Abstract
Objective
We evaluated the feasibility and acceptability of a tailored evidence‐based intervention, consisting of a leaflet and a letter, to encourage timely help‐seeking for dementia in Black elders.
Methods
Participating GP surgeries were randomised to send either the intervention or a control leaflet about ageing well to Black patients aged ≥50 years old without known dementia. We interviewed patients 2 weeks later about the intervention's acceptability using closed and open‐ended questions, and they completed a Theory‐of‐Planned‐behaviour questionnaire about what they would do if they developed memory problems, which they also completed 4 months later.
Results
Five of 26 surgeries approached agreed to invite patients. Sixty‐five patients responded, of whom 61 (93.8%) agreed to participate. At 2 weeks, we consented and interviewed 47/61 (77%), of whom 24 received the intervention, and at 4 months we followed up 43/47 (91.5%). At 2 weeks, 44/47 (93.6%) found either intervention acceptable to receive by post, including 23/24 of the intervention. Nineteen of 24 (79.2%) reported reading the intervention leaflet compared with 13/23 (56.5%) controls. The intervention leaflet made 16/24 (66.7%) think about visiting their doctor for memory problems and led 4 to help‐seeking behaviour. We calculated that 191 patients and 24 surgeries are required for an efficacy trial.
Conclusions
Given the intervention is acceptable, inexpensive, and unlikely to cause harm, we judge it appropriate to disseminate it without a full‐scale trial. Recruitment attainment, retention, and projected sample size calculation indicated feasibility for a larger trial.
Keywords: African‐Caribbean, BME, dementia, help‐seeking, intervention, trial
Key points.
Black elders present late to dementia services.
We present a targeted, evidence‐based, inexpensive intervention to improve timely help‐seeking for dementia in Black elders.
This intervention is feasible and acceptable.
This pilot study provides data for future trials and resources for routine practice.
1. INTRODUCTION
National and international strategies prioritise early detection of dementia,1 which have led to a significant increase in investment, research, training, and the number of people being diagnosed.2 However, Black and Minority Ethnic (BME) populations access dementia services later in their illness,3, 4, 5, 6, 7 despite being at higher risk of dementia than their White counterparts.8 Timely diagnosis of dementia benefits patient and carer through better care planning, delayed care home entry, prolonged autonomy, access to social care, and reduced crises and costs.9, 10, 11
Black African and Caribbean elders (BACe) tend to seek help first from their immediate social network, including close relatives and friends,12 and delay help‐seeking from health and social care providers until crisis strikes or they can no longer cope.4, 13 Their dementia care pathway also appears to differ from that of their White counterparts, with a lesser likelihood of receiving anti‐dementia medication, or taking part in research trials of novel therapies.4
Barriers to help‐seeking for dementia are diverse and include failing to identify or acknowledge symptoms, concerns about outcomes of receiving a diagnosis, conception of dutiful family care; attitudes towards service providers, ability, and willingness to divulge private or sensitive information.7, 14 Some of these are not specific to dementia, but others about the meaning of memory loss, the idea that dementia is only a disease of white women, and the outcomes of a diagnosis are.14 UK Black adults regard memory problems as a private and stigmatising matter that should be kept in the family and find it a difficult subject to broach with their General Practitioner (GP), possibly because of being brought up with the idea that it was meritorious not to divulge these symptoms. Some even view dementia as a “White person's illness” that does not exist in the Black population.14
As the process of help‐seeking for dementia has culture specific aspects,7 tailoring health interventions and services to cultural groups is recommended.13, 15
2. THE PRESENT STUDY
This trial was part of a larger research project with the overall aim of improving dementia care in Black African and Caribbean populations.
The first stage was a qualitative study with 50 Black adults to better understand how Black families respond to memory problems, a possible sign of dementia.14
We used these findings to (1) develop a new intervention to facilitate timely diagnosis of dementia in BACe, and (2) evaluated its acceptability and (3) its delivery in a feasibility cluster randomised controlled trial (RCT). We also used the APEND, a validated Theory‐of‐Planned‐behaviour (TPB) questionnaire16 developed to evaluate intention to seek help for dementia in Minority Ethnic elders.
3. METHODS
National Research Ethics Service Committee Cambridgeshire and Hertfordshire gave ethics committee approval, and we obtained Research and Development (R&D) permission from the areas in which the GP practices for the RCT were located.
3.1. Developing the intervention
3.1.1. Intervention
We used findings from earlier systematic reviews6, 17 and our qualitative study14 to develop a leaflet entitled “Getting help for forgetfulness” (Appendix 1, found in the Supporting Information), tailored to overcome barriers to help‐seeking specific to BACe.
