Abstract
The multinational, observational CVD‐REAL study recently showed that initiation of sodium‐glucose co‐transporter‐2 inhibitors (SGLT‐2i) was associated with significantly lower rates of death and heart failure vs other glucose‐lowering drugs (oGLDs). This sub‐analysis of the CVD‐REAL study sought to determine the association between initiation of SGLT‐2i vs oGLDs and rates of myocardial infarction (MI) and stroke. Medical records, claims and national registers from the USA, Sweden, Norway and Denmark were used to identify patients with T2D who newly initiated treatment with SGLT‐2i (canagliflozin, dapagliflozin or empagliflozin) or oGLDs. A non‐parsimonious propensity score was developed within each country to predict initiation of SGLT‐2i, and patients were matched 1:1 in the treatment groups. Pooled hazard ratios (HRs) and 95% CIs were generated using Cox regression models. Overall, 205 160 patients were included. In the intent‐to‐treat analysis, over 188 551 and 188 678 person‐years of follow‐up (MI and stroke, respectively), there were 1077 MI and 968 stroke events. Initiation of SGLT‐2i vs oGLD was associated with a modestly lower risk of MI and stroke (MI: HR, 0.85; 95%CI, 0.72‐1.00; P = .05; Stroke: HR, 0.83; 95% CI, 0.71‐0.97; P = .02). These findings complement the results of the cardiovascular outcomes trials, and offer additional reassurance with regard to the cardiovascular effects of SGLT‐2i, specifically as it relates to ischaemic events.
Keywords: cardiovascular disease, observational study, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Despite advances in prevention, cardiovascular disease remains the leading cause of mortality and morbidity in patients with type 2 diabetes (T2D). Two large randomized controlled trials (RCTs) of sodium‐glucose co‐transporter‐2 inhibitors (SGLT‐2i; empagliflozin and canagliflozin) have shown significant reductions in major adverse cardiac events, as well as in hospitalizations for heart failure.1, 2 The rates of non‐fatal myocardial infarction (MI) were numerically lower with both empagliflozin and canagliflozin vs placebo; the point estimates for non‐fatal stroke numerically favoured placebo vs empagliflozin in the EMPA‐REG OUTCOME trial, and canagliflozin vs placebo in the CANVAS Program, although none of these differences was statistically significant.1, 2
Recently, the The Comparative Effectiveness of Cardiovascular Outcomes (CVD‐REAL) study, a multinational, observational study of over 300 000 patients, found that initiation of SGLT‐2i was associated with a significant reduction in death and heart failure when compared to other glucose‐lowering drugs (oGLDs).3 Observational data from Nordic countries (CVD‐REAL Nordic) has shown non‐significant point estimates in favour of SGLT‐2i for MI and stroke, using somewhat different statistical methods, and with dapagliflozin dominating the SGLT‐2i group.4, 5 However, the effects of SGLT‐2i on atherothrombotic events in the larger CVD‐REAL cohort, including patients from the USA, with a broader representation of SGLT‐2i compounds, have not been explored previously. Accordingly, in this analysis of global CVD‐REAL data, we sought to determine the association between initiation of SGLT‐2i vs oGLDs and MI and stroke events.
2. METHODS
The CVD‐REAL study design has been described previously.3 For this analysis, adult patients with T2D who newly initiated treatment with SGLT‐2i (canagliflozin, dapagliflozin or empagliflozin) or oGLDs were identified from medical records, claims and national registers collected from 4 countries (USA, Sweden, Norway and Denmark). Because of small numbers of patients and events in Germany and the United Kingdom, we elected not to include data from these countries in this analysis. A non‐parsimonious propensity score was developed separately within each country to predict the likelihood of receiving a prescription for an SGLT‐2i, and patients were matched 1:1 in the 2 treatment groups. In the main on‐treatment analysis, patients were followed from the index date (initiation of the SGLT‐2i or oGLD) until completion of treatment, occurrence of an outcome event, death or censoring. The endpoints of interest were time to MI and stroke. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) for each endpoint were generated using Cox regression models, with inverse variance weighting for each country. In a sensitivity analysis, an intent‐to‐treat (ITT) approach was used, in which patients were followed after discontinuation of index treatment. Analyses of de‐identified data were conducted in accordance with local laws and regulations, and received approvals from the respective Scientific/Ethics/Data Protection Committees.
