2113. 10.1038/npp.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, S. M. (2001). The effects of menstrual cycle on electromyography and mechanomyography during isotonic muscle actions and fatigue.
- Drake, S. M. , Evetovich, T. , Eschbach, C. , & Webster, M. (2003). A pilot study on the effect of oral contraceptives on electromyography and mechanomyography during isometric muscle actions. Journal of Electromyography and Kinesiology, 13(3), 297–301. [DOI] [PubMed] [Google Scholar]
- Drasbek, K. R. , & Jensen, K. (2006). THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor‐mediated conductance in mouse neocortex. Cerebral Cortex, 16(8), 1134–1141. 10.1093/cercor/bhj055 [DOI] [PubMed] [Google Scholar]
- Epperson, C. N. , Haga, K. , Mason, G. F. , Sellers, E. , Gueorguieva, R. , Zhang, W. , … Krystal, J. H. (2002). Cortical gamma‐aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Archives of General Psychiatry, 59(9), 851–858. 10.1001/archpsyc.59.9.851 [DOI] [PubMed] [Google Scholar]
- Epperson, C. N. , O'Malley, S. , Czarkowski, K. A. , Gueorguieva, R. , Jatlow, P. , Sanacora, G. , … Mason, G. F. (2005). Sex, GABA, and nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biological Psychiatry, 57(1), 44–48. 10.1016/j.biopsych.2004.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehring, R. J. , Schneider, M. , & Raviele, K. (2006). Variability in the phases of the menstrual cycle. Journal of Obstetric, Gynecologic, & Neonatal Nursing, 35(3), 376–384. 10.1111/j.1552-6909.2006.00051.x [DOI] [PubMed] [Google Scholar]
- Feshchenko, V. A. , Veselis, R. A. , & Reinsel, R. A. (1997). Comparison of the EEG effects of midazolam, thiopental, and propofol: The role of underlying oscillatory systems. Neuropsychobiology, 35(4), 211–220. [DOI] [PubMed] [Google Scholar]
- Friston, K. , Bastos, A. M. , Pinotsis, D. , & Litvak, V. (2015). LFP and oscillations—what do they tell us?. Current Opinion in Neurobiology, 31(Supplement C), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz, W. , Roberts, T. P. , Singh, K. D. , & Muthukumaraswamy, S. D. (2012). Functional and structural correlates of the aging brain: Relating visual cortex (V1) gamma band responses to age‐related structural change. Human Brain Mapping, 33(9), 2035–2046. 10.1002/hbm.21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, M. I. , Kilner, J. M. , Kiebel, S. J. , Stephan, K. E. , Baldeweg, T. , & Friston, K. J. (2009). Repetition suppression and plasticity in the human brain. NeuroImage, 48(1), 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, M. I. , Kilner, J. M. , Stephan, K. E. , & Friston, K. J. (2009). The mismatch negativity: A review of underlying mechanisms. Clinical Neurophysiology, 120(3), 453–463. 10.1016/j.clinph.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani, A. R. , Petraglia, F. , Bernardi, F. , Casarosa, E. , Salvestroni, C. , Tonetti, A. , … Luisi, M. (1998). Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. Journal of Clinical Endocrinology and Metabolism, 83(6), 2099–2103. 10.1210/jcem.83.6.4905 [DOI] [PubMed] [Google Scholar]
- Girdler, S. S. , Straneva, P. A. , Light, K. C. , Pedersen, C. A. , & Morrow, A. L. (2001). Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biological Psychiatry, 49(9), 788–797. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Burgos, G. , & Lewis, D. A. (2008). GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin, 34(5), 944–961. 10.1093/schbul/sbn070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S. D. , Barnes, G. R. , Furlong, P. L. , Seri, S. , & Hillebrand, A. (2010). Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco‐magnetoencephalography. Human Brain Mapping, 31(4), 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattemer, K. , Knake, S. , Reis, J. , Rochon, J. , Oertel, W. H. , Rosenow, F. , & Hamer, H. M. (2007). Excitability of the motor cortex during ovulatory and anovulatory cycles: a transcranial magnetic stimulation study. Clinical Endocrinology, 66(3), 387–393. [DOI] [PubMed] [Google Scholar]
- Jensen, O. , Goel, P. , Kopell, N. , Pohja, M. , Hari, R. , & Ermentrout, B. (2005). On the human sensorimotor‐cortex beta rhythm: Sources and modeling. NeuroImage, 26(2), 347–355. [DOI] [PubMed] [Google Scholar]