Content of the leaflet
We included illustrations and anonymised quotes from Black adults whom we interviewed in the qualitative study14 and covered the following themes:
How to overcome barriers to help‐seeking for memory problems
Frequent memory lapses are a potential sign of illnesses like dementia
Dementia is a physical illness that affects people of all ethnicities
Seeking professional help early for memory problems is important
Concerns about confidentiality, loss of autonomy, medication, and time allocated for consultation
Seeking medical advice for memory problems is consistent with dutiful family care
Where to find more information and help.
We improved and refined the leaflet content, layout, and illustration through consultation with people with mild dementia recruited from memory clinics, family carers, dementia experts, clinicians, volunteers from initial focus groups and from the public, and professional graphic designers to ensure acceptability, understandability, and clarity.
Accompanying letter
We developed a personalised letter to accompany the leaflet which was addressed on each surgery's headed paper to individual patients and signed by their GP by refining a letter from previous projects.18 The letter encouraged patients to read the leaflet as it contained information about dealing with memory problems relevant to BACe, and reassured them that receiving it was not indicative of suspicion by the GP that they, or a family member had memory problems. It distinguished potential symptoms from occasional lapses in memory by their persistence and severity and advised patients to make an appointment to see a doctor if they are worried about their memory (Appendix 2, found in the Supporting Information).
3.1.2. Control intervention
We selected a leaflet of similar length and layout about keeping physically active and ageing well (Appendix 3, found in the Supporting Information), which was not specifically tailored to a Black audience or about dementia. It did not contain images of Black individuals, nor did it include quotes or address concerns specific to Black families. We obtained written authorisation and printed copies from the relevant owner. The control leaflet was accompanied with a minimally modified version of the personalised GP letter (Appendix 4, found in the Supporting Information).
3.1.3. Exploratory cluster randomised controlled trial
We aimed to evaluate feasibility of recruitment (GPs and patients), acceptability of the new intervention, and its delivery in a feasibility cluster RCT. We pre‐specified that if feasible and acceptable, then ≥70% of patients who expressed an interest in the study would consent and ≥80% of those who enrolled initially would participate in the follow‐up phase. We also aimed for a rating of the intervention as acceptable by ≥80% of participants. The trial was registered with the International Standard for RCT (http://www.isrctn.com/ISRCTN67199930).
3.1.4. Participants
Patients were Black African, Caribbean, and British, aged ≥50 years and were registered with one of the GP practices we had recruited. We excluded patients who had a known diagnosis of dementia, or were being assessed for dementia, those who lacked capacity to consent to the study, and people living in a care home.
3.1.5. Design
This was a multisite, parallel group, cluster randomised controlled feasibility, and acceptability trial. We aimed to recruit 5 GPs from areas of Greater London with a high density of residents from Black backgrounds based on reports from the Office for National Statistics.19
3.1.6. Procedure
We searched NHS official websites for contacts of GP practices in these areas and where we had R&D permission. We linked with the primary care research network in North Thames and South London to help with the recruitment of GP surgeries. We then approached those surgeries and asked staff to search their records for registered patients and to invite them to the study by mail. The letter of invitation sent by GP staff between November 2015 and June 2016 included information about a voucher incentive of £20 as a token of appreciation for participation and a return response slip for patients to complete with their contact details and send to the researchers if they wished to participate. The letter asked whether they would agree to participate in a study with UCL. It did not specify the research was about dementia care in Black elders (Appendix 5, found in the Supporting Information).
We telephoned patients who responded to discuss the study and sent them written information by post if they agreed to participate. Twenty‐four hours or more after they were deemed to have received the information, we asked GP staff to send them the new tailored or the control intervention depending on the group their GP practice was allocated.
3.1.7. Randomisation
GP practices were randomly allocated to intervention (n = 2) or control (n = 3) group using “ralloc” procedure in Stata version 14 by an independent statistician within CLAHRC (Collaboration for Leadership in Applied Health Research and Care) North Thames who was not involved in the remainder of the trial.
3.1.8. Assessment
Two weeks after the leaflet was sent out, we contacted each participant to arrange a face‐to‐face interview during which they completed a consent form. Participants were excluded from the study if they had not agreed to a date for interview within 4 weeks of being contacted.
Interviews
Participants provided sociodemographic information, including ethnicity, sex, age, religion, country of birth, year of arrival in UK, education, employment status, and marital status.
We asked all participants closed and open‐ended questions about what they thought of the intervention (or control); whether they found it acceptable to receive them in the post; whether they read them; and to provide any additional comments they had about them. We used this information to assess acceptability by summing up their answers to “yes and no” questions and by analysing their answers to open‐ended questions for rating of the intervention.