3. RESULTS
After propensity‐score matching, 205 160 patients were included in the analysis (102 580 in each group), and baseline characteristics were well balanced between the 2 groups. Among participants, the mean age was 57 years, 43% were female, 14% had documented cardiovascular disease before SGLT‐2i or oGLD initiation. In the SGLT‐2i group, of the total exposure time, 49% of patients received dapagliflozin, 44% canagliflozin and 7% empagliflozin. There was significant geographical variation with regard to the specific SGLT‐2i used, with canagliflozin used predominately (75%) in the USA, and dapagliflozin used predominately (90%) in Europe. In patients initiating treatment with oGLDs, the most commonly used classes were insulin (34%), dipeptidyl peptidase‐4 (DPP‐4) inhibitors (18%), sulfonylureas (17%), glucagon‐like peptide‐1 receptor agonists (14%) and metformin (12%).
For the on‐treatment analysis, the mean follow‐up time was 254 days in the SGLT‐2i group and 232 days in the oGLD group. Over 136 524 and 136 626 person‐years of follow‐up (MI and stroke, respectively), there were 779 MIs and 674 strokes (event rate [ER], 0.57/ and 0.49/100 person‐years, respectively). Initiation of treatment with SGLT‐2i vs oGLD was associated with a lower risk of MI and stroke (MI: ER, 0.49/100 person‐years for SGLT‐2i vs 0.66/100 person‐years for oGLD; HR, 0.78; 95% CI, 0.65‐0.95; P = .01 [Figure 1A]; Stroke: ER, 0.42/100 person‐years for SGLT‐2i vs 0.58/100 person‐years for oGLD; HR, 0.80; 95%CI, 0.66‐0.97; P = .02) [Figure 1B], with no evidence of heterogeneity by country.
Figure 1.
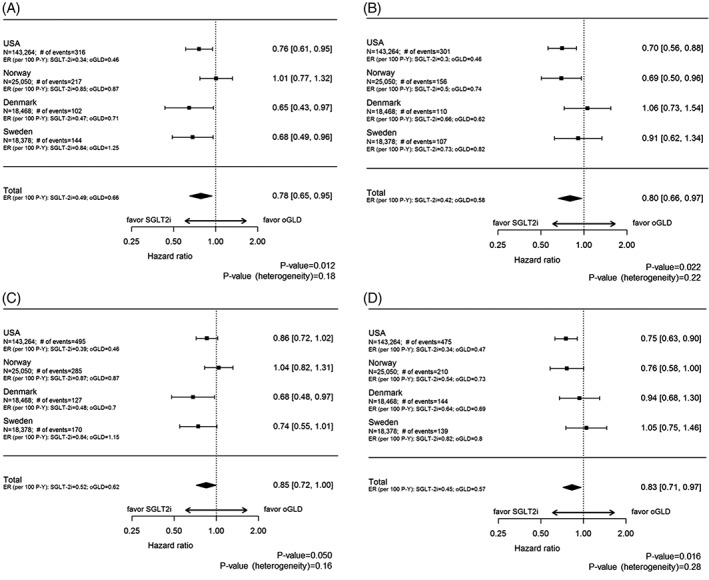
Event rates, unadjusted hazard ratios and 95% CIs for acute myocardial infarction (A) and stroke (B) in the on‐treatment population, and for acute myocardial infarction (C) and stroke (D) in the ITT population. Abbreviations: ER, event rate; oGLD, other glucose‐lowering drug; P‐Y, person‐years; SGLT‐2i, sodium‐glucose transporter‐2 inhibitors
In the ITT analysis, mean follow‐up time was 339 days in the SGLT‐2i group and 332 days in the oGLD group. Over 188 551 and 188 678 person‐years of follow‐up (MI and stroke, respectively), there were 1077 MIs and 968 strokes (ER, 0.57/ and 0.51/100 person‐years, respectively). Initiation of treatment with SGLT‐2i vs oGLD was associated with a lower risk of MI and stroke (MI: ER, 0.52/100 person‐years for SGLT‐2i vs 0.62/100 person‐years for oGLD; HR, 0.85; 95% CI, 0.72‐1.00; P = .05; [Figure 1C]; Stroke: ER, 0.45/100 person‐years for SGLT‐2i vs 0.57/100 person‐years for oGLD; HR, 0.83; 95% CI, 0.71‐0.97; P = .02 [Figure 1D]), with no evidence of heterogeneity by country.
