Abstract
In patients with immune thrombocytopenia who do not adequately respond to first‐line therapy, there is no clear consensus on which second‐line therapy to initiate and when. This situation leads to suboptimal approaches, including prolonged exposure to treatments that are not intended for long‐term use (eg, corticosteroids) and overuse of off‐label therapies (eg, rituximab) while approved, more efficacious options exist. These approaches may not only fail to address symptoms and burden of disease, but may also worsen health‐related quality of life. A better understanding of available second‐line treatments may ensure best use of therapeutic options and thereby optimize patient outcomes.
1. INTRODUCTION
Immune thrombocytopenia (ITP) is characterized by a reduction in platelet count that may be associated with severe bleeding in some patients.1 The disease may have a substantial impact on the patient's quality of life, resulting, at least in part, from significant treatment burden. Indeed, health‐related quality of life for patients with ITP may be worse than that of patients with many other chronic conditions, including hypertension and arthritis.2
First‐line treatments for ITP include corticosteroids (prednisone, dexamethasone), intravenous immunoglobulin (IVIg), and anti‐D (Rh0) immunoglobulin.3 These therapies are used as upfront treatment in newly diagnosed patients and also as rescue therapies in patients with established ITP, with the goal of rapidly elevating platelet counts and preventing or controlling bleeding events.4 While corticosteroids and immunoglobulins produce an initial response in most patients (60%‐70% and 90%, respectively), the response is usually transient (<6 months and 2–4 weeks, respectively) and the medications must be readministered if the patient's platelet count does not stabilize.5 Repeated or prolonged administration of first‐line therapies is typically not suitable due to significant adverse effects (eg, osteoporosis, diabetes, cataracts, weight gain, infections) with corticosteroids and high cost, inconvenience of frequent infusions, and debilitating post‐infusion headache with immunoglobulins.4, 6, 7
Spontaneous remissions in patients who have never received treatment are uncommon in adults with ITP (9%). In many patients, the disease becomes persistent (3–12 months duration) or chronic (>12 months duration), and second‐line treatment may be needed.3, 8, 9
2. SECOND‐LINE TREATMENT OPTIONS
Second‐line or maintenance therapy in persistent or chronic ITP (hereafter referred to as ITP) aims to establish a durable platelet response and to minimize bleeding events with a treatment that is safe, tolerable, and convenient for long‐term management. Splenectomy, thrombopoietin receptor agonists (TPO‐RAs), and rituximab are the standard second‐line treatment options in current use.3, 4 Before initiation of a second‐line therapy, it is advisable to confirm the diagnosis of primary ITP by excluding potential non‐immune causes of thrombocytopenia as well as causes of secondary ITP if these have not been previously ruled out.
2.1. Splenectomy
Conventionally, splenectomy has been the principal option for long‐term management of ITP because of its potential to induce long‐term remission. Splenectomy provides a high initial response rate (85%); however, up to 30% of responders will relapse during the 10 years following an initial response (typically, within 2 years after splenectomy), and there is no widely available and reliable means of predicting whether an individual patient will respond.10 In addition, splenectomy is associated with serious short‐ and long‐term risks. Surgical complications were reported in 10% of patients in the 30‐day period following splenectomy, even when less‐invasive laparoscopic methods were used.10 Furthermore, lack of splenic function may result in infections, thromboembolism, and possibly an increased incidence of malignancy, which result in an increased risk of death that persists for >10 years after surgery.11 Based on a study of 8149 US veterans who had splenectomy for any indication, the risk of death due to certain events was 3‐ to 4‐fold higher in patients who had undergone splenectomy versus those with intact spleens (the risks of septicemia, pulmonary embolism, and non‐Hodgkin lymphoma were 3.02‐, 4.53‐, and 4.69‐fold higher, respectively).11 In addition, another study in 9976 patients with ITP revealed a 2.7‐fold increased risk of venous thromboembolism and a 1.6‐ to 3.1‐fold increased risk of sepsis (depending on timing and comorbidities) ≥90 days after splenectomy (median follow‐up of 120 months), but an increase in the risk of malignancy was not reported.12 Thus, splenectomized patients need lifelong management to prevent sepsis, such as vaccinations and prophylactic antibiotics, as well as surveillance for relapse. The advent of pharmaceutical second‐line treatment options has significantly decreased the use of splenectomy in ITP.13, 14 However, splenectomy may still be preferred by some patients who desire independence from medications.15
2.2. Thrombopoietin receptor agonists
Thrombopoietin receptor agonists are approved for the treatment of patients with chronic ITP who had an insufficient response to either a first‐line therapy or splenectomy.16, 17