- Jensen, O. , & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience, 4, 186 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonge, X. , Boot, C. , Thom, J. , Ruell, P. , & Thompson, M. (2001). The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. Journal of Physiology, 530(1), 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner, M. , Brainard, D. , Pelli, D. , Ingling, A. , Murray, R. , & Broussard, C. (2007). What's new in Psychtoolbox‐3. Perception, 36(14), 1. [Google Scholar]
- Kossioni, A. E. , & Karkazis, H. C. (1993). Reproducibility of the human masseteric jaw‐jerk reflex in association with the menstrual cycle. Archives of Oral Biology, 38(12), 1099–1105. [DOI] [PubMed] [Google Scholar]
- Kujala, J. , Jung, J. , Bouvard, S. , Lecaignard, F. , Lothe, A. , Bouet, R. , … Jerbi, K. (2015). Gamma oscillations in V1 are correlated with GABAA receptor density: A multi‐modal MEG and Flumazenil‐PET study. Scientific Reports, 5, 1 10.1038/srep16347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmroth, V. , Lee, W. S. , & Moskowitz, M. A. (1996). GABAA‐receptor‐mediated effects of progesterone, its ring‐A‐ reduced metabolites and synthetic neuroactive steroids on neurogenic oedema in the rat meninges. British Journal of Pharmacology, 117(1), 99–104. 10.1111/j.1476-5381.1996.tb15160.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick, T. A. , Griffiths, J. L. , Dunn, S. M. J. , & Martin, I. L. (2005). Changes in GABAA receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience, 131(2), 397–405. [DOI] [PubMed] [Google Scholar]
- Lozano‐Soldevilla, D. , ter Huurne, N. , Cools, R. , & Jensen, O. (2014). GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Current Biology, 24(24), 2878–2887. 10.1016/j.cub.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Magazzini, L. , Muthukumaraswamy, S. D. , Campbell, A. E. , Hamandi, K. , Lingford‐Hughes, A. , Myers, J. F. , … Singh, K. D. (2016). Significant reductions in human visual gamma frequency by the GABA reuptake inhibitor tiagabine revealed by robust peak frequency estimation. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, J. L. , Stell, B. M. , Rafizadeh, M. , & Mody, I. (2005). Ovarian cycle‐linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neuroscience, 8(6), 797. [DOI] [PubMed] [Google Scholar]
- Maier, A. , Adams, G. K. , Aura, C. , & Leopold, D. A. (2010). Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Frontiers in System Neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska, M. D. , Harrison, N. L. , Schwartz, R. D. , Barker, J. L. , & Paul, S. M. (1986). Steroid hormone metabolites are barbiturate‐like modulators of the GABA receptor. Science, 232(4753), 1004–1007. [DOI] [PubMed] [Google Scholar]
- Mansour, D. , Korver, T. , Marintcheva‐Petrova, M. , & Fraser, I. S. (2008). The effects of Implanon® on menstrual bleeding patterns. European Journal of Contraception & Reproductive Health Care, 13(supp1), 13–28. 10.1080/13625180801959931 [DOI] [PubMed] [Google Scholar]
- Metcalf, M. G. , & Mackenzie, J. A. (1980). Incidence of ovulation in young women. Journal of Biosocial Science, 12(3), 345–352. [DOI] [PubMed] [Google Scholar]
- Moran, R. J. , Pinotsis, D. A. , & Friston, K. (2013). Neural masses and fields in dynamic causal modeling. Frontiers in Computational Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, R. J. , Stephan, K. E. , Seidenbecher, T. , Pape, H. C. , Dolan, R. J. , & Friston, K. J. (2009). Dynamic causal models of steady‐state responses. NeuroImage, 44(3), 796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy, S. D. (2013). High‐frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Frontiers in Human Neuroscience, 7, 138 10.3389/fnhum.2013.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy, S. D. (2014). The use of magnetoencephalography in the study of psychopharmacology (pharmaco‐MEG). Journal of Psychopharmacology, 28(9), 815–829. 10.1177/0269881114536790 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy, S. D. , & Singh, K. D. (2013). Visual gamma oscillations: The effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. NeuroImage, 69, 223–230. 10.1016/j.neuroimage.2012.12.038 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy, S. D. , Singh, K. D. , Swettenham, J. B. , & Jones, D. K. (2010). Visual gamma oscillations and evoked responses: Variability, repeatability and structural MRI correlates. NeuroImage, 49(4), 3349–3357. 10.1016/j.neuroimage.2009.11.045 [DOI] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova, E. V. , Butorina, A. V. , Sysoeva, O. V. , Prokofyev, A. O. , Nikolaeva, A. Y. , & Stroganova, T. A. (2015). Frequency of gamma oscillations in humans is modulated by velocity of visual motion. Journal of Neurophysiology, 114(1), 244–255. 10.1152/jn.00232.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge, W. M. , & Mietus, L. J. (1979). Transport of steroid hormones through the rat blood–brain barrier: Primary role of albumin‐bound hormone. Journal of Clinical Investigation, 64(1), 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. [PubMed] [Google Scholar]
