Abstract
The aim of this study was to investigate the protective effect of fluorofenidone (5‐methyl‐1‐[3‐fluorophenyl]‐2‐[1H]‐pyridone, AKF‐PD) on ultraviolet (UV)‐A‐induced senescence in human dermal fibroblasts (HDF) and examine the mechanisms involved. HDF were treated with AKF‐PD. Senescence‐associated (SA)‐β‐galactosidase level, cell viability and expression of p16 were evaluated. In addition, UV‐A‐irradiated HDF were treated with AKF‐PD, rapamycin and MHY1485; SA‐β‐galactosidase staining, 3‐(4 5‐dimethylthiazol‐2‐yl)‐2 5‐diphenyltetrazolium bromide assay and western blot for SIRT1 were performed; and phosphorylated mammalian target of rapamycin (p‐mTOR) expression and reactive oxygen species (ROS) levels were measured. Intracellular ROS was detected by the 2′,7′‐dichlorofluroescein diacetate probe. Our results showed that AKF‐PD substantially attenuated the changes of p16 expression, SA‐β‐galactosidase staining and cellular proliferation induced by UV‐A irradiation in HDF. AKF‐PD rescued the increased mTOR phosphorylation and reduced SIRT1 expression induced by UV‐A irradiation in HDF. AKF‐PD and rapamycin together had a synergistic effect on p‐mTOR reduction and SIRT1 increase. mTOR activator MHY1485 partly blocked the above effects. Moreover, intracellular ROS level induced by UV‐A irradiation could partly decrease by AKF‐PD, and MHY1485 could reduce this effect. Our results indicated that AKF‐PD could alleviate HDF senescence induced by UV‐A‐irradiation by inhibiting the p‐mTOR and increasing SIRT1. Moreover, AKF‐PD may be a potential treatment material for skin.
Keywords: fluorofenidone, human dermal fibroblasts, mammalian target of rapamycin, SIRT1, skin photoaging
Introduction
Repeat exposure to ultraviolet (UV) radiation contributes to skin photoaging. Clinical changes of skin photoaging include formation of fine and coarse wrinkles, increased skin thickness, dryness, laxity and pigmentation.1 Histological features of photoaging may include hypertrophy of the epidermis and dermis and occasionally stratum corneum hyperkeratosis. Increased thickness of the basal membrane and irregular distribution of melanocytes along the basal membrane are also observed. UV light, depending on its wavelength, penetrates into the skin to different extents and interacts with skin cells.2, 3, 4 UV‐A (320–400 nm) is able to cross the epidermis and reach the dermis, contributing to skin photoaging.5, 6, 7 UV radiation (UV‐A and ‐B) act on melanocytes and other skin cells, such as keratinocytes, leading to DNA damage through oxidative stress and generation of reactive oxygen species (ROS). ROS activate signaling pathways associated with cell/tissue growth, differentiation, senescence and photoaging.8, 9, 10 ROS generated in UV‐A‐irradiated human skin cells are primarily responsible for photoaging.1, 11
Mammalian target of rapamycin (mTOR) is a member of the phosphatidylinositol 3‐kinase (PI3K)‐related protein kinase subfamily and plays a critical role in the regulation of various cellular events such as cell growth, proliferation and aging.TOR complex (TORC)1 and TORC2 differ in subunit compositions and biologic functions. TORC1 is sensitive to rapamycin. Rapamycin‐sensitive mTOR is associated with controlling aging and other aspects of nutrient‐related physiology.12, 13 Previous researchers showed that rapamycin treatment decreased ROS in normal diploid fibroblasts and increased the viability of human diploid fibroblasts.14 SIRT1 is a nucleocytoplasmic protein that belongs to the nicotinamide adenine dinucleotide+‐dependent enzymes family. Recent studies have demonstrated that SIRT1 regulates cell survival and reduces cell aging. It is generally believed that SIRT1 inhibition is a hallmark of aging.15, 16 A growing body of evidence supports the hypothesis that the aging process is regulated by a continuous cross‐talk between ROS and SIRT1.17, 18 In cardiomyocytes, mice neurocytes and rat endothelial cells, SIRT1 activation protected the cells from oxidative stress and decreased ROS production.18, 19, 20 Taken together, we suspected a relationship between SIRT1 and the mTOR pathways for controlling ROS production and the aging process.
