Summary
The Mirror Neuron System (MNS) plays a crucial role in action perception and imitative behavior, which is suggested to be impaired in Autism Spectrum Disorders (ASDs). In this review, we discuss the plausibility and empirical evidence of a neural interaction between the MNS, action perception, empathy, imitative behavior, and their impact on social decision making in ASDs. To date, there is no consensus regarding a particular theory in ASDs and its underlying mechanisms. Some theories have completely focused on social difficulties, others have emphasized sensory aspects. Based on the current studies, we suggest a multilayer neural network model including the MNS on a first layer and transforming this information to a higher layer network responsible for reasoning. Future studies with ASD participants combining behavioral tasks with neuroimaging methods and transcranial brain stimulation as well as computational modeling can help validate and complement this suggested model. Moreover, we propose applying the behavioral paradigms, and the neurophysiological markers mentioned in this review article for evaluating psychiatric treatment approaches in ASDs. The investigation of modulating effects of different treatment approaches on the neurophysiological markers of the MNS can help find specific subgroups of ASDs patients and support tailored psychiatric interventions.
Keywords: autism spectrum disorders, basal ganglia, mirror neuron system, motor cortex, social decision making
1. AUTISM SPECTRUM DISORDERS
Autism Spectrum Disorders (ASDs) are neurodevelopmental disorders characterized by impairment in communication and social interactions, and restricted repetitive and stereotyped behaviors.1, 2, 3 According to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5), people with ASD have difficulty with communication and interaction with other people, restricted interests, and repetitive behaviors and symptoms that hurt the person's ability to function properly in school, work, and other areas of life. Autism is known as a “spectrum” disorder because there is wide variation in the type and severity of symptoms people experience.4 Table 1 provides an overview of the DSM‐5 criteria for ASD with examples. The criteria for obtaining the severity level for ASD are shown in Table 2. In contrast to DSM‐4 under the DSM‐5 criteria, individuals with ASD must show symptoms from early childhood, even if those symptoms are not recognized until later. This criteria change encourages earlier diagnosis of ASD and also allows people whose symptoms may not be fully recognized until social demands exceed their capacity to receive the diagnosis. The earliest symptoms in ASDs include the lack of attention to faces,5 imitative behaviors,6 and motor impairments.7
Table 1.
Overview of the DSM‐5 criteria for autism spectrum disorders (ASD) with examples
| A. Persistent deficits in social communication and social interaction across multiple contexts, as manifested by the following, currently or by history |
| 1. Deficits in social‐emotional reciprocity, ranging, for example, from abnormal social approach and failure of normal back‐and‐forth conversation; to reduced sharing of interests, emotions, or affect; to failure to initiate or respond to social interactions |
| 2. Deficits in nonverbal communicative behaviors used for social interaction, ranging, for example, from poorly integrated verbal and nonverbal communication; to abnormalities in eye contact and body language or deficits in understanding and use of gestures; to a total lack of facial expressions and nonverbal communication |
| 3. Deficits in developing, maintaining, and understanding relationships, ranging, for example, from difficulties adjusting behavior to suit various social contexts; to difficulties in sharing imaginative play or in making friends; to absence of interest in peers |
|
Specify current severity:
Severity is based on social communication impairments and restricted, repetitive patterns of behavior (see Table 2) |
| B. Restricted, repetitive patterns of behavior, interests, or activities, as manifested by at least two of the following, currently or by history |
| 1. Stereotyped or repetitive motor movements, use of objects, or speech (eg, simple motor stereotypes, lining up toys or flipping objects, echolalia, idiosyncratic phrases) |
| 2. Insistence on sameness, inflexible adherence to routines, or ritualized patterns of verbal or nonverbal behavior (eg, extreme distress at small changes, difficulties with transitions, rigid thinking patterns, greeting rituals, need to take same route or eat same food every day) |
| 3. Highly restricted, fixated interests that are abnormal in intensity or focus (eg, strong attachment to or preoccupation with unusual objects, excessively circumscribed or perseverative interests) |
| 4. Hyper‐ or hypo‐reactivity to sensory input or unusual interest in sensory aspects of the environment (eg, apparent indifference to pain/temperature, adverse response to specific sounds or textures, excessive smelling or touching of objects, visual fascination with lights or movement) |
|
Specify current severity:
Severity is based on social communication impairments and restricted, repetitive patterns of behavior (see Table 2) |
| C. Symptoms must be present in the early developmental period (but may not become fully manifest until social demands exceed limited capacities, or may be masked by learned strategies in later life |
| Early primary caregivers report no longer essential |
| “Early Childhood” approximately age 8 and younger |
| D. Symptoms cause clinically significant impairment in social, occupational, or other important areas of current functioning |
| Select one severity level specifier for Social Communication and one for Restricted Interests and Repetitive Behaviors |
| Minimal social impairments: “without supports in place, deficits in social communication cause noticeable impairments. Has difficulty initiating social interactions and demonstrates clear examples of atypical or unsuccessful responses to social overtures of others. May appear to have decreased interest in social interactions.” (from DSM 5 severity rating) |
| Minimal RRB impairments: “Rituals and repetitive behaviors (RRB's) cause significant interference with Functioning in one or more contexts. Resists attempts by others to interrupt RRB's or to be redirected from fixated interest.” (from DSM 5 severity rating) |
| E. These disturbances are not better explained by intellectual disability (intellectual developmental disorder) or global developmental delay. Intellectual disability and autism spectrum disorder frequently co‐occur; to make comorbid diagnoses of autism spectrum disorder and intellectual disability, social communication should be below that expected for general developmental level |
Table 2.