Thrombopoietin receptor agonists were developed after the US clinical trials of recombinant human thrombopoietin (rhTPO) were withdrawn because injection of a pegylated, truncated, and nonglycosylated fragment of TPO (pegylated recombinant human megakaryocyte growth and development factor) caused anti‐thrombopoietin antibody development that resulted in severe thrombocytopenia.18 While the same theoretical risk exists with full‐length recombinant TPO, this treatment is still being used in China and there are no published cases of neutralizing anti‐TPO antibody development with this agent.19, 20 TPO‐RAs share no structural analogy with endogenous TPO and thus are not expected to induce formation of anti‐TPO antibodies.21 Moreover, they have improved pharmacological properties compared with rhTPO including more potent activation of the TPO receptor and convenience of administration.16, 17, 18, 22
In Western countries, TPO‐RAs are the only widely available drug class that directly and specifically improves platelet counts by increasing platelet production in the bone marrow.23 There are 2 TPO‐RAs with unique mechanisms of action in clinical use for patients with ITP: eltrombopag and romiplostim.22 Both are well tolerated, even with long‐term use,24, 25 and elicit durable responses in most patients. In clinical trials, TPO‐RAs increased platelet counts in 70%‐80% of patients, and 85%‐95% of these patients responded at least once in the long‐term extension studies with a median TPO‐RA exposure of approximately 2 years (range, 2 days to 8.8 years).24, 26, 27, 28 With an effective starting dose, initial responses to TPO‐RAs are typically observed within 1–2 weeks of treatment.27, 29 Clinical studies also demonstrated that TPO‐RAs may reduce bleeding events and the need for emergency treatments, as well as improving the quality of life in patients with chronic ITP.30, 31 Real‐world experience with TPO‐RAs are consistent with the results of clinical trials.32, 33
Thrombopoietin receptor agonists are conventionally considered to be lifelong therapies that need to be used continuously to maintain response. However, accumulating data suggest that some patients who use TPO‐RAs may achieve long‐term remissions that are sustained off treatment. Although randomized, placebo‐controlled, long‐term studies that would directly demonstrate the remission‐inducing potential of TPO‐RAs are not available, it appears unlikely that remissions observed in the TPO‐RA discontinuation studies are purely spontaneous. Earlier studies showed that the rate of spontaneous remissions in adult patients is only about 9%,9 while the overall rate of long‐term (≥6 months) remission in TPO‐RA studies is nearly 30%.34, 35, 36, 37 Second, spontaneous remissions are less frequent following the first year after diagnosis, but most patients who achieved remission in TPO‐RA studies had ITP for >1 year, and some had been living with ITP for much longer (up to 54 years).1, 34, 36, 37, 38, 39 The authors speculated that this putative disease‐modifying activity of TPO‐RAs could be linked to the TPO‐RA–mediated restoration of impaired regulatory T‐cell function and immune tolerance that was observed in patients with ITP.37, 39, 40
The most common adverse events (≥5% and greater than placebo) in clinical trials in adult patients with ITP were nausea, diarrhea, upper respiratory tract infection, vomiting, increased alanine aminotransferase, myalgia, and urinary tract infection with eltrombopag16; and arthralgia, dizziness, insomnia, myalgia, pain in extremity, abdominal pain, shoulder pain, dyspepsia, and paresthesia with romiplostim.17
Potential risks of TPO‐RAs include rebound thrombocytopenia after discontinuation, thromboembolic events, and bone marrow reticulin formation.4, 16, 17 Although an increased risk of thromboembolic events was not confirmed in placebo‐controlled trials of TPO‐RAs,25, 41 it is prudent to be cautious, especially in patients with preexisting thrombotic risk factors. Similarly, because of its association with hepatobiliary laboratory abnormalities and hepatotoxicity, it is advisable to use eltrombopag with caution in patients with liver disease with close monitoring of liver function tests. Bone marrow reticulin formation, which is more common in patients with ITP than in hematologically normal patients at baseline, occurred in studies with TPO‐RAs.42 However, these events were typically mild, asymptomatic, and reversible upon treatment interruption.42, 43 In toxicology studies of rodents exposed to suprapharmacologic concentrations of eltrombopag, cataract events were noted in juvenile animals.16 In the eltrombopag extension study, however, the rate of cataract formation was not greater than the expected rate in the general adult population.44 Preliminary data in patients with myelodysplastic syndromes suggested a potential risk of progression to leukemia with TPO‐RAs.16, 17, 45, 46 Neutralizing antibodies against romiplostim have been detected in patients receiving romiplostim in clinical trials; however no neutralizing activity against endogenous TPO has been observed.17
Until platelet counts are stabilized, they should be monitored weekly in all patients receiving TPO‐RAs. For patients receiving eltrombopag, a baseline liver enzyme test and ocular examination with regular follow‐up monitoring is recommended.16 In addition, peripheral blood smears may be periodically reviewed to monitor for changes that could potentially indicate bone marrow reticulin formation.
