Abstract
Heterogeneity in pluripotent stem cell (PSC) aggregation leads to variability in mass transfer and signaling gradients between aggregates, which results in heterogeneous differentiation and therefore variability in product quality and yield. We have characterized a chemical‐based method to control aggregate size within a specific, tunable range with low heterogeneity, thereby reducing process variability in PSC expansion. This method enables controlled, scalable, stirred suspension‐based manufacturing of PSC cultures that are critical for the translation of regenerative medicine strategies to clinical products.
Keywords: aggregation, bioreactor: pluripotent stem cell (PSC), process variability, stem cells
1. INTRODUCTION
Pluripotent stem cells (PSC) are a promising cell source for creating therapeutics because these cells can be expanded indefinitely and differentiated into all cell types in the body. Industrializing these therapies will require the development of scalable and controlled suspension bioreactors for PSC‐based cell therapy manufacturing. The main culture methods used to translate laboratory adherent culture (Figure 1a‐i) to suspension‐based expansion of PSC are as cell aggregates in stirred suspension (Figure 1a‐ii; Amit et al., 2011; Kehoe, Jing, Lock, & Tzanakakis, 2010; Zweigerdt, Olmer, Singh, Haverich, & Martin, 2011) or using microcarriers as a substrate for cell attachment (Figure 1a‐iii; Chen, Chen, Choo, Reuveny, & Oh, 2011; Oh et al., 2009; Phillips et al., 2008). Aggregate‐based expansion methods provide simplicity and a reduction in the number of processing steps required. Microcarriers have been adapted to bioreactor expansion as an adherent surface with a high surface area for cell growth (Oh et al., 2009), but adhesion and removal of cells remain challenging. Most aggregate PSC bioreactor expansion strategies to date rely on bulk seeding of single cells in suspension, which form aggregates with heterogeneous sizes. Aggregate size control has emerged as a critical parameter in understanding and directing cell fate transitions, as cells sense and respond to cues in their environment that are influenced by geometry both in vitro and in vivo. When recapitulating developmental processes in vitro to study cell biology and create cell therapies, controlling aggregate size is essential for the homogeneity, reproducibility, and efficiency of the desired protocol (Bauwens et al., 2011).
Figure 1.
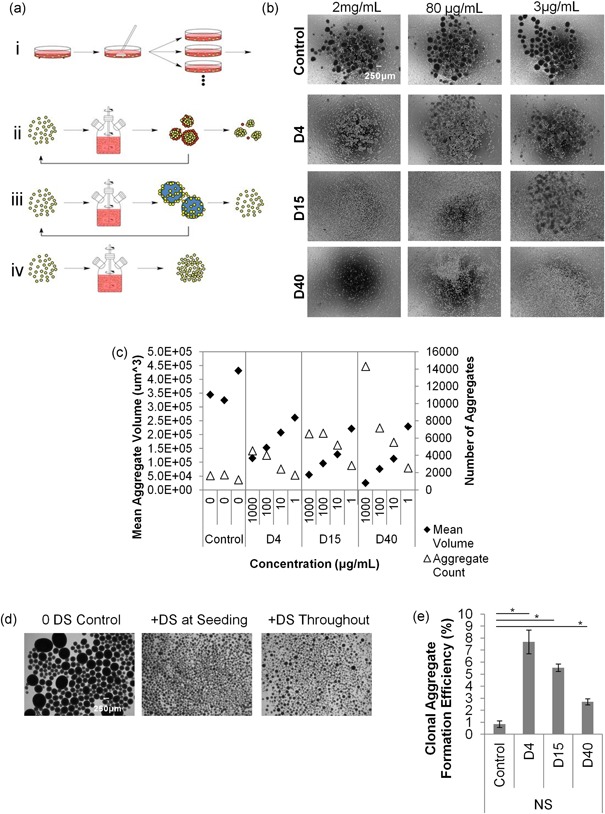
DS controls aggregation. (a) The adherent growth format (i) typically used in laboratory PSC culture is translated to suspension bioreactor technologies for industrialization. (ii) Suspension aggregate‐based culture can lead to heterogeneity in aggregation and serial passaging challenges. (iii) Microcarrier strategies are also used and have unique challenges with serial passaging. (iv) Moving toward continuous single cell bioreactor expansion is an important future direction in the field. (b) Aggregate formation in orbital shaker suspension culture at different concentrations of DS after 24 hr in the presence of rock inhibitor. (c) Average aggregate volume and count in PSC suspension expansion after 4 days in culture. (Representative experiment is shown. Aggregate counts in the conditions shown from left to right are 1,654; 1,741; 1,182; 4,515; 4,023; 2,412; 1,722; 6,510; 6,587; 5,195; 2,823; 14,303; 7,182; 5,516; and 2,557). (d) Addition of D40 to suspension cultures at seeding only compared to daily addition. (e) Aggregate formation efficiency of PSC seeded at clonal densities in suspension in Nutristem medium (n = 4, p < 0.05, t‐test). DS, dextran sulfate; PSC, pluripotent stem cell
