Summary
Weekly ixazomib with lenalidomide‐dexamethasone (Rd) is feasible and has shown activity in newly diagnosed multiple myeloma (NDMM) patients. This phase 1/2 study (NCT01383928) evaluated the recommended phase 2 dose (RP2D), pharmacokinetics, safety and efficacy of twice‐weekly ixazomib plus Rd in NDMM; 64 patients were enrolled across both phases. Patients received twice‐weekly oral ixazomib 3·0 or 3·7 mg plus lenalidomide 25 mg and dexamethasone 20 mg (10 mg in cycles 9–16) for up to sixteen 21‐day cycles, followed by maintenance with twice‐weekly ixazomib alone. No dose‐limiting toxicities were reported in cycle 1; the RP2D was 3·0 mg based on overall tolerability across multiple cycles. In 62 evaluable patients, the confirmed overall response rate was 94% (68% ≥very good partial response; 24% complete response). Median progression‐free survival was 24·9 months. Responses (median duration 36·9 months for patients receiving the RP2D) deepened during treatment. Grade 3 drug‐related adverse events (AEs) occurred in 64% of patients, including: rash, 13%; peripheral neuropathy, 8%; hyperglycaemia, 8%. There were no grade 4 drug‐related AEs. Thirteen patients discontinued due to AEs. Twice‐weekly ixazomib‐Rd offers substantial activity with promising long‐term outcomes in NDMM patients but may be associated with greater toxicity compared with weekly ixazomib‐Rd in this setting.
Keywords: ixazomib, oral, newly diagnosed, multiple myeloma, twice‐weekly
Introduction
The treatment of multiple myeloma (MM) has advanced over the past two decades with the introduction of proteasome inhibitors and immunomodulatory drugs, leading to improved survival for patients (Kumar et al, 2008, 2014a). Data from phase 3 randomized trials in patients with newly diagnosed MM (NDMM) or relapsed/refractory MM (RRMM) have typically demonstrated the benefit of a triplet regimen compared with a doublet regimen, particularly triplet regimens containing a proteasome inhibitor (e.g., bortezomib, carfilzomib or ixazomib) and an immunomodulatory drug (e.g., thalidomide or lenalidomide) (Cavo et al, 2010, 2012; Garderet et al, 2012; Rosinol et al, 2012; Stewart et al, 2015; Moreau et al, 2016; Durie et al, 2017; Kumar et al, 2017). Additionally, early‐phase studies in NDMM patients have demonstrated favourable tolerability and efficacy with a triplet regimen comprising carfilzomib plus lenalidomide and dexamethasone (KRd) (Jakubowiak et al, 2012; Dytfeld et al, 2014; Korde et al, 2015; Roussel et al, 2016).
Although MM remains generally incurable despite recent treatment advances, patient outcomes, including progression‐free survival (PFS) and overall survival (OS), have improved, and thanks to improved regimens and the increasing number of active treatment options, MM is becoming more of a chronic condition for many patients, with increasing 10‐year survival rates (Pulte et al, 2011; Barlogie et al, 2014; Katodritou et al, 2016). An associated development is that treatment strategies are substantially moving towards continuous, long‐term therapeutic approaches, which have been shown to improve patient outcomes compared with fixed‐duration therapy options (Benboubker et al, 2014; Palumbo et al, 2014, 2015; Guglielmelli & Palumbo, 2015; Katodritou et al, 2016). In this context, there is a need for additional active, safe and convenient regimens that are feasible for long‐term administration, offer reduced patient burden and maintain quality of life (Baz et al, 2015; Guglielmelli & Palumbo, 2015; Dowling et al, 2016; Delforge & Ludwig, 2017).
The oral proteasome inhibitor ixazomib is approved in various countries, including the United States and in Europe, in combination with lenalidomide and dexamethasone (Rd) for the treatment of patients with MM who have received at least one prior therapy. Ixazomib approval was based on the findings of the global, randomized, double‐blind, placebo‐controlled phase 3 TOURMALINE‐MM1 study (NCT01564537). In adult patients with RRMM, the combination of weekly ixazomib‐Rd demonstrated significantly longer PFS, with limited additional toxicity, compared with placebo‐Rd; responses to ixazomib‐Rd were rapid and durable, deepening with increasing duration of treatment (Moreau et al, 2016).
The triplet regimen of weekly ixazomib‐Rd administered in 28‐day cycles has also been investigated in NDMM (NCT01217957); the overall response rate (ORR) was high (92%), with 58% of patients achieving at least a very good partial response (VGPR). Responses deepened with an increasing number of treatment cycles, and the combination was well tolerated (Kumar et al, 2014b). Data from this study also showed the feasibility of long‐term maintenance treatment with single‐agent ixazomib (Kumar et al, 2014c). A parallel study has investigated ixazomib‐Rd in NDMM patients but using a twice‐weekly ixazomib dosing schedule; here, we report the findings from this study (NCT01383928) after a median follow‐up of almost 4 years.
Methods
Patients
Adult patients with NDMM (transplant eligible or ineligible) and measurable disease (defined as: serum M‐protein ≥10 g/l, urine M‐protein ≥200 mg/24 h, or involved free light chain (FLC) ≥100 mg/l if the serum FLC ratio was abnormal), Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and adequate renal, hepatic, cardiac and haematological function were eligible. Patients were excluded if they had: grade ≥2 peripheral neuropathies not elsewhere classified (NEC; high‐level term including peripheral neuropathy [PN] and peripheral sensory neuropathy); major surgery or an infection necessitating antibiotics within 14 days of commencing study treatment; prior treatment for MM; uncontrolled cardiovascular disorders within the past 6 months; previous deep‐vein thrombosis (DVT); prolonged QT interval; or known human immunodeficiency virus/hepatitis infection. For detailed eligibility criteria see Table SI. All patients provided written, informed consent at the time of enrolment.
Study design and objectives
This open‐label, multicentre (15 sites in the United States) phase 1/2 clinical trial (NCT01383928) was conducted in association with the Multiple Myeloma Research Consortium. The study was performed in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki and appropriate regulatory requirements, and with approval of Institutional Review Boards at individual enrolling institutions.
The phase 1 primary objective was to determine the safety, tolerability, maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of twice‐weekly ixazomib in combination with Rd. Phase 1 secondary objectives were to characterize ixazomib plasma pharmacokinetics in combination with Rd, and to evaluate response rates. Phase 2 primary objectives were to determine the combined rate of complete response (CR) plus VGPR, and to further evaluate tolerability and toxicity. Phase 2 secondary objectives included further assessment of response rates, and determination of time to response, duration of response (DOR), PFS and OS.
Patients received oral ixazomib [administered on an empty stomach, ≥1 h before or ≥2 h after a meal (Gupta et al, 2016a)] on days 1, 4, 8 and 11 of a 21‐day cycle, in combination with oral lenalidomide 25 mg on days 1–14 and oral dexamethasone 20 mg (10 mg in cycles 9–16) on days 1, 2, 4, 5, 8, 9, 11 and 12, for up to 16 cycles in the absence of disease progression or unacceptable toxicity. Doses of ixazomib, lenalidomide and dexamethasone could be individually held or reduced in order to manage toxicities. While receiving Rd, all patients were required to take concurrent aspirin 81–325 mg/day (or enoxaparin 40 mg/day subcutaneously, or its equivalent) as anticoagulation prophylaxis. At the discretion of the investigator, patients could undergo stem cell collection after a minimum of four treatment cycles, and patients deemed transplant eligible by the treating physician could elect to stop treatment after a minimum of eight treatment cycles to proceed to autologous stem cell transplantation (ASCT) off study. Patients in both phases who were in response or had stable disease after 16 cycles of induction with ixazomib‐Rd could proceed to receive maintenance therapy with single‐agent ixazomib on the same schedule and at the last‐tolerated dose during induction until disease progression or unacceptable toxicity. Patients who underwent ASCT did not receive ixazomib maintenance therapy post‐transplant.
Patients were not permitted to receive concomitant systemic treatment with strong inhibitors of cytochrome P450 (CYP) 1A2 or strong inhibitors of CYP3A. Excluded foods and dietary supplements included St John's wort and ginkgo biloba.
Dose escalation and determination of the MTD and RP2D
In phase 1, two fixed doses of ixazomib, 3·0 mg and 3·7 mg, were assessed. The starting dose was determined based on dose escalation in a phase 1 study of twice‐weekly single‐agent ixazomib in patients with RRMM (Richardson et al, 2014), with the body surface area‐based dosing converted to fixed dosing based on results from population pharmacokinetics analysis (Gupta et al, 2015a). Dose escalation proceeded via a standard 3 + 3 scheme, based on dose‐limiting toxicities (DLTs; see Table SII) observed in cycle 1. Patients who did not receive all ixazomib doses during cycle 1 for reasons other than DLTs were replaced in the DLT‐evaluable cohort. The MTD was defined as the highest tolerated ixazomib dose at which no more than 1 of 6 DLT‐evaluable patients experienced a DLT in cycle 1. In phase 2, patients received ixazomib at the RP2D, which was selected based on all available data from the phase 1 portion including, but not limited to: analyses of efficacy results (response rates: CR and VGPR) and toxicity characterization (grade 3/4 adverse events [AEs], serious AEs [SAEs], all‐grade PN and treatment discontinuation).