Pyridone‐derivative fluorofenidone (5‐methyl‐1‐[3‐fluorophenyl]‐2‐[1H]‐pyridone, AKF‐PD) and its analogs have been reported to exert strong antioxidative activity in several cell lines, tissues and animal models, including neurocytes, kidney tissue and mice and rat models.21, 22, 23 UV‐A irradiation activates oxidative stress and induces ROS production in human skin cells, resulting in skin photoaging. Therefore, we hypothesized that AKF‐PD can protect the skin from photoaging by reducing oxidative stress. However, the effect of AKF‐PD on skin photoaging has not been studied. The goals of this study were to evaluate the protective effect of AKF‐PD on UV‐A‐irradiated skin and examine the underlying mechanisms involved.
Methods
Reagents
The AKF‐PD powder used in this study were gifts from the School of Pharmacy, Central South University (Changsha, Hunan, China).
The following antibodies were used for western blotting: rabbit anti‐phospho‐mTOR Ser2448 and mTOR (both from Cell Signaling Technology, Danvers, MA, USA), rabbit anti‐SIRT1 (Abcam, Cambridge, MA, USA) and mouse anti‐β‐actin (Sigma‐Aldrich, St Louis, MO, USA). The following polymerase chain reaction (PCR) primers for SIRT1 were used in this study: forward, 50‐ acaacttgta cgacgaagac‐30, and reverse, 50‐aggaggagta gtgaaagtgt‐30(SangonBiotech, Shanghai, China). Primers specific to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) RNA were used to standardize the amount of RNA in each sample.
Rapamycin was purchased from Beyotime Institute of Biotechnology, China. MHY1485 was purchased from Sigma‐Aldrich.
Cell culture
Primary human dermal fibroblasts (HDF) were obtained from circumcised foreskins of healthy human donors aged 5–20 years and cultured at 37°C in a humidified incubator with 5% CO2, maintained in Dulbecco's modified Eagle medium (Gibco, Grand Island, NY, USA) with penicillin (100 U/mL), streptomycin (100 ng/mL) and 10% fetal bovine serum (Gibco).
All procedures involving human subjects were approved by the Clinical Research Ethics Committee, Xiang Ya Hospital, Central South University Changsha, China. Informed written consent was obtained from all human subjects.
SA β‐gal staining
Senescence‐associated β‐galactosidase (SA‐β‐gal) activity was measured by a β‐galactosidase staining kit (BioVision, Mountain View, CA, USA) according to the manufacturer's instructions. Cells in subconfluent cultures were washed with phosphate‐buffered saline (PBS), fixed in fixing solution for 15 min at 24°C and incubated overnight at 37°C in staining solution. Blue‐stained cells were counted in at least five fields at ×400 magnification and relative SA‐β‐gal activity under each studied condition was expressed as the percentage of positive cells.
MTT assays
Cell viability was determined using the 3‐(4 5‐dimethylthiazol‐2‐yl)‐2 5‐diphenyltetrazolium bromide (MTT) assay. Cells (2 × 103 cells/well) were seeded into 96‐well plates and incubated at 37°C. After adhesion, cells were exposed to UV‐A irradiation and grown in complete medium or complete medium with AKF‐PD, rapamycin or MHY1485. Cells were incubated for 4 h in fresh medium containing 0.5 mg/mL MTT (Sigma‐Aldrich) 24, 72 or 120 h after irradiation. After removing the MTT solution, dimethylsulfoxide (150 μL/well) was added to each well to dissolve the formazan crystals. The absorbance was measured at 570 nm using a Synergy 2 Multi‐Mode Microplate Reader (BioTek, Seattle, WA, USA). All experiments were performed in triplicate and the data presented are the means of three independent experiments ± standard deviation.
Cell UV‐A irradiation
Before UV‐A irradiation, cells were washed and coated with a thin layer of PBS to prevent UV‐A absorption by components of the medium. A Philips UV‐A lamp with an emission spectrum between 320 and 400 nm was used for the experiment. The dose of UV‐A irradiation was 10 J/cm2 per day, which was verified with a UV light meter (Sigma, Shanghai, China) for 3 days. After UV‐A irradiation, cells were incubated in complete medium and maintained with indicated compounds.
RNA extraction and miRNA real‐time quantitative PCR
RNA were extracted from primary HDF using TRIzol. miRNA transcription was performed using a TaqMan miRNA reverse transcription kit according to the manufacturer's instructions with Eppendorf Master Cycler Nexus. The resultant first‐strand cDNA was synthesized using a high‐capacity RNA‐to‐cDNA kit to examine the expression levels of genes of interest. Quantitative PCR was performed using SYBR Green (Applied Biosystems, Foster City, CA, USA) on a 7500 Real Time PCR System (Applied Biosystems). GAPDH was used as an internal control. The comparative threshold (DCt) method was used to analyze the results of three independent experiments.