- Peng, H.‐Y. , Chen, G.‐D. , Lee, S.‐D. , Lai, C.‐Y. , Chiu, C.‐H. , Cheng, C.‐L. , … Lin, T.‐B. (2009). Neuroactive steroids inhibit spinal reflex potentiation by selectively enhancing specific spinal GABA A receptor subtypes. Pain, 143(1), 12–20. [DOI] [PubMed] [Google Scholar]
- Phillips, S. K. , Sanderson, A. G. , Birch, K. , Bruce, S. A. , & Woledge, R. C. (1996). Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. Journal of Physiology, 496(2), 551–557. 10.1113/jphysiol.1996.sp021706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, D. S. (2004). Role of neurosteroids in catamenial epilepsy. Epilepsy Research, 62(2–3), 99–118. 10.1016/j.eplepsyres.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Rowland, L. M. , Edden, R. A. , Kontson, K. , Zhu, H. , Barker, P. B. , & Hong, L. E. (2013). GABA predicts inhibition of frequency‐specific oscillations in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences, 25(1), 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar, R. , Niclos, B. B. , & Rutherford, O. M. (1996). Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. Journal of Physiology, 493(1), 267–272. 10.1113/jphysiol.1996.sp021381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, A. , Hudson, M. , Du, X. , Wu, Y. W. C. , Nakamura, J. , van den Buuse, M. , … Hill, R. A. (2017). Estradiol and raloxifene modulate hippocampal gamma oscillations during a spatial memory task. Psychoneuroendocrinology, 78(Supplement C), 85–92. [DOI] [PubMed] [Google Scholar]
- Semyanov, A. , Walker, M. C. , Kullmann, D. M. , & Silver, R. A. (2004). Tonically active GABAA receptors: Modulating gain and maintaining the tone. Trends in Neurosciences, 27(5), 262–269. [DOI] [PubMed] [Google Scholar]
- Shaw, A. D. , Moran, R. J. , Muthukumaraswamy, S. D. , Brealy, J. , Linden, D. E. , Friston, K. J. , & Singh, K. D. (2017). Neurophysiologically‐informed markers of individual variability and pharmacological manipulation of human cortical gamma. NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. , Adams, L. F. , Schmidt, P. J. , Rubinow, D. R. , & Wassermann, E. M. (2002). Effects of ovarian hormones on human cortical excitability. Annals of Neurology, 51(5), 599–603. [DOI] [PubMed] [Google Scholar]
- Smith, M. , Keel, J. , Greenberg, B. , Adams, L. , Schmidt, P. , Rubinow, D. , & Wassermann, E. M. (1999). Menstrual cycle effects on cortical excitability. Neurology, 53(9), 2069–2069. [DOI] [PubMed] [Google Scholar]
- Smith, S. , Shen, H. , Gong, Q. H. , & Zhou, X. (2007). Neurosteroid regulation of GABAA receptors: Focus on the α4 and δ subunits. Pharmacology & Therapeutics, 116(1), 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís‐Ortiz, S. , Ramos, J. , Arce, C. , Guevara, M. , & Corsi‐Cabrera, M. (1994). EEG oscillations during menstrual cycle. International Journal of Neuroscience, 76(3–4), 279–292. [DOI] [PubMed] [Google Scholar]
- Stagg, C. J. , Bachtiar, V. , & Johansen‐Berg, H. (2011). What are we measuring with GABA magnetic resonance spectroscopy? Communicative & Integrative Biology, 4(5), 573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell, B. M. , Brickley, S. G. , Tang, C. , Farrant, M. , & Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit‐containing GABAA receptors. Proceedings of the National Academy of Sciences, 100(24), 14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom, I. , Andersson, A. , Nyberg, S. , Ashbrook, D. , Purdy, R. H. , & Bäckström, T. (1998). Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology, 67(2), 126–138. [DOI] [PubMed] [Google Scholar]
- Sundstrom, I. , Nyberg, S. , & Bäckström, T. (1997). Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology, 17(6), 370–381. 10.1016/S0893-133X(97)00086-9 [DOI] [PubMed] [Google Scholar]
- Swettenham, J. B. , Muthukumaraswamy, S. D. , & Singh, K. D. (2009). Spectral properties of induced and evoked gamma oscillations in human early visual cortex to moving and stationary stimuli. Journal of Neurophysiology, 102(2), 1241–1253. 10.1152/jn.91044.2008 [DOI] [PubMed] [Google Scholar]
