Abstract
This study aimed to identify the optimal dose of the endothelin‐1 receptor antagonist atrasentan with maximal albuminuria reduction and minimal signs of sodium retention, as manifested by increase in bodyweight. Data from the RADAR‐JAPAN studies were used, evaluating the effect of 0.75 or 1.25 mg/d of atrasentan in 161 patients with type 2 diabetes and kidney disease. Individual pharmacokinetic parameters were estimated using a population pharmacokinetic approach. Subsequently, changes in the urinary albumin‐to‐creatinine ratio (UACR) and bodyweight from baseline after 2 weeks' exposure were modelled as a function of the pharmacokinetic parameters. The 0.75 and 1.25 mg doses showed a mean UACR reduction of 34.0% and 40.1%, whereas mean bodyweight increased by 0.9 and 1.1 kg, respectively. A large variation between individuals was observed in the UACR and bodyweight responses. Individual pharmacokinetic parameters correlated significantly with both individual UACR and bodyweight responses (P < .01). The individual response curves for UACR and bodyweight crossed at approximately the mean trough concentration of 0.75 mg atrasentan, indicating that 0.75 mg/d of atrasentan is the optimal dose for kidney protection with maximal efficacy (albuminuria reduction) and safety (minimal sodium retention).
Keywords: diabetic nephropathy, dose‐finding, endothelin receptor antagonist, pharmacodynamics, pharmacokinetics
1. INTRODUCTION
Defining the dose of a drug with optimal efficacy and safety is important for a drug's development programme and its use in clinical practice. This is especially important for drugs with a narrow therapeutic window, or drugs for which the efficacy and safety exposure‐response curves overlap.
Endothelin receptor antagonists (ERAs) are an example of a class of drugs with a narrow therapeutic window. The class is tested for cardiovascular protection, including reducing the progression of kidney disease.1, 2, 3, 4 Albuminuria lowering is believed to be an efficacy biomarker that reflects the drug's efficacy to delay progression of kidney disease, whereas the sodium retention during endothelin receptor antagonism is a biomarker for unwanted side effects. The optimal dose of an ERA for kidney protection is a balance between maximal albuminuria lowering and minimal sodium retention.
Atrasentan is an ERA that has been shown to decrease albuminuria at relatively low doses of 0.75 and 1.25 mg/d in the dose‐finding phase 2 RADAR trial.5 However, even at these low doses, atrasentan also caused sodium retention as manifested by increases in bodyweight. The aim of this study is to employ exposure‐response analyses to identify the optimal atrasentan dose with maximal albuminuria reduction and minimal sodium retention.
2. METHODS
2.1. Clinical trial design and patient population
Data from 161 participants in the RADAR (NCT01356849) and JAPAN (NCT01424319) trials were used. The RADAR and JAPAN trials assessed the effect of atrasentan on albuminuria reduction. The design and primary results of both trials were previously published.5 To be eligible, participants were required to have a urinary albumin‐to‐creatinine ratio (UACR) within 300 to 3500 mg/g and an estimated glomerular filtration rate (eGFR) of 30 to 75 mL/min/1.73 m2. As per protocol, all participants received the maximum tolerated labeled daily dose of a renin‐angiotensin‐aldosterone‐system (RAAS) inhibitor. Patients were randomly allocated to 12 weeks of treatment with atrasentan at doses of 0.75 or 1.25 mg/d, or a placebo using a double‐blind design. The primary endpoint of the trial was the change in UACR over time.
Three consecutive first‐void urine specimens were collected at baseline and every 2 weeks thereafter to determine urinary albumin and creatinine concentrations. Blood samples were sparsely collected to determine plasma atrasentan exposure. In line with previous reports of this trial, changes in bodyweight were used as proxy for sodium retention. Analyses focused on changes in sodium retention after 2 weeks of atrasentan therapy in order to maximise detection of atrasentan on sodium retention.
2.2. Pharmacokinetic and pharmacodynamic analyses
The population pharmacokinetic model was previously published.6 The original data file and model results were combined to generate a simulation dataset (data transformations and visualisations were performed in R3.4.2 [R Foundation for Statistical Computing, Vienna, Austria]). For each individual, the simulation dataset contained dosing information, demographics and post‐hoc Bayesian pharmacokinetic parameter estimates (e.g. individual absorption rates, clearances and volumes of distribution). For simulation purposes, all available pharmacokinetic observations were set at missing. In order to obtain additional individual pharmacokinetic parameters (e.g. area‐under‐the‐plasma‐concentration time curve [AUC]), atrasentan exposure was simulated on day 14 after first dosing in time steps of 0.1 hour. Simulations were run in NONMEM 7.3 (ICON Development Solutions, Ellicott City, MD, USA) using the individual post‐hoc Bayesian parameter estimates and the original model structure. The simulated pharmacokinetic profiles per individual were used to obtain the following individual pharmacokinetic parameters on day 14: maximum plasma atrasentan concentration (Cmax), trough concentration (Ctrough [on day 15]), and average steady state concentration (Css). The individual AUC for day 14 (AUCd14) was calculated by the amount of administered/individual clearance. Subsequently, regression analyses were performed to assess the association between the change from baseline in log‐transformed UACR and bodyweight after 2 weeks with Ctrough, Cmax, Css and AUC d14.