Western blotting
Cells were lysed in lysis buffer, and total cell lysates containing equal amounts of proteins were loaded onto 4–12% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS‐PAGE) gels for electrophoresis and then transferred to polyvinylidene difluoride membranes (Pierce, Rockford, IL, USA). The membranes were blocked with 5% skim milk dissolved in PBS containing 0.02% Tween (Sigma‐Aldrich) and incubated overnight at 4°C with specific primary antibodies. The membranes were subsequently incubated with specific horseradish peroxidase (HRP)‐conjugated secondary antibody for 1 h at room temperature. Bands of interest in western blots were visualized with a western blot HRP substrate (Millipore, Billerica, MA, USA).
ROS scavenging assay
Intracellular ROS scavenging assays were performed by measuring the fluorescence intensity of the 2′,7′‐dichlorofluroescein diacetate (DCF‐DA) probe, which was proportional to the amount of ROS produced. Cells were given different treatment separately: UV‐A, UV‐A + AKF‐PD and UV‐A + MHY1485 + AKF‐PD. Then, the cells were harvested and mixed with DCF‐DA solution and incubated at 37°C for 1 h. Fluorescence intensity was measured using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data were collected and analyzed to obtain the mean and standard deviation for three independent experiments. Differences between groups were analyzed by one‐way anova, followed by further analysis by the least significant difference test. P < 0.05 was considered statistically significant.
Results
AKF‐PD protects HDF from UV‐A‐induced senescence
To determine the effects of AKF‐PD on UV‐A‐induced cellular senescence, we measured SA‐β‐gal activity and the expression of a cellular senescence hallmark p16. Our data showed that the percentage of senescent cells (blue‐stained SA‐β‐gal+ cells) substantially increased in UV‐A‐irradiated HDF, compared with that in the non‐irradiated control, and that AKF‐PD pretreatment suppressed this phenomenon in a dose‐dependent manner (Fig. 1A). Western blot of p16 showed that UV‐A increased p16 expression in HDF and that pretreatment with AKF‐PD partly decreased this effect in a dose‐dependent manner (Fig. 1B), which is in accordance with the results of SA‐β‐gal staining. These results indicated that 10 J/cm2 UV‐A irradiation for 3 days was a suitable irradiation dose for establishing a HDF senescence model under our experimental conditions and that AKF‐PD improved this senescence state. MTT assays showed that UV‐A irradiation decreased HDF proliferation compared with control and that AKF‐PD treatment partially increased HDF proliferation (Fig. 1C).
Figure 1.
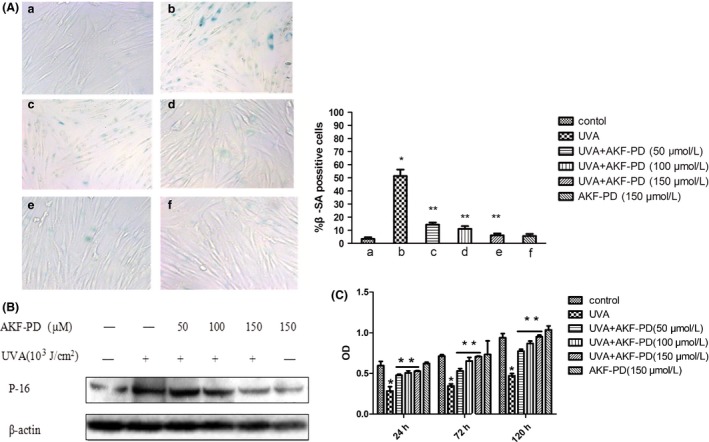
Protective effect of pyridone‐derivative fluorofenidone (5‐methyl‐1‐[3‐fluorophenyl]‐2‐[1H]‐pyridone, AKF‐PD) on ultraviolet (UV)‐A‐irradiated human dermal fibroblast (HDF) senescence. (A) AKF‐PD pretreatment improved senescence HDF induced by UV‐A (stain, SA‐β‐galactosidase, original magnification ×400). (B) AKF‐PD pretreatment partly suppressed the increased p16 expression induced by UV‐A. (C) AKF‐PD treatment partially increased HDF proliferation. *Significant differences between the UV‐A‐irradiated group and the non‐irradiated control. **Significant differences between the AKF‐PD + UV‐A groups and UV‐A‐irradiated group (n = 3, P < 0.01).