In addition to developmental aspects associated with aggregate size control, there are also significant bioprocess manufacturing considerations. Robust bioprocesses are those that produce a consistent output in spite of variability in input parameters. Subtle variability in cell culture conditions leads to batch‐to‐batch variability in adhesion molecule expression and other influential parameters. This can lead to inter‐ and intrabatch heterogeneity in the aggregate formation that poses a significant challenge to process development (Lipsitz, Timmins, & Zandstra, 2016). In particular, aggregate size control can be used to minimize nutrient gradients within the aggregate, supporting the maintenance of a viable, undifferentiated, and homogeneous PSC population. We previously described a method for mechanically controlling aggregate size at the laboratory scale (Ungrin et al., 2012; Ungrin, Joshi, Nica, Bauwens, & Zandstra, 2008); however, no analogous technologies have been developed for scalable aggregate manufacturing. This study aimed to develop a technique to control single‐cell human PSC aggregation in stirred suspension.
2. RESULTS
A review of biopharmaceutical manufacturing literature led us to identify dextran sulfate (DS)‐based chemistries as a potential chemical method for controlling human PSC aggregation properties without loss of pluripotency. DS is a well‐characterized polysulfated compound used to prevent aggregation of cells in biopharmaceutical manufacturing (Dee, Shuler, & Wood, 1997; Hyoung Park, Sin Lim, Rang Woo, Won Kim, & Min Lee, 2016; Renner, Jordan, Eppenberger, & Leist, 1993). To guide stirred tank bioreactor studies, we first varied the concentration of DS in small‐scale orbital shaker suspension culture using three‐molecular‐weight DS: 4,000 kDA (D4), 15,000 kDA (D15), and 40,000 kDA (D40). In each of these conditions, we observed changes in PSC aggregation properties compared with the PSC medium control (Figure 1b). The addition of DS to the medium resulted in the formation of aggregates with significantly reduced diameters in a dose‐dependent fashion (Figure 1c and Supporting Information Figure S1b). In addition, more homogeneous aggregate sizes were observed in the presence of DS, as evidenced by reduced standard deviations and lower coefficients of variation of aggregate volume (Supporting Information Figure S1a). However, in the absence of DS, large, heterogeneous aggregates formed, with a small number of very large aggregates (diameter 150–700 µm) that contained a large fraction of the cells (Figure 1c and Supporting Information Figures S1b and S1c).
To maintain homogeneous aggregate sizes throughout the culture period, DS treatment is only required during PSC seeding. Similar aggregate sizes were observed at each time point in both conditions where PSC were treated with D40 either daily or only at cell seeding (Figure 1d). In biopharmaceutical applications, it has been suggested that DS acts as an antiapoptotic agent during the exponential growth phase of cell culture (Jing et al., 2011; Zanghi, Renner, Bailey, & Fussenegger, 2000). To test the antiapoptotic effect of DS, we seeded PSC into static suspension conditions at low densities typically used for clonal expansion. In the presence of 100 μg/ml all DS compounds, we observed enhanced cell survival and aggregate formation over untreated conditions (Figure 1e).
We next evaluated the capacity for small, homogeneous DS aggregates to expand in culture over a 6‐day culture period. In suspension culture on an orbital shaker, small aggregates formed in D40 remained small and homogeneous throughout the 6‐day expansion relative to the large and heterogeneous untreated aggregates (Figure 2a). Translating these orbital shaker findings to scalable stirred suspension bioreactors for PSC culture confirmed that D40 at seeding was associated with reduced aggregate size throughout the process relative to untreated PSC (Figure 2b). After 6 days in stirred suspension, untreated PSC displayed large, heterogeneous aggregate morphologies in both bioreactor geometries. Furthermore, in stirred tank bioreactors seeded with DS, PSC were successfully expanded for three passages while maintaining high expression of pluripotency markers Oct4 and Sox2 (>85%; Supporting Information Figure S1d). Importantly, after nine passages (five passages in adherent conditions, four passages in suspension), DS‐treated PSC retained a normal karyotype (Supporting Information Figure S1e) and maintained the ability to differentiate into cell types from all three germ layers (Supporting Information Figure S1f). These findings indicate that DS treatment enables uniform aggregate formation without causing a loss of pluripotency or introducing karyotypic instability.