2.3. Anti‐CD20 antibody
The anti‐CD20 antibody rituximab is used in patients with refractory ITP primarily based on experience in other autoimmune diseases, as well as uncontrolled studies in ITP. It is licensed for use in certain hematological malignancies and rheumatoid arthritis, but not in ITP.47 The optimal dosing of rituximab for ITP has not been defined. It is typically administered at a dose of 375 mg/m2 over 4 consecutive weekly infusions, although lower doses may be sufficient.48, 49
Based on a meta‐analysis of randomized controlled trials, the complete response rate to a single course of rituximab by 6 months is 47% versus 32.5% with standard of care, however, no statistically significant difference was found in overall response rate (P = .11) and reduction in bleeding events (P = .44).50 Long‐term studies of rituximab have also shown disappointing results. A placebo‐controlled long‐term study found no significant benefit of rituximab beyond 1.5 years compared with placebo (P = .65).51 In a retrospective analysis of adults with an initial response to rituximab, the response rate at 5 years was only 21%.52
However, for certain patient populations and in combination with corticosteroids, rituximab may provide durable remissions.53, 54 Most recently, a small study in 49 patients found that among adult females with newly diagnosed or persistent ITP (disease duration of <1 year) who had shown an initial response to rituximab and high‐dose dexamethasone, a remarkable percentage of patients (79%) achieved a durable remission (>48 months), whereas remission rates in other populations were dramatically lower (0%‐21%).54 Although these results require confirmation, they suggest that response rates to rituximab are influenced by gender and disease duration.
Rituximab is usually well tolerated in patients with ITP, but infusion reactions (rash, urticaria, fever, myalgia, headache, and transient hypertension) are relatively common (≥20%).55 Particularly among patients who receive multiple courses of rituximab, there is a risk of hypogammaglobulinemia, and monitoring serum immunoglobulin levels before and periodically after rituximab may be needed.56, 57 Rituximab has also been associated with rare but potentially fatal complications, including severe mucocutaneous reactions, reactivation of hepatitis B, and multifocal leukoencelopathy.10, 47 In addition, a meta‐analysis of the safety population in the uncontrolled rituximab trials in ITP revealed a relatively high death rate (3%), but it was not clear if reported deaths were attributable to rituximab.58
2.4. Other treatments
Other agents, including mycophenolate mofetil, azathioprine, danazol, dapsone, vinca alkaloids, sirolimus, cyclosporine, and cyclophosphamide have been used in ITP as alternative treatments since they are less expensive compared with rituximab and the TPO‐RAs.4, 59 However, unlike standard second‐line treatments, these agents have not been subject to randomized‐controlled trials to establish their safety and efficacy in ITP. In addition, limited clinical data on their safety and efficacy suggest that they have lower response rates and greater toxicity compared with approved treatments. Thus, current guidelines recommend that they be reserved for patients who do not respond to or cannot tolerate standard second‐line therapies.3, 4 These agents may play an important role in the second‐line treatment of ITP in countries where access to standard second‐line therapies is limited.
3. CHALLENGES IN THE TRANSITION TO SECOND‐LINE TREATMENT
In patients who require additional treatment after first‐line therapy, there is no consensus on when to stop first‐line treatment and switch to a therapy more suitable for maintaining a long‐term response. In addition, there is no consensus on which second‐line option to try first. Perhaps because of this uncertainty, several common suboptimal treatment approaches have emerged in clinical practice.