Inhibition of SIRT1 expression and activation of mTOR pathway induced by UV‐A are reduced by AKF‐PD treatment
We observed that UV‐A irradiation was able to attenuate the protein expression of SIRT1 compared with control and that administration of AKF‐PD significantly reduced the inhibition in a dose‐dependent manner (Fig. 2A), suggesting that SIRT1 expression may contribute to the protective effect of AKF‐PD on UV‐A‐induced HDF senescence. Previous studies demonstrated that inhibition of phosphorylated (p)‐mTOR reduced cellular ROS production.24 In this study, UV‐A irradiation activated p‐mTOR and AKF‐PD treatment attenuated this activation in a dose‐dependent manner, similar to the effect on SIRT1 expression (Fig. 2B).
Figure 2.
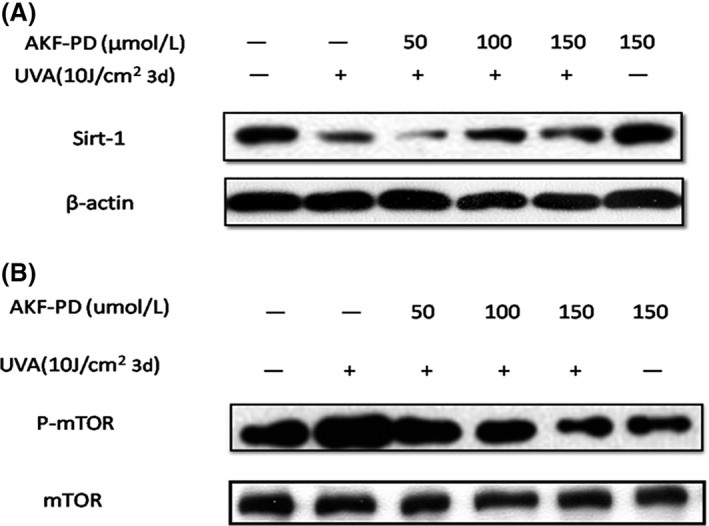
Ultraviolet (UV)‐A irradiation‐induced mammalian target of rapamycin (mTOR) pathway activation and SIRT1 protein expression inhibition reversed by pyridone‐derivative fluorofenidone (5‐methyl‐1‐[3‐fluorophenyl]‐2‐[1H]‐pyridone, AKF‐PD) treatment in human dermal fibroblasts (HDF). (A) AKF‐PD significantly reduced the inhibition of SIRT1 induced by UV‐A. (B) AKF‐PD treatment attenuated phosphorylated mammalian target of rapamycin (p‐mTOR) activation.
AKF‐PD increases UV‐A‐induced SIRT1 inhibition and improved HDF senescence via inhibition of the mTOR pathways
Experiments described in last section showed that UV‐A‐induced mTOR pathway activation and inhibition of SIRT1 expression were reversed by AKF‐PD treatment. SA‐β‐gal staining showed that the percentage of senescent cells decreased after AKF‐PD treatment. In addition, percentage of senescent cells significantly decreased after AKF‐PD and rapamycin treatment in UV‐A‐irradiated cells compared with that reported for AKF‐PD treatment alone (Fig. 3A). MHY1485 treatment suppressed the AKF‐PD protective effect and increased the percentage of senescent cells (Fig. 3A). In accordance with the above results, the MTT assay demonstrated that AKF‐PD + rapamycin treatment increased cell proliferation rate compared with AKF‐PD treatment alone. AKF‐PD + MHY1485 treatment decreased cell activity compared with AKF‐PD treatment in UV‐A‐irradiated HDF (Fig. 3B). Moreover, our data indicate that AKF‐PD treatment remitted the UV‐A‐induced inhibition of SIRT1 mRNA and protein expression and had a synergistic effect on these processes with the mTOR inhibitor, rapamycin. Treated with activator of mTOR, MHY1485, the effect of AKF‐PD on SIRT1 expression reduced (Fig.3 C,D). In addtion, we found AKF‐PD decreased the UV‐A radiate induced intracellular ROS. This effect is also reduced when pre‐treated with MHY1485(Fig. 3E). The data suggested that AKF‐PD attenuates UV‐A‐induced SIRT1 inhibition, increased ROS level and HDF senescence by inhibiting the mTOR pathways in HDF.
Figure 3.