-
They also completed the APEND—Attitudes of People from Ethnic Minorities to Help‐Seeking for Dementia 16 TPB questionnaire and answered questions about whether the intervention influenced their attitudes towards help‐seeking for memory problems from their doctor, and whether it led them to contact their GP.
The APEND questionnaire is a validated 19‐item scale with each statement scored on a 7‐level Likert scale from 1 (strongly disagree) to 7 (strongly agree), with higher scores denoting favourable attitudes towards help‐seeking. It was developed to measure attitudes of people from minority ethnic groups to help‐seeking for dementia and evaluate the impact of interventions seeking to promote intention to seek formal help for memory problems.16 We used this to evaluate behavioural intention by summing up the 3 items of the scale measuring intention (Table 2).
Participants also answered questions about whether they have known, cared for, or worked with someone with dementia.
Four‐month follow‐up
Participants were interviewed 4 months after they received the intervention and asked to complete the APEND questionnaire again.
4. ANALYSIS
We described percentages of GP practices who agreed to be randomised, patients who responded to GPs' invitations to enrol, those who consented to the study, and retention rates at follow‐up. We described the demographics of the participants and classified occupation by the ONS SOC Group (2010)—Office for National Statistics Standard Occupation Classification.20 We analysed the answers to questions about acceptability using Excel for Windows. We calculated the percentage of patients who accessed and rated the new tailored intervention and compared it with patients' reaction to the control intervention. We also calculated projected sample size for a future main trial, including the number of patients and GP surgeries to contact to reach the sample target. Mixed effects linear models were used to assess the differences in intention to seek help between groups over time of testing.
5. RESULTS
The flow of participants is shown in the consort diagram (Figure 1). Of the 26 surgeries approached, 17 (65.4%) did not respond after 3 or more attempts and 4 (15.4%) declined to participate. The 5 (19.2%) GP practices randomised to the trial were in outer and inner London (Lambeth, Barking and Dagenham, and Ilford). There were a total of 1674 eligible registered patients, but we only sent invitation letters to 787 of these as we aimed to recruit 30 to 40 participants. We received 65 responses, of whom 93.8% (61/65) agreed to receive the intervention. Two weeks later, 77% (47/61) consented to the study at the time of first interview. Four months later, 91.5% (43/47) of participants completed the follow‐up interviews.
Figure 1.
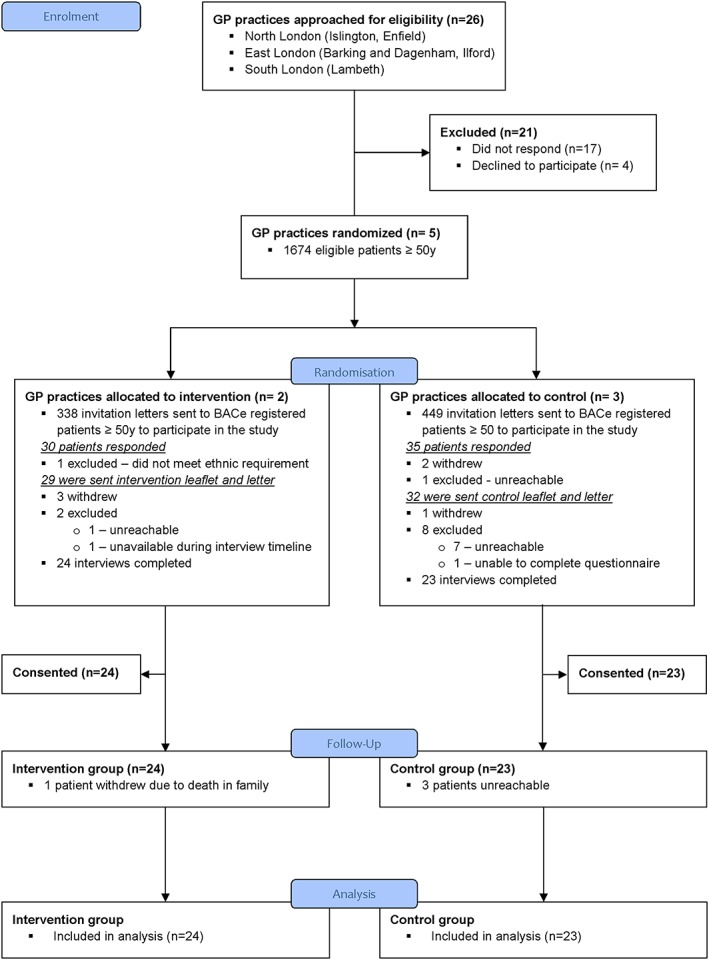
CONSORT flow diagram [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1 shows and compares the demographic characteristics of the 47 patients who consented to the study according to randomisation group. Twenty‐six (55.3%) participants were female, and the mean age was 57.6 (SD = 11.8) ranging from 50 to 89 years. Nearly half (23/47, 48.9%) self‐identified as African and had lived in UK an average of 30.9 years (SD = 15.5) ranging from 5 to 56 years. Eleven (23.4%) were UK born. Nearly two thirds (29/47, 61.7%) had post‐secondary education and were from a wide range of occupational backgrounds. The majority religion was Christianity (38/47, 80.9%). The control group had a greater proportion of African participants (60.9% vs 37.5%), had more people who were married or living with a partner (47.8% vs 29.2%), and included more male participants (56.5% vs 33.3%) than in the intervention group.