3.1. Excessive duration of corticosteroid treatment
As corticosteroid treatment is a familiar, low‐cost, and efficacious option for many patients, some clinicians maintain their patients on corticosteroids for months or even years before transitioning to a second‐line therapy. This practice may increase not only the risk of bleeding due to poor management of thrombocytopenia as the corticosteroid dose is tapered, but also the risk of adverse events associated with prolonged exposure to corticosteroids, such as weight gain, diabetes, osteoporosis, cataracts, and infections.6
Patients with ITP already experience a high burden of disease; thus, best efforts should be made to provide treatment options that do not augment the overall burden. Inadequate management of thrombocytopenia may cause emergencies that exacerbate a patient's fear of bleeding. Hospitalizations for emergencies or infusion treatments may also impact the patient's day‐to‐day functioning and be disruptive for work or school. Adverse events associated with corticosteroids are particularly burdensome. Brown et al reported that nearly all patients who received corticosteroids for ITP experienced adverse events, most commonly weight gain or increased appetite and personality/mood changes. Among patients who received corticosteroids, 53% were very highly bothered by the adverse events, compared to 9%‐11% of patients receiving other ITP treatments.60
3.2. Premature splenectomy
Splenectomy may induce remission and eliminate the need for further treatment, and thus it is sometimes considered soon after diagnosis and before the exhaustion of other treatment options. However, splenectomy is an invasive and irreversible process that leads to a loss of multiple hematological and immunological functions.10 Therefore, unless there is an urgent need to raise the platelet count and the patient does not respond to or cannot tolerate second‐line medical therapies, splenectomy should generally be reserved for patients in whom remission is unlikely (ie, those with a disease duration of >1 year).61
3.3. Underuse of TPO‐RAs
Several factors may have curtailed the use of TPO‐RAs for ITP in clinical practice. First, TPO‐RAs remain a relatively new treatment option that may be less familiar to hematologists than conventional ITP therapies such as corticosteroids and splenectomy. Second, low‐cost, short‐term treatment options such as corticosteroids may be prescribed in preference to TPO‐RAs based on an expectation of reduced overall treatment costs. However, this analysis does not take into consideration that fewer bleeding events and reduced concomitant medications associated with TPO‐RA use could confer significant cost savings. For example, a US cost consequence model showed that from a payer perspective TPO‐RA use may significantly reduce the overall management costs of non‐splenectomized patients with chronic ITP compared with a watch‐and‐rescue approach.62
Another possible reason for reluctance in starting TPO‐RA therapy could be that it is perceived as a lifelong commitment for continuous therapy. However, as noted above, as many as 30% of patients with ITP using TPO‐RAs may achieve long‐term remission34, 35, 36, 37 and be able to discontinue treatment. For those patients who do relapse after TPO‐RA therapy is stopped, TPO‐RAs may be resumed without loss of efficacy.29
3.4. Overuse of rituximab
Rituximab is commonly used in patients with refractory ITP in the absence of clear supporting evidence from controlled trials with long‐term follow‐up. Familiarity with the drug due to its approved hematological indications and the possibility of reimbursement for infusion therapy may contribute to the use of rituximab. In addition, use may have been driven by the perception that rituximab was the only nonsurgical treatment option for ITP with long‐term efficacy. However, the role of rituximab in the treatment of ITP requires reevaluation now that other long‐term treatment options are available, and in light of accumulating data showing no significant long‐term benefits.51
As discussed, efficacy of rituximab also appears to be affected by age, sex, and duration of ITP. In male patients and patients who have had ITP for >1 year, rituximab may have reduced efficacy.54 Considering potential risks associated with rituximab, a variable and unpredictable time to response (1–8 weeks3, 5), and limited long‐term benefits in most patients, indiscriminate use of rituximab should be avoided.
3.5. Use of other off‐label agents
The agents listed above as “other treatments” have been utilized in ITP as alternative treatments due to cost and availability considerations. However, these agents may produce highly variable responses in individual patients and may require weeks or months to be effective.3 Thus, they may potentially be of use as adjunct therapy in patients with insufficient response to second‐line therapy, but they are not recommended for most patients unless available second‐line options have been exhausted. An important exception is patients in countries where access to TPO‐RAs and rituximab may be limited.
4. BEST PRACTICES
4.1. Transitioning to second‐line therapy
My approach to selecting patients for second‐line therapy is depicted in Figure 1. If a first‐line therapy has produced a response that lasted for >6 months and was tolerable, the patient could be re‐treated with it. However, I generally discontinue first‐line therapy and switch to second‐line therapy if any of the following criteria are met: (1) the patient cannot tolerate first‐line treatment; (2) the patient does not respond to first‐line treatment within 2–4 weeks; (3) the patient's response to the last course of therapy is lost within 6 months, including a failure to taper corticosteroids to a low dose (ie, prednisone ≤5 mg/day) due to loss of response. In addition to these criteria, patient preferences and treatment burden should also be incorporated in decision making.
Figure 1.
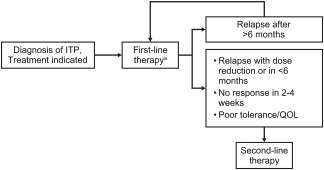
Selection of patients for second‐line treatment of ITP. General management suggestions are shown. Clinicians should make individualized treatment decisions that take into account the patient's comorbidities, lifestyle, and personal values and preferences. ITP, immune thrombocytopenia; QOL, quality of life. aCorticosteroids are standard first‐line therapy. They may be combined with intravenous immunoglobulin (IVIg) when a more rapid response is required. Either IVIg or anti‐D may be used as first‐line treatment if corticosteroids are contraindicated. Anti‐D should be considered only in nonsplenectomized, Rh+ patients who have a negative direct antiglobulin test3
4.2. Selection of second‐line therapy and beyond
The risks and benefits of all second‐line management options should be discussed with the patient before making a treatment decision. My approach to selecting optimal second and subsequent lines of treatment for patients with ITP is shown in Figure 2.