Criteria for obtaining the severity level for autism spectrum disorders (ASD)
| Severity level for ASD | Social communication | Restricted interests & repetitive behaviors |
|---|---|---|
| Level 3: Requiring very substantial support | Severe deficits in verbal and nonverbal social communication skills cause severe impairments in functioning; very limited initiation of social interactions and minimal response to social overtures from others | Preoccupations, fixated rituals, and/or repetitive behaviors markedly interfere with functioning in all spheres. Marked distress when rituals or routines are interrupted; very difficult to redirect from fixated interest or returns to it quickly |
| Level 2: Requiring substantial support | Marked deficits in verbal and nonverbal social communication skills; social impairments apparent even with supports in place; limited initiation of social interactions and reduced or abnormal response to social overtures from others | RRBs and/or preoccupations or fixated interests appear frequently enough to be obvious to the casual observer and interfere with functioning in a variety of contexts. Distress or frustration is apparent when RRB's are interrupted; difficult to redirect from fixated interest |
| Level 1: Requiring support | Without supports in place, deficits in social communication cause noticeable impairments. Has difficulty initiating social interactions and demonstrates clear examples of atypical or unsuccessful responses to social overtures of others. May appear to have decreased interest in social interactions | Rituals and repetitive behaviors (RRB's) cause significant interference with functioning in one or more contexts. Resists attempts by others to interrupt RRB's or to be redirected from fixated interest |
In ASDs, a variety of neural structures are affected, ranging from the brain stem to the cerebellum and cerebral cortex.8, 9, 10, 11 As far as the cortical abnormalities are concerned, of particular interest is the connectivity deficit found in the parieto‐frontal network, ie, in the circuit whose areas are endowed with the mirror mechanism.12 It has been proposed that a malfunctioning of the mirror mechanism is one of the factors underlying the cognitive aspects of the deficit.13
Osterling et al (2002) addressed whether autism can be distinguished from mental retardation by 1 year of age.14 They used home videotapes of first birthday parties from 20 infants later diagnosed with ASDs, 14 infants later diagnosed with mental retardation (without autism), and 20 typically developing infants. Results indicated that 1‐year olds with ASDs can be distinguished from 1‐year olds with typical development (TD) and those with mental retardation. The infants with ASDs looked at others and oriented to their names less frequently than infants with mental retardation. The infants with ASDs and those with mental retardation used gestures and looked to objects held by others less frequently and engaged in repetitive motor actions more frequently than typically developing infants. These results demonstrate that ASDs can be distinguished from mental retardation and typical development already by 1 year of age.14
Although several studies have shed light on specific facets of ASDs, there is still no consensus on a universally accepted theory to explain its underlying mechanisms. While some theories have exclusively focused on sensory aspects, others have emphasized social difficulties and deficits in imitating and understanding actions of others. However, sensory, motor, and social processes in ASDs might be interconnected to a higher degree than what has been traditionally thought.15
2. ACTION PERCEPTION AND IMITATIVE BEHAVIOR IN ASDS
Imitation plays a central role in human development and learning of motor, communicative, and social skills.16, 17 Nevertheless, the neural basis of imitation and its functional mechanisms in ASDs remain elusive. In their pioneering study, di Pellegrino et al (1992) reported for the first time that in the premotor cortex of the macaque monkey (area F5) there are neurons that fire both when the monkey performs an action and when it observes an individual making a similar action.18 Based on this finding, Iacoboni et al (1999) hypothesized that imitation may be based on a mechanism directly matching the observed action onto an internal motor representation of that action (“direct matching hypothesis”).17 To test this hypothesis, human participants were asked to observe and imitate a finger movement and to perform the same movement after spatial or symbolic cues. Brain activity was measured with functional magnetic resonance imaging (fMRI). They found brain regions (the left inferior frontal cortex and the right superior parietal lobule) become active during finger movement, regardless of how it is evoked; however, their activation increased when the same movement was elicited by the observation of an identical movement made by another individual.17 In a recent seminal review article, Rizzolatti et al (2014) discussed the role that the cortical motor system plays in understanding and imitating the behavior of others.19 Their notion is that there is a mechanism, the mirror neuron mechanism (MNM), located in the motor system that plays a fundamental role in action understanding. Empirical findings supporting this notion have led to a paradigmatic conceptual shift in cognitive neuroscience and psychology. As summarized by Jeannerod: “…the observation of actions performed by other agents generates in the brain of the observer representations similar to those of the agents. This circular process, from the self to action and from action to other selves, has as a consequence that action representations can be shared by 2 or more people. These new findings have radically changed the traditional view of the motor system as an executive system that merely follows instructions elaborated somewhere else. Motor system now stands as a system that allows understanding the behavior of others and even as a probe that explores the external world for interacting with other people and gathering new knowledge”.20
Intriguingly, the mu rhythm has also been used as a physiological indicator of the human mirror neuron system (MNS).21, 22 Mu rhythm is an EEG measure of resting motor neurons, which is normally suppressed by input because of action observation or movement execution.23
Oberman et al (2005) investigated MNS sensitivity by examining mu suppression to familiarity, ie, the degree to which the observer is able to identify with the actor on the screen using familiar versus unfamiliar actors.24 They used 4 conditions: an unfamiliar hand grasping an object (“stranger”), the hand of the child's relatives performing the same action (“familiar”), the participant's own hand (“own”), and, finally, bouncing balls (“control condition”). Their results show that mu suppression was sensitive to the degree of familiarity. Both typically developing participants and those with ASD showed greater suppression to familiar hands compared to those of strangers. These findings suggest that the MNS responds to observed actions in individuals with ASD, but only when individuals can identify in some personal way with the stimuli.