Table 1.
Demographic characteristics of patients who consented to the study according to randomisation group
| Demographic | Intervention n = 24 | Control n = 23 | All n = 47 | |
|---|---|---|---|---|
| Ethnicity (%) | Black African | 9 (37.5) | 14 (60.9) | 23 (48.9) |
| Black Caribbean | 6 (25.0) | 5 (21.7) | 11 (23.4) | |
| Black British | 8 (33.3) | 3 (13.0) | 11 (23.4) | |
| Asian Caribbean | 1 (4.2) | 1 (4.2) | 2 (4.3) | |
| Sex (%) | Male | 8 (33.3) | 13 (56.5) | 21 (44.7) |
| Age | Range (mean; SD) | (60.3; 10.0) | (58.4; 7.7) | 50 to 89 (57.6; 11.8) |
| Marital status (%) | Married or living with partner | 7 (29.2) | 11 (47.8) | 18 (38.3) |
| Single | 7 (29.2) | 5 (21.7) | 12 (25.5) | |
| Divorced | 3 (12.5) | 3 (13.0) | 6 (12.8) | |
| Separated | 2 (8.3) | 2 (8.7) | 4 (8.5) | |
| Widowed | 2 (8.3) | 1 (4.3) | 3 (6.4) | |
| Prefer not to say | 3 (12.5) | 1 (4.3) | 4 (6.4) | |
| Religion (%) | Christian | 19 (79.2) | 19 (82.6) | 38 (80.9) |
| Muslim | 3 (12.5) | 1 (4.3) | 4 (8.5) | |
| No religion | 2 (8.3) | 2 (8.7) | 4 (8.5) | |
| Rasta | ‐ | 1 (4.3) | 1 (2.1) | |
| Arrival in UK | Years in UK—range (mean; SD) | 16 to 56 | 5 to 55 | 5 to 56 (30.9; 15.5) |
| Education (%) | Post‐secondary | 13 (54.2) | 16 (69.6) | 29 (61.7) |
| Secondary | 6 (25.0) | 7 (30.4) | 13 (27.7) | |
| Primary | 1 (4.2) | ‐ | 1 (2.1) | |
| Current | 2 (8.3) | ‐ | 2 (4.3) | |
| No education | 1 (4.2) | ‐ | 1 (2.1) | |
| Unknown | 1 (4.2) | ‐ | 1 (2.1) | |
| Employment status (%) | Employed | 12 (50.0) | 13 (56.5) | 25 (53.2) |
| Unemployed | 2 (8.3) | 4 (17.4) | 6 (12.8) | |
| Retired | 6 (25.0) | 6 (21.7) | 11 (23.4) | |
| Unable to work | 3 (12.5) | 1 (4.3) | 4 (8.5) | |
| Prefer not to say | 1 (4.2) | ‐ | 1 (2.1) | |
| SOCa group (2010) (%) | (N = 23) | (N = 20) | (N = 43) | |
| 1. Managers, directors, and senior officials | ‐ | 2 (8.7) | 2 (4.3) | |
| 2. Professional occupations | 4 (16.7) | 4 (17.4) | 8 (17.0) | |
| 3. Associate professional and technical occupations | 1 (4.2) | ‐ | 1 (2.1) | |
| 4. Administrative and secretarial occupations | 2 (8.3) | 5 (21.7) | 7 (14.9) | |
| 5. Skilled trades occupations | 4 (16.7) | 3 (13.0) | 7 (14.9) | |
| 6. Caring, leisure, and other service occupations | 6 (25.0) | ‐ | 6 (12.8) | |
| 7. Sales and customer service occupations | 2 (8.3) | ‐ | 2 (4.3) | |
| 8. Process, plant, and machine operatives | ‐ | ‐ | ‐ | |
| 9. Elementary occupations | 4 (16.7) | 6 (26.1) | 10 (21.3) |
Standard occupational classification.