Figure 2.
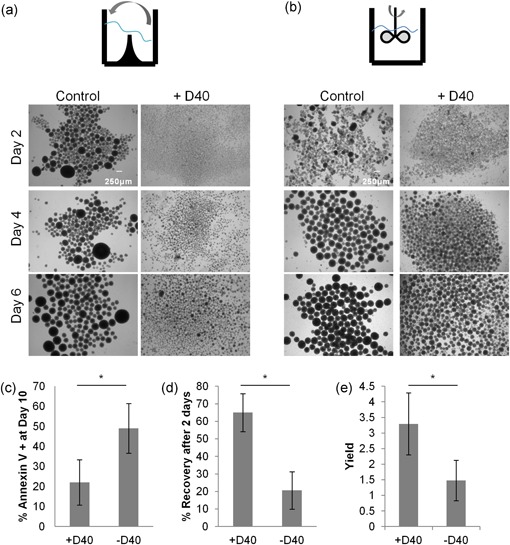
Aggregation control and culture robustness. (a) Representative phase contrast images of PSC aggregates in orbital shaker suspension culture and stirred tank bioreactor culture (b) in the presence and absence of 100 μg/ml D40 at seeding. (c) Expression level of early apoptotic marker annexin V after 10 days in orbital shaker culture (n = 4, p < 0.05, the t‐test). (d) Cell recovery measured 2 days after seeding of passage 2 of PSC in suspension, in the presence and absence of 100 μg/ml D40 (n = 4, p < 0.05, t‐test). (e) Suspension cell yield in the presence and absence of 100 μg/ml D40 after two passages in suspension (n = 6, p < 0.05, t‐test). PSC, pluripotent stem cell
We next investigated the fate of large PSC aggregates that arise in control cultures to understand the benefit that DS conveys to PSC suspension expansion. By extending the suspension culture period to 10 days, untreated PSC cultures were observed to contain primarily large, heterogeneous aggregates, whereas D40‐treated conditions were observed to contain small, homogeneous aggregates (Supporting Information Figure S1g). The untreated, large, dark aggregates exhibited elevated expression of early apoptotic marker annexin V (Figure 2c). When dissociated and reseeded in suspension, D40‐treated cells again formed small, homogeneous aggregates, whereas control cells formed large heterogeneous aggregates. The cell recovery following dissociation after 2 days in culture was significantly enhanced in the D40‐treated PSC (Figure 2d). Heterogeneous aggregation in the first passage preceded reduced cell recovery, lower pluripotency marker expression, and, ultimately, lower cell yield after 6 days of a second passage (Figure 2e). It appears that DS enhances process robustness by mitigating the risk that variable and difficult‐to‐control input factors cause large aggregate formation. These large aggregates would otherwise lead to reduced cell viability and expansion potential and may impact downstream differentiation capacity. In addition to the HES2 line, DS is broadly applicable for aggregation control across different cell lines and culture conditions. We cultured the H9 PSC line in a medium containing DS in both conventional PSC culture conditions and conditions supporting a “naïve” pluripotent state (Gafni et al., 2013) and found that the DS is able to control H9 PSC aggregation in both pluripotent states (Supporting Information Figure S1h).
3. DISCUSSION
Polysulfated compounds, including heparin (Li et al., 2011), polyvinyl sulfate (Zanghi et al., 2000), suramin (Zanghi et al., 2000), and DS (Hyoung Park et al., 2016), have long been used in the biopharmaceutical industry to inhibit yield‐ and quality‐reducing cell aggregation in bioreactors (Hyoung Park et al., 2016). Similar to the effects of DS, we observed that heparin and pentosan polysulfate also displayed aggregate control properties in PSC (data not shown). Antiapoptotic activity, cell surface charge modulation (Dee et al., 1997), and heparan sulfate‐based syndecan signaling have all been suggested as mechanisms of action for these molecules (Stanley, Liebersbach, Liu, Anhalt, & Sanderson, 1995), and may play a role in the aggregation control effects that DS has on PSC. In our study, we observed that although 1,000 μg/ml D15 and D40 lead to the largest number of small aggregates, the total cell number is reduced in these conditions (Supporting Information Figure S1i). This finding suggests that in addition to antiapoptotic effects of DS, tradeoffs should be considered between the increased growth potential of small PSC aggregates, inhibitory maximum DS concentrations, the availability of prosurvival signals received by cells in small aggregates, and additional mechanisms of action.
