Abstract
Treatment with 5α‐reductase inhibitors has been associated with sexual adverse events such as impotence (erectile dysfunction) and decreased libido. The primary objective of this study was to evaluate adverse events related to sexual function, based on their frequency, duration, persistence and associated treatment discontinuations, in men treated with dutasteride for androgenetic alopecia. Participants were randomized to receive double‐blind dutasteride 0.5 mg or placebo once daily for 24 weeks, followed by open‐label dutasteride 0.5 mg for an additional 24 weeks. Sexual adverse events were followed up until resolution or for up to 24 weeks after the last dose. Overall, 117 men, 23–50 years of age, were randomized. The incidence of sexual adverse events was approximately twofold higher in the dutasteride group (16%) than the placebo group (8%) during the double‐blind period; the overall incidence of sexual adverse events was lower (5%) during the open‐label period. All adverse events were mild to moderate in severity and considered treatment‐related. The adverse events resolved while on study treatment or after the end of treatment and did not lead to treatment discontinuation. A limitation of this study was the small sample size. The sexual adverse events of impotence, decreased libido and ejaculation disorders reported in this study were expected and reversible.
Keywords: androgenetic alopecia, dutasteride, erectile dysfunction, libido, sexual function
Introduction
Dutasteride is a 5α‐reductase inhibitor (5‐ARI) used to treat benign prostatic hyperplasia (BPH) and male androgenetic alopecia (AGA). Treatment with 5‐ARI, including dutasteride and finasteride, in men has been associated with adverse events (AE) related to sexual function, such as impotence (erectile dysfunction) and decreased libido.1 There have been reports of persistent, irreversible sexual AE with finasteride2, 3, 4 but causality remains uncertain and it is unknown whether this also occurs with dutasteride. Furthermore, sexual AE with dutasteride are not well‐characterized in men with AGA, who are generally younger (aged 18–50 years) than men with BPH (generally aged ≥45 years).
This study assessed sexual AE following dutasteride treatment over 24 and 48 weeks in men with AGA.
Methods
Participants were sexually active men aged 18–50 years with AGA who had been in a stable heterosexual relationship for 6 months prior to screening and expected to maintain that relationship throughout the study. Exclusion criteria are described in Appendix S1.
This international, multicenter, parallel‐group study comprised a 4‐week participant‐blinded placebo run‐in period and a 24‐week double‐blind (DB) treatment period where participants were randomized (1:1 ratio) using a centralized computer system to once‐daily dutasteride 0.5 mg or placebo. This was followed by a 24‐week open‐label (OL) active treatment period in which all participants received once‐daily dutasteride 0.5 mg, and a 4‐week post‐treatment follow‐up visit (Fig. S1). Participants with an ongoing sexual AE at the end of the treatment period and those who discontinued study treatment due to an AE related to sexual function entered a targeted follow‐up period until resolution of the sexual AE or 24 weeks after the last dose of the study treatment, whichever occurred first.
The primary objective was to evaluate the frequency, duration and persistence of AE related to sexual function (decreased libido, impotence and ejaculation disorders), and associated treatment discontinuations in men treated with dutasteride for AGA. A protocol amendment changed the primary objective from change in sexual function (measured by the International Index of Erectile Function [IIEF] – Erectile Function domain [EF]) to the incidence of sexual AE. The amendment also added the 24‐week OL period to extend monitoring of on‐treatment AE reversibility.
Other objectives included overall safety; changes in sexual function, measured by the IIEF questionnaire; participant‐perceived changes in sexual function, measured by global assessment questions; participant satisfaction with hair growth with dutasteride treatment for male AGA, measured by the Hair Growth Satisfaction Scale; and participant‐perceived impact of alopecia on health‐related quality of life (QoL), measured by the Dermatology Life Quality Index (DLQI). Additional details regarding these assessment tools and the statistical analyses can be found in Appendix S1.
This study (NCT02014584; registered 12 December 2013) was approved by the applicable institutional review board/ethics committee (registration date, 21 March 2014; registration no. 201401094MSB) for each country and study center. Written informed consent was obtained from each participant prior to participation.