Assessments
Patients were assessed for response after cycles 1, 2, 3 and 4, and then every 2 cycles during induction and maintenance. Responses were determined according to International Myeloma Working Group criteria (Durie et al, 2006; Rajkumar et al, 2011), incorporating the additional categories of near CR (nCR) and minimal response (MR). Bone marrow biopsy and aspirate were obtained prior to starting therapy and presence of minimal residual disease (MRD) was assessed by flow cytometry (sensitivity of available assay: 10−4) in bone marrow aspirate obtained to confirm a potential CR.
AEs were evaluated throughout the treatment period and for 30 days after the final dose of study treatment and graded according to the National Cancer Institute's Common Terminology Criteria for AEs, version 4·03 (https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Patients reported their experience of neurotoxicity using the Functional Assessment of Cancer Therapy/Gynecology Oncology Group Neurotoxicity (FACT/GOG‐Ntx) questionnaire (Calhoun et al, 2003), which was completed on day 1 of every treatment cycle and at the end‐of‐treatment visit.
Serial blood samples were collected during phase 1 for determination of ixazomib plasma pharmacokinetics (see Data SI).
Statistical analysis
Phase 1 enrolment was expected to be 12 patients. The sample size calculation for the phase 2 component was designed to test the null hypothesis of a CR + VGPR rate of 35% and the alternative hypothesis of a CR + VGPR rate of >35%. With 80% power to reject the null hypothesis if the true CR + VGPR rate was 50% and a one‐sided significance level of α = 0·10, the required sample size was 48 patients. Patients enrolled at the RP2D in the phase 1 part of the study were included in the phase 2 part.
The safety population included all patients who received ≥1 dose of any study drug. The DLT‐evaluable population included patients in phase 1 who received all cycle 1 doses of ixazomib and completed all cycle 1 procedures, or who experienced a DLT during cycle 1. The response‐evaluable population comprised patients who had received ≥1 dose of study drug, had measurable disease at baseline and had ≥1 post‐baseline response assessment. Safety and efficacy were assessed among all patients, and in phase 2 patients, as well as in the modified intent‐to‐treat (mITT) population, which comprised phase 2 patients plus phase 1 patients treated at the RP2D. There was no formal statistical testing or analyses; all outcomes are reported with descriptive statistical analyses. DOR, PFS and OS were assessed using Kaplan–Meier methodology.
The pharmacokinetic‐evaluable population comprised all phase 1 patients with sufficient dosing and ixazomib concentration–time data to permit calculation of ixazomib plasma pharmacokinetic parameters, which were calculated on days 1 and 11 of cycle 1 using non‐compartmental analysis methods (Phoenix WinNonlin version 6·2, Pharsight, a Certara Company, Princeton, NJ, USA).
Results
Patients and disposition
Sixty‐four patients (phase 1, n = 14; phase 2, n = 50) were enrolled between 31 October 2011 and 12 December 2013. Patient demographics and baseline disease characteristics are summarized in Table 1: median age was 63·5 years (range 34–82), with 9% of patients aged ≥75 years; 52% had International Staging System (ISS) disease stage II or III; 81% had normal renal function or mild renal impairment (calculated creatinine clearance >60 ml/min); and 9% had high‐risk cytogenetic abnormalities.
Table 1.
Patient demographics and baseline disease characteristics among all patients, by study phase, and in the mITT population
| Phase 1 n = 14 | Phase 2 n = 50 | mITT n = 57 | Total N = 64 | |
|---|---|---|---|---|
| Median age, years (range) | 63 (42–78) | 63·5 (34–82) | 64 (34–82) | 63·5 (34–82) |
| Male/female, n (%) | 9 (64)/5 (36) | 31 (62)/19 (38) | 36 (63)/21 (37) | 40 (63)/24 (38) |
| Race, n (%) | ||||
| White | 12 (86) | 42 (84) | 47 (82) | 54 (84) |
| Black or African American | 1 (7) | 7 (14) | 8 (14) | 8 (13) |
| Other/not reported | 1 (7)/0 | 0/1 (2) | 1 (2)/1 (2) | 1 (2)/1 (2) |
| ECOG performance status, n (%) | ||||
| 0 | 8 (57) | 27 (54) | 30 (53) | 35 (55) |
| 1 | 5 (36) | 21 (42) | 25 (44) | 26 (41) |
| 2 | 1 (7) | 2 (4) | 2 (4) | 3 (5) |
| ISS disease stage at diagnosis, n (%) | ||||
| I | 8 (57) | 23 (46) | 25 (44) | 31 (48) |
| II | 5 (36) | 17 (34) | 21 (37) | 22 (34) |
| III | 1 (7) | 10 (20) | 11 (19) | 11 (17) |
| MM disease subtype, n (%) | ||||
| IgG | 7 (50) | 31 (62) | 34 (60) | 38 (59) |
| IgA | 3 (21) | 13 (26) | 15 (26) | 16 (25) |
| Light chain | 3 (21) | 4 (8) | 6 (11) | 7 (11) |
| Biclonal | 1 (7) | 1 (2) | 1 (2) | 2 (3) |
| Unknown | 0 | 1 (2) | 1 (2) | 1 (2) |
| Creatinine clearance, ml/min, median (range) | 70·6 (31·68–140·61) | 74·9 (40·94–180·50) | 74·6 (31·68–180·50) | 74·6 (31·68–180·50) |
| Cytogenetic testing,a n (%) | ||||
| Conventional/karyotype | 0 | 2 (4) | 2 (4) | 2 (3) |
| Molecular/FISH | 12 (86) | 14 (28) | 20 (35) | 26 (41) |
| Both | 2 (14) | 34 (68) | 35 (61) | 36 (56) |
| Patients with cytogenetic abnormality, n (%) | 11 (79) | 31 (62) | 36 (63) | 42 (66) |
| Patients with high‐risk cytogenetics,b n (%) | 1 (7) | 5 (10) | 6 (11) | 6 (9) |
| Monosomy 17/del17p | 0 | 3 (6) | 3 (5) | 3 (5) |
| t(4;14) | 1 (7) | 2 (4) | 3 (5) | 3 (5) |
ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in‐situ hybridisation; Ig, immunoglobulin; ISS, International Staging System; mITT, modified intent to treat; MM, multiple myeloma.
Cytogenetics were evaluated locally.
High‐risk cytogenetics included del17, t(4;14), t(14;16).
At the data cut‐off (18 October 2016), 60 patients (94%) had discontinued study treatment: 20 (31%) to undergo ASCT; 13 (20%) due to an AE; 13 (20%) due to progressive disease; 4 (6%) due to withdrawal of consent; 1 (2%) due to unsatisfactory therapeutic response; and 9 (14%) for other reasons (patient [n = 4] or investigator [n = 2] decision to discontinue treatment but continue follow‐up, health complications unrelated to study drug, patient removed, lost to follow‐up [each n = 1]). Four patients (6%) remained on study treatment (phase 1, n = 1; phase 2, n = 3).
DLTs, MTD and RP2D
In phase 1, 7 patients received ixazomib 3·0 mg and 7 patients received ixazomib 3·7 mg (1 patient at each dose was not DLT evaluable: at ixazomib 3·7 mg, cycle 1 was not completed; at ixazomib 3·0 mg, lenalidomide was taken for 21 days instead of 14 days per protocol). There were no DLTs reported at either dose; thus, with no further dose escalation, the MTD was 3·7 mg. For estimation of the RP2D, cycle 1 data from 12 evaluable patients were used in addition to the available clinical data supporting tolerance over multiple treatment cycles. AEs that met DLT criteria or that led to dose modification in cycle 2 and beyond were considered when determining the RP2D. Treatment exposure data showed that the median relative dose intensity (RDI) of lenalidomide (defined as the dose received divided by the dose prescribed, as a percentage) was 73·3% and 97·4% in patients who received ixazomib 3·7 and 3·0 mg, respectively. The RDI of lenalidomide was impacted by the higher dose of ixazomib as a result of overlapping toxicities such as rash, thrombocytopenia and gastrointestinal toxicities (http://media.celgene.com/content/uploads/revlimid-pi.pdf, Kumar et al, 2014d). Based on this and a review of safety (grade 3/4 AEs, SAEs, all‐grade PN and treatment discontinuation) and efficacy (CR and VGPR) information, the RP2D was determined as 3·0 mg.
Treatment exposure and safety profile
Among all 64 patients, the median number of treatment cycles received was 9 (range 1–75); 77% received ≥8 cycles; 39% ≥12 cycles; 30% ≥16 cycles; 14% ≥32 cycles. Forty‐five (70%) patients discontinued on or before cycle 16. Eighteen patients were treated in cycle 17 and beyond with single‐agent ixazomib maintenance therapy (phase 1, n = 5; phase 2, n = 13; mITT, n = 16). Treatment exposure is summarized in Table 2. The overall median dose intensities (defined as the proportion of planned dose of drug across all treated cycles that was actually received) were 92·9%, 94·9% and 94·5% for ixazomib, lenalidomide and dexamethasone, respectively.
Table 2.