3. RESULTS
Baseline characteristics of patients assigned to 0.75 and 1.25 mg doses of atrasentan were reported previously.5 The mean Ctrough (2.5th to 97.5th Percentile [P]) of atrasentan at week 2 was 1.7 ng/mL (0.4‐4.7) and 3.4 ng/mL (1.0‐10.0) for the 0.75 and 1.25 mg doses, respectively. After 2 weeks of treatment with either 0.75 or 1.25 mg of atrasentan, UACR decreased by 34.0% (P < .01) and 40.1% (P < .01), respectively, compared to baseline, with a large variation among individuals (2.5th to 97.5th P: −68.4 to 70.5 and −76.2 to 12.3). The mean increase in bodyweight [2.5th to 97.5th P] with 0.75 and 1.25 mg of atrasentan was 0.9 kg [−1.0 to 3.0] and 1.1 kg [−1.0 to 4.0], respectively. The individually predicted values for Ctrough, Css, Cmax and AUCd14 correlated significantly to both individual UACR and bodyweight responses (Table 1). Figure 1 shows that the exposure‐response curves for albuminuria and bodyweight crossed at a mean Ctrough corresponding to approximately 0.75 mg of atrasentan per day. At the mean Ctrough of the 1.25 mg dose, a slightly larger albuminuria response was observed, at the expense of a larger increase in bodyweight probably because of a larger degree of sodium retention. Results were similar when Css, Cmax and AUCd14 were modelled (Figure S1).
Table 1.
Associations between pharmacokinetic parameters and albuminuria and bodyweight response at 2 weeks
| Mean (95% CI) % change in UACR per 2‐fold increase in atrasentan concentration | P | Mean (95% CI) change in bodyweight in kg per 2‐fold increase in atrasentan concentration | P | |
|---|---|---|---|---|
| Ctrough | −9.8 (−15.7 to −3.5) | .003 | 0.31 (0.10‐0.52) | .004 |
| Css | −10.2 (−16.3 to −3.6) | .003 | 0.30 (0.08‐0.52) | .008 |
| Cmax | −10.3 (−16.6 to −3.5) | .004 | 0.28 (0.05‐0.51) | .019 |
| 24‐hour AUCd14 | −9.9 (−16.1 to −3.3) | .004 | 0.30 (0.08‐0.52) | .008 |
Figure 1.
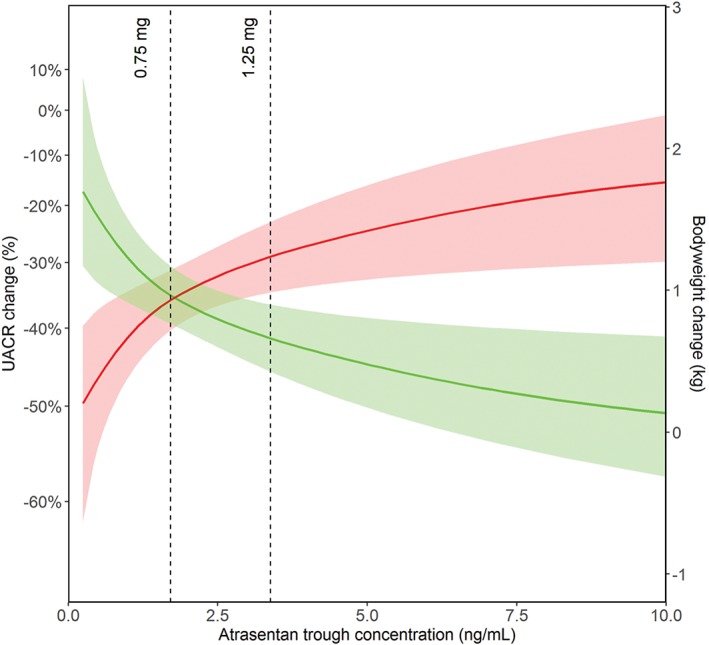
Predicted atrasentan trough concentration versus predicted albuminuria (green) and predicted bodyweight response (red). The mean predicted response (solid line) is shown for the 95% prediction intervals (shaded areas). The dotted lines represent the mean atrasentan trough concentrations for 0.75 and 1.25 mg doses
4. DISCUSSION
This study showed a large individual variation in albuminuria and sodium retention (bodyweight) response after 2 weeks of treatment with a low dose of atrasentan. The observed variation in albuminuria and bodyweight response correlated to the variation in the estimated individual pharmacokinetic parameters of atrasentan. At the atrasentan Ctrough equivalent to the administration of 0.75 mg of atrasentan, a significant and clinically relevant reduction in albuminuria was observed with fewer signs of sodium retention in comparison to a Ctrough equivalent to the administration of 1.25 mg of atrasentan.