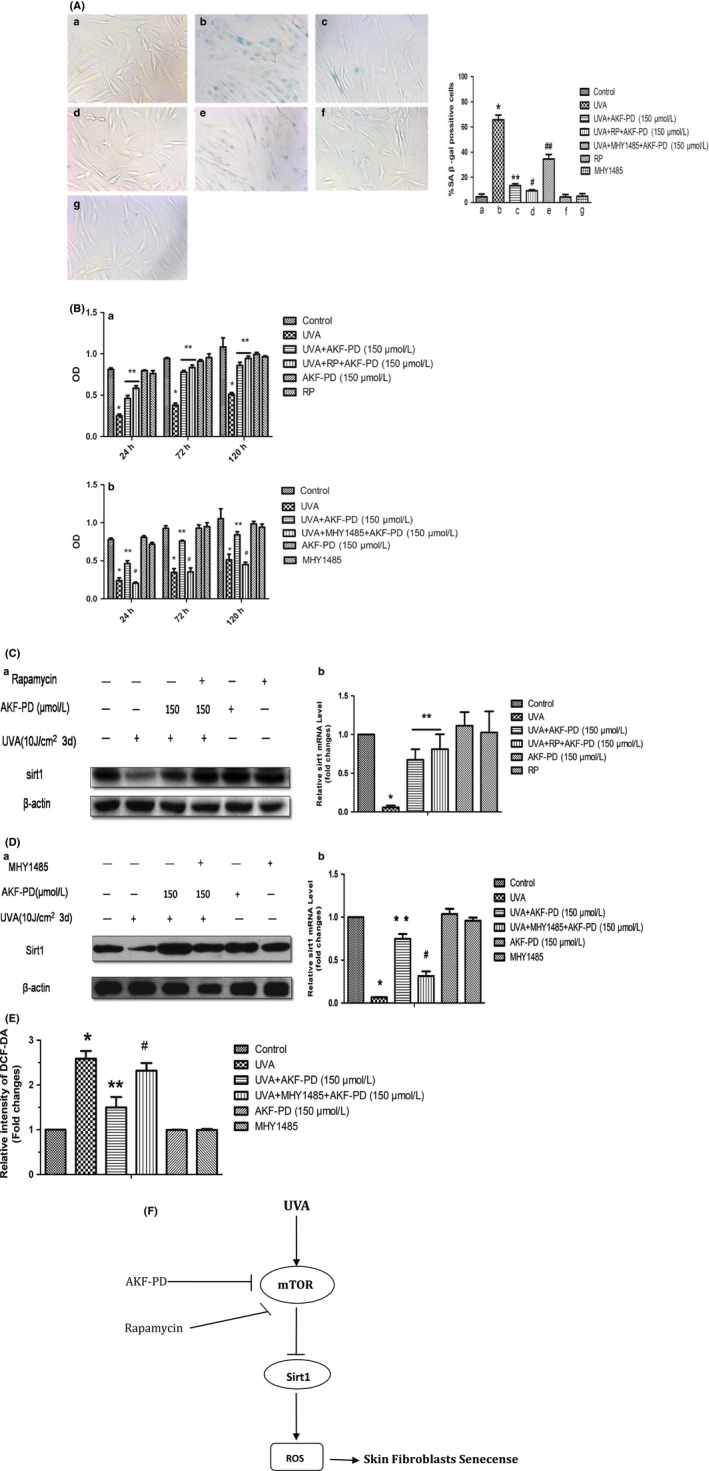
Pyridone‐derivative fluorofenidone (5‐methyl‐1‐[3‐fluorophenyl]‐2‐[1H]‐pyridone, AKF‐PD) increased ultraviolet (UV)‐A irradiation‐induced SIRT1 inhibition and improved human dermal fibroblast (HDF) senescence by inhibiting the mTOR‐dependent pathways. (A) AKF‐PD decreased the HDF senescence through inhibiting p‐mTOR (stain, SA‐β‐galactosidase, original magnification ×400). Significant difference between the *UV‐A irradiated group and the non‐irradiated control, **UV‐A + AKF‐PD group and UV‐A‐irradiated group, #UV‐A +rapamycin (RP) + AKF‐PD and UV‐A + AKF‐PD group, and ##UV‐A + MHY1485 + AKF‐PD and UV‐A + AKF‐PD group (n = 3, P < 0.01). (B) AKF‐PD increased the cell proliferation by inhibiting phosphorylated mammalian target of rapamycin (p‐mTOR). Significant differences between the *UV‐A‐irradiated group and non‐irradiated control, **UV‐A + AKF‐PD group and UV‐A‐irradiated group, and #UV‐A + MHY1485 + AKF‐PD and UV‐A + AKF‐PD group (n = 3, P < 0.01). (C,D) AKF‐PD increased SIRT1 expression by inhibiting p‐mTOR. (E) AKF‐PD decreased intracellular reactive oxygen species (ROS) by inhibiting p‐mTOR. Significant differences between the *UV‐A‐irradiated group and non‐irradiated control, **UV‐A + AKF‐PD group and UV‐A‐irradiated group, #UV‐A + MHY1485 + AKF‐PD and UV‐A + AKF‐PD group (n = 3, P < 0.01). (F) AKF‐PD inhibits senescence in human skin fibroblasts via the mTOR‐dependent SIRT1 pathway.