Treatment exposure
| Phase 1 n = 14 | Phase 2 n = 50 | mITT n = 57 | Patients who did not proceed to ASCT n = 41 | Patients who received maintenance n = 18 | All N = 64 | |
|---|---|---|---|---|---|---|
| Cycles received, median (range) | 10·5 (1–75) | 8·5 (1–61) | 9 (1–75) | 13 (1–75) | 31·5 (17–75) | 9 (1–75) |
| Cycles received, n (%) | ||||||
| ≥8 | 10 (71) | 39 (78) | 45 (79) | 29 (71) | 18 (100) | 49 (77) |
| ≥12 | 6 (43) | 19 (38) | 23 (40) | 22 (54) | 18 (100) | 25 (39) |
| ≥16 | 5 (36) | 14 (28) | 17 (30) | 18 (44) | 18 (100) | 19 (30) |
| ≥32 | 2 (14) | 7 (14) | 9 (16) | 9 (22) | 9 (50) | 9 (14) |
| Median relative dose intensity,a % | ||||||
| Ixazomib | 88·8 | 93·8 | 93·8 | 89·8 | 88·3 | 92·9 |
| Lenalidomide | 73·5 | 97·4 | 97·1 | 89·4 | 91·4 | 94·9 |
| Dexamethasone | 93·7 | 94·5 | 93·8 | 95·3 | 93·2 | 94·5 |
| Patients remaining on treatment, n (%) | 1 (7) | 3 (6) | 4 (7) | 4 (10) | 4 (22) | 4 (6) |
ASCT, autologous stem cell transplantation; mITT, modified intent to treat.
Dose received as a percentage of the total planned dose over all treated cycles.
Table 3 summarizes the overall safety profile of twice‐weekly ixazomib‐Rd. All patients except one experienced drug‐related AEs, which could have been related to any of the treatment drugs; 89% of patients who received single‐agent ixazomib maintenance experienced new‐onset drug‐related AEs during maintenance. Drug‐related grade 3 AEs were reported in 64% of patients, with new‐onset events reported in 28% of patients during maintenance. There were no drug‐related grade 4 AEs. Common drug‐related AEs are summarized in Table 4; common AEs regardless of attribution are shown in Table SIII. The most common drug‐related AEs were PN NEC (59%), fatigue (48%), and rashes, eruptions and exanthems NEC (high‐level term incorporating the preferred terms of rash, macular rash, papular rash and maculo‐papular rash; 47%); new‐onset rates of these drug‐related AEs during single‐agent ixazomib maintenance were 28%, 11% and 17%, respectively. The most common drug‐related grade 3 events were rashes, eruptions and exanthems NEC (13%), hyperglycaemia (8%) and PN NEC (8%); respective new‐onset rates during maintenance were 6%, 0% and 6%. Changes in FACT/GOG‐Ntx score did not follow a consistent pattern (data not shown).
Table 3.
Overall safety profile of twice‐weekly ixazomib plus lenalidomide and dexamethasone
| AE, n (%) | Phase 1 n = 14 | Phase 2 n = 50 | mITT n = 57 | Patients who received maintenancea n = 18 | Total N = 64 |
|---|---|---|---|---|---|
| Any AE | 14 (100) | 50 (100) | 57 (100) | 16 (89) | 64 (100) |
| Any drug‐related AE | 14 (100) | 49 (98) | 56 (98) | 16 (89) | 63 (98) |
| Any grade ≥3 AE | 11 (79) | 37 (74) | 43 (75) | 8 (44) | 48 (75) |
| Any drug‐related grade ≥ 3 AE | 10 (71) | 31 (62) | 36 (63) | 5 (28) | 41 (64) |
| Any SAE | 7 (50) | 23 (46) | 28 (49) | 2 (11) | 30 (47) |
| Any drug‐related SAE | 5 (36) | 16 (32) | 19 (33) | 2 (11) | 21 (33) |
| AE resulting in any study drug dose reduction | 11 (79) | 31 (62) | 37 (65) | 6 (33) | 42 (66) |
| AE resulting in discontinuation | 3 (21) | 10 (20) | 11 (19) | 3 (17) | 13 (20) |
| On‐study deaths | 0 | 1 (2) | 1 (2) | 0 | 1 (2) |
AE, adverse event; mITT, modified intent to treat; SAE, serious adverse event.
New‐onset AEs during cycle ≥17.
Table 4.
Drug‐relateda AEs reported at any grade in ≥15% of the total population and/or at grade 3 in ≥5% of the total population (no drug‐related grade 4 AEs were reported)
| AE, n (%) | Phase 1, n = 14 | Phase 2, n = 50 | mITT, n = 57 | Patients who received maintenance,b n = 18 | Total, N = 64 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All‐grade | Grade 3 | All‐grade | Grade 3 | All‐grade | Grade 3 | All‐grade | Grade 3 | All‐grade | Grade 3 | |
| PN NECc | 10 (71) | 2 (14) | 28 (56) | 3 (6) | 33 (58) | 4 (7) | 5 (28) | 1 (6) | 38 (59) | 5 (8) |
| Fatigue | 9 (64) | 1 (7) | 22 (44) | 1 (2) | 27 (47) | 2 (4) | 2 (11) | 0 | 31 (48) | 2 (3) |
| Rashes, eruptions and exanthems NECd | 7 (50) | 3 (21) | 23 (46) | 5 (10) | 25 (44) | 6 (11) | 3 (17) | 1 (6) | 30 (47) | 8 (13) |
| Nausea | 5 (36) | 0 | 17 (34) | 1 (2) | 19 (33) | 1 (2) | 6 (33) | 1 (6) | 22 (34) | 1 (2) |
| Diarrhoea | 5 (36) | 0 | 15 (30) | 1 (2) | 18 (32) | 1 (2) | 5 (28) | 1 (6) | 20 (31) | 1 (2) |
| Peripheral oedema | 7 (50) | 1 (7) | 13 (26) | 1 (2) | 17 (30) | 2 (4) | 2 (11) | 0 | 20 (31) | 2 (3) |
| Dysgeusia | 3 (21) | 0 | 17 (34) | 0 | 18 (32) | 0 | 0 | 0 | 20 (31) | 0 |
| Insomnia | 6 (43) | 1 (7) | 13 (26) | 0 | 15 (26) | 0 | 0 | 0 | 19 (30) | 1 (2) |
| Constipation | 4 (29) | 0 | 14 (28) | 0 | 15 (26) | 0 | 0 | 0 | 18 (28) | 0 |
| Dizziness | 2 (14) | 0 | 12 (24) | 2 (4) | 14 (25) | 2 (4) | 1 (6) | 0 | 14 (22) | 2 (3) |
| Vomiting | 3 (21) | 0 | 8 (16) | 1 (2) | 9 (16) | 1 (2) | 3 (17) | 1 (6) | 11 (17) | 1 (2) |
| Anxiety | 3 (21) | 0 | 9 (18) | 1 (2) | 11 (19) | 1 (2) | 0 | 0 | 12 (19) | 1 (2) |
| Muscle spasms | 3 (21) | 0 | 9 (18) | 0 | 11 (19) | 0 | 0 | 0 | 12 (19) | 0 |
| Muscular weakness | 3 (21) | 0 | 7 (14) | 1 (2) | 9 (16) | 1 (2) | 2 (11) | 1 (6) | 10 (16) | 1 (2) |
| Hyperglycaemia | 4 (29) | 3 (21) | 4 (8) | 2 (4) | 8 (14) | 5 (9) | 2 (11) | 0 | 8 (13) | 5 (8) |
| Hyponatraemia | 1 (7) | 1 (7) | 3 (6) | 3 (6) | 3 (5) | 3 (5) | 0 | 0 | 4 (6) | 4 (6) |
| ALT increased | 2 (14) | 2 (14) | 4 (8) | 1 (2) | 4 (7) | 1 (2) | 1 (6) | 0 | 6 (9) | 3 (5) |
| Pneumonia | 1 (7) | 1 (7) | 4 (8) | 3 (6) | 5 (9) | 4 (7) | 0 | 0 | 5 (8) | 4 (6) |
| Neutrophil count decreased | 0 | 0 | 3 (6) | 3 (6) | 3 (5) | 3 (5) | 1 (6) | 1 (6) | 3 (5) | 3 (5) |
| Neutropenia | 1 (7) | 1 (7) | 4 (8) | 2 (4) | 5 (9) | 3 (5) | 0 | 0 | 5 (8) | 3 (5) |
| Thrombocytopenia | 2 (14) | 2 (14) | 2 (4) | 1 (2) | 3 (5) | 2 (4) | 0 | 0 | 4 (6) | 3 (5) |
AE, adverse event; ALT, alanine aminotransferase; mITT, modified intent to treat; NEC, not elsewhere classified; PN, peripheral neuropathy.
Drug‐related defined as related to any drug in the combination.
New‐onset AEs during cycle ≥17.
High‐level term, includes PN and peripheral sensory neuropathy.
High‐level term, includes rash, macular rash, papular rash and maculo‐papular rash.