Results
Demographics and baseline characteristics
The study commenced on 2 July 2014 and terminated on 19 March 2016. In total, 117 male participants aged 23–50 years (mean, 39 years) were randomized (Table S1, Fig. S2). The majority were Asian (84%). Hair loss had begun from 13–43 years of age and was ongoing in nearly all cases. Participants had high mean IIEF scores at baseline: 66.4–67.1 for total IIEF and 28.9–29.2 for the IIEF‐EF domain, indicating few problems. The impact of male AGA on QoL was small at baseline.
Safety
AE were experienced by approximately one‐third of participants; incidences were similar between the placebo (31%) and dutasteride (33%) groups and during the 24‐week OL period (29%). Approximately half of participants treated with dutasteride for 48 weeks reported one or more AE. Nasopharyngitis and erectile dysfunction were the only AE reported by more than 5% of participants in any study period. The investigator considered the majority of AE to be mild or moderate in intensity. Two participants (one during DB placebo and one during OL dutasteride treatment) experienced a severe AE (neither was considered treatment‐related).
There were no deaths or AE leading to treatment discontinuation or study withdrawal in this study. Three participants experienced serious AE: two events of severe intervertebral disc protrusion (one during DB placebo and one during OL dutasteride treatment) and one event of mild hypokalemia (DB dutasteride treatment). All three serious AE resolved within 3–25 days; none were considered treatment‐related. There were no reported AE related to suicidality, breast disorders, prostate cancer or cardiovascular events of interest.
Treatment‐related AE during the DB period were reported by more participants in the dutasteride group (19%) than the placebo group (8%) (Table S2); the majority were associated with sexual function.
The incidence of sexual AE was approximately twofold higher in the dutasteride group (16%) than the placebo group (8%) during the DB period, but lower (5%) in the OL period. All sexual AE were mild to moderate in severity and considered treatment‐related. The onset of most sexual AE occurred within the first 3 months of dutasteride treatment.
Overall, 20 participants experienced one or more sexual AE: 14 participants during dutasteride treatment (nine DB and five OL), five with placebo and one after placebo was discontinued (Fig. 1, Table 1). Of note, nine out of ten impotence AE during the DB period were reported at a single study center (representing 56% of the 16 participants enrolled at that center) and four of six decreased libido AE were reported at a different center (representing 17% of 23 participants).
Figure 1.
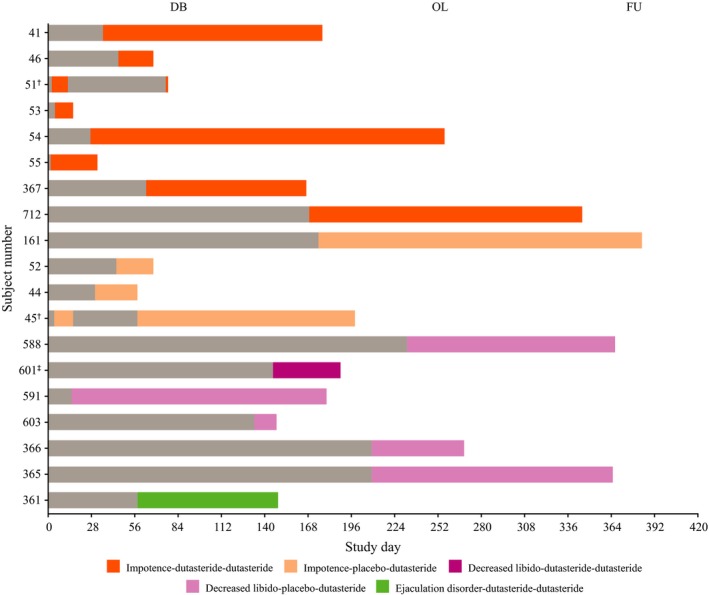
Onset and duration of on‐treatment sexual adverse events (AE). †Participant had two occurrences of AE during the study. ‡Participant 601 did not enter the open‐label (OL) period; therefore, his AE resolution was post‐treatment. One additional participant (no. 707) experienced an AE of impotence with onset after discontinuing placebo. DB, double‐blind period; FU, follow up.
Table 1.