Regulatory agencies have developed rigorous guidelines on how to use dose‐response data to support dose selection and drug registration.7, 8 Despite these rigorous guidelines, dose‐finding studies to determine the optimal therapeutic dose are hampered by various factors. Firstly, dose‐finding studies often include only a small number of patients per drug‐dose arm. Combined with the consideration that the individual exposure and response to many drugs vary substantially among patients,9 the small sample size compromises accurate and precise determination of the optimal dose. Secondly, the patient population included in the dose‐finding studies is not always representative of the population enrolled in confirmatory clinical trials and those who will eventually be treated in clinical practice; this is because the latter population is often more heterogeneous with varying degrees of renal or hepatic function, multiple comorbidities, and the use of many concomitant medications. Each of these factors can alter dose‐exposure‐response relationships.
A further problem in determining the optimal therapeutic dose is that its selection is based on an inadequate balance between efficacy and safety. Traditionally, dose finding is based on the drug's efficacy in modifying a single risk factor that the drug is targeting—for example, blood pressure for an antihypertensive drug. The safety is mainly established from a fixed set of parameters. However, many drugs have effects on other parameters (off‐target effects), which may also be risk factors that contribute to clinical outcomes, either in a positive or a negative way. The sodium retention effect of ERAs is one such off‐target effect that contributes to clinical outcomes in a negative way. Therefore, dose selection should be based on the balance of drug effects on multiple parameters, both on those that contribute to protection and those that induce harm.
These problems in selecting the optimal therapeutic dose for an ERA are illustrated by the ERA avosentan. A phase III trial (ASCEND) with avosentan was terminated early because of an increased incidence of congestive heart failure probably caused by the sodium‐retaining effects.10 In hindsight, the increased sodium retention and congestive heart failure could have been expected, because the high doses of 25 and 50 mg used in the phase III trial were associated with significant sodium retention and peripheral edema in an earlier dose‐finding trial.11 Despite the high incidence of edema, the 25 and 50 mg doses were selected for the phase III outcome trial. This highlights the importance of careful dose selection when balancing maximal albuminuria reduction and minimal sodium retention.
Additionally, the high doses used in the ASCEND trial are not the only explanation for the increased edema and heart failure, but also the difference in populations studied in the phase III outcome trial and the dose‐finding study. In the phase III trial, patients with overt diabetic nephropathy were enrolled; they had a mean eGFR of 33 mL/min/1.73 m2.3 These patients are prone to sodium retention. However, in the dose‐finding study, patients who are less prone to sodium retention, with an estimated creatinine clearance of ~80 mL/min, were enrolled.12 This finding also highlights the importance of strictly monitoring patients with diabetes and impaired kidney function for signs of sodium retention.
For the development of the ERA atrasentan, the main inclusion and exclusion criteria for the phase II and III trials were kept similar, and the sodium‐retaining effects of atrasentan were carefully analysed during the dose selection process. However, the sample size of the atrasentan phase II dose‐finding study was small, thus limiting the accuracy and precision of the dose‐finding analyses.
In conclusion, the exposure‐response analysis showed that 0.75 mg/d of atrasentan as an adjunct to RAAS inhibition is the optimal dose for renal protection with maximal albuminuria reduction while minimising sodium retention.
Supporting information
FIGURE S1. Predicted atrasentan steady state (A), maximum (B) concentration and area under the curve (C) versus predicted albuminuria (green) and predicted bodyweight response (red). The mean predicted response (solid line) is displayed with the 95% prediction intervals (shaded areas). The dotted lines represent the mean atrasentan trough concentrations for 0.75 and 1.25 mg doses. The mean (2.5th to 97.5th) steady state atrasentan 0.75 and 1.25 mg concentrations were 1.9 ng/mL (0.5‐5.2) and 3.3 ng/mL (1.2‐11.3).The mean (2.5th to 97.5th) maximal atrasentan for 0.75 and 1.25 mg were 2.3 (0.7‐6.9) and 3.9 (1.7‐14.8). The mean (2.5th to 97.5th) area under the curve for atrasentan 0.75 and 1.25 mg were 52.2 (15.9‐173) and 83.4 (29.5‐294).