AEs led to dose reduction of any study drug in 66% of all patients, including new‐onset AEs resulting in dose reductions in 33% of patients who received ixazomib maintenance (Table 3). The most common AEs resulting in dose reductions included PN NEC (n = 10, 16%), rashes, eruptions and exanthems NEC (n = 9, 14%), anxiety (n = 7, 11%), peripheral oedema (n = 5, 8%) and fatigue (n = 4, 6%). Thirty (47%) patients had a treatment‐emergent SAE, with pneumonia (n = 4, 6%), lung infections (n = 2, 3%), atrial fibrillation (n = 2, 3%) and cellulitis (n = 2, 3%) the only events reported in >1 patient. AEs leading to discontinuation of study treatment were reported in 13 (20%) patients, with only PN NEC (n = 3, 5%) reported in >1 patient. Twelve (19%) patients (all with pre‐existing cardiac risk factors) reported AEs within the cardiac disorders system organ class, with only 4 of these patients reporting grade ≥3 AEs, and only 1 discontinuing treatment. Pre‐existing risk factors for these 4 patients with grade ≥3 AEs included: Patient 1, hyperlipidaemia, hypertension, history of coronary artery bypass graft, and sinus bradycardia; Patient 2, fatigue, diabetes, hypertension, obstructive sleep apnoea and history of transient ischaemic attack; Patient 3 (discontinued treatment), atrial fibrillation, coronary artery disease, diastolic dysfunction, dyspnoea, high cholesterol and sinus tachycardia; Patient 4: obesity, hypertension, diabetes, hypercholesterolaemia, dyspnoea, fatigue and atrial fibrillation. Five patients (8%) had peripheral embolism and thrombosis (grade 3, n = 1). One patient in the phase 2 cohort died on study due to cardio‐respiratory arrest, which was probably secondary to a pulmonary embolism (PE), that was considered related to lenalidomide treatment.
Response and outcomes
Among all response‐evaluable patients, the ORR was 94%, including: CR + VGPR, 68%; CR, 24% (Table 5). In mITT response‐evaluable patients, the ORR was 95%, including: CR + VGPR, 68%; CR, 27%. A waterfall plot of best M‐protein responses (mITT population) is shown in Fig 1. Of 6 patients with high‐risk cytogenetic abnormalities, 4 achieved a CR (duration, 38·5–51·3 months in the 3 patients with available follow‐up) and 1 achieved a partial response (PR). In the mITT population, the median time to first response was 0·7 months, and the median time to best response among patients achieving ≥PR, ≥VGPR and ≥CR was 1·9, 3·4 and 4·2 months, respectively. With 12 of 53 responding patients in the mITT population having had progression events, the median DOR was 36·9 months. Responses deepened over the course of treatment (Fig 2). Among 18 patients receiving ixazomib maintenance therapy, 4 (22%) had improvement in depth of response during this phase (1 CR to stringent complete response [sCR]; 1 VGPR to CR; 2 VGPR to nCR).
Table 5.
Confirmed best response to treatment among response‐evaluable patients, by study phase and overall
| Response rate, n (%) [95% CI] | Phase 1 n = 13 | Phase 2 n = 49 | mITT n = 56 | Patients who entered the maintenance period n = 18 | Total N = 62 |
|---|---|---|---|---|---|
| ORR (≥PR) |
12 (92) [64, 100] |
46 (94) [83, 99] |
53 (95) [85, 99] |
17 (94) [73, 100] |
58 (94) [84, 98] |
| CR + VGPR |
10 (77) [46, 95] |
32 (65) [50, 78] |
38 (68) [54, 80] |
16 (89) [65, 99] |
42 (68) [55, 79] |
| CR |
1 (8) [0, 36] |
14 (29) [17, 43] |
15 (27) [16, 40] |
8 (44) [22, 69] |
15 (24) [14, 37] |
| sCR | 0 | 11 (22) | 11 (20) | 5 (28) | 11 (18) |
| VGPR | 9 (69) | 18 (37) | 23 (41) | 8 (44) | 27 (44) |
| nCR | 2 (15) | 5 (10) | 6 (11) | 4 (22) | 7 (11) |
| PR | 2 (15) | 14 (29) | 15 (27) | 1 (6) | 16 (26) |
| MR | 1 (8) | 3 (6) | 3 (5) | 1 (6) | 4 (6) |
| Stable disease | 0 | 0 | 0 | 0 | 0 |
| Progressive disease | 0 | 0 | 0 | 0 | 0 |
CI, confidence interval; CR, complete response; mITT, modified intent to treat; MR, minimal response; nCR, near complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
62 of 64 patients were evaluable for response (2 patients did not have post‐baseline response assessments; thus were not evaluable). Data are n (%) or n (%) [95% CI].
Figure 1.
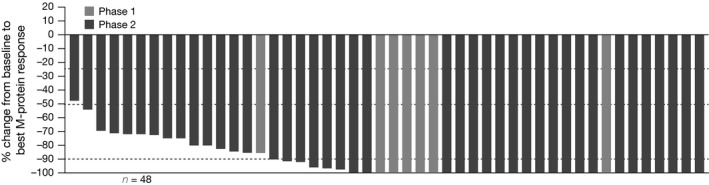
Waterfall plot of best M‐protein response, among response‐evaluable patients treated at the recommended phase 2 dose (modified intent‐to‐treat population).
Figure 2.
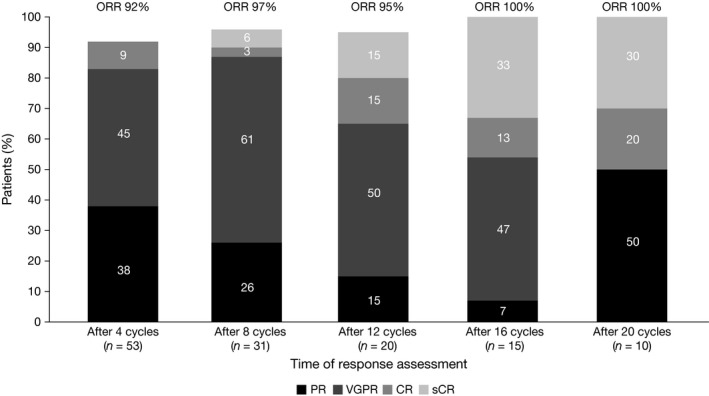
Increasing depth of response with increasing duration of therapy in response‐evaluable patients treated at the recommended phase 2 dose (modified intent‐to‐treat population). CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
A total of 27 patients in the overall study population had an MRD assessment at the time of suspected CR. Responses after bone marrow assessment were: CR or sCR, 33%; VGPR or nCR, 56%; PR, 7%; and MR, 4%. MRD assessment was successfully performed in all 27 patients. Among all 62 response‐evaluable patients, 23% had MRD‐negative status: 7 out of 9 patients in CR or sCR (11%) and 7 out of 15 patients in VGPR or nCR (11%). Two patients achieved MRD‐negative status during the maintenance period.
Among all 64 patients, 18 (28%) had a PFS event at the time of data cut‐off, and 46 (72%) were censored; patients who proceeded to ASCT were censored at their last response assessment. Median PFS was 24·9 months (95% confidence interval [CI], 17·4–40·5). In the mITT population, 17 (30%) of 57 patients had a PFS event, and median PFS was 29·7 months (95% CI, 17·4–not estimable) (Fig 3). With a median follow‐up for survival of 46·9 months, median OS was not reached in the overall or mITT populations; the respective 3‐year OS estimates were 91% and 90%.
Figure 3.
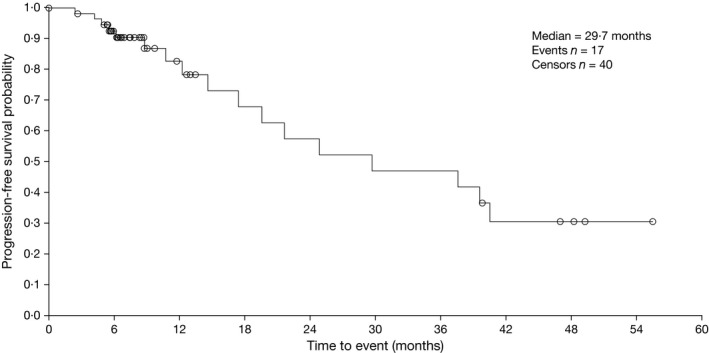
Kaplan–Meier analysis of progression‐free survival in the modified intent‐to‐treat population (mITT) population.
Stem cell mobilization and engraftment
Stem cell mobilization was conducted according to individual site protocol and was successful, with no failures, for 31 patients after a median of 7 (range 4–13) induction cycles; 18 (58%) and 13 (42%) patients underwent 1 and 2 apheresis procedures, respectively. The most commonly used mobilization regimen was granulocyte colony‐stimulating factor (G‐CSF) plus plerixafor (n = 14, 45%), followed by G‐CSF plus cyclophosphamide (n = 6, 19%) and G‐CSF alone (n = 5, 16%). The median number of harvested CD34 + stem cells was 9·5 × 106/l (range 4–52 × 106/l). Twenty‐four patients then proceeded to early ASCT and engraftment kinetics were as expected; median time to neutrophil engraftment (n = 11; absolute neutrophil count [ANC] >0·5 × 109/l) was 14 days (range 11–42) and to platelet engraftment (n = 13; >20 × 109/l) was 15 days (range 12–111).
Pharmacokinetics
Thirteen patients were evaluable for pharmacokinetics. Ixazomib was rapidly absorbed; the median first observed time of maximum plasma concentration (C max) was 1 h after dosing on both days 1 and 11. At the RP2D, the geometric mean (%CV [coefficient of variation]) C max was 33·5 (57) ng/ml and 58·7 (32) ng/ml on days 1 and 11, respectively. The corresponding values for the area under the plasma concentration versus time curve from 0–72 h post‐dose (AUC0–72) were 315 (23) h*ng/ml and 1110 (39) h*ng/ml, respectively.