Many participants had some experience of dementia, either having known (19/47, 40.4%), cared for (10/47, 21%), or worked with (10/47, 21%) someone with dementia.
5.1. Acceptability
Forty‐four out of 47 (93.6%) said they found it acceptable to receive an intervention through the post, including 23 of the 24 in the intervention group. One participant in the intervention group who had experience working with people with dementia thought it should only be sent to patients expressing concerns about their memory. The 2 others who found it unacceptable to receive interventions through the post were from the control group. One found the enclosed letter worrying and did not read the leaflet, and the other did not explain.
A total of 79.2% (19/24) of participants reported reading the intervention leaflet in part or fully, compared with 56.5% (13/23) of the controls. Also, 66.7% (16/24) in the intervention group said it made them think that either they or a relative or friend should visit their doctor about memory problems, compared with 39.1% (9/23) in the control group. Twenty‐three‐point five percent (4/17) in the intervention group reported seeking help, compared with 11.1% (1/9) in the control group. Qualitative analysis of participants' responses to the open‐ended questions revealed that the intervention was found to be relevant, useful, helpful, informative, and educational.
5.2. APEND
The distribution of ratings for all items to the APEND questionnaire at 2 weeks was negatively skewed with skewness ranging between −0.853 to −2.728 (SE: 0.347) and median values between 5.00 and 7.00, suggesting favourable attitudes towards seeking help for memory problems from a doctor (Table 2). The middle 50% of ratings fell between 4 (neutral) and 7 (strongly agree).
Table 2.
Summary of average ratings of intention to seek help at 2 weeks using the APEND questionnaire
| Questionnaire Items | Median | IQR | ||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| Intention to seek help (IN) | ||||
| IN1 If I had memory problems like Mrs Abraham, I would seek help from my doctor: | 7.0 | 7.0 | 6.0–7.0 | 4.0–7.0 |
| IN2 I would expect to go to see my doctor for help, if I had memory problems: | 6.5 | 7.0 | 6.0–7.0 | 5.0–7.0 |
| IN3 I would want to see my doctor if I had memory problems: | 7.0 | 7.0 | 6.0–7.0 | 6.0–7.0 |
| Perceived behavioural control (PBC) | ||||
| PBC1 It would be easy to seek help from my doctor for memory problems: | 6.0 | 6.0 | 4.25–7.0 | 4.0–7.0 |
| PBC2 It would be my decision whether or not to see my doctor for memory problems: | 7.0 | 6.0 | 6.0–7.0 | 5.0–7.0 |
| PBC3 I am confident that I would be able to see my doctor for memory problems if I wanted to: | 7.0 | 6.0 | 6.0–7.0 | 6.0–7.0 |
| Subjective norms (SN) | ||||
| SN1 Most people who are important to me would approve of seeking help from my doctor for memory problems: | 7.0 | 7.0 | 6.0–7.0 | 5.0–7.0 |
| SN2 It would be expected of me that I would see my doctor for memory problems: | 6.0 | 6.0 | 5.25–7.0 | 5.0–7.0 |
| Behavioural attitudes (BA)a | ||||
| BA1 Overall, I think seeking help from my doctor for memory problems would be: | 7.0 | 7.0 | 6.0–7.0 | 6.0–7.0 |
| Useless 1 2 3 4 5 6 7 valuable | ||||
| BA2 Overall, I think seeking help from my doctor for memory problems would be: | 7.0 | 7.0 | 6.0–7.0 | 6.0–7.0 |
| Bad 1 2 3 4 5 6 7 good | ||||
| Behavioural beliefs (BB) | ||||
| BB1 My doctor would be able to provide treatments to help with memory problems: | 6.0 | 5.0 | 4.5–7.0 | 4.0–6.0 |
| BB2 My doctor would be able to tell me what the cause of memory problems is: | 5.5 | 5.0 | 4.0–7.0 | 4.0–7.0 |
| BB3 My doctor would be able to tell me what services are available to help with memory problems: | 7.0 | 6.0 | 6.0–7.0 | 6.0–7.0 |
| Outcome evaluations (OE) | ||||
| OE1 For memory problems, a treatment to help would be desirable: | 7.0 | 7.0 | 6.0–7.0 | 6.0–7.0 |
| OE2 For memory problems, finding out about the cause would be desirable: | 7.0 | 7.0 | 6.0–7.0 | 7.0–7.0 |
| OE3 For memory problems, finding out about what services are available to help would be desirable: | 7.0 | 7.0 | 6.0–7.0 | 6.0–7.0 |
| Normative beliefs (NB) | ||||
| NB1 Getting help from my doctor for memory problems would be embarrassing: | 6.5 | 7.0 | 4.25–7.0 | 6.0–7.0 |
| NB2 My family would think that I should seek help from my doctor for memory problems: | 6.5 | 6.0 | 6.0–7.0 | 6.0–7.0 |
| Motivation to comply (MC) | ||||
| MC1 What my family thinks I should do is important to me: | 6.0 | 6.0 | 5.0–7.0 | 5.0–7.0 |
Rating scales were different for BA items.