Summary of special interest adverse events related to sexual function
| DB period | OL period | Combined periods | ||||
|---|---|---|---|---|---|---|
| Placebo n = 59 | Dutasteride n = 58 | Placebo DB‐dutasteride OL n = 49 | Dutasteride‐DB/OL n = 48 | Total n = 97 | Dutasteride combined n = 48 | |
| Impotence (erectile dysfunction) | ||||||
| Participants, n (%) | 3 (5) | 7 (12) | 1 (2) | 1 (2)† | 2 (2) | 8 (17)† |
| Maximum intensity | ||||||
| Mild | 2 (67) | 6 (86) | 1 (100) | 1 (100) | 2 (100) | 7 (88) |
| Moderate | 1 (33) | 1 (14) | 0 | 0 | 0 | 1 (13) |
| Onset | ||||||
| 0–3 months | 3 (100) | 7 (100) | 1 (100) | 1 (100) | 2 (100) | 7 (88) |
| >3 months | 0 | 0 | 0 | 0 | 0 | 1 (13)‡ |
| Duration, median (range) | 28 days (24–153) | 30 days (12–228) | 181 days | 176 days | 178.5 days (176–181) | 67 days (12–228) |
| Period of resolution | DB = 3 | DB = 5, OL = 2 | TFU | OL | OL = 1, TFU = 1 | DB = 5, OL = 2, TFU = 1 |
| Decreased libido | ||||||
| Participants, n (%) | 2 (3) | 1 (2) | 2 (4) | 1 (2) | 3 (3) | 1 (2) |
| Maximum intensity | ||||||
| Mild | 2 (100) | 1 (100) | 2 (100) | 0 | 2 (67) | 0 |
| Moderate | 0 | 0 | 0 | 1 (100) | 1 (33) | 1 (100) |
| Onset | ||||||
| 0–3 months | 1 (50) | 0 | 2 (100) | 1 (100) | 3 (100) | 0 |
| >3 months | 1 (50) | 1 (100) | 0 | 0 | 0 | >9 months |
| Duration, median (range) | 88.5 days (15–162) | 44 days | 77.5 days (27–128) | 135 days | 128 days (27–135) | 135 days |
| Period of resolution | DB = 2 | TFU | OL = 1, TFU = 1 | TFU | OL = 1, TFU = 2 | TFU |
| Ejaculation disorder | ||||||
| Participants, n (%) | 0 | 1 (2) | 0 | 0 | 0 | 1 (2) |
| Maximum intensity | ||||||
| Mild | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) |
| Onset | ||||||
| 0–3 months | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) |
| Duration, median (range) | 0 | 63 days | 0 | 0 | 0 | 63 days |
| Period of resolution | 0 | DB | 0 | 0 | 0 | DB |
Represents first occurrence of the specified adverse event (AE) in each period of the study (for participants with more than one event). †Includes organic erectile dysfunction. ‡Participant completed 3 months of double‐blind (DB) dutasteride treatment, and then experienced impotence during the first 3 months of open‐label (OL) treatment. AE, adverse event; TFU, targeted follow‐up (period).
Five participants entered the targeted follow‐up period. Four completed the follow up and were all receiving dutasteride when the sexual AE began. The frequency of sexual AE before treatment discontinuation was reported as “rarely” and the frequency after discontinuation was considered “intermittent”. The AE resolved within 2–6 weeks after the last dutasteride dose. The fifth participant experienced a sexual AE nearly 3 months after discontinuing placebo; the AE was resolving at the time of study withdrawal.
Health outcomes
There were minimal mean changes from baseline in IIEF domain scores for intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction (range, −0.4 to 0.2). The IIEF‐EF domain mean scores in the placebo group remained stable (mean change, −0.5), while there was a trend towards worsening of erectile function in the dutasteride group from baseline to DB week 12 (mean change, −1.3) and 24 (mean change, −1.2).
There were large variations in individual participant scores across groups and time points (e.g. change from baseline of −26 at a single visit, and 0 to −5 at preceding and following visits). The proportion of participants with an IIEF‐EF score decrease of 4 units or more from baseline to DB week 12 and 24 was higher with dutasteride (13% and 12%, respectively) than the placebo group (7% and 5%, respectively) (week 12 treatment difference, 6.4% [95% confidence interval [CI], −4.7% to 17.6%]; week 24 difference, 6.3% [95% CI, −4.2% to 16.7%]). In the OL period, only four participants switching from placebo to dutasteride experienced a decrease of 4 units or more.
The majority of participants (75−86%) reported “no change” to the global assessment questions on sexual function. Ability to achieve/maintain an erection was reported as “a little worse” for 9% in the placebo group and 13% in the dutasteride group (one additional participant reported “much worse”) at DB week 24 and for 12% of participants overall at OL week 24.