Discussion
The RP2D of twice‐weekly oral ixazomib plus Rd in patients with NDMM was 3·0 mg. At this dose level the ORR was 95%, including 68% CR + VGPR and 27% CR. Responses were rapid, deep and durable, deepening over the course of treatment. Median PFS was 29·7 months. The combination had no deleterious effects on the ability to collect stem cells for transplant, and engraftment kinetics, where available, did not suggest any adverse effect. This was the first study to evaluate twice‐weekly ixazomib‐Rd for 16 cycles (48 weeks), followed by a period of single‐agent ixazomib maintenance. The regimen was generally well tolerated and long‐term therapy was feasible with 28% of patients continuing on therapy beyond 16 cycles and proceeding to maintenance. Among all 64 patients, 64% had grade 3 drug‐related AEs; there were no grade 4 drug‐related AEs. New‐onset drug‐related grade 3 AEs during maintenance therapy with single‐agent ixazomib were limited. Pharmacokinetic data were similar to other studies suggesting no pharmacokinetic interaction of ixazomib with Rd (http://media.celgene.com/content/uploads/revlimid-pi.pdf, Gupta et al, 2017a).
While the MTD for twice‐weekly ixazomib‐Rd in patients with NDMM was determined to be 3·7 mg, the RP2D was 3·0 mg based on overall tolerability across multiple cycles, including an elevated rate of rash with 3·7 mg (Kumar et al, 2014d). Additionally, the RDI of lenalidomide was impacted by the higher 3·7 mg dose of ixazomib as a result of overlapping toxicities, such as rash, thrombocytopenia and gastrointestinal toxicities (http://media.celgene.com/content/uploads/revlimid-pi.pdf, Kumar et al, 2014d). This finding is similar to results from a parallel study investigating weekly ixazomib‐Rd in patients with NDMM in which the lenalidomide RDI was 96% and 84·6% in patients who received ixazomib at an approximate fixed dose of 4·0 mg and 5·5 mg, respectively (Kumar et al, 2014b; Gupta et al, 2017b). Consequently, 4·0 mg ixazomib was the dose selected for phase 3 evaluation of weekly ixazomib‐Rd (Gupta et al, 2017b). The RP2D of 3·0 mg determined here for twice‐weekly ixazomib‐Rd is lower than the RP2D of 4·0 mg reported in the parallel study investigating weekly ixazomib‐Rd in patients with NDMM (Kumar et al, 2014b). Although the twice‐weekly regimen represents a more intensive ixazomib dosing schedule compared with weekly dosing, the 21‐day cycle used in the present study represents a less intensive schedule for Rd, with lenalidomide dosed on days 1–14 compared with days 1–21 in the weekly ixazomib‐Rd study (Kumar et al, 2014b).
At the RP2D, twice‐weekly ixazomib‐Rd was associated with a high ORR (95%) in patients with NDMM; in particular, the CR + VGPR rate (phase 2 primary endpoint) was 68%. Responses were rapid in onset and deepened with increasing duration of treatment. Approximately one‐third of patients discontinued early to undergo ASCT, thereby potentially limiting further deepening of responses in these patients. Response rates with twice‐weekly ixazomib‐Rd are similar to those seen with weekly ixazomib‐Rd (ORR, 92%; ≥VGPR 58%) (Kumar et al, 2014b). Although cross‐trial comparisons should be interpreted with caution, these response rates and PFS are also similar to those seen with other triplet regimens incorporating a proteasome inhibitor and an immunomodulatory drug in NDMM (Richardson et al, 2010; Kumar et al, 2012; Dytfeld et al, 2014; Durie et al, 2017). Furthermore, responses were long‐lasting, with a median DOR of 36·9 months. The overall rate of MRD negativity in response‐evaluable patients who achieved CR, sCR, VGPR or nCR was 23%. Other studies in NDMM have reported MRD‐negativity rates of around 40% for triplet regimens including a proteasome inhibitor and an immunomodulatory agent. In NDMM patients treated with KRd, with median follow‐up of 13 months and median treatment duration of 12 cycles, MRD‐negativity was seen in 20/22 patients with CR or suspected CR (including nCR) for an MRD‐negativity rate in all response‐evaluable patients (n = 53) of 38% (Jakubowiak et al, 2012). In 98 NDMM patients treated with bortezomib in combination with thalidomide and dexamethasone with or without cyclophosphamide, with median follow‐up of approximately 65 months, 42 patients (43%) with >PR, including 34 patients (35%) with bone‐marrow confirmed CR, were MRD‐negative (Ludwig et al, 2015). However, any comparison of these findings must be interpreted with caution given differences in treatment regimen, duration of follow‐up, timing of MRD assessment, the numbers of patients tested and the sensitivity of the MRD assays used. For example, considering treatment regimen, in the Jakubowiak et al study, patients could receive maintenance therapy with KRd for up to 24 cycles, and in the Ludwig et al study 90% of patients underwent ASCT following initial treatment with 4 cycles of bortezomib plus thalidomide and dexamethasone +/‐ cyclophosphamide (Jakubowiak et al, 2012; Ludwig et al, 2015).
Patients received a median of 9 treatment cycles with twice‐weekly ixazomib‐Rd, with median treatment duration limited by one‐third of patients discontinuing to undergo ASCT. Nevertheless, 30% and 14% of patients remained on ixazomib therapy for approximately 1 and 2 years, respectively. Together with the lack of cumulative toxicity seen with long‐term administration, including the limited new‐onset toxicity in patients receiving single‐agent ixazomib maintenance, these data demonstrate the tolerability and support the feasibility of long‐term treatment with this regimen. While the role of early versus late transplant requires clarification, evidence suggests that prolonged treatment may benefit both transplant‐ineligible and ‐eligible patients (Palumbo et al, 2010; Attal et al, 2012; Mateos et al, 2012; Benboubker et al, 2014).
Drug‐related grade 3 AEs were experienced by 64% of all patients, with no grade 4 drug‐related AEs reported; only 28% of patients proceeding to maintenance reported new‐onset drug‐related grade 3 AEs during maintenance with single‐agent ixazomib. AEs led to dose reductions (of any agent) or discontinuation in 66% and 20% of patients, respectively. AE rates reported here appear similar to those seen in the parallel study of weekly ixazomib‐Rd in patients with NDMM, but dose reductions and discontinuations due to AEs were lower with weekly ixazomib dosing (57% and 8%, respectively) (Kumar et al, 2014b). The AE profile reported here was largely expected based on clinical experience with each drug in this triplet regimen. The most common drug‐related AEs were PN NEC (high‐level term), fatigue, rashes, eruptions and exanthems NEC (high‐level term) and nausea; the majority of these AEs were grade 1 or 2.
Rashes, eruptions and exanthems NEC were seen in a substantial number of patients, but were mainly low grade, were not reported as DLTs and proved manageable with dose modifications (including reductions or holding of either lenalidomide or ixazomib, whichever was the causative agent) and oral or topical antihistamines, as required. The occurrence of rash events probably represents an overlapping effect of lenalidomide (Tinsley et al, 2015) and ixazomib (Kumar et al, 2014d; Richardson et al, 2014; Gupta et al, 2016b). The rate of 47% for drug‐related, any‐grade rashes, eruptions and exanthems reported here is higher than the rate of 29% reported for this high‐level term in the parallel phase 1/2 study investigating weekly ixazomib‐Rd in patients with NDMM (Takeda, unpublished data, February 2017). It is difficult to put these data into context with safety findings for other proteasome inhibitors due to differences in how rash events have been reported (in aggregate, or as individual preferred terms). For example, a phase 1/2 study of bortezomib‐Rd in patients with NDMM reported the incidence of all‐grade and grade 3 rash/desquamation as 36% and 2%, respectively (Richardson et al, 2010).
Drug‐related PN NEC was reported in 59% of patients but was grade 3 in 8%. These rates of drug‐related PN NEC are higher than reported with weekly ixazomib‐Rd in NDMM (all grades, 38%; grade ≥3, 6%) (Kumar et al, 2014b). PN is an AE of clinical interest with respect to some proteasome inhibitors, and these findings should be viewed in the context of available data for other agents. Phase 3 evaluation of bortezomib‐Rd in patients with NDMM without intent for immediate ASCT reported 33% of patients with treatment‐related grade ≥3 neurological events (Durie et al, 2017). In a phase 2 study of bortezomib‐Rd in patients with NDMM, grade 1/2 PN occurred in 55% of patients, and grade ≥3 neuropathy in 17% of patients (Kumar et al, 2012). Phase 1/2 evaluation of bortezomib‐Rd in patients with NDMM reported sensory neuropathy in 80% of patients (2% grade 3) and motor neuropathy in 18% of patients (2% grade 3) (Richardson et al, 2010). A phase 1/2 study of KRd in NDMM reported treatment‐emergent PN (all grade ≤2) during induction (cycles 1–8) in 23% of patients; during maintenance, PN remained limited (11%; all grade ≤2); most neuropathic events were attributed to lenalidomide (Jakubowiak et al, 2012). Limited additional PN was seen with ixazomib‐Rd versus placebo‐Rd in the TOURMALINE‐MM1 study in RRMM, consistent with lenalidomide being associated with neuropathic events (Moreau et al, 2016).