Figure 2.
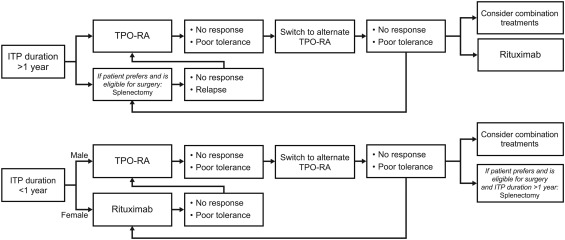
Approach to selection of second‐line treatment of ITP. General management suggestions are shown. Clinicians should make individualized treatment decisions that take into account the patient's comorbidities, lifestyle, and personal values and preferences. ITP, immune thrombocytopenia; TPO‐RA, thrombopoietin receptor agonist
For most patients who have had ITP for >1 year, TPO‐RAs should be considered in the second‐line setting. Splenectomy is also an important option for eligible patients who prefer surgical treatment. Before making a treatment decision, the patient should be informed of the long‐term risks of splenectomy that might require lifelong medical attention, including delayed relapse, infection, thromboembolism, and possibly cancer. The possibility of long‐term remission or treatment holidays with TPO‐RAs or rituximab should be discussed with the patient. However, it is important to clarify that these medications are neither intended nor confirmed to cure the disease, and that the possibility of long‐term platelet response after discontinuation of therapy is much higher with splenectomy.
For patients who have had ITP for <1 year, TPO‐RAs remain a viable option regardless of age and gender. For adult women who have had ITP for <1 year, rituximab with high‐dose dexamethasone could also be considered in the second line. In that case, TPO‐RAs may be used as bridge therapy to compensate for the variable time to response with rituximab. In a trial of rituximab combined with recombinant TPO or placebo, recombinant TPO significantly reduced the median time to response (7 days vs. 28 days, respectively; P < .01).20
In patients who have an inadequate response with TPO‐RA monotherapy, combination therapy with an immunosuppressive agent (eg, low‐dose corticosteroids, danazol, mycophenolate mofetil, etc.) that reduces platelet destruction may allow for synergistic outcomes.4 In a small study of patients with multi‐refractory ITP who had not achieved a stable response to first‐ or second‐line treatments, only 1 of 14 patients responded to immunosuppressive therapy alone, whereas 7 of 10 patients responded to a combination of a TPO‐RA and an immunosuppressive agent.63
Patients who do not respond to or cannot tolerate one second‐line therapy may be considered for another second‐line option. In patients with chronic ITP who do not sufficiently benefit from TPO‐RAs, rituximab remains an option. If all second‐line pharmaceutical agents and their combinations fail to achieve a response, splenectomy should be considered in patients who have had ITP for >1 year.
4.3. Optimal use of TPO‐RAs in the second line
The minimum TPO‐RA dose necessary to maintain a target platelet count and prevent bleeding should be used. If a patient achieves a platelet count within or above the target range at the lowest recommended dose of TPO‐RA (ie, eltrombopag 12.5 mg/day or romiplostim 1 mcg/kg/week), I hold the TPO‐RA and follow the platelet count closely off therapy to monitor for potential remissions.
Patients who do not respond to or do not tolerate a TPO‐RA may switch to the alternate TPO‐RA. The majority of patients who switch TPO‐RA treatment respond to the alternate TPO‐RA, even if the reason for switching was lack of efficacy with the initial TPO‐RA.32, 64, 65
Abrupt interruptions of TPO‐RAs or excessive dose adjustments may cause platelet fluctuations and should be avoided. Platelet fluctuations are more common with romiplostim and in that case could be resolved by switching to eltrombopag.64, 65 Platelet fluctuations may also be more common in splenectomized patients, potentially due to the absence of normal splenic functions, including platelet sequestration.64, 66
5. CONCLUSIONS
Overall familiarity of the physician with the therapy, rapidity of response, and drug cost appear to play a decisive role in long‐term management of some patients with ITP, but other factors should also be considered when determining the best management approach. As discussed, the prolonged use of corticosteroids as a first‐line therapy may increase the risk of bleeding and side effects including weight gain, osteoporosis, cataracts, and infections. Similarly, whereas the potential to induce long‐term remission with splenectomy makes it an attractive option, the long‐term risks of asplenia and the potential for non‐response or relapse should be discussed with the patient and incorporated in decision making. The lack of significant benefit with long‐term use of rituximab should be considered as should the effects of age, sex, and duration of ITP on efficacy, which should limit use in male patients and in those who have had ITP for >1 year.