Hair growth satisfaction was higher with dutasteride than placebo at DB weeks 12 and 24, with the adjusted mean change from baseline at DB week 24 showing improvement with dutasteride (3.2 units) versus placebo (−0.1 units) (treatment difference, 3.3 units [95% CI, 1.1–5.6]). During the OL period, the mean change was 4.5 units. Satisfaction with hair growth continued improving throughout the study (mean change from baseline, 7.6 units at OL week 24).
Mean changes from baseline on the DLQI to DB weeks 12 and 24 were 0.2 and 0.7 units for the placebo group and −0.4 and 0.2 units for the dutasteride group, respectively. There were no notable between‐group differences.
Discussion
The potential for sexual AE with 5‐ARI may require careful consideration in younger men initiating AGA treatment. Sexual AE were the most commonly reported treatment‐related AE across studies of dutasteride for male AGA.5, 6, 7, 8 However, most events were mild, occurred early and resolved during continuing treatment or upon treatment cessation.6 The frequency of sexual AE across studies of 5‐ARI has been inconsistent, with more recent studies (ARI1142635 and ARI114264)8 reporting higher incidences than earlier studies (ARIA20046 and ALO106377).7 Some variation may be attributed to different coding of events or how AE were elicited.
The incidence of impotence reported in the dutasteride group during the 24‐week DB period was higher in this study (12%) than the 24‐week DB period of study ARI114263 (5%), but was similar in the placebo groups of both studies (5% vs 4%, respectively).5 Reports of decreased libido and ejaculation disorders with dutasteride were similar in both studies (2−5%).5
In the combined dutasteride group (median 345 days exposure, 97% compliance), the overall incidence of sexual AE was 21%, which is higher than reported over 52 weeks of treatment in study ARI114264 (16%).8 The IIEF‐targeted questioning into specific aspects of sexual function might have factored into the higher incidence of sexual AE in this study. The incidence of impotence (erectile dysfunction) AE was higher in this study (17%) than study ARI114264 (12%);8 however, this does not hold true for decreased libido and ejaculation disorders, which were less frequent in this study (2% each) than in ARI114264 (12% and 5%, respectively).8 Impotence had a faster onset (45 days) and shorter duration (91 days) in this study than in ARI114264 (81 days onset, 193 days duration).8
In the current study, most sexual AE resolved while participants remained on treatment. The four AE ongoing at the end of treatment resolved within 6 weeks after the last dose of dutasteride. The sexual AE profile of dutasteride treatment for male lower urinary tract symptoms/BPH has been consistent in previous randomized control trials.9, 10 Interestingly, most sexual AE occur in the first 6 months of dutasteride treatment in BPH clinical trials9, 10 and the likelihood of patients experiencing such AE even decreases over time. Furthermore, it has previously been reported in the combAT study9 that impotence was the most frequently reported sexual AE in patients with BPH who received treatment with dutasteride. In a study of patients with androgenetic alopecia (ARI114264 study), sexual AE resolved while on treatment in six participants and resolved within 6 months after the end of treatment in 13 participants.8 The resolution pattern of sexual AE in the AGA population of the current study seems to be analogous to the findings previously reported. These results also support the reversibility of sexual AE that occur with dutasteride treatment.
The majority of impotence AE (9/12 participants) were reported at a single study center; thus, variability in reporting sexual AE also appears to be influenced by a human component. The inter‐site and inter‐study variability could be linked to the nocebo phenomenon, where occurrence of symptoms is related to a patient's negative expectations.11, 12 This phenomenon was shown in a randomized controlled study evaluating sexual dysfunction during treatment with finasteride for BPH. The rate of sexual dysfunction was higher in participants who were informed of possible sexual AE than in those who were not informed (44% vs 15%).13 The authors concluded that the physician–patient relationship was fundamental with respect to reporting of sexual AE. A similar phenomenon has been documented in studies of atenolol for coronary heart disease.12
Minimal changes in IIEF domain scores for intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction were observed. Some of the extreme drops in IIEF scores might have been caused by external factors (e.g. extended time out of town and a fight with their partner were reported) and were not necessarily correlated with occurrence of sexual AE. Only 13 of the 30 participants with a worsening of IIEF‐EF of 4 units or more had a corresponding AE of impotence or decreased libido.