Awareness of cardiovascular toxicity associated with proteasome inhibitors is increasing (Koulaouzidis & Lyon, 2017). Integrated nonclinical and clinical risk assessment has previously reported no clinically meaningful effects of ixazomib on QTc or heart rate (Gupta et al, 2015b). The incidence of cardiovascular AEs in patients with no pre‐existing cardiac risk factors was limited in the present study and included 2 patients (3%) with atrial fibrillation reported as a treatment‐emergent SAE. Twelve patients (19%) reported AEs within the cardiac disorders system organ class; however, these patients all had pre‐existing cardiac risk factors. Cardiovascular AEs, including heart failure (Roussel et al, 2016), have previously been reported with KRd in NDMM patients. Cardiovascular toxicities have also been reported with bortezomib‐Rd in NDMM patients, including: treatment‐related cardiac arrhythmia (5%) and general cardiac events (21%) (Durie et al, 2017), and QTc interval (6%); and atrial fibrillation (2%) (Richardson et al, 2010). The prescribing information for both carfilzomib (https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202714s015lbl.pdf) and bortezomib (http://www.velcade.com/files/PDFs/VELCADE_PRESCRIBING_INFORMATION.pdf) notes cardiac toxicities under warnings and precautions reflective of these safety concerns, but this is not noted in the ixazomib prescribing information (https://www.ninlaro.com/prescribing-information.pdf; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003844/WC500217620.pdf) and our data support no such safety concerns with ixazomib‐based combinations.
We demonstrate that twice‐weekly ixazomib‐Rd offers substantial activity and promising outcomes in NDMM patients but appears to be associated with a somewhat higher toxicity burden than weekly ixazomib‐Rd in this setting. Further investigation of twice‐weekly dosing is potentially warranted to define a subset of patients who are able to tolerate, and benefit from, more intensive therapy.
Author contributions
P.G.R., E.L., N.G., A.D.B., D.B. and R.B. contributed to the study concept and design; all authors collected and assembled the data, and/or provided the study materials or patients; P.G.R., D.H.V., J.G.B., A.C., C.B., E.L., N.G., A.D.B., J.E., D.B. and R.B. analysed and interpreted the data; P.G.R. and D.B. drafted the manuscript, all authors contributed to critically revising the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict‐of‐interest disclosures
P.G.R. reports advisory board participation for Celgene, Novartis and Takeda. C.C.H. reports research funding from Takeda, Celgene, ARNO Therapeutics, Janssen Pharmaceuticals, Karyopharm and Merck, and advisory board participation for Thrassos and InCyte. C.A.R. reports independent review committee membership for Millennium/Takeda clinical trial NCT01564537, advisory committee membership for Millennium, and consultancy fees from Celgene and Bristol Myers Squibb. D.H.V. reports stock ownership in Amgen, and honoraria from Celgene, Takeda, Janssen and Amgen. J.G.B. reports research funding from AbbVie, Acetylon, Amgen, Bluebird, Bristol Myers Squibb, Calithera, Celgene, Constellation, Curis, Epizyme, Janssen, Karyopharm, Kesios, Novartis, Onyx, Takeda and Tragara. M.L. reports research funding from Celgene and Takeda, and advisory board participation for Takeda. A.C. reports research funding from and advisory board participation for Millennium/Takeda, Celgene, Array Bio Pharma, Novartis Pharmaceuticals and Onyx, research funding from Janssen Pharmaceuticals and Bristol Myers Squibb, and consultancy fees from Janssen Pharmaceuticals and Bristol Myers Squibb. S.D.S. reports research funding from Seattle Genetics, Merck Sharp and Dohme Corp, Janssen Research and Development, LLC, Acerta Pharma BV, Pharmacyclics, Genentech and Portola Pharmaceuticals. N.R. reports consultancy for Onyx, Amgen, Millennium and Celgene. C.B., N.G. and D.B. report employment by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. E.L. reports previous employment by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. A.D.B. and J.E. report stock ownership in and employment by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. R.B. reports honoraria from Celgene and research funding (to his institution) from Celgene, Takeda, Karyopharm, Merck, Bristol Myers Squibb, Novartis and Signal Genetics. M.H. and D.L. declare no competing financial interests.
Supporting information
Data S1. Supplementary methods.
Table SI. Eligibility criteria.
Table SII. Definitions of dose‐limiting toxicities.
Table SIII. Treatment‐emergent AEs* reported at any grade in ≥25% of the total population and/or at grade 3 in ≥5% of the total population.
Acknowledgments
The authors would like to acknowledge writing support from Helen Wilkinson and Rachel Moir of FireKite, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 ethical guidelines (Battisti et al, 2015). This work was supported by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
References
- Attal, M. , Lauwers‐Cances, V. , Marit, G. , Caillot, D. , Moreau, P. , Facon, T. , Stoppa, A.M. , Hulin, C. , Benboubker, L. , Garderet, L. , Decaux, O. , Leyvraz, S. , Vekemans, M.C. , Voillat, L. , Michallet, M. , Pegourie, B. , Dumontet, C. , Roussel, M. , Leleu, X. , Mathiot, C. , Payen, C. , Avet‐Loiseau, H. , Harousseau, J.L. & Investigators, I.F.M. (2012) Lenalidomide maintenance after stem‐cell transplantation for multiple myeloma. New England Journal of Medicine, 366, 1782–1791. [DOI] [PubMed] [Google Scholar]
- Barlogie, B. , Mitchell, A. , van Rhee, F. , Epstein, J. , Morgan, G.J. & Crowley, J. (2014) Curing myeloma at last: defining criteria and providing the evidence. Blood, 124, 3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti, W.P. , Wager, E. , Baltzer, L. , Bridges, D. , Cairns, A. , Carswell, C.I. , Citrome, L. , Gurr, J.A. , Mooney, L.A. , Moore, B.J. , Peña, T. , Sanes‐Miller, C.H. , Veitch, K. , Woolley, K.L. & Yarker, Y.E. & International Society for Medical Publication Professionals . (2015) Good publication practice for communicating company‐sponsored medical research: GPP3. Annals of Internal Medicine, 163, 461–464. [DOI] [PubMed] [Google Scholar]
- Baz, R. , Lin, H.M. , Hui, A.M. , Harvey, R.D. , Colson, K. , Gallop, K. , Swinburn, P. , Laubach, J. , Berg, D. & Richardson, P. (2015) Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health‐related quality of life. Supportive Care in Cancer, 23, 2789–2797. [DOI] [PubMed] [Google Scholar]
- Benboubker, L. , Dimopoulos, M.A. , Dispenzieri, A. , Catalano, J. , Belch, A.R. , Cavo, M. , Pinto, A. , Weisel, K. , Ludwig, H. , Bahlis, N. , Banos, A. , Tiab, M. , Delforge, M. , Cavenagh, J. , Geraldes, C. , Lee, J.J. , Chen, C. , Oriol, A. , de la Rubia, J. , Qiu, L. , White, D.J. , Binder, D. , Anderson, K. , Fermand, J.P. , Moreau, P. , Attal, M. , Knight, R. , Chen, G. , Van Oostendorp, J. , Jacques, C. , Ervin‐Haynes, A. , Avet‐Loiseau, H. , Hulin, C. & Facon, T. (2014) Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. New England Journal of Medicine, 371, 906–917. [DOI] [PubMed] [Google Scholar]
- Calhoun, E.A. , Welshman, E.E. , Chang, C.H. , Lurain, J.R. , Fishman, D.A. , Hunt, T.L. & Cella, D. (2003) Psychometric evaluation of the functional assessment of cancer therapy/gynecologic oncology group‐neurotoxicity (Fact/GOG‐Ntx) questionnaire for patients receiving systemic chemotherapy. International Journal of Gynecological Cancer, 13, 741–748. [DOI] [PubMed] [Google Scholar]
- Cavo, M. , Tacchetti, P. , Patriarca, F. , Petrucci, M.T. , Pantani, L. , Galli, M. , Di Raimondo, F. , Crippa, C. , Zamagni, E. , Palumbo, A. , Offidani, M. , Corradini, P. , Narni, F. , Spadano, A. , Pescosta, N. , Deliliers, G.L. , Ledda, A. , Cellini, C. , Caravita, T. , Tosi, P. & Baccarani, M. (2010) Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet, 376, 2075–2085. [DOI] [PubMed] [Google Scholar]