We have shown that PSC in large aggregates rapidly lose pluripotency, undergo increased apoptosis, and lose the ability to be successfully passaged in suspension. These findings underscore the importance of developing methods to form small, homogeneous aggregates that are suitable for implementation in scalable bioreactors. The large numbers of cells contained in even a single large aggregate have the potential to skew culture outcome. Comparatively, seeding 2 × 105 cells/ml can result in approximately 1.3 × 104 aggregates with a diameter of 50 μm or 8 aggregates with a diameter of 600 μm. Methods to prevent the formation of large aggregates mitigate the risk that variability in input parameters will cause unexpected outcomes. Poor repeatability in PSC aggregation is a widespread concern in the field (Hookway, Butts, Lee, Tang, & McDevitt, 2016). To date, the factors that affect human PSC aggregate formation properties are not well understood; however, cell health, confluence, dissociation timing, and other complex factors likely all play a role in this critical quality attribute. Here, we have demonstrated that DS reduces the formation of large aggregates, thereby promoting culture homogeneity, providing a mechanism for potential improvements to process robustness. Future experiments should analyze batch‐to‐batch variability across multiple production runs to quantify the extent to which DS reduces variability in product output parameters including yield and pluripotency marker expression across batches. DS could also enable strategies for the scalable translation of differentiation protocols requiring precise control of aggregate size. Encouragingly, DS requires no pretreatment or adaptation for efficacy. DS consistently leads to homogeneous aggregation across different bioreactor geometries, suggesting that it may mitigate geometry‐associated process translation challenges caused by different mixing, shear, and initial aggregation properties. Further optimization of this system may enable single cell suspension culture of PSC (Figure 1a‐iv), which would resolve many of the issues around growth of adhesion‐dependent cells in suspension bioreactors. We note that the presence of residual quantities of DS in media formulations arising from unpurified, supernatant proteins could contribute to the challenges of reproducing key studies in our field, especially those involving passaging or suspension culture. Improvements in process robustness through aggregate control will simplify and enhance PSC‐based cell therapy scale‐up and manufacturing, contributing to broader patient access to life‐saving therapies.
4. MATERIALS AND METHODS
4.1. PSC culture
PSC were cultured as described previously. The hESC lines were provided by G. Keller (McEwen Centre for Regenerative Medicine/University Health Network). In brief, HES2 were cultured on the Geltrex LDEV‐Free Reduced Growth Factor Basement Membrane Matrix (Thermo Fisher, Waltham, MA; 1:50 dilution in Dulbecco’s Modified Eagle Medium, Thermo Fisher) coated plates in Nutristem hESC XF Culture Medium (Biological Industries, Beit Haemek, Israel, “NS”) or E8 Medium (Thermo Fisher), supplemented with 1× penicillin–streptomycin (Life Technologies). Plates were coated with Geltrex for 30 min at 37°C. Cells were passaged 1:24 every 6 days by dissociation with TrypLE Express (Thermo Fisher) at 37°C for 4–5 min. All cell line stocks were confirmed negative for mycoplasma contamination. Conversion to a human “naïve” PSC state was performed as described previously (Gafni et al., 2013). DS compounds (Sigma‐Aldrich, St Louis, MO) were prepared by dilution in deionized water at a stock concentration of 100 mg/ml followed by filter sterilization.