4. DISCUSSION
In summary, in this multinational study involving over 200 000 patients, seen within real‐world clinical practice, with a very large number of ischaemic events, initiation of treatment with SGLT‐2i vs oGLD was associated with modestly lower rates of MI and stroke. Although our patient population differed from the EMPA‐REG OUTCOME trial and the CANVAS Program, with a lower prevalence of cardiovascular disease, and thus lower event rates, our results were directionally and numerically consistent with both studies with respect to MI,1, 2 with a difference in width of the confidence intervals that is probably related to the greater absolute number of events in our study. Our data were also directionally and numerically consistent with the CANVAS Program with respect to stroke.1 Our study offers important incremental information with regard to the association between SGLT‐2i use and atherothrombotic events, including MI and stroke, in a broad population of patients with T2D from routine clinical practice. Although prior observational data from Nordic countries (CVD‐REAL Nordic) examined these relationships, and showed numerically lower, non‐significant point estimates for MI and stroke favouring SGLT‐2i vs oGLDs, as well as vs DPP‐4 inhibitors, those investigations evaluated smaller patient samples and numbers of events, used somewhat different statistical approaches, and dapagliflozin dominated the SGLT‐2i group.4, 5 Our study substantially expands these findings in the much larger CVD‐REAL cohort, with a greater number of events, with patients from the USA, and with a broader representation of SGLT‐2i compounds. Collectively, our findings complement the results of the completed cardiovascular outcomes trials of SGLT‐2i, and prior observational analyses, and offer additional reassurance with regard to the cardiovascular effects of SGLT‐2i, specifically as it relates to ischaemic events, especially stroke, for which concerns had been raised previously based on a small numerical excess of stroke events with empagliflozin vs placebo, which was not statistically significant.2
The results of our study should be considered in the context of several potential limitations. First, given the observational nature of the analyses, and despite robust statistical techniques, including 1:1 propensity matching, a possibility of residual, unmeasured confounding cannot be definitively excluded. Second, despite a large number of accrued patient‐years of follow up, the average duration of follow‐up was relatively limited, as SGLT‐2i use in real‐world practice is still recent; longer‐term follow‐up will be needed to evaluate whether effects are sustained over time. Finally, given that CVD‐REAL is a large, multinational pharmaco‐epidemiologic comparative effectiveness study, it was not designed to examine the potential mechanisms linking the use of SGLT‐2i and associated cardiovascular benefits. However, it is highly unlikely that glucose lowering per se is behind the lower risk of cardiovascular events. As an example, prior analyses from the EMPA‐REG OUTCOME trial have demonstrated little mediation effect of HbA1c on the cardiovascular benefits of empagliflozin.6 Potential mechanisms may involve reductions in oxidative stress, improvement in endothelial function, neuro‐hormonal modulation and anti‐inflammatory effects, among others.7, 8, 9, 10, 11 A metabolic hypothesis has also been proposed, suggesting that a shift in myocardial metabolism from glucose and free fatty acids to ketones may contribute to the cardiovascular benefits of SGLT‐2i.12 Importantly, this knowledge gap is being examined by mechanistic investigations across the SGLT‐2i class, with more information forthcoming in the near future.
Supporting information
CVD‐REAL Investigator and Study Group
ACKNOWLEDGMENTS
Data from Norway were obtained from the Norwegian Cause of Death Registry, the Norwegian Patient Registry and The Norwegian Prescription Registry. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian patient register is intended, nor should be inferred. This study is based, in part, on data from the Clinical Practice Research Datalink (CPRD) obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the National Health Service as part of their care and support. This CPRD study also used data from the Office for National Statistics (ONS), provided by ONS, and from the Hospital Episode Statistics (HES); Copyright © 2018, re‐used with permission of The Health & Social Care Information Centre (all rights reserved). The study was approved by the Independent Scientific Advisory Committee (ISAC) of CPRD; protocol 16_064RAR. Data from The Health Improvement Network (THIN) from the UK was also used and the independent Scientific Review Committee (SRC) approved the study (protocol 16THIN027A1). The interpretation and conclusions contained in this study are those of the authors alone.