Since the sensory‐motor cortex has neurons that are active during perception and execution,25, 26 we hypothesize that a perturbation of M1 impairs the MNS leading to deficits in imitating behavior in ASDs. Evidence for this notion can be derived from studies using transcranial magnetic stimulation (TMS) to induce motor‐evoked potentials (MEPs). Theoret et al (2005) investigated the neural mechanism matching action observation and execution in adults with ASD and normal controls.27 They applied TMS over the primary motor cortex (M1) during observation of finger movements. They showed that the overall modulation of M1 excitability during action observation was significantly lower in individuals with ASD compared with matched controls. A further TMS study also found reduced excitability of the motor cortex in ASD patients compared to controls and, remarkably, also found a negative correlation between the activity of the motor cortex and self‐reported social impairment.28
Most studies on the relationship between mirror mechanism and autism have employed EEG and MEG techniques. They revealed that the cortical rhythms that desynchronize in typically developing children (TD) during both execution and observation of hand movements do desynchronize in children with autism only during active hand movements.24, 29, 30
3. THE MIRROR NEURON MECHANISM AND ACTION UNDERSTANDING
The ability to understand and control motor actions is an important facet of everyday life. For example, the ability to mimic the body language of others can influence both social interactions and personal relationships.31, 32 Previous studies have shown that participants with ASDs have deficits in imitating the actions of others 33 and in learning motor actions (eg, simple body movements).13 Therefore, learning motor actions has been associated with mimicking the actions of others and understanding the purpose of the action.13 Also, this capability enables us to identify when and where to perform a certain social action, such as a wave or a handshake.34
When we observe a motor action (eg grasping a cup) done by another individual, we usually extract 2 types of information: the goal (grasping) and the intention underlying it (eg grasping for drinking). Boria et al (2009) examined whether children with ASD are able to understand these 2 aspects of motor actions.35 In their study, one group of high‐functioning children with ASD and one of TD children were presented with pictures showing hand‐object interactions and asked what the individual was doing and why. In half of the “why” trials, the observed grip was congruent with the function of the object (“why‐use” trials); in the other half it corresponded to the grip typically used to move that object (“why‐place” trials). The results showed that children with ASD have no difficulties in reporting the goals of individual motor acts. In contrast, they made several errors in the why task with all errors occurring in the “why‐place” trials. Thus, children with ASD have no deficit in the second type of understanding (ie, what action is being executed or performed), while they have difficulties in understanding others’ intentions when they have to rely exclusively on motor cues.35
Some authors argue for the involvement of the MNS in understanding what action to perform and the purpose of producing the action.36, 37 However, the mirror neuron theory of action understanding has also been criticized.38, 39, 40 Hickock and Hauser (2010) argue that the interpretation of mirror neurons as supporting action understanding was a wrong turn at the start, and that a more appropriate interpretation was lying in wait with respect to sensorimotor learning.40 They suggest interpreting “mirror neurons” as sensorimotor association cells relevant to action selection, just like object‐oriented cells. According to their point of view, empirical findings favor the sensorimotor account by showing that action understanding and motor system function dissociate, that motor actions alone are insufficient to explain action understanding, and that animals comprehend many actions that they cannot execute.38, 40
Conversely, other researchers cast doubt on one of this critique by demonstrating a correlation between the activity of the MNS and the observed action of the monkeys’ extrapersonal space, rather than their peripersonal space.41 These findings demonstrate that neuronal responses to an object rely on the actual possibility for the monkey to interact with the observed stimulus.