Discussion
Skin aging is a complicated process influenced by intrinsic and extrinsic factors.6 Extrinsic aging develops through several environmental factors, among which UV radiation is the most important, contributing up to 80%. Many in vitro studies have shown that UV‐A irradiation produces ROS and induces long‐term growth arrest and alterations in cell morphology (post‐mitotic phenotypes) and increases SA‐β‐gal activity, indicating cell senescence.25, 26 Two layers, the epidermis and dermis, make up human skin. The dermis is composed of connective tissues, including fibroblasts, matrix proteins and other substances. It is reported that fibroblasts, which are more vulnerable to UV‐A exposure than keratinocytes, are the dominating components of the dermis and contribute to producing connective tissues. UV‐A photons can induce fibroblasts to synthesize metalloproteinase 1, which stimulates degradation of the collagenous extracellular matrix and accounts for most of the connective tissue damage.27, 28 Cellular senescence of human skin fibroblasts has been reported as one of the most important causes of skin aging.29 UV‐A can induce senescence of human skin fibroblasts which will lead to loose, fragile of skin appearance and account for skin aging.
One of the main factors of cellular senescence is p16, which inhibits cyclin‐dependent kinases, leading to G1 cell cycle arrest. In our in vitro study, HDF exposed to 10 J/cm2 UV‐A per day for 3 days demonstrated increased SA‐β‐gal activity and p16 expression, and long‐term growth arrest, indicating that this UV‐A dose was sufficient for inducing HDF senescence. While AKF‐PD significantly reduced increased SA‐β‐gal activity and p16 expression and decreased proliferation rate of UV‐A‐irradiated HDF, indicating that AKF‐PD may be an effective agent for inhibiting HDF senescence, the underlying mechanism is unknown.
Pyridone derivatives, AKF‐PD and pirfenidone (PFD), have antifibrotic and anti‐inflammatory properties. Recently, an increasing number of studies have focused on the antioxidative activity of AKF‐PD and PFD.30, 31 Qin et al.21, 32 demonstrated that AKF‐PD served as an antioxidative agent by blocking nicotinamide adenine dinucleotide phosphate oxidase‐dependent oxidative stress in rat proximal tubular epithelial cells, and as an anti‐Nox‐mediated oxidative stress agent in AKF‐PD‐treated rats. AKF‐PD was reported to have anti‐oxidative activity in experimental rats, protecting hippocampal neurons and hepatocytes in cirrhosis,4, 22, 23 and exerting anti‐oxidative activity in pulmonary interstitial cells.31
In most cases, UV‐A irradiation acts indirectly on skin photoaging through generation of ROS. mTOR and SIRT1 play an important role in regulating the aging process and adjusting cellular oxidative status. Rapamycin treatment of aged oocytes decreased ROS activity and DNA fragmentation compared with untreated control. Zhuge et al.33 demonstrated that activation of SIRT1 could rescue retinal pigment epithelial (RPE) cells from oxidative stress‐induced senescence. In accordance with our hypothesis, our study showed that AKF‐PD alleviated UV‐A‐irradiated HDF senescence by upregulating SIRT1 and inhibiting mTOR. We used a p‐mTOR inhibitor, rapamycin, and a p‐mTOR activator, MHY1485, to confirm the effect of AKF‐PD on UV‐A‐induced HDF. When mTOR was inhibited, the percentage of senescent cells decreased, cell proliferation increased and SIRT1 expression increased after AKF‐PD treatment. Activation of mTOR yielded opposite results. In our study, AKF‐PD may regulate SIRT1 expression via an mTOR‐dependent pathway in UV‐A‐irradiated HDF. The interaction between SIRT1 and the mTOR pathways is complex. In different cell lines, tissues, organs or physiological and pathological processes, different regulation mechanisms are at work. Zhang et al.24 showed that rapamycin arrested the senescence of mesangial cells induced by high glucose and increased SIRT1 expression. Back et al.34 demonstrated that inhibition of SIRT1 by mTOR fostered the survival of DNA damage‐induced prematurely senescent squamous cell carcinoma cells. However, SIRT1 was reported to regulate the mTOR pathway in autophagy processes,35 Alzheimer's disease model,36 inhibition of acute and chronic pain and cell survival.37
In our study, we reported that AKF‐PD inhibited UV‐A‐induced senescence and increased SIRT1 expression in HDF via the mTOR‐dependent signaling pathway (Fig. 3F). Recently, a study has been carried out to improve the pharmacokinetics of AKF‐PD, by incorporating AKF‐PD in β‐cyclodextrin and hydroxypropyl‐β‐cyclodextrin to increase dissolution rate.38 Therefore, AKF‐PD may be developed as a potential cosmetic agent for prevention of skin photoaging.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by grants from the China Hunan Provincial Science and Technology Department (2013FJ6065, 2013SK3005), the National Natural Science Foundation of China (81573074, 81502750), and Training Program of the Major Research Plan of the National Natural Science Foundation of China(91749114). We thank Li Yong for critical reading of the manuscript.