Rapid response may be important for the treatment of acute disease, but durability of response and long‐term safety are critical for patients with chronic ITP. TPO‐RAs are well‐tolerated treatments suitable for long‐term management of chronic ITP that have shown efficacy in randomized controlled trials. They stably increase the platelet count, reduce bleeding, reduce the need for rescue therapy, and improve quality of life in adults with chronic ITP.
Familiarity with TPO‐RAs may be limited by their relatively narrow range of indications, but they are the only therapy specifically developed to treat ITP. Additionally, consideration of drug cost in comparison to corticosteroids may have prevented the more widespread use of TPO‐RAs for ITP in clinical practice, but whereas corticosteroids or a watch‐and‐rescue approach may minimize drug cost, overall costs may be higher when the expenses associated with increased emergency hospitalizations and missed days from school or work are included. Finally, the perception that use of TPO‐RAs constitutes a lifelong commitment for continuous treatment can be offset by the knowledge that as many as 30% of patients with ITP using TPO‐RAs may achieve long‐term remission off therapy.
Patients with ITP experience a high burden of disease, and it is important to offer management options that will improve quality of life. When possible, treatment options that are compatible with the personal preferences and lifestyle of the patient should be offered. Oral treatments taken once a day may be preferred by some patients, but others may favor injections that are administered less frequently. For patients who want to minimize their medication and monitoring needs, splenectomy may be the most desirable option.
An in‐depth understanding of second‐line treatment options will help optimize management of ITP and patient outcomes by ensuring the best use of available therapeutic options.
DISCLOSURE STATEMENT
AC has received research funding from Alexion, Bayer, Bioverativ, Novo Nordisk, Shire, and Spark and has served as a consultant for Kedrion, Stago, and Synergy.
ACKNOWLEDGMENTS
ClinicalThinking, NJ, USA provided medical writing and editorial support for the preparation of this manuscript, which was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Cuker A. Transitioning patients with immune thrombocytopenia to second‐line therapy: Challenges and best practices. Am J Hematol. 2018;93:816–823. 10.1002/ajh.25092
Funding information Novartis Pharmaceuticals Corporation
REFERENCES
- 1. Rodeghiero F, Stasi R, Gernsheimer T. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. [DOI] [PubMed] [Google Scholar]
- 2. McMillan R, Bussel JB, George JN, Lalla D, Nichol JL. Self‐reported health‐related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol. 2008;83(2):150–154. [DOI] [PubMed] [Google Scholar]
- 3. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. [DOI] [PubMed] [Google Scholar]
- 4. Cuker A, Neunert CE. How I treat refractory immune thrombocytopenia. Blood. 2016;128(12):1547–1554. [DOI] [PubMed] [Google Scholar]
- 5. Arnold DM. Positioning new treatments in the management of immune thrombocytopenia. Pediatr Blood Cancer. 2013;60(S1):S19–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris E, Tiganescu A, Tubeuf S, Mackie SL. The prediction and monitoring of toxicity associated with long‐term systemic glucocorticoid therapy. Curr Rheumatol Rep. 2015;17(6):513‐015‐0513‐4. [DOI] [PubMed] [Google Scholar]
- 7. Sandler SG, Novak SC, Roland B. The cost of treating immune thrombocytopenic purpura using intravenous Rh immune globulin versus intravenous immune globulin. Am J Hematol. 2000;63(3):156–158. [DOI] [PubMed] [Google Scholar]
- 8. Reid MM. Chronic idiopathic thrombocytopenic purpura: incidence, treatment, and outcome. Arch Dis Child. 1995;72(2):125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stasi R, Stipa E, Masi M, et al. Long‐term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98(5):436–442. [DOI] [PubMed] [Google Scholar]
- 10. Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second‐line treatment. Blood. 2012;120(5):960–969. [DOI] [PubMed] [Google Scholar]
- 11. Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long‐term risks after splenectomy among 8,149 cancer‐free American veterans: a cohort study with up to 27 years follow‐up. Haematologica. 2014;99(2):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyle S, White RH, Brunson A, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood. 2013;121(23):4782–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodeghiero F, Ruggeri M. Is splenectomy still the gold standard for the treatment of chronic ITP? Am J Hematol. 2008;83(2):91. [DOI] [PubMed] [Google Scholar]
- 14. Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol. 2016;91(4):E267–E272. [DOI] [PubMed] [Google Scholar]
- 15. Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131(11):1172–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Promacta (eltrombopag) [Package Insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 17. Nplate (romiplostim). [Prescribing Information]. Thousand Oaks, CA: Amgen, Inc; 2014. [Google Scholar]
- 18. Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109(11):4607–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.3SBio.Inc. TPIAO: Recombinant human thrombopoietin (rhTPO) injection. http://www.3sbio.com/en/products/oncology/tpiao. Accessed January, 2018.