A further line of evidence comes from studies transiently disrupting cortical excitability by repetitive transcranial magnetic stimulation (rTMS). Remarkably, it has been shown that people are less accurate in identifying hand action goals after receiving continuous theta‐burst stimulation (cTBS) over the hand area than after receiving cTBS over the lip area of the left ventral premotor cortex (PMv), whereas they are less accurate in identifying mouth action goals after receiving cTBS over the lip area than over the hand area.42 Given that the application of cTBS over motor areas diminishes the excitability of cortical tissues, these findings suggest that PMv might have a causal role in understanding others’ actions.43
In their review article, Rizzolatti & Sinigaglia conclude that the mirror mechanism might allow others’ actions, emotions, or vitality forms to be understood from the inside.43 However, this does not imply that the mirror‐based processing is enough to fully understand them. Fully understanding an action, an emotion, or a vitality form is a multilevel process. The first level involves identifying which outcome is the goal of the observed action and which emotion or vitality form other individuals are displaying. Further levels may involve representing others’ mental states (for example, beliefs, desires, intentions, and so on). Reasoning about others’ mental states mainly activates a putative “mind‐reading network” that is formed by the prefrontal cortex (PFC), anterior cingulate cortex (ACC), and the temporoparietal junction (TPJ).44, 45, 46
4. SOCIAL DECISION MAKING IN ASDS
Autism Spectrum Disorders are associated with deficits in social‐emotional reciprocity, social communication, and social interaction across multiple contexts (see Table 1). Remarkably, several brain regions shown to be affected in ASD have also been shown to be involved in decision making. Neurobiological theories of ASD emphasize functional abnormalities in the amygdala, prefrontal cortex, superior temporal sulcus, and fusiform gyrus; together, these regions are thought to comprise the “social brain”.47, 48, 49 An overlapping set of regions, including the amygdala, prefrontal cortex, and ventral striatum, are implicated in decision making according to both functional neuroimaging 50 and lesion studies.51, 52
Previous studies have also shown that autonomic arousal differs in ASD patients relative to controls.53, 54 For example, Hirstein et al (2001) found that an autistic group did not show difference in skin conductance responses (SCRs) when making eye contact with their mothers compared with looking at a cup, whereas the control group showed significantly greater SCRs during eye contact.54
Johnson et al (2006) examined choice behavior and SCRs during the Iowa Gambling Task (IGT) in young adults with Asperger's disorder (ASP) and healthy controls.55 This decision making task requires participants to learn to make advantageous choices on the basis of feedback in the form of monetary gains and losses.
Consistent with the known heterogeneity in ASD, the authors found a subgroup of ASD participants (40%) which attended only to losses and demonstrated very low choice consistency, ie individuals in this subgroup were more motivated by avoidance of negative outcomes than by positive reinforcement. Moreover, SCR results revealed reduced responsiveness in the ASP group during the IGT compared with the control group.55 Further studies have also shown that participants with ASDs frequently switch between their decisions regardless of whether the outcome of the decision is positive or negative.56, 57 Therefore, participants with ASDs do not modulate their choice despite their understanding of the motivating purpose of the stimuli.
When viewing emotional expressions, adults spontaneously and quickly activate congruent facial muscles (ie they smile to a smile and scowl to a scowl). Automatic mimicry of facial expressions occurs even when expressions are presented without instructions to mimic and facilitates social interaction, supporting interpersonal rapport, emotional contagion, and emotion recognition.58, 59 Using facial electromyography (EMG), McIntoch et al (2006) showed that ASD participants did not automatically mimic facial expressions, whereas TD participants did.58 In contrast, ASD participants and TD participants were equally successful on voluntary mimicry. Their data suggest that autism is associated with an impairment of a basic automatic social‐emotion process affecting emotional contagion and social cognition.58
Moreover, several studies have shown that individuals with ASD have not only deficits in recognizing emotional facial expressions60, 61, 62 but have also deficits in making a wide range of social judgments from faces, including decisions related to threat (such as judgments of approachability) and decisions not related to physical threat (such as judgments of intelligence).60, 63
Along with the understanding of the goal of an action (“what” is done) and the intention underlying it (“why” it is done),35 social interactions largely depend on the appraisal of the action from the dynamics of the movement: “how” it is performed (its “vitality form”).64 Action dynamics enable the observer to understand the cognitive/emotional state of the agent of the performed action. For instance, a minute variation in the temporal contour, force, or direction of the actions may help the observer understand whether the agent is gentle or angry, whether he or she performs the action willingly or hesitating, and so on. The dynamics of action carrying this kind of information is called “vitality forms”.65
Rochat et al (2013) show that, unlike TD individuals, individuals with autism reveal severe deficits in recognizing vitality forms, and their capacity to appraise them does not improve with age.64
Further studies have combined fMRI with the Facial Action Coding System (FACS;66) and revealed the somatotopic organization of emotional facial expressions in the brain.67 Pathophysiological alteration of the cortical excitability associated with deficits in recognizing facial emotional expressions and theory of mind skills in neuropsychiatric disorders has been shown using TMS‐based motor‐evoked potentials and functional magnetic resonance imaging.44, 68, 69
Taken together, our ability to understand (i) the social context, (ii) emotions of others, (iii) the intent of the motor actions, and (iv) the vitality forms is necessary to make appropriate social decisions.
Despite these remarkable findings, it remains unclear whether there is a link between social decision making and the impairment of the MNS. Approaching this question remains elusive due to our limited knowledge of the neural mechanisms involved in the interaction between the MNS, motor control, and social decision making. Based on the current knowledge, we assume that participants with ASDs experience (i) MNS impairments, (ii) deficits in automatic mimicry of facial expressions but not in voluntary mimicry, (iii) deficits in recognizing emotional facial expressions, (iv) deficits in understanding action intention and its vitality form, which may overall cause (v) severe deficits in social interaction and social decision making. It is therefore plausible to assume a neurophysiological interaction between the pathways responsible for the MNS, imitative behavior, motor action, and social decision making.