- Cavo, M. , Pantani, L. , Petrucci, M.T. , Patriarca, F. , Zamagni, E. , Donnarumma, D. , Crippa, C. , Boccadoro, M. , Perrone, G. , Falcone, A. , Nozzoli, C. , Zambello, R. , Masini, L. , Furlan, A. , Brioli, A. , Derudas, D. , Ballanti, S. , Dessanti, M.L. , De Stefano, V. , Carella, A.M. , Marcatti, M. , Nozza, A. , Ferrara, F. , Callea, V. , Califano, C. , Pezzi, A. , Baraldi, A. , Grasso, M. , Musto, P. & Palumbo, A. (2012) Bortezomib‐thalidomide‐dexamethasone is superior to thalidomide‐dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood, 120, 9–19. [DOI] [PubMed] [Google Scholar]
- Delforge, M. & Ludwig, H. (2017) How I manage the toxicities of myeloma drugs. Blood, 129, 2359–2367. [DOI] [PubMed] [Google Scholar]
- Dowling, M. , Kelly, M. & Meenaghan, T. (2016) Multiple myeloma: managing a complex blood cancer. British Journal of Nursing, 25, S18–S28. [DOI] [PubMed] [Google Scholar]
- Durie, B.G. , Harousseau, J.L. , Miguel, J.S. , Blade, J. , Barlogie, B. , Anderson, K. , Gertz, M. , Dimopoulos, M. , Westin, J. , Sonneveld, P. , Ludwig, H. , Gahrton, G. , Beksac, M. , Crowley, J. , Belch, A. , Boccadaro, M. , Cavo, M. , Turesson, I. , Joshua, D. , Vesole, D. , Kyle, R. , Alexanian, R. , Tricot, G. , Attal, M. , Merlini, G. , Powles, R. , Richardson, P. , Shimizu, K. , Tosi, P. , Morgan, G. & Rajkumar, S.V. & International Myeloma Working Group . (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Durie, B.G. , Hoering, A. , Abidi, M.H. , Rajkumar, S.V. , Epstein, J. , Kahanic, S.P. , Thakuri, M. , Reu, F. , Reynolds, C.M. , Sexton, R. , Orlowski, R.Z. , Barlogie, B. & Dispenzieri, A. (2017) Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem‐cell transplant (SWOG S0777): a randomised, open‐label, phase 3 trial. Lancet, 389, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dytfeld, D. , Jasielec, J. , Griffith, K.A. , Lebovic, D. , Vesole, D.H. , Jagannath, S. , Al‐Zoubi, A. , Anderson, T. , Detweiler‐Short, K. , Stockerl‐Goldstein, K. , Ahmed, A. , Jobkar, T. , Durecki, D.E. , McDonnell, K. , Mietzel, M. , Couriel, D. , Kaminski, M. , Vij, R. & Jakubowiak, A.J. (2014) Carfilzomib, lenalidomide, and low‐dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica, 99, e162–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garderet, L. , Iacobelli, S. , Moreau, P. , Dib, M. , Lafon, I. , Niederwieser, D. , Masszi, T. , Fontan, J. , Michallet, M. , Gratwohl, A. , Milone, G. , Doyen, C. , Pegourie, B. , Hajek, R. , Casassus, P. , Kolb, B. , Chaleteix, C. , Hertenstein, B. , Onida, F. , Ludwig, H. , Ketterer, N. , Koenecke, C. , van Os, M. , Mohty, M. , Cakana, A. , Gorin, N.C. , de Witte, T. , Harousseau, J.L. , Morris, C. & Gahrton, G. (2012) Superiority of the triple combination of bortezomib‐thalidomide‐dexamethasone over the dual combination of thalidomide‐dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005‐04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Journal of Clinical Oncology, 30, 2475–2482. [DOI] [PubMed] [Google Scholar]
- Guglielmelli, T. & Palumbo, A. (2015) Multiple myeloma: is a shift toward continuous therapy needed to move forward? Expert Review of Hematology, 8, 253–256. [DOI] [PubMed] [Google Scholar]
- Gupta, N. , Zhao, Y. , Hui, A.M. , Esseltine, D.L. & Venkatakrishnan, K. (2015a) Switching from body surface area‐based to fixed dosing for the investigational proteasome inhibitor ixazomib: a population pharmacokinetic analysis. British Journal of Clinical Pharmacology, 79, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Huh, Y. , Hutmacher, M.M. , Ottinger, S. , Hui, A.M. & Venkatakrishnan, K. (2015b) Integrated nonclinical and clinical risk assessment of the investigational proteasome inhibitor ixazomib on the QTc interval in cancer patients. Cancer Chemotherapy and Pharmacology, 76, 507–516. [DOI] [PubMed] [Google Scholar]
- Gupta, N. , Hanley, M.J. , Venkatakrishnan, K. , Wang, B. , Sharma, S. , Bessudo, A. , Hui, A.M. & Nemunaitis, J. (2016a) The effect of a high‐fat meal on the pharmacokinetics of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. Journal of Clinical Pharmacology, 56, 1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Labotka, R. , Liu, G. , Hui, A.M. & Venkatakrishnan, K. (2016b) Exposure‐safety‐efficacy analysis of single‐agent ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma: dose selection for a phase 3 maintenance study. Investigational New Drugs, 34, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Diderichsen, P.M. , Hanley, M.J. , Berg, D. , van de Velde, H. , Harvey, R.D. & Venkatakrishnan, K. (2017a) Population pharmacokinetic analysis of ixazomib, an oral proteasome inhibitor, including data from the phase III TOURMALINE‐MM1 study to inform labelling. Clinical Pharmacokinetics, 56, 1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Yang, H. , Hanley, M.J. , Zhang, S. , Liu, R. , Kumar, S. , Richardson, P.G. , Skacel, T. & Venkatakrishnan, K. (2017b) Dose and schedule selection of the oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma: clinical and model‐based analyses. Target Oncology, 12, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak, A.J. , Dytfeld, D. , Griffith, K.A. , Lebovic, D. , Vesole, D.H. , Jagannath, S. , Al‐Zoubi, A. , Anderson, T. , Nordgren, B. , Detweiler‐Short, K. , Stockerl‐Goldstein, K. , Ahmed, A. , Jobkar, T. , Durecki, D.E. , McDonnell, K. , Mietzel, M. , Couriel, D. , Kaminski, M. & Vij, R. (2012) A phase 1/2 study of carfilzomib in combination with lenalidomide and low‐dose dexamethasone as a frontline treatment for multiple myeloma. Blood, 120, 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katodritou, E. , Papadaki, S. , Konstantinidou, P. & Terpos, E. (2016) Is it possible to cure myeloma without allogeneic transplantation? Transfusion and Apheresis Science, 54, 63–70. [DOI] [PubMed] [Google Scholar]
- Korde, N. , Roschewski, M. , Zingone, A. , Kwok, M. , Manasanch, E.E. , Bhutani, M. , Tageja, N. , Kazandjian, D. , Mailankody, S. , Wu, P. , Morrison, C. , Costello, R. , Zhang, Y. , Burton, D. , Mulquin, M. , Zuchlinski, D. , Lamping, L. , Carpenter, A. , Wall, Y. , Carter, G. , Cunningham, S.C. , Gounden, V. , Sissung, T.M. , Peer, C. , Maric, I. , Calvo, K.R. , Braylan, R. , Yuan, C. , Stetler‐Stevenson, M. , Arthur, D.C. , Kong, K.A. , Weng, L. , Faham, M. , Lindenberg, L. , Kurdziel, K. , Choyke, P. , Steinberg, S.M. , Figg, W. & Landgren, O. (2015) Treatment with carfilzomib‐lenalidomide‐dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncology, 1, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulaouzidis, G. & Lyon, A.R. (2017) Proteasome inhibitors as a potential cause of heart failure. Heart Failure Clinics, 13, 289–295. [DOI] [PubMed] [Google Scholar]
- Kumar, S.K. , Rajkumar, S.V. , Dispenzieri, A. , Lacy, M.Q. , Hayman, S.R. , Buadi, F.K. , Zeldenrust, S.R. , Dingli, D. , Russell, S.J. , Lust, J.A. , Greipp, P.R. , Kyle, R.A. & Gertz, M.A. (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood, 111, 2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Flinn, I. , Richardson, P.G. , Hari, P. , Callander, N. , Noga, S.J. , Stewart, A.K. , Turturro, F. , Rifkin, R. , Wolf, J. , Estevam, J. , Mulligan, G. , Shi, H. , Webb, I.J. & Rajkumar, S.V. (2012) Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood, 119, 4375–4382. [DOI] [PubMed] [Google Scholar]
- Kumar, S.K. , Dispenzieri, A. , Lacy, M.Q. , Gertz, M.A. , Buadi, F.K. , Pandey, S. , Kapoor, P. , Dingli, D. , Hayman, S.R. , Leung, N. , Lust, J. , McCurdy, A. , Russell, S.J. , Zeldenrust, S.R. , Kyle, R.A. & Rajkumar, S.V. (2014a) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia, 28, 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S.K. , Berdeja, J.G. , Niesvizky, R. , Lonial, S. , Laubach, J.P. , Hamadani, M. , Stewart, A.K. , Hari, P. , Roy, V. , Vescio, R. , Kaufman, J.L. , Berg, D. , Liao, E. , Di Bacco, A. , Estevam, J. , Gupta, N. , Hui, A.M. , Rajkumar, V. & Richardson, P.G. (2014b) Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open‐label phase 1/2 study. Lancet Oncology, 15, 1503–1512. [DOI] [PubMed] [Google Scholar]