6. RANDOMIZED CONTROLLED TRIAL
The sum and average score of ratings for intention to seek help at 2 weeks and 4 months showed no differences between intervention and control groups (Table 3).
Table 3.
Average score of ratings for behavioural intention for each group at 2 weeks and 4 months
| Group | 2 weeks | 4 months | Mean |
|---|---|---|---|
| All patients | |||
| Intervention mean (SD) | 18.4 (3.8), n = 24 | 18.3 (3.1), n = 23 | 18.35 |
| Control mean (SD) mean difference | 18.3 (4.0), n = 23 0.1 | 19.5 (2.1), n = 20–1.2 | 18.9 |
| Patients who accessed the intervention | |||
| Intervention mean (SD) | 18.2 (4.1), n = 18 | 17.8 (3.2), n = 18 | 18.0 |
| Control mean (SD) mean difference | 18.2 (3.1), n = 11 0.0 | 19.2 (2.7), n = 11–1.4 | 18.7 |
Linear mixed model analyses with intention to seek help as main outcome with fixed effect for time and study groups and a random effect for subject, adjusted for gender, age, ethnicity, and employment status showed no differences in behavioural intention between intervention and control groups over time (mean combined APEND intention score, intervention group = 18.35, 95% CI [17.14, 19.56], SD = 2.80); controls = 19.0, 95% CI [17.92, 20.18], SD = 2.42; Parameter estimate = 0.66, 95% CI [−.94, 2.25], P = .414).
6.1. Post‐hoc analysis
Post‐hoc analysis using Mann‐Whitney U test of the subgroup of the 29 patients who accessed both interventions and completed both legs of the trial with exact probability measure revealed no significant differences on intention to seek help between the intervention and control groups, at 2 weeks (mean difference of 0, U = 95.00, z = −.19, P = .877) and 4 months, (mean difference of −1.4, U = 75.50, z = −1.11, P = .296) (Table 3).
6.2. Cost calculation
The service support cost per GP practice was £566, comprising: 1 hour of site initiation for 1 GP (£70), 1‐hour practice manager time (£28), and 1‐hour administrator time (£15), as well as time for database searches, contacting and mailing eligible patients at administrator rate (£15/hour), and mailing list screen at GP hourly rate.
The price of printing the leaflets was £0.24 per item (£589 per 2500 leaflets), plus postage, and packing costs (approximately £0.65 each).
7. NUMBER NEEDED FOR A FULL TRIAL
We calculated that a total sample size of 126 participants, with 63 per group, will be needed for an effect of 0.5 point difference on the behavioural intention subscale of the APEND between groups (SD = 1) to be detected in a full trial (α = 5% and power = 80%). Because in the current feasibility trial, we lost 28% (47/65) of initial recruits, we need to inflate the sample to account for this and the 6% (44/47) attrition observed at follow‐up, which bring the sample set to 191. Only 8% (65/787) responded to invitations to participate in the current trial, which suggests that in the main trial and under the same conditions, we would need to approach approximately 2388 GP patients to attain the target recruitment number. This sample size does not correct for clustering.
We calculated that we would need 24 (191/8) GP practices to agree to randomization, using the lower end of 8 to 10 patients to be recruited per practice. Approximately 19% of practices we contacted in this trial agreed to participate in the study, giving an estimated 126 GP surgeries to contact to achieve the target of 24 practices.
8. DISCUSSION
We demonstrated the feasibility and acceptability of developing and testing a tailored intervention to encourage timely help‐seeking for dementia in BACe. All criteria for feasibility and acceptability were met: consent rate, retention rate at follow‐up, and rating of acceptability. The intervention, which targeted situations and concerns Black families have about getting help for dementia, was well received by BACe who found it acceptable to receive by post, relevant to their community and needs, and helpful in making them think they should seek help from a doctor for early signs of dementia. This trial provides data for planning of a superiority trial, including calculations of projected sample size and estimates of the number of patients and GP practices to approach.
We did not find any differences in intention to seek help for memory problems between the 2 groups; however, the study was not powered to evaluate efficacy or detect differences between groups.