Based on the current studies, we suggest a multilayer neural network model including the MNS on a first layer and transforming this information to a higher layer network responsible for reasoning (cf. Figure 1). Reasoning about others’ mental states mainly activates a putative “mind‐reading network” that is formed by the prefrontal cortex, anterior cingulate cortex (ACC), and the temporoparietal junction (TPJ).43, 44, 45, 46, 70 Future studies with ASD participants combining behavioral tasks with neuroimaging methods and transcranial brain stimulation as well as computational modeling can help validate and complement this suggested model.
Figure 1.
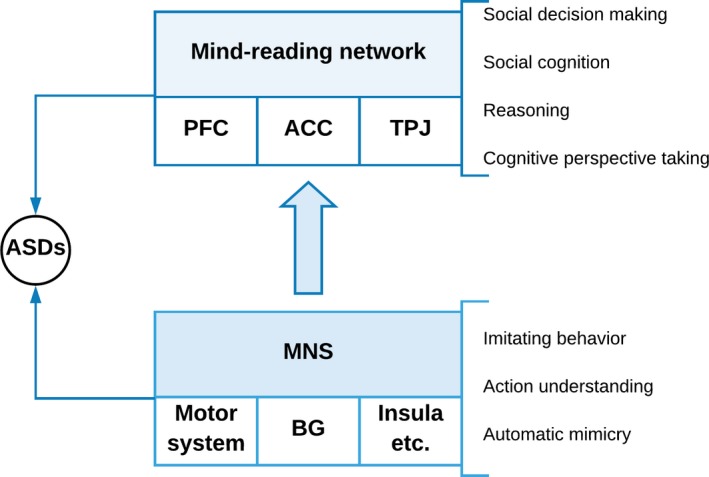
A schematic sketch of the proposed dynamical interaction between Autism Spectrum Disorders (ASDs), Social Decision Making, and the Mirror Neuron System (MNS). The proposed multilayer neural network model includes the MNS on a first layer and transforming this information to a higher layer network responsible for reasoning. The MNS involves the motor system, the basal ganglia (BG), the insula, and other brain regions revealing the mirror neuron mechanism. Reasoning about others’ mental states mainly activates a putative “mind‐reading network” that is formed by the prefrontal cortex, the anterior cingulate cortex (ACC), and the temporoparietal junction (TPJ)43, 44, 45, 46, 70
5. USING BEHAVIORAL PARADIGMS COMBINED WITH NEUROPHYSIOLOGICAL MARKERS FOR TREATMENT EVALUATION OF PATIENTS WITH ASDS
We agree with Rizzolati et al's assumption that psychiatry will be one of the most promising fields in the research of the MNS.19 Therapeutic options to improve clinical deficits in ASDs include pharmacological interventions71, 72 cognitive behavioral therapy,44, 73 and experimental approaches using noninvasive transcranial brain stimulation.74, 75 In psychiatric departments, diagnosis and treatment evaluation of ASDs is usually based on the DSM V or the ICD‐10 as well as psychometric scales.73 However, the specific behavioral paradigms and the neurophysiological markers mentioned in this review article are usually ignored. We therefore propose applying the behavioral paradigms and the neurophysiological markers mentioned in this review article for evaluating the treatment process. Table 3 provides an overview of the proposed behavioral and neurophysiological measures. Moreover, the investigation of modulating effects of different treatment approaches on the neurophysiological markers of the MNS can help find specific subgroups of patients and support tailored psychiatric interventions, eg, investigating on a neurophysiological level which patients show EEG‐based mu rhythm alterations,23, 24, 29 or hypoactivity of the prefrontal cortex during theory of mind tasks based on neuroimaging 26, 44 or reduced automatic mimicry of facial expressions using facial electromyography.58, 59 Taking the wide range of ASDs into account, future studies investigating the effects of specific treatment approaches on specific neurophysiological markers of the MNS might reveal diagnostic subgroups of ASDs patients. Thus, each neurophysiologically defined subgroup might need a specific treatment approach.
Table 3.