- Kumar, S.K. , Berdeja, J.G.B. , Niesvizky, R. , Lonial, S. , Laubach, J.P.H. , Hamadani, M. , Stewart, A.K. , Hari, P.H. , Roy, V. , Vescio, R. , Kaufman, J.L. , Berg, D. , Liao, E. , Hui, A.M. , Rajkumar, S.V. & Richardson, P.G. (2014c) Long‐term ixazomib maintenance is tolerable and improves depth of response following ixazomib‐lenalidomide‐dexamethasone induction in patients (Pts) with previously untreated multiple myeloma (MM): phase 2 study results. Blood, 124, 82. [Google Scholar]
- Kumar, S.K. , Bensinger, W.I. , Zimmerman, T.M. , Reeder, C.B. , Berenson, J.R. , Berg, D. , Hui, A.M. , Gupta, N. , Di Bacco, A. , Yu, J. , Shou, Y. & Niesvizky, R. (2014d) Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood, 124, 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S.K. , Callander, N.S. , Alsina, M. , Atanackovic, D. , Biermann, J.S. , Chandler, J.C. , Costello, C. , Faiman, M. , Fung, H.C. , Gasparetto, C. , Godby, K. , Hofmeister, C. , Holmberg, L. , Holstein, S. , Huff, C.A. , Kassim, A. , Liedtke, M. , Martin, T. , Omel, J. , Raje, N. , Reu, F.J. , Singhal, S. , Somlo, G. , Stockerl‐Goldstein, K. , Treon, S.P. , Weber, D. , Yahalom, J. , Shead, D.A. & Kumar, R. (2017) Multiple myeloma, Version 3.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 15, 230–269. [DOI] [PubMed] [Google Scholar]
- Ludwig, H. , Greil, R. , Masszi, T. , Spicka, I. , Shpilberg, O. , Hajek, R. , Dmoszynska, A. , Paiva, B. , Vidriales, M.B. , Esteves, G. , Stoppa, A.M. , Robinson, D. Jr , Chaturvedi, S. , Ataman, O. , Enny, C. , Feng, H. , van de Velde, H. & Viterbo, L. (2015) Bortezomib, thalidomide and dexamethasone, with or without cyclophosphamide, for patients with previously untreated multiple myeloma: 5‐year follow‐up. British Journal of Haematology, 171, 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos, M.V. , Oriol, A. , Martinez‐Lopez, J. , Gutierrez, N. , Teruel, A.I. , Lopez de la Guia, A. , Lopez, J. , Bengoechea, E. , Perez, M. , Polo, M. , Palomera, L. , de Arriba, F. , Gonzalez, Y. , Hernandez, J.M. , Granell, M. , Bello, J.L. , Bargay, J. , Penalver, F.J. , Ribera, J.M. , Martin‐Mateos, M.L. , Garcia‐Sanz, R. , Lahuerta, J.J. , Blade, J. & San‐Miguel, J.F. (2012) Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood, 120, 2581–2588. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Masszi, T. , Grzasko, N. , Bahlis, N.J. , Hansson, M. , Pour, L. , Sandhu, I. , Ganly, P. , Baker, B.W. , Jackson, S.R. , Stoppa, A.M. , Simpson, D.R. , Gimsing, P. , Palumbo, A. , Garderet, L. , Cavo, M. , Kumar, S. , Touzeau, C. , Buadi, F.K. , Laubach, J.P. , Berg, D.T. , Lin, J. , Di Bacco, A. , Hui, A.M. , van de Velde, H. , Richardson, P.G. & TOURMALINE‐MM1 Study Group . (2016) Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. New England Journal of Medicine, 374, 1621–1634. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Bringhen, S. , Rossi, D. , Cavalli, M. , Larocca, A. , Ria, R. , Offidani, M. , Patriarca, F. , Nozzoli, C. , Guglielmelli, T. , Benevolo, G. , Callea, V. , Baldini, L. , Morabito, F. , Grasso, M. , Leonardi, G. , Rizzo, M. , Falcone, A.P. , Gottardi, D. , Montefusco, V. , Musto, P. , Petrucci, M.T. , Ciccone, G. & Boccadoro, M. (2010) Bortezomib‐melphalan‐prednisone‐thalidomide followed by maintenance with bortezomib‐thalidomide compared with bortezomib‐melphalan‐prednisone for initial treatment of multiple myeloma: a randomized controlled trial. Journal of Clinical Oncology, 28, 5101–5109. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Cavallo, F. , Gay, F. , Di Raimondo, F. , Ben Yehuda, D. , Petrucci, M.T. , Pezzatti, S. , Caravita, T. , Cerrato, C. , Ribakovsky, E. , Genuardi, M. , Cafro, A. , Marcatti, M. , Catalano, L. , Offidani, M. , Carella, A.M. , Zamagni, E. , Patriarca, F. , Musto, P. , Evangelista, A. , Ciccone, G. , Omede, P. , Crippa, C. , Corradini, P. , Nagler, A. , Boccadoro, M. & Cavo, M. (2014) Autologous transplantation and maintenance therapy in multiple myeloma. New England Journal of Medicine, 371, 895–905. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Gay, F. , Cavallo, F. , Di Raimondo, F. , Larocca, A. , Hardan, I. , Nagler, A. , Petrucci, M.T. , Hajek, R. , Pezzatti, S. , Delforge, M. , Patriarca, F. , Donato, F. , Cerrato, C. , Nozzoli, C. , Yu, Z. , Boccadifuoco, L. , Caravita, T. , Benevolo, G. , Guglielmelli, T. , Vincelli, D. , Jacques, C. , Dimopoulos, M.A. , Ciccone, G. , Musto, P. , Corradini, P. , Cavo, M. & Boccadoro, M. (2015) Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. Journal of Clinical Oncology, 33, 3459–3466. [DOI] [PubMed] [Google Scholar]
- Pulte, D. , Gondos, A. & Brenner, H. (2011) Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist, 16, 1600–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar, S.V. , Harousseau, J.L. , Durie, B. , Anderson, K.C. , Dimopoulos, M. , Kyle, R. , Blade, J. , Richardson, P. , Orlowski, R. , Siegel, D. , Jagannath, S. , Facon, T. , Avet‐Loiseau, H. , Lonial, S. , Palumbo, A. , Zonder, J. , Ludwig, H. , Vesole, D. , Sezer, O. , Munshi, N.C. & San Miguel, J. for the International Myeloma Workshop Consensus Panel . (2011) Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood, 117, 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Weller, E. , Lonial, S. , Jakubowiak, A.J. , Jagannath, S. , Raje, N.S. , Avigan, D.E. , Xie, W. , Ghobrial, I.M. , Schlossman, R.L. , Mazumder, A. , Munshi, N.C. , Vesole, D.H. , Joyce, R. , Kaufman, J.L. , Doss, D. , Warren, D.L. , Lunde, L.E. , Kaster, S. , Delaney, C. , Hideshima, T. , Mitsiades, C.S. , Knight, R. , Esseltine, D.L. & Anderson, K.C. (2010) Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood, 116, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Baz, R. , Wang, M. , Jakubowiak, A.J. , Laubach, J.P. , Harvey, R.D. , Talpaz, M. , Berg, D. , Liu, G. , Yu, J. , Gupta, N. , Di Bacco, A. , Hui, A.M. & Lonial, S. (2014) Phase 1 study of twice‐weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood, 124, 1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinol, L. , Oriol, A. , Teruel, A.I. , Hernandez, D. , Lopez‐Jimenez, J. , de la Rubia, J. , Granell, M. , Besalduch, J. , Palomera, L. , Gonzalez, Y. , Etxebeste, M.A. , Diaz‐Mediavilla, J. , Hernandez, M.T. , de Arriba, F. , Gutierrez, N.C. , Martin‐Ramos, M.L. , Cibeira, M.T. , Mateos, M.V. , Martinez, J. , Alegre, A. , Lahuerta, J.J. , San Miguel, J. & Blade, J. (2012) Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood, 120, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Roussel, M. , Lauwers‐Cances, V. , Robillard, N. , Belhadj, K. , Facon, T. , Garderet, L. , Escoffre, M. , Pegourie, B. , Benboubker, L. , Caillot, D. , Fohrer, C. , Moreau, P. , Leleu, X. , Avet‐Loiseau, H. & Attal, M. (2016) Frontline therapy with carfilzomib, lenalidomide, and dexamethasone (KRd) induction followed by autologous stem cell transplantation, Krd consolidation and lenalidomide maintenance in newly diagnosed multiple myeloma (NDMM) patients: primary results of the intergroupe francophone du myélome (IFM) Krd Phase II Study. Blood, 128, 1142. [Google Scholar]
- Stewart, A.K. , Rajkumar, S.V. , Dimopoulos, M.A. , Masszi, T. , Spicka, I. , Oriol, A. , Hajek, R. , Rosinol, L. , Siegel, D.S. , Mihaylov, G.G. , Goranova‐Marinova, V. , Rajnics, P. , Suvorov, A. , Niesvizky, R. , Jakubowiak, A.J. , San‐Miguel, J.F. , Ludwig, H. , Wang, M. , Maisnar, V. , Minarik, J. , Bensinger, W.I. , Mateos, M.V. , Ben‐Yehuda, D. , Kukreti, V. , Zojwalla, N. , Tonda, M.E. , Yang, X. , Xing, B. , Moreau, P. , Palumbo, A. & Investigators, A. (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. New England Journal of Medicine, 372, 142–152.25482145 [Google Scholar]
- Tinsley, S.M. , Kurtin, S.E. & Ridgeway, J.A. (2015) Practical management of lenalidomide‐related rash. Clinical Lymphoma, Myeloma and Leukemia, 15, S64–S69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary methods.
Table SI. Eligibility criteria.
Table SII. Definitions of dose‐limiting toxicities.
Table SIII. Treatment‐emergent AEs* reported at any grade in ≥25% of the total population and/or at grade 3 in ≥5% of the total population.