- 20. Zhou H, Xu M, Qin P, et al. A multicenter randomized open‐label study of rituximab plus rhTPO vs rituximab in corticosteroid‐resistant or relapsed ITP. Blood. 2015;125(10):1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98(1):10–23. [DOI] [PubMed] [Google Scholar]
- 22. Raslova H, Vainchenker W, Plo I. Eltrombopag, a potent stimulator of megakaryopoiesis. Haematologica. 2016;101(12):1443–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raj AB. Immune thrombocytopenia: pathogenesis and treatment approaches. J Hematol Transfus. 2017;5:1056. [Google Scholar]
- 24. Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long‐term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. [DOI] [PubMed] [Google Scholar]
- 25. Rodeghiero F, Stasi R, Giagounidis A, et al. Long‐term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: a pooled analysis of 13 clinical trials. Eur J Haematol. 2013;91(5):423–436. [DOI] [PubMed] [Google Scholar]
- 26. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. [DOI] [PubMed] [Google Scholar]
- 27. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet. 2008;371(9610):395–403. [DOI] [PubMed] [Google Scholar]
- 28. Kuter DJ, Bussel JB, Newland A, et al. Long‐term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411–423. [DOI] [PubMed] [Google Scholar]
- 29. Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short‐term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP). Br J Haematol. 2013;160(4):538–546. [DOI] [PubMed] [Google Scholar]
- 30. George JN, Mathias SD, Go RS, et al. Improved quality of life for romiplostim‐treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo‐controlled trials. Br J Haematol. 2009;144(3):409–415. [DOI] [PubMed] [Google Scholar]
- 31. Khelif A, Saleh MN, Salama A, et al. Patient‐reported health‐related quality of life improves over time in patients with chronic immune thrombocytopenia receiving long‐term treatment with eltrombopag. Blood. 2016;128:3750–3750. [Google Scholar]
- 32. Kuter DJ, Macahilig C, Grotzinger KM, et al. Treatment patterns and clinical outcomes in patients with chronic immune thrombocytopenia (ITP) switched to eltrombopag or romiplostim. Int J Hematol. 2015;101(3):255–263. [DOI] [PubMed] [Google Scholar]
- 33. Mazza P, Minoia C, Melpignano A, et al. The use of thrombopoietin‐receptor agonists (TPO‐RAs) in immune thrombocytopenia (ITP): a “real life” retrospective multicenter experience of the Rete Ematologica Pugliese (REP). Ann Hematol. 2016;95(2):239–244. [DOI] [PubMed] [Google Scholar]
- 34. Bussel J, Shah KM, Brigstocke S, Torneten S. Tapering eltrombopag in patients with chronic ITP: How successful is this and in whom does it work? Blood. 2015;126(1054). [Google Scholar]
- 35. Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172(2):262–273. [DOI] [PubMed] [Google Scholar]
- 36. Leven E, Miller A, Boulad N, Haider A, Bussel JB. Successful discontinuation of eltrombopag treatment in patients with chronic ITP. Blood. 2012;120(1085). [Google Scholar]
- 37. Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion (Paris). 2013;53(11):2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Červinek L, Mayer J, Doubek M. Sustained remission of chronic immune thrombocytopenia after discontinuation of treatment with thrombopoietin‐receptor agonists in adults. Int J Hematol. 2015;102(1):7–11. [DOI] [PubMed] [Google Scholar]
- 39. González‐López TJ, Sánchez‐González B, Pascual C, et al. Sustained response after discontinuation of short‐and medium‐term treatment with eltrombopag in patients with immune thrombocytopenia. Platelets. 2015;26(1):83–86. [DOI] [PubMed] [Google Scholar]
- 40. Bao W, Heck S, Karpoff M, Bussel JB, Yazdanbakhsh K, Improved Regulatory T. Cell activity in patients with chronic immune thrombocytopenia purpura treated with thrombopoietic agents. Blood. 2009;114:684–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bussel J, Cheng G, Saleh M, Mayer B, Vasey S, Brainsky A. Incidence of thromboembolic events across eltrombopag clinical trials in chronic immune thrombocytopenia (ITP). Blood. 2010;116 [Abstract 70]. [Google Scholar]
- 42. Brynes RK, Wong RSM, Thein MM, et al. A 2‐year, longitudinal, prospective study of the effects of eltrombopag on bone marrow in patients with chronic immune thrombocytopenia. Acta Haematol. 2017;137(2):66–72. [DOI] [PubMed] [Google Scholar]