- Tan, H. R. M. , Gross, J. , & Uhlhaas, P. J. (2016). MEG sensor and source measures of visually induced gamma‐band oscillations are highly reliable. NeuroImage, 137, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga, P. , & Sejnowski, T. J. (2009). Cortical enlightenment: Are attentional gamma oscillations driven by ING or PING? Neuron, 63(6), 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen Möller, A. , Bäckström, T. , Söndergaard, H. , Kushnir, M. , & Bergquist, J. (2016). Diurnal variations of endogenous steroids in the follicular phase of the menstrual cycle. Journal of Neuroscience and Neuropharmacology ‐ Open Access, 2(109), 2. [Google Scholar]
- Timby, E. , Bäckström, T. , Nyberg, S. , Stenlund, H. , Wihlbäck, A.‐C. N. , & Bixo, M. (2016). Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls—a pilot study. Psychopharmacology (Berlin), 233(11), 2109–2117. 10.1007/s00213-016-4258-1 [DOI] [PubMed] [Google Scholar]
- Türkmen, Ş. , Bäckström, T. , Wahlström, G. , Andreen, L. , & Johansson, I. M. (2011). Tolerance to allopregnanolone with focus on the GABA‐A receptor. British Journal of Pharmacology, 162(2), 311–327. 10.1111/j.1476-5381.2010.01059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier, H. , Drinkenburg, W. H. I. M. , van Eeten, Y. J. W. , & Coenen, A. M. L. (2004). Effects of diazepam and zolpidem on EEG beta frequencies are behavior‐specific in rats. Neuropharmacology, 47(2), 163–174. [DOI] [PubMed] [Google Scholar]
- van Pelt, S. , Boomsma, D. I. , & Fries, P. (2012). Magnetoencephalography in twins reveals a strong genetic determination of the peak frequency of visually induced gamma‐band synchronization. Journal of Neuroscience, 32(10), 3388–3392. 10.1523/jneurosci.5592-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen, B. D. , Van Drongelen, W. , Yuchtman, M. , & Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering, 44(9), 867–880. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Seippel, L. , Purdy, R. H. , & Bäckström, T. (1996). Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5 alpha‐pregnane‐3, 20‐dione and 3 alpha‐hydroxy‐5 alpha‐pregnan‐20‐one. Journal of Clinical Endocrinology & Metabolism, 81(3), 1076–1082. [DOI] [PubMed] [Google Scholar]
- Whitham, E. M. , Pope, K. J. , Fitzgibbon, S. P. , Lewis, T. , Clark, C. R. , Loveless, S. , … Willoughby, J. O. (2007). Scalp electrical recording during paralysis: Quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clinical Neurophysiology, 118(8), 1877–1888. 10.1016/j.clinph.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Whittington, M. A. , Traub, R. D. , Kopell, N. , Ermentrout, B. , & Buhl, E. H. (2000). Inhibition‐based rhythms: Experimental and mathematical observations on network dynamics. International Journal of Psychophysiology, 38(3), 315–336. [DOI] [PubMed] [Google Scholar]
- Wójtowicz, T. , & Mozrzymas, J. W. (2010). Estradiol and GABAergic transmission in the hippocampus. Vitamins & Hormones, 82, 279–300. [DOI] [PubMed] [Google Scholar]
- Xing, D. , Yeh, C.‐I. , Burns, S. , & Shapley, R. M. (2012). Laminar analysis of visually evoked activity in the primary visual cortex. Proceedings of the National Academy of Sciences, 109(34), 13871–13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomaku, D. , Numakawa, T. , Numakawa, Y. , Suzuki, S. , Matsumoto, T. , Adachi, N. , … Hatanaka, H. (2003). Estrogen enhances depolarization‐induced glutamate release through activation of phosphatidylinositol 3‐kinase and mitogen‐activated protein kinase in cultured hippocampal neurons. Molecular Endocrinology, 17(5), 831–844. [DOI] [PubMed] [Google Scholar]
- Yuval‐Greenberg, S. , Tomer, O. , Keren, A. S. , Nelken, I. , & Deouell, L. Y. (2008). Transient induced gamma‐band response in EEG as a manifestation of miniature saccades. Neuron, 58(3), 429–441. [DOI] [PubMed] [Google Scholar]
- Zhu, D. , Wang, M. D. , Bäckström, T. , & Wahlström, G. (2001). Evaluation and comparison of the pharmacokinetic and pharmacodynamic properties of allopregnanolone and pregnanolone at induction of anaesthesia in the male rat. British Journal of Anaesthesia, 86(3), 403–412. 10.1093/bja/86.3.403 [DOI] [PubMed] [Google Scholar]
- Zoghi, M. , Vaseghi, B. , Bastani, A. , Jaberzadeh, S. , & Galea, M. P. (2015). The Effects of Sex Hormonal Fluctuations during Menstrual Cycle on Cortical Excitability and Manual Dexterity (a Pilot Study). PLoS One, 10(8), e0136081 10.1371/journal.pone.0136081 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