Contributor Information
Dan Jian, Email: 569085332@qq.com.
Ji Li, Email: liji_xy@csu.edu.cn.
References
- 1. Kim MR, Lee HS, Choi HS et al Protective effects of ginseng leaf extract using enzymatic extraction against oxidative damage of UVA‐irradiated human keratinocytes. Appl Biochem Biotechnol 2014; 173(4): 933–945. [DOI] [PubMed] [Google Scholar]
- 2. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 2004; 195(3): 298–308. [DOI] [PubMed] [Google Scholar]
- 3. Debacq‐Chainiaux F, Leduc C, Verbeke A et al UV, stress and aging. Dermatoendocrinol 2012; 4(3): 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berneburg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photoimmunol Photomed 2000; 16(6): 239–244. [DOI] [PubMed] [Google Scholar]
- 5. Puizina‐Ivić N. Skin aging. Acta Dermatovenerol Alp Pannonica Adriat 2008; 17(2): 47–54. [PubMed] [Google Scholar]
- 6. Yang TH, Lai YH, Lin TP et al Chronic exposure to Rhodobacter sphaeroides extract Lycogen™ prevents UVA‐induced malondialdehyde accumulation and procollagen I down‐regulation in human dermal fibroblasts. Int J Mol Sci 2014; 15(2): 1686–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie H, Liu F, Liu L et al Protective role of AQP3 in UVA‐induced NHSFs apoptosis via Bcl2 up‐regulation. Arch Dermatol Res 2013; 305(5): 397–406. [DOI] [PubMed] [Google Scholar]
- 8. Ichihashi M, Ueda M, Budiyanto A et al UV‐induced skin damage. Toxicology 2003; 189(2): 21–39. [DOI] [PubMed] [Google Scholar]
- 9. Fan J, Zhuang Y, Li B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013; 5(1): 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryu J, Park SJ, Kim IH et al Protective effect of porphyra‐334 on UVA‐induced photoaging in human skin fibroblasts. Int J Mol Med 2014; 34(3): 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwon OS, Jung SH, Yang BS. Topical administration of manuka oil prevents UV‐B irradiation‐induced cutaneous photoaging in mice. Evid Based Complement Alternat Med 2013; 2013(6): 697–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124(3): 471–484. [DOI] [PubMed] [Google Scholar]
- 13. Inoki K. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth: cell. Cell 2006; 126(5): 955–968. [DOI] [PubMed] [Google Scholar]
- 14. Nacarelli T, Azar A, Sell C. Inhibition of mTOR prevents ROS production initiated by ethidium bromide‐induced mitochondrial DNA depletion. Front Endocrinol 2014; 5: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang B, Chen J, Cheng AS et al Depletion of sirtuin 1 (SIRT1) leads to epigenetic modifications of telomerase (TERT) gene in hepatocellular carcinoma cells. PLoS One 2014; 9(1): e84931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang J, Gan Q, Han L et al SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS One 2008; 3(3): e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 2007; 404(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salminen A, Kaarniranta K, Kauppinen A et al Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 2013; 14(2): 3834–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan RS, Dine K, Sarma JD et al SIRT1 activating compounds reduce oxidative stress mediated neuronal loss in viral induced CNS demyelinating disease. Acta Neuropathol Commun 2014; 2(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zarzuelo MJ, López‐Sepúlveda R, Sánchez M et al SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 2013; 85(9): 1288–1296. [DOI] [PubMed] [Google Scholar]
- 21. Qin J, Xie YY, Huang L et al Fluorofenidone inhibits nicotinamide adeninedinucleotide phosphate oxidase via PI3K/Akt pathway in the pathogenesis of renal interstitial fibrosis. Nephrology 2013; 18(10): 690–699. [DOI] [PubMed] [Google Scholar]