4.2. Suspension expansion of PSC
Six‐well culture plates were precoated with 5% Pluronic F‐68 (Thermo Fisher) for 30 min at 37°C or overnight at 4°C. Dissociation of PSC to single cells was carried out by TrypLE Express (Thermo Fisher) treatment for 5 min at room temperature. Single cells were seeded into the Pluronic F‐68 coated six‐well plates that were then placed on an orbital shaker with 10 μM Y‐27632 (Reagents Direct, Encinitas, CA) in normoxic (21% O2) conditions. Cells were seeded at densities of 2 × 105 cells/ml unless otherwise noted. The medium was exchanged by angling the plate at 45° and allowing aggregates to settle onto the bottom edge. Two days after seeding, half of the growth medium was removed and replaced each day. Cells were harvested at the end of culture by dissociation with a 5‐min TrypLE treatment at 37°C, and cell counts were performed by Trypan Blue exclusion (Thermo Fisher). Bioreactor experiments were conducted following the manufacturer’s instructions for operating the Ambr‐24 bioreactor system (Sartorius, Gottingen, Germany). Cell dissociation and medium exchange were performed as described above. Bioreactors were operated at 450 rpm and seeded with 4 × 105 cells/ml unless otherwise noted. Aggregates were analyzed using the Mastersizer 3000 particle analyzer (Malvern, Westborough, MA) according to the manufacturer’s instructions. Unless otherwise noted, the 560‐μm aperture was used for analysis. Aggregates were dissociated and seeded into adherent conditions after three passages in suspension with DS. Directed differentiation into all three germ layers was performed as described previously for endoderm (Ungrin et al., 2012), mesoderm (Bauwens et al., 2011), and ectoderm (Fluri et al., 2012) lineages. G‐Band karyotyping was performed by WiCell (Madison, WI).
4.3. Flow cytometry and immunocytochemistry
Apoptosis analysis was conducted using Annexin V staining. Dissociated cells were resuspended in Hank’s Buffered Saline Solution (Thermo Fisher) supplemented with 2% fetal bovine serum (HF; Thermo Fisher). Cells were washed once in HF and then resuspended in the Annexin V Binding Buffer (BD Biosciences) solution with the antibody (Annexin V‐647, 1:100 dilution; BD Biosciences, San Jose, CA) for 15 min. Cells were washed in binding buffer again and resuspended in 7AAD (100 μg/ml; Thermo Fisher) viability stain to distinguish necrotic cells from apoptotic cells. For intracellular staining, dissociated cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 10 min and permeabilized with 0.1% triton X‐100 (Sigma‐Aldrich). Cells were then stained with the primary antibody for 25 min at 4°C followed by the secondary antibody for 25 min at 4°C. Three wash steps with HF were included between each step. Cells were analyzed on a FACS Canto II (BD) or FACS Fortessa (BD) flow cytometer. Analysis was performed with FlowJo (Tree Star, Ashland, OR). For immunocytochemistry, undissociated samples were prepared as described for intracellular staining and imaged on an EVOS microscope (Thermo Fisher).
4.4. Statistical analysis
Statistical analysis was performed in Excel software (Microsoft, Redmond, WA). The Student t test was used to compare treatments in Figures 1e and 2c–e. Coefficient of variation was calculated by estimating the volume of each aggregate from its diameter, calculating the standard deviation of each treatment, and normalizing to the mean. Two‐sided F‐test was used to compare coefficients of variation. Error bars represent standard deviation. * denotes p < 0.05 unless otherwise noted.
Supporting information
Supporting information
Supporting information
Lipsitz YY, Tonge PD, Zandstra PW. Chemically controlled aggregation of pluripotent stem cells. Biotechnology and Bioengineering. 2018;119:2061–2066. 10.1002/bit.26719
References
REFERENCES
- Amit, M. , Laevsky, I. , Miropolsky, Y. , Shariki, K. , Peri, M. , & Itskovitz‐Eldor, J. (2011). Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nature Protocols, 6(5), 572–579. [DOI] [PubMed] [Google Scholar]
- Bauwens, C. L. , Song, H. , Thavandiran, N. , Ungrin, M. , Massé, S. , Nanthakumar, K. , … Zandstra, P. W. (2011). Geometric control of cardiomyogenic induction in human pluripotent stem cells. Tissue Engineering. Part A, 17(15–16), 1901–1909. [DOI] [PubMed] [Google Scholar]
- Chen, A. K. L. , Chen, X. , Choo, A. B. H. , Reuveny, S. , & Oh, S. K. W. (2011). Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Research, 7(2), 97–111. [DOI] [PubMed] [Google Scholar]