- 43. Vishnu P, Aboulafia DM. Long‐term safety and efficacy of romiplostim for treatment of immune thrombocytopenia. J Blood Med. 2016;7:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong RSM, Khelif A, Salama A, Saleh MN. Occurrence and management of cataracts in adult patients (pts) with chronic immune thrombocytopenia (cITP) during long‐term treatment with eltrombopag (EPAG): results from the extend study. Blood. 130(Suppl. 1):1053. [Google Scholar]
- 45. Kantarjian H, Fenaux P, Sekeres MA, et al. Safety and efficacy of romiplostim in patients with lower‐risk myelodysplastic syndrome and thrombocytopenia. JCO. 2010;28(3):437–444. [DOI] [PubMed] [Google Scholar]
- 46. Dickinson M, Cherif H, Fenaux P, et al. Eltrombopag in combination with azacitidine for first‐line treatment of MDS patients with thrombocytopenia: the randomized, placebo‐controlled, phase III, support study. Leuk Res. 55:S23–S24. [Google Scholar]
- 47. Rituxan (Rituximab) [Prescribing Information]. San Francisco, CA: Genentech, Inc; 2016. [Google Scholar]
- 48. Tran H, Brighton T, Grigg A, et al. A multi‐centre, single‐arm, open‐label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R‐ITP1000 study). Br J Haematol. 2014;167(2):243–251. [DOI] [PubMed] [Google Scholar]
- 49. Zaja F, Vianelli N, Volpetti S, et al. Low‐dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–334. [DOI] [PubMed] [Google Scholar]
- 50. Chugh S, Darvish‐Kazem S, Lim W, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta‐analysis. Lancet Haematol. 2015;2(2):e75–e81. [DOI] [PubMed] [Google Scholar]
- 51. Ghanima W, Khelif A, Waage A, et al. Rituximab as second‐line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet Lond Engl. 2015;385(9978):1653–1661. [DOI] [PubMed] [Google Scholar]
- 52. Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bussel JB, Lee CS, Seery C, et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica. 2014;99(7):1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chapin J, Lee CS, Zhang H, Zehnder JL, Bussel JB. Gender and duration of disease differentiate responses to rituximab‐dexamethasone therapy in adults with immune thrombocytopenia. Am J Hematol. 2016;91(9):907–911. [DOI] [PubMed] [Google Scholar]
- 55. Godeau B, Porcher R, Fain O, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter phase 2 study. Blood. 2008;112(4):999–1004. [DOI] [PubMed] [Google Scholar]
- 56. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levy R, Mahevas M, Galicier L, et al. Profound symptomatic hypogammaglobulinemia: a rare late complication after rituximab treatment for immune thrombocytopenia. Report of 3 cases and systematic review of the literature. Autoimmun Rev. 2014;13(10):1055–1063. [DOI] [PubMed] [Google Scholar]
- 58. Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146(1):25–33. [DOI] [PubMed] [Google Scholar]
- 59. Audia S, Godeau B, Bonnotte B. Is there still a place for “old therapies” in the management of immune thrombocytopenia? Rev Med Interne Fondee Par Soc Natl Francaise Med Interne. 2016;37(1):43–49. [DOI] [PubMed] [Google Scholar]
- 60. Brown TM, Horblyuk RV, Grotzinger KM, Matzdorff AC, Pashos CL. Patient‐reported treatment burden of chronic immune thrombocytopenia therapies. BMC Hematol. 2012;12(1):2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. [DOI] [PubMed] [Google Scholar]
- 62. Li X, Sharma A, Zhang X, et al. Cost per treatment success of thrombopoietin receptor agonists vs “watch and rescue” strategy for treating adult non‐splenectomized patients with chronic immune thrombocytopenia: a US payer perspective. Blood. 2015;126:4461. [Google Scholar]
- 63. Mahévas M, Gerfaud‐Valentin M, Moulis G, et al. Characteristics, outcome, and response to therapy of multirefractory chronic immune thrombocytopenia. Blood. 2016;128(12):1625–1630. [DOI] [PubMed] [Google Scholar]
- 64. Cantoni S, Carpenedo M, Mazzucconi MG, et al. Alternate use of thrombopoietin receptor agonists in adult primary immune thrombocytopenia patients: a retrospective collaborative survey from Italian hematology centers. Am J Hematol. 2018;93(1):58–64. [DOI] [PubMed] [Google Scholar]
- 65. Gonzalez‐Porras JR, Mingot‐Castellano ME, Andrade MM, et al. Use of eltrombopag after romiplostim in primary immune thrombocytopenia. Br J Haematol. 2015;169(1):111–116. [DOI] [PubMed] [Google Scholar]
- 66. Abrams C. Thrombocytopenia In: Goldman L, Schafer A, eds. Goldman‐Cecil Medicine. Philadelphia, PA: Saunders Elsevier; 2011:1124–1131. [Google Scholar]