Overview of useful behavioral and neurophysiological markers for the evaluation of psychiatric treatment approaches of autism spectrum disorders (ASD) patients
| Behavioral paradigms | Neurophysiological markers |
|---|---|
|
Action observation and imitative behavior (eg imitating finger movements)17
Understanding the intention of actions35 Recognizing vitality forms of actions.64 |
fMRI‐based BOLD response in brain regions during observation and imitation17, 19
EEG‐based mu rhythm alterations21, 22, 23 MEG‐based power increase in beta activity (beta rebound, 21) TMS‐based measurement of motor cortical excitability by investigating modulations of motor‐evoked potentials (MEPs)27, 28 Disrupting motor cortical excitability by rTMS43 |
| Automatic mimicry of facial expressions58, 59 | Facial electromyography (EMG)58, 59 |
|
Choice behavior during the Iowa Gambling task55
Making eye contact with a close relative compared with looking at a cup54 |
Skin conductance response (SCR) 54, 55 |
|
Recognition of facial expressions44, 60, 62
Social judgments based on facial perception60, 63 |
fMRI‐based BOLD response (eg in the Amygdala and medial prefrontal cortex44, 62, 63
EEG‐based coherence analyses45, 77 |
6. CONCLUDING REMARKS
In this review, we discuss the plausibility and empirical evidence of neural interaction between the MNS, action perception, imitative behavior, and their impact on social decision making in ASDs. Several studies in humans using TMS of the motor cortex, EEG‐based mu rhythm, and other neuroimaging techniques as well as facial electromyography convincingly demonstrate the involvement of the MNS in action understanding and imitative behavior (for a review see 43, 76). However, in ASDs, also impairment of higher cognitive functions such as theory of mind skills and complex social cognition has been observed (for a review see 44). Based on the current studies, we therefore suggest a multilayer neural network model including the MNS on a first layer and transforming this information to a higher layer network responsible for reasoning (cf. Figure 1). Reasoning about others’ mental states mainly activates a putative “mind‐reading network” that is formed by the prefrontal cortex, anterior cingulate cortex (ACC), and the temporoparietal junction (TPJ).43, 44, 45, 46, 70 Future studies with ASD participants combining behavioral tasks with neuroimaging methods and transcranial brain stimulation as well as computational modeling can help validate and complement this suggested model. Moreover, we propose applying the behavioral paradigms and the neurophysiological markers mentioned in this review article for evaluating psychiatric treatment approaches in ASDs (see Table 3). The investigation of modulating the effects of different pharmacological, neurostimulation, or psychotherapeutic treatment approaches on the neurophysiological markers of the MNS can help find specific subgroups of ASDs patients and support tailored psychiatric interventions.
Khalil R, Tindle R, Boraud T, Moustafa AA, Karim AA. Social decision making in autism: On the impact of mirror neurons, motor control, and imitative behaviors. CNS Neurosci Ther. 2018;24:669–676. 10.1111/cns.13001
Contributor Information
Radwa Khalil, Email: radwakhalil@hotmail.com.
Ahmed A. Karim, Email: ahmed.karim@uni-tuebingen.de
REFERENCES
- 1. DiCicco‐Bloom E, Lord C, Zwaigenbaum L, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897‐6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103‐111. [DOI] [PubMed] [Google Scholar]
- 3. Hamilton AFDC. Cognitive underpinnings of social interaction. Q J Exp Psychol. 2015;68:417‐432. [DOI] [PubMed] [Google Scholar]
- 4. American Psychiatric Association . Task Force on DSM 5. Diagnostic and Statistical Manual of Mental Disorders DSM‐5. DSM‐5. Philadelphia: American Psychiatric Association; 2013. [Google Scholar]
- 5. Hadjikhani N, Joseph RH, Snyder J, Tager‐Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28:441‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nebel MB, Joel SE, Muschelli J, et al. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 2014;35:567‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Staples KL, Reid G. Fundamental movement skills and autism spectrum disorders. J Autism Dev Disord. 2010;40:209‐217. [DOI] [PubMed] [Google Scholar]
- 8. Breveglieri R, Galletti C, Gamberini M, Passarelli LFP. Somatosensory cells in area PEc of macaque posterior parietal cortex. J Neurosci. 2006;26:3679‐3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269‐278. [DOI] [PubMed] [Google Scholar]
- 10. Frith U. Autism Explaining the Enigma. Hoboken, NJ: Blackwell Publishing; 2003. [Google Scholar]
- 11. Müller RA, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Abnormal variability and distribution of functional maps in autism: An fMRI study of visuomotor learning. Am J Psychiatry. 2003;160:1847‐1862. [DOI] [PubMed] [Google Scholar]
- 12. Hadjikhani N, Joseph RM, Snyder J, Tager‐Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276‐1282. [DOI] [PubMed] [Google Scholar]
- 13. Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25:287‐295. [DOI] [PubMed] [Google Scholar]
- 14. Osterling JA, Dawson G, Munson JA. Early recognition of 1‐year‐old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14:239‐251. [DOI] [PubMed] [Google Scholar]
- 15. Bolis D, Schilbach L. Observing and participating in social interactions: action perception and action control across the autistic spectrum. Dev Cogn Neurosci. 2017;29:168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piaget J. Play, Dreams and Imitation in Childhood. New York: Norton; 1962. [Google Scholar]