- Dee, K. U. , Shuler, M. L. , & Wood, H. A. (1997). Inducing single‐cell suspension of BTI‐TN5B1‐4 insect cells: I. The use of sulfated polyanions to prevent cell aggregation and enhance recombinant protein production. Biotechnology and Bioengineering, 54(3), 191–205. [DOI] [PubMed] [Google Scholar]
- Fluri, D. A. , Tonge, P. D. , Song, H. , Baptista, R. P. , Shakiba, N. , Shukla, S. , … Zandstra, P. W. (2012). Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nature Methods, 9(5), 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni, O. , Weinberger, L. , Mansour, A. A. , Manor, Y. S. , Chomsky, E. , Ben‐Yosef, D. , … Hanna, J. H. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature, 504(7479), 282–286. [DOI] [PubMed] [Google Scholar]
- Hookway, T. A. , Butts, J. C. , Lee, E. , Tang, H. , & McDevitt, T. C. (2016). Aggregate formation and suspension culture of human pluripotent stem cells and differentiated progeny. Methods, 101, 11–20. [DOI] [PubMed] [Google Scholar]
- Hyoung Park, J. , Sin Lim, M. , Rang Woo, J. , Won Kim, J. , & Min Lee, G. (2016). The molecular weight and concentration of dextran sulfate affect cell growth and antibody production in CHO cell cultures. Biotechnology Progress, 32(5), 1113–1122. [DOI] [PubMed] [Google Scholar]
- Jing, Y. , Egan, S. E. , Qian, Y. , Borys, M. C. , Abu‐Absi, N. R. , & Li, Z. J. (2011). Dextran sulfate inhibits staurosporine‐induced apoptosis in Chinese hamster ovary (CHO) cells: Involvement of the mitochondrial pathway. Process Biochemistry, 46(1), 427–432. [Google Scholar]
- Kehoe, D. E. , Jing, D. , Lock, L. T. , & Tzanakakis, E. S. (2010). Scalable stirred‐suspension bioreactor culture of human pluripotent stem cells. Tissue Engineering. Part A, 16(2), 405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Qin, J. , Feng, Q. , Tang, H. , Liu, R. , Xu, L. , & Chen, Z. (2011). Heparin promotes suspension adaptation process of CHO‐TS28 cells by eliminating cell aggregation. Molecular Biotechnology, 47(1), 9–17. [DOI] [PubMed] [Google Scholar]
- Lipsitz, Y. Y. , Timmins, N. E. , & Zandstra, P. W. (2016). Quality cell therapy manufacturing by design. Nature Biotechnology, 34(4), 393–400. [DOI] [PubMed] [Google Scholar]
- Oh, S. K. W. , Chen, A. K. , Mok, Y. , Chen, X. , Lim, U. M. , Chin, A. , … Reuveny, S. (2009). Long‐term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Research, 2(3), 219–230. [DOI] [PubMed] [Google Scholar]
- Phillips, B. W. , Horne, R. , Lay, T. S. , Rust, W. L. , Teck, T. T. , & Crook, J. M. (2008). Attachment and growth of human embryonic stem cells on microcarriers. Journal of Biotechnology, 138(1–2), 24–32. [DOI] [PubMed] [Google Scholar]
- Renner, W. A. , Jordan, M. , Eppenberger, H. M. , & Leist, C. (1993). Cell–cell adhesion and aggregation: Influence on the growth behavior of CHO cells. Biotechnology and Bioengineering, 41(2), 188–193. [DOI] [PubMed] [Google Scholar]
- Stanley, M. J. , Liebersbach, B. F. , Liu, W. , Anhalt, D. J. , & Sanderson, R. D. (1995). Heparan sulfate‐mediated cell aggregation: Syndecans‐1 and ‐4 mediate intercellular adhesion following their transfection in to human B lymphoid cells. Journal of Biological Chemistry, 270(10), 5077–5083. [DOI] [PubMed] [Google Scholar]
- Ungrin, M. D. , Clarke, G. , Yin, T. , Niebrugge, S. , Nostro, M. C. , Sarangi, F. , … Zandstra, P. W. (2012). Rational bioprocess design for human pluripotent stem cell expansion and endoderm differentiation based on cellular dynamics. Biotechnology & Bioengineering, 109(4), 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungrin, M. D. , Joshi, C. , Nica, A. , Bauwens, C. , & Zandstra, P. W. (2008). Reproducible, ultra high‐throughput formation of multicellular organization from single cell suspension‐derived human embryonic stem cell aggregates. PLoS One, 3(2), e1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanghi, J. A. , Renner, W. A. , Bailey, J. E. , & Fussenegger, M. (2000). The growth factor inhibitor suramin reduces apoptosis and cell aggregation in protein‐free CHO cell batch cultures. Biotechnology Progress, 16(3), 319–325. [DOI] [PubMed] [Google Scholar]
- Zweigerdt, R. , Olmer, R. , Singh, H. , Haverich, A. , & Martin, U. (2011). Scalable expansion of human pluripotent stem cells in suspension culture. Nature Protocols, 6(5), 689–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