- 22. Salazar‐Montes A, Ruiz‐Corro L, López‐Reyes A, Castrejón‐Gómez E, Armendáriz‐Borunda J. Potent antioxidant role of Pirfenidone in experimental cirrhosis. Eur J Pharmacol 2008; 595(1–3): 69–77. [DOI] [PubMed] [Google Scholar]
- 23. Castro‐Torres RD, Chaparro‐Huerta V, Flores‐Soto ME et al A single dose of pirfenidone attenuates neuronal loss and reduces lipid peroxidation after kainic acid‐induced excitotoxicity in the pubescent rat hippocampus. J Mol Neurosci 2014; 52(2): 193–201. [DOI] [PubMed] [Google Scholar]
- 24. Zhang S, Cai G, Fu B et al SIRT1 is required for the effects of rapamycin on high glucose‐inducing mesangial cells senescence. Mech Ageing Dev 2012; 133(6): 387–400. [DOI] [PubMed] [Google Scholar]
- 25. Herrmann G, Brenneisen P, Wlaschek M et al Psoralen photoactivation promotes morphological and functional changes in fibroblasts in vitro reminiscent of cellular senescence. J Cell Sci 1998; 111: 759–767. [DOI] [PubMed] [Google Scholar]
- 26. Wang B, Xie HF, Li WZ et al Asymmetrical dimethylarginine promotes the senescence of human skin fibroblasts via the activation of a reactive oxygen species‐p38 MAPK‐microRNA‐138 pathway. J Dermatol Sci 2015; 78(2): 161–164. [DOI] [PubMed] [Google Scholar]
- 27. Hahn HJ, Kim KB, Bae S et al Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet A irradiation. Ann Dermatol 2016; 28: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Li S. Dandelion extracts protect human skin fibroblasts from UVB damage and cellular senescence. Oxid Med Cell Longev 2015; 2015: 619560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang B, Du R, Xiao X et al Microrna‐217 modulates human skin fibroblast senescence by directly targeting DNA methyltransferase 1. Oncotarget 2017; 8(20): 33475–33486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng J, Zou Y, Hu C et al Fluorofenidone attenuates bleomycin‐induced pulmonary inflammation and fibrosis in mice via restoring caveolin 1 expression and inhibiting mitogen‐activated protein kinase signaling pathway. Shock 2012; 38(5): 567–573. [DOI] [PubMed] [Google Scholar]
- 31. Takeda Y, Tsujino K, Kijima T et al Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer Adherence 2014; 8(8): 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peng ZZ, Gao‐Yun HU, Shen H et al Fluorofenidone attenuates collagen I and transforming growth factor‐beta1 expression through a nicotinamide adenine dinucleotide phosphate oxidase‐dependent way in NRK‐52E cells. Nephrology 2009; 14(6): 565–572. [DOI] [PubMed] [Google Scholar]
- 33. Zhuge CC, Xu JY, Zhang J et al Fullerenol protects retinal pigment epithelial cells from oxidative stress‐induced premature senescence via activating SIRT1. Invest Ophthalmol Vis Sci 2014; 55(7): 4628–4638. [DOI] [PubMed] [Google Scholar]
- 34. Back JH, Rezvani HR, Zhu Y et al Cancer cell survival following DNA damage‐mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)‐dependent Inhibition of sirtuin 1. J Biol Chem 2011; 286(21): 19100–19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ai TW, Kitada M, Kanasaki K et al SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP‐1 cells. Biochem Biophys Res Comm 2012; 427(1): 191–196. [DOI] [PubMed] [Google Scholar]
- 36. Braidy N, Jayasena T, Poljak A et al Sirtuins in cognitive ageing and Alzheimer's disease. Curr Opin Psychiatry 2012; 25(3): 226–230. [DOI] [PubMed] [Google Scholar]
- 37. Tillu DV, Melemedjian OK, Asiedu MN et al Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision‐induced acute and chronic pain. Mol Pain 2012; 8(2): 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang S, Ding Y, Yao Y. Inclusion complexes of fluorofenidone with beta‐cyclodextrin and hydroxypropyl‐beta‐cyclodextrin. Drug Dev Ind Pharm 2009; 35(35): 808–813. [DOI] [PubMed] [Google Scholar]