We recruited our predefined sample of BACe patients on target although response rates to enrol were low. Only 8% (65/787) of those contacted by GP staff responded to the letter inviting them to enter the study. However, the large majority of those who expressed interest enrolled and completed the follow‐up phase. The barriers to recruiting BME in health research are not clearly understood but appear to be multifactorial and multilevel, and begin even before the actual initiation of the research study.21 Regulations in the US have helped with increasing the number of minority ethnic participants included in trials.22 In the UK where there is no such legislation, the participation of ethnic minorities in trials is lagging far behind American trials.23
We also met our target number of GP practices. Over 19% of those we approached agreed to randomisation. Recruitment was facilitated by utilizing existing relationships with practices willing to take part in dementia research, linking with primary care research networks, and offering monetary incentive24 to cover service costs.
The distribution of responses on the APEND questionnaire suggested that Black older patients have favourable attitudes towards help‐seeking for dementia from doctors. We cannot conclude causality between the intervention effect and intention to seek help. However, these results are interesting as they show that, contrary to popular beliefs, Black elders are willing to seek help for dementia from their GP, and their late presentation to dementia services may be driven by other factors than culture and may include failings in health care service provision. A recent RCT to facilitate memory clinics' referral in the general population found that a significant increase in GP consultations for memory problems did not translate into a significant increase in referral to memory clinic,18 thus, suggesting that the obstacles to entering dementia services may reside at service provider level rather than patient level.
9. LIMITATIONS OF THE STUDY
We were unable for ethical reasons to have any information of those potential participants who did not agree to be approached. Nearly 80% of the people in the intervention group said they read the leaflet and letter in part or fully, but we do not know whether they did so and as is the nature of a feasibility study whether it changed outcomes. In addition, the voucher incentive while a normal part of such research may have contributed to the high number of patients that reportedly read the leaflet. While we assumed that both groups were the same at baseline before receiving the leaflet, we do not know whether this is true in terms of intentions. A full efficacy trial should include a method of assessing how well patients engage with the intervention. Other recruitment channels may also be advisable as recruiting only via GP practices may be affected by doctor‐patient relationships when the relationship is weak or broken, because doctors play an important role in patients decision making about research trial participation.25 Furthermore, the APEND questionnaire is a relatively new scale, developed for UK‐based South Asian people, which may need to be adapted to this study population. Finally, the questionnaires were completed during an interview with a member of the research team who was aware of participants' study group allocation. Therefore, there was a potential for observer bias26 and social desirability bias27 in that participants may have responded in a manner that they perceived would be viewed favourably by the interviewer.
10. CONCLUSIONS AND RECOMMENDATIONS
These findings suggest that a full‐scale trial would be feasible. Future trials should develop appropriate interventions and evaluate their efficacy on BACe intention to seek formal help for dementia. Hitherto, public health resources and the media have largely excluded BME people when presenting dementia, perhaps contributing to the belief among some minority ethnic groups that dementia only affects White populations.14
Given the “Getting help for forgetfulness” leaflet is unlikely to cause harm, we have made it available for dissemination to health and social care service providers, community, and voluntary organisations, and charities without carrying out a further trial. Nevertheless, future larger trials of complex interventions to improve dementia care in BME people are desirable. Whilst research in the factors influencing help‐seeking for dementia in BME people is growing, robust trials of effective interventions are still lacking in comparison to what is known about the beliefs and attitudes of BME people about dementia.
DESCRIPTION OF AUTHORS' ROLES
G. Livingston and N. Mukadam designed and supervised the study and revised the article after the first draft.
M. Roche collected the data, carried out the statistical analysis, and wrote the first draft of the article.
S. Adelman assisted with writing the article.
CONFLICT OF INTEREST
None declared.
Supporting information
Data S1. Supporting info item
ACKNOWLEDGEMENTS
This research was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research (Grant No. 508926) and Care North Thames at Barts Health NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
N.M. is funded by the NIHR, grant number DRF‐2012‐05‐141 and G.L. is supported by the UCLH NIHR Biomedical Research Centre.
We would like thanks to all participants, patients, carers, GPs, and research colleagues who contributed to the development and evaluation of the intervention and permitted the successful completion of this trial based on their responses and input.