- 17. Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526‐2528. [DOI] [PubMed] [Google Scholar]
- 18. di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176‐180. [DOI] [PubMed] [Google Scholar]
- 19. Rizzolatti G, Cattaneo L, Fabbri‐Destro M, Rozzi S. Cortical mechanisms underlying the organization of goal‐directed actions and mirror neuron‐based action understanding. Physiol Rev. 2014;94:655‐706. [DOI] [PubMed] [Google Scholar]
- 20. Jeannerod M. Oxford psychology series. Motor cognition: What actions tell the self. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 21. Honaga E, Ishii R, Kurimoto R, et al. Post‐movement beta rebound abnormality as indicator of mirror neuron system dysfunction in autistic spectrum disorder: an MEG study. Neurosci Lett. 2010;478:141‐145. [DOI] [PubMed] [Google Scholar]
- 22. Bimbi M, Festante F, Coudé G, Vanderwert RE, Fox NA, Ferrari PF. Simultaneous scalp recorded EEG and local field potentials from monkey ventral premotor cortex during action observation and execution reveals the contribution of mirror and motor neurons to the mu‐rhythm. NeuroImage. 2018;175:22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braadbaart L, Williams JHG, Waiter GD. Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? Int J Psychophysiol. 2013;89:99‐105. [DOI] [PubMed] [Google Scholar]
- 24. Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn Brain Res. 2005;24:190‐198. [DOI] [PubMed] [Google Scholar]
- 25. Avenanti A, Annella L, Candidi M, Urgesi C, Aglioti SM. Compensatory plasticity in the action observation network: virtual lesions of STS enhance anticipatory simulation of seen actions. Cereb Cortex. 2013;23:570‐580. [DOI] [PubMed] [Google Scholar]
- 26. Schulte‐Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face‐to‐face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19:1345‐1372. [DOI] [PubMed] [Google Scholar]
- 27. Théoret H, Halligan E, Kobayashi M, Fregni F, Tager‐Flusberg H, Pascual‐Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol. 2005;15:R84‐R85. [DOI] [PubMed] [Google Scholar]
- 28. Enticott PG, Kennedy HA, Rinehart NJ, et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry. 2012;71:427‐433. [DOI] [PubMed] [Google Scholar]
- 29. Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: is the mirror neuron system involved? Int J Psychophysiol. 2008;68:35‐40. [DOI] [PubMed] [Google Scholar]
- 30. Ramachandran VS, Oberman LM. Broken mirrors: a theory of autism. Sci Am. 2006;17:20‐29. [DOI] [PubMed] [Google Scholar]
- 31. Kobayashi H. Mimicry as social glue: spontaneous mimicry in autism spectrum disorder. Jpn Psychol Rev. 2007;50:89‐95. [Google Scholar]
- 32. Hess U, Fischer A. Emotional mimicry as social regulation. Pers Soc Psychol Rev. 2013;17:142‐157. [DOI] [PubMed] [Google Scholar]
- 33. Smith IM, Bryson SE. Imitation and action in autism: a critical review. Psychol Bull. 1994;116:259‐273. [DOI] [PubMed] [Google Scholar]
- 34. Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653‐670. [DOI] [PubMed] [Google Scholar]
- 35. Boria S, Fabbri‐Destro M, Cattaneo L, et al. Intention understanding in autism. PLoS ONE. 2009;4:e5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;4:e5596. [DOI] [PubMed] [Google Scholar]
- 37. Rizzolatti G, Fabbri‐Destro M, Cattaneo L. Mirror neurons and their clinical relevance. Nat Clin Pract Neurol. 2009;5:24‐34. [DOI] [PubMed] [Google Scholar]
- 38. Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J Cogn Neurosci. 2009;21:1229‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hickok G. Do mirror neurons subserve action understanding? Neurosci Lett. 2013;540:56‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hickok G, Hauser M. (Mis)understanding mirror neurons. Curr Biol. 2010;20:R593‐R594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G. Ventral premotor neurons encoding representations of action during self and others’ inaction. Curr Biol. 2014;24:1611‐1614. [DOI] [PubMed] [Google Scholar]
- 42. Michael J, Sandberg K, Skewes J, et al. Continuous theta‐burst stimulation demonstrates a causal role of premotor homunculus in action understanding. Psychol Sci. 2014;25:963‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rizzolatti G, Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci. 2016;17:757‐765. [DOI] [PubMed] [Google Scholar]
- 44. Krippl M, Karim AA. “Theory of mind” and its neuronal correlates in forensically relevant disorders. Nervenarzt. 2011;82:843‐852. [DOI] [PubMed] [Google Scholar]
- 45. Daltrozzo J, Kotchoubey B, Gueler F, Karim AA. Effects of transcranial magnetic stimulation on body perception: no evidence for specificity of the right temporo‐parietal junction. Brain Topogr. 2016;29:704‐715. [DOI] [PubMed] [Google Scholar]
- 46. Corradi‐Dell'Acqua C, Hofstetter C, Vuilleumier P. Cognitive and affective theory of mind share the same local patterns of activity in posterior temporal but not medial prefrontal cortex. Soc Cogn Affect Neurosci 2014;9:1175‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baron‐Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355‐364. [DOI] [PubMed] [Google Scholar]
- 48. Brothers L. Brain mechanisms of social cognition. J Psychopharmacol. 1996;10:2‐8. [DOI] [PubMed] [Google Scholar]
- 49. Schultz RT, Romanski LM, Tsatsanis KD. Neurofunctional models of autistic disorder and Asperger syndrome: clues from neuroimaging. Asperger Syndr. 2000;1:172‐209. [Google Scholar]