ACKNOWLEDGMENTS
The authors thank all site investigators and patients who participated in the RADAR trial. H. J. L. H. is supported by a VIDI grant from the Netherlands Organisation for Scientific Research (917.15.306). J. S. is supported by a grant from the Novo Nordisk Foundation, Grant Number NNF14SA0003.
Conflict of interest
J. K. and J. S. report no conflicts of interest. H.‐H. P. has equity in Merck and Novo Nordisk and has received consulting and lecture fees from AstraZeneca, Abbott, Novartis, and Reata. D. d. Z. is a consultant for and received honoraria (to employer) from AbbVie, Astellas, Bayer, Boehringer Ingelheim, Novo Nordisk, Fresenius, Janssen, and Mitsubishi Tanabe. H. J. L. H. is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Janssen, and Merck; he has a policy of all honoraria being paid to his employer. N. M. is an employee of AbbVie and may own stock or stock options.
Author contributions
J. K. and H. J. L. H. wrote the draft of this report. N. M., J. S. and J. K. performed statistical analyses. All the authors contributed to interpretation and critical revision of the publication. H. J. L. H. takes full responsibility for this report.
Koomen JV, Stevens J, Mostafa NM, Parving H‐H, de Zeeuw D, Heerspink HJL. Determining the optimal dose of atrasentan by evaluating the exposure‐response relationships of albuminuria and bodyweight. Diabetes Obes Metab. 2018;20:2019–2022. 10.1111/dom.13312
Funding information This study was supported by AbbVie Inc.
REFERENCES
- 1. Heerspink HJL, Andress DL, Bakris G, et al. Rationale and protocol of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018. [Epub ahead of print]. 10.1111/dom.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Komers R, Gipson DS, Nelson P, et al. Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET). Kidney Int Rep. 2017;2:654‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heerspink HJL, Andress DL, Bakris G, et al. Baseline characteristics and enrichment results of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial. Diabetes Obes Metab. 2018. [Epub ahead of print]. 10.1111/dom.13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin CW, Mostafa NM, L Andress D, J Brennan J, Klein CE, Awni WM. Relationship between Atrasentan concentrations and urinary albumin to creatinine ratio in western and Japanese patients with diabetic nephropathy. Clin Ther. 2017;40:242‐251. [DOI] [PubMed] [Google Scholar]
- 7. European Medicines Agency . ICH Topic E 4: Dose Response Information to Support Drug Registration. European Medicines Agency: London, England; 1994. [Google Scholar]
- 8. U.S. Department of Health and Human Services, Food and Drug Administration . Guidance for Industry: Exposure‐Response relationships ‐ Study Design, Data Analysis, and Regulatory Applications. Rockville, MD, United States; 2003.
- 9. Heerspink HJL, Oberbauer R, Perco P, et al. Drugs meeting the molecular basis of diabetic kidney disease: bridging from molecular mechanism to personalized medicine. Nephrol Dial Transplant. 2015;30(Suppl 4):105‐112. [DOI] [PubMed] [Google Scholar]
- 10. Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin‐1 inhibits sodium reabsorption by ET(A) and ET(B) receptors in the mouse cortical collecting duct. Am J Physiol Renal Physiol. 2013;305:F568‐F573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smolander J, Vogt B, Maillard M, et al. Dose‐dependent acute and sustained renal effects of the endothelin receptor antagonist avosentan in healthy subjects. Clin Pharmacol Ther. 2009;85:628‐634. [DOI] [PubMed] [Google Scholar]
- 12. Wenzel RR, Littke T, Kuranoff S, et al. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20:655‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Predicted atrasentan steady state (A), maximum (B) concentration and area under the curve (C) versus predicted albuminuria (green) and predicted bodyweight response (red). The mean predicted response (solid line) is displayed with the 95% prediction intervals (shaded areas). The dotted lines represent the mean atrasentan trough concentrations for 0.75 and 1.25 mg doses. The mean (2.5th to 97.5th) steady state atrasentan 0.75 and 1.25 mg concentrations were 1.9 ng/mL (0.5‐5.2) and 3.3 ng/mL (1.2‐11.3).The mean (2.5th to 97.5th) maximal atrasentan for 0.75 and 1.25 mg were 2.3 (0.7‐6.9) and 3.9 (1.7‐14.8). The mean (2.5th to 97.5th) area under the curve for atrasentan 0.75 and 1.25 mg were 52.2 (15.9‐173) and 83.4 (29.5‐294).