Roche M, Mukadam N, Adelman S, Livingston G. The IDEMCare Study—Improving Dementia Care in Black African and Caribbean Groups: A feasibility cluster randomised controlled trial. Int J Geriatr Psychiatry. 2018;33:1048–1056. 10.1002/gps.4891
REFERENCES
- 1. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 2. Donegan K, Fox N, Black N, Livingston G, Banerjee S, Burns A. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Health. 2017;2(3):e149‐e156. [DOI] [PubMed] [Google Scholar]
- 3. Tuerk R, Sauer J. Dementia in a black and minority ethnic population: characteristics of presentation to an inner London memory service. B J Psych Bull. 2015;39(4):162‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper C, Tandy AR, Balamurali TB, Livingston G. A systematic review and meta‐analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193‐203. [DOI] [PubMed] [Google Scholar]
- 5. Moriarty J, Sharif N, Robinson J. Black and Minority Ethnic People with Dementia and their Access to Support and Services. London: Social Care Institute for Excellence; 2011. [Google Scholar]
- 6. Mukadam N, Cooper C, Livingston G. A systematic review of ethnicity and pathways to care in dementia. Int J Geriatr Psychiatry. 2011;26(1):12‐20. [DOI] [PubMed] [Google Scholar]
- 7. Mukadam N, Cooper C, Basit B, Livingston G. Why do ethnic elders present later to UK dementia services? A qualitative study. Int Psychogeriatr. 2011;23(7):1070‐1077. [DOI] [PubMed] [Google Scholar]
- 8. Adelman S, Blanchard M, Rait G, Leavey G, Livingston G. Prevalence of dementia in African‐Caribbean compared with UK‐born white older people: two‐stage cross‐sectional study. Br J Psychiatry. 2011;199(2):119‐125. [DOI] [PubMed] [Google Scholar]
- 9. Prince M, Bryce R, Ferri C. World Alzheimer Report 2011: the benefits of early diagnosis and intervention. Alzheimer's Disease International; 2011. [Google Scholar]
- 10. Knapp M, Prince M, Albanese E, et al. The full report In: London: Alzheimer's Society; 2007. [Google Scholar]
- 11. Burns A. The benefits of early diagnosis of. Dementia. 2012. [DOI] [PubMed] [Google Scholar]
- 12. Werner P, Goldstein D, Karpas DS, Chan L, Lai C. Help‐seeking for dementia: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2014;28(4):299‐310. [DOI] [PubMed] [Google Scholar]
- 13. Mukadam N, Cooper C, Livingston G. Improving access to dementia services for people from minority ethnic groups. Curr Opin Psychiatry. 2013;26(4):409‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berwald S, Roche M, Adelman S, Mukadam N, Livingston G. Black African and Caribbean British Communities' perceptions of memory problems: “We don't do dementia.”. PLoS One. 2016;11(4):e0151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72‐83. [DOI] [PubMed] [Google Scholar]
- 16. Hailstone J, Mukadam N, Owen T, Cooper C, Livingston G. The development of attitudes of people from ethnic minorities to help‐seeking for dementia (APEND): a questionnaire to measure attitudes to help‐seeking for dementia in people from south Asian backgrounds in the UK. Int J Geriatr Psychiatry. 2016. [DOI] [PubMed] [Google Scholar]
- 17. Mukadam N, Cooper C, Kherani N, Livingston G. A systematic review of interventions to detect dementia or cognitive impairment. Int J Geriatr Psychiatry. 2015;30(1):32‐45. [DOI] [PubMed] [Google Scholar]
- 18. Livingston G, Baio G, Sommerlad A, et al. Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: cluster randomised controlled trial. PLoS Med. 2017;14(3):e1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ethnicity and National Identity in England and Wales—Office for National Statistics. 2011; https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11.
- 20. Elias P, Birch M. SOC2010: revision of the standard occupational classification. Economic & Labour Market Review. 2010;4(7):48‐55. [Google Scholar]
- 21. Brown G, Marshall M, Bower P, Woodham A, Waheed W. Barriers to recruiting ethnic minorities to mental health research: a systematic review. Int J Methods Psychiatr Res. 2014;23(1):36‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Association AA . Response to OMB directive 15: race and ethnic standards for federal statistics and administrative reporting. Retrieved October 1997;23:2006. [Google Scholar]
- 23. Hussain‐Gambles M, Atkin K, Leese B. Why ethnic minority groups are under‐represented in clinical trials: a review of the literature. Health Soc Care Community. 2004;12(5):382‐388. [DOI] [PubMed] [Google Scholar]
- 24. Pit SW, Vo T, Pyakurel S. The effectiveness of recruitment strategies on general practitioner's survey response rates—a systematic review. BMC Med Res Methodol. 2014;14(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King NM, Churchill LR. Clinical research and the physician‐patient relationship: the dual roles of physician and researcher In: The Cambridge Textbook of Bioethics; 2008:214. [Google Scholar]
- 26. Bruce NG, Shaper AG, Walker M, Wannamethee G. Observer bias in blood pressure studies. J Hypertens. 1988;6(5):375‐380. [PubMed] [Google Scholar]
- 27. Grimm P. Social desirability bias In: Wiley International Encyclopedia of Marketing; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting info item