Editorial support was provided by Nicola Truss PhD of inScience Communications, Springer Healthcare, and was funded by AstraZeneca.
Conflict of interest
M. K. has received research grants from AstraZeneca and Boehringer Ingelheim; has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Sanofi, Glytec, Novo Nordisk, ZS Pharma, Janssen, Merck (Diabetes) and Novartis; has served as a consultant for AstraZeneca, Boehringer Ingelheim, Sanofi, GSK, Janssen, Intarcia, Merck (Diabetes), Novo Nordisk, Glytec and ZS Pharma. K. I. B. has received grants to his institution from AstraZeneca for this study and fees for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. M. A. C. has received personal fees from AstraZeneca, Merck, Sanofi‐Aventis, Chiesi and research support (non‐salary) from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, The Medicines Company, Merck and Takeda. A. Z. F. has received grants from AstraZeneca and Merck and personal fees from Asclepius Analytics and Complete HEOR Solutions. J. P. W. has received lecture fees from Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, Orexigen, Sanofi; has received consultancy (Institutional) fees from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly and Orexigen; has received grants to his institution from Takeda, Novo Nordisk and AstraZeneca. K. K. has served as a consultant and speaker for AstraZeneca, Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Janssen and Boehringer Ingelheim; has received grants in support of investigator and investigator‐initiated trials from AstraZeneca, Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, Boehringer Ingelheim, Merck Sharp & Dohme and Roche; has served on advisory boards for Astra Zeneca, Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Janssen and Boehringer Ingelheim; is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC EM). R. W. H. has received grants from AstraZeneca. A. N. has received personal fees from AstraZeneca for this study and honoraria for lectures and advisory board meetings from Novo Nordisk, Boehringer Ingelheim and Lilly. M. E. J. is a shareholder in Novo Nordisk; was employed by Steno Diabetes Center A/S, a research hospital within the Danish National Health Service and owned by Novo Nordisk A/S, until December 31, 2016; and has received grants from AstraZeneca. M. T. is an employee of Statisticon, which was under contract with AstraZeneca for this study. J. B., E. T. W., N. H. and P. F. are employees of AstraZeneca.
Author contributions
M. K., N. H. and P. F. were involved in the study design and the protocol was finalized with contributions from all authors. N. H. and M. T. were involved in data collection and all authors were involved in analysis and interpretation of the data. M. K. wrote the manuscript and all authors reviewed and critically revised the draft. All authors provided final approval for submission.
Kosiborod M, Birkeland KI, Cavender MA, et al. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT2‐inhibitors versus other glucose‐lowering agents in real‐world clinical practice: Results from the CVD‐REAL study. Diabetes Obes Metab. 2018;20:1983–1987. 10.1111/dom.13299
Funding information This work was supported by AstraZeneca.
REFERENCES
- 1. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 3. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study. Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20:344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2007;5:709‐717. [DOI] [PubMed] [Google Scholar]
- 6. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41:356‐363. [DOI] [PubMed] [Google Scholar]
- 7. Inzucchi SE, Zinman B, Wanner C, et al. SGLT‐2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oelze M, Kroller‐Schon S, Welschof P, et al. The sodium‐glucose co‐transporter 2 inhibitor empagliflozin improves diabetes‐induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One. 2014;9:e112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752‐772. [DOI] [PubMed] [Google Scholar]
- 10. Marx N, McGuire DK. Sodium‐glucose cotransporter‐2 inhibition for the reduction of cardiovascular events in high‐risk patients with diabetes mellitus. Eur Heart J. 2016;37:3192‐3200. [DOI] [PubMed] [Google Scholar]
- 11. Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA‐REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a "Thrifty Substrate" hypothesis. Diabetes Care. 2016;39:1108‐1114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CVD‐REAL Investigator and Study Group