- 50. Ernst M, Bolla K, Mouratidis M, et al. Decision‐making in a risk‐taking task: a PET study. Neuropsychopharmacology. 2002;26:682‐691. [DOI] [PubMed] [Google Scholar]
- 51. Bar‐On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence In: Cacioppo JT, Berntson GG, eds. Social Neuroscience: key Readings. New York, NY: Psychology Press; 2003. [DOI] [PubMed] [Google Scholar]
- 52. Bechara A. The role of emotion in decision‐making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30‐40. [DOI] [PubMed] [Google Scholar]
- 53. Althaus M, Van Roon AM, Mulder LJM, Mulder G, Aarnoudse CC, Minderaa RB. Autonomic response patterns observed during the performance of an attention‐demanding task in two groups of children with autistic‐type difficulties in social adjustment. Psychophysiology. 2004;41:893‐904. [DOI] [PubMed] [Google Scholar]
- 54. Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proc R Soc B Biol Sci. 2001;268:1883‐1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson SA, Yechiam E, Murphy RR, Queller S, Stout JC. Motivational processes and autonomic responsivity in Asperger's disorder: evidence from the Iowa Gambling Task. J Int Neuropsychol Soc. 2006;12:668‐676. [DOI] [PubMed] [Google Scholar]
- 56. Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16:237‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shafritz KM, Bregman JD, Ikuta T, Szeszko PR. Neural systems mediating decision‐making and response inhibition for social and nonsocial stimuli in autism. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:1112‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McIntosh DN, Reichmann‐Decker A, Winkielman P, Wilbarger JL. When the social mirror breaks: deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Dev Sci. 2006;9:295‐302. [DOI] [PubMed] [Google Scholar]
- 59. Dimberg U. Facial reactions to facial expressions. Psychophysiology. 1982;19:643‐647. [DOI] [PubMed] [Google Scholar]
- 60. Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232‐240. [DOI] [PubMed] [Google Scholar]
- 61. Hobson RP, Ouston J, Lee A. What's in a face? The case of autism. Br J Psychol. 1988;79:441‐453. [DOI] [PubMed] [Google Scholar]
- 62. Howard MA, Cowell PE, Boucher J, et al. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. NeuroReport. 2000;11:2931‐2935. [DOI] [PubMed] [Google Scholar]
- 63. Hall J, Whalley HC, McKirdy JW, et al. A common neural system mediating two different forms of social judgement. Psychol Med. 2010;40:1183‐1192. [DOI] [PubMed] [Google Scholar]
- 64. Rochat MJ, Veroni V, Bruschweiler‐Stern N, et al. Impaired vitality form recognition in autism. Neuropsychologia. 2013;51:1918‐1924. [DOI] [PubMed] [Google Scholar]
- 65. Stern DN. Forms of Vitality: Exploring Dynamic Experience in Psychology, The Arts, Psychotherapy, and Development. Case studies in clinical psychological science: bridging the gap from science to practice. Oxford: Oxford University Press; 2010. [Google Scholar]
- 66. Ekman P, Friesen WV, Hager JC. Facial Action Coding System. Manual and Investigator's Guide. Salt Lake City, UT: Research Nexus; 2002. [Google Scholar]
- 67. Krippl M, Karim AA, Brechmann A. Neuronal correlates of voluntary facial movements. Front Hum Neurosci. 2015;9:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karim A, Schneider M, Kipping J, et al. Psychopathic traits and the psychophysiology of deception: a transcranial direct current stimulation (tDCS) study. Clin Neurophysiol. 2013;124:e57‐e58. [Google Scholar]
- 69. Khedr EM, El Fetoh NA, El Bieh E, Ali AM, Karim AA. Altered cortical excitability in anorexia nervosa. Neurophysiol Clin. 2014;44:291‐299. [DOI] [PubMed] [Google Scholar]
- 70. de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454‐457. [DOI] [PubMed] [Google Scholar]
- 71. Riikonen R. Treatment of autistic spectrum disorder with insulin‐like growth factors. Eur J Paediatr Neurol. 2016;20:816‐823. [DOI] [PubMed] [Google Scholar]
- 72. Deb S, Farmah BK, Arshad E, Deb T, Roy M, Unwin GL. The effectiveness of aripiprazole in the management of problem behaviour in people with intellectual disabilities, developmental disabilities and/or autistic spectrum disorder ‐ A systematic review. Res Dev Disabil. 2014;35:711‐725. [DOI] [PubMed] [Google Scholar]
- 73. Matson JL, ed. Handbook of Assessment and Diagnosis of Autism Spectrum Disorder. Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 74. Nobusako S, Nishi Y, Nishi Y, et al. Transcranial direct current stimulation of the temporoparietal junction and inferior frontal cortex improves imitation‐inhibition and perspective‐taking with no effect on the autism‐spectrum quotient score. Front Behav Neurosci. 2017;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gómez L, Vidal B, Maragoto C, et al. Non‐invasive brain stimulation for children with autism spectrum disorders: a short‐term outcome study. Behav Sci (Basel). 2017;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rizzolatti G, Fogassi L. The mirror mechanism: recent findings and perspectives. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130420‐20130420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jaime M, McMahon CM, Davidson BC, Newell LC, Mundy PC, Henderson HA. Brief report: reduced temporal‐central EEG alpha coherence during joint attention perception in adolescents with autism spectrum disorder. J Autism Dev Disord. 2016;46:1477‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]