As expected, hair growth satisfaction during the study was higher in the dutasteride than in the placebo group.5, 6, 7, 8
The impact of alopecia on QoL was minimal at baseline. The DLQI may not be the most appropriate tool because the impact on QoL of alopecia is primarily on self‐esteem rather than daily activities. The small sample size in this study may be a limitation. The study was not powered to detect a significant difference between groups but was adequate to assess the persistence and reversibility of sexual AE.
In conclusion, no new safety signals were identified for dutasteride in this study. The sexual AE of impotence, decreased libido and ejaculation disorders associated with dutasteride treatment were expected, tolerable and reversible. Most sexual AE were mild or moderate, occurred early and resolved while participants remained on treatment. Dutasteride was not associated with any persistent sexual dysfunction following treatment cessation.
Conflict of Interest
S. J., B. B. and B. O. are former employees and stock holders of GSK. P. K. and Z. L. are current employees of GSK and are also stock holders of GSK. B. B. is employed by PAREXEL as a contract organization on behalf of GSK. T.‐F. T., G. S. C., B. J. K., M.‐B. K. and C. F. N. served as investigators for GSK. This study was approved by the applicable institutional review board/ethics committees for each country and study center. Written informed consent was obtained from each participant prior to the performance of any study‐specific procedures. ClinicalTrials.gov Identifier: NCT02014584.
Supporting information
Appendix S1. Additional materials and methods.
Table S1. Demographics and baseline characteristics (intention‐to‐treat).
Table S2. Incidence of treatment‐related adverse events.
Figure S1. Study design.
Figure S2. Disposition flow chart.
Acknowledgments
Editorial support was provided by Lisa Auker, Ph.D., Fishawack Communications, Oxford, UK. This study (NCT02014584) was funded by GlaxoSmithKline (GSK) Research & Development.
References
- 1. Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5alpha‐reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med 2011; 8(3): 872–884. [DOI] [PubMed] [Google Scholar]
- 2. Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med 2011; 8(6): 1747–1753. [DOI] [PubMed] [Google Scholar]
- 3. Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med 2012; 9(11): 2927–2932. [DOI] [PubMed] [Google Scholar]
- 4. Ali AK, Heran BS, Etminan M. Persistent sexual dysfunction and suicidal ideation in young men treated with low‐dose finasteride: a pharmacovigilance study. Pharmacotherapy 2015; 35(7): 687–695. [DOI] [PubMed] [Google Scholar]
- 5. Gubelin Harcha W, Barboza Martinez J, Tsai TF et al A randomized, active‐ and placebo‐controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol 2014; 70(3): 489–498.e3. [DOI] [PubMed] [Google Scholar]
- 6. Olsen EA, Hordinsky M, Whiting D et al The importance of dual 5alpha‐reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo‐controlled study of dutasteride versus finasteride. J Am Acad Dermatol 2006; 55(6): 1014–1023. [DOI] [PubMed] [Google Scholar]
- 7. Eun HC, Kwon OS, Yeon JH et al Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double‐blind, placebo‐controlled, phase III study. J Am Acad Dermatol 2010; 63(2): 252–258. [DOI] [PubMed] [Google Scholar]
- 8. Tsunemi Y, Irisawa R, Yoshiie H et al Long‐term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J Dermatol 2016; 43(9): 1051–1058. [DOI] [PubMed] [Google Scholar]
- 9. Roehrborn CG, Siami P, Barkin J et al The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4‐year results from the CombAT study. Eur Urol 2010; 57(1): 123–131. [DOI] [PubMed] [Google Scholar]
- 10. Roehrborn CG, Manyak MJ, Palacios‐Moreno JM et al A prospective randomised placebo‐controlled study of the impact of dutasteride/tamsulosin combination therapy on sexual function domains in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int 2018; 121: 647–658. [DOI] [PubMed] [Google Scholar]
- 11. Planès S, Villier C, Mallaret M. The nocebo effect of drugs. Pharmacol Res Perspect 2016; 4(2): e00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hauser W, Hansen E, Enck P. Nocebo phenomena in medicine: their relevance in everyday clinical practice. Dtsch Arztebl Int 2012; 109(26): 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mondaini N, Gontero P, Giubilei G et al Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med 2007; 4(6): 1708–1712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Additional materials and methods.
Table S1. Demographics and baseline characteristics (intention‐to‐treat).
Table S2. Incidence of treatment‐related adverse events.
Figure S1. Study design.
Figure S2. Disposition flow chart.


