Abstract
Aim
The SONAR trial uses an enrichment design based on the individual response to the selective endothelin receptor antagonist atrasentan on efficacy (the degree of the individual response in the urinary albumin‐to‐creatinine ratio [UACR]) and safety/tolerability (signs of sodium retention and acute increases in serum creatinine) to assess the effects of this agent on major renal outcomes. The patient population and enrichment results are described here.
Methods
Patients with type 2 diabetes with an estimated glomerular filtration rate (eGFR) within 25 to 75 mL/min/1.73 m2 and UACR between 300 and 5000 mg/g were enrolled. After a run‐in period, eligible patients received 0.75 mg/d of atrasentan for 6 weeks. A total of 2648 responder patients in whom UACR decreased by ≥30% compared to baseline were enrolled, as were 1020 non‐responders with a UACR decrease of <30%. Patients who experienced a weight gain of >3 kg and in whom brain natriuretic peptide exceeded ≥300 pg/mL, or who experienced an increase in serum creatinine >20% (0.5 mg/dL), were not randomized.
Results
Baseline characteristics were similar for atrasentan responders and non‐responders. Upon entry to the study, median UACR was 802 mg/g in responders and 920 mg/g in non‐responders. After 6 weeks of treatment with atrasentan, the UACR change in responders was −48.8% (95% CI, −49.8% to −47.9%) and in non‐responders was −1.2% (95% CI, −6.4% to 3.9%). Changes in other renal risk markers were similar between responders and non‐responders except for a marginally greater reduction in systolic blood pressure and eGFR in responders.
Conclusions
The enrichment period has successfully identified a population with a profound UACR reduction without clinical signs of sodium retention in whom a large atrasentan effect on clinically important renal outcomes is possible. The SONAR trial aims to establish whether atrasentan confers renal protection.
Keywords: atrasentan, diabetic kidney disease, endothelin receptor antagonist, precision medicine, randomized controlled clinical trial
1. INTRODUCTION
It has become increasingly apparent that the effects of treatment can vary among subgroups of patients with diabetic kidney disease.1, 2 Biomarker‐driven enrichment clinical trials are aimed at identifying individual patients who are more likely to benefit from new therapies and less likely to experience side effects; consequently, they have the potential to increase the success rate of confirmatory clinical trials and promote personalized medicine.
As described in the accompanying design manuscript, the Study Of diabetic Nephropathy with AtRasentan (SONAR trial) is a clinical trial that aims to test the renoprotective effects of the selective endothelin receptor antagonist atrasentan in patients at high risk of progressing to end‐stage renal disease.3, 4, 5 The trial design includes a biomarker‐based enrichment period to attempt to identify individuals most likely to benefit from the study drug prior to randomization. In the case of atrasentan, this means identifying patients based on significant clinical albuminuria reduction with minimal sodium retention. To achieve this, all those patients who fulfilled the entry criteria of the trial entered an enrichment period and received 0.75 mg/d of atrasentan for 6 weeks. Patients with a significant albuminuria response to atrasentan (≥30% reduction in urinary albumin‐to‐creatinine ratio [UACR]) without significant sodium and fluid retention proceeded to randomization, and they comprised the primary population for assessing the efficacy and safety of atrasentan. Approximately a thousand non‐responders (UACR reduction of <30% from baseline) were also randomized to double‐blind treatment in a parallel study stratum in order to determine if atrasentan delays progression of renal function decline in patients with a modest albuminuria reduction.3
This article describes the baseline characteristics of the atrasentan responder and non‐responder populations and the results of the enrichment period.
2. METHODS
2.1. Study population
The detailed design, rationale, and study endpoints of the SONAR trial are described in the accompanying publication.3 Briefly, the trial is being conducted at 795 clinical sites in 41 countries. All the patients are aged 18 to 85 years old with type 2 diabetes (T2D) and an estimated glomerular filtration rate (eGFR) of 25 to 75 mL/min/1.73 m2, a UACR ≥300 mg/g and creatinine <5000 mg/g, and a B‐type natriuretic peptide (BNP) ≤ 200 pg/mL. The main exclusion criteria were type 1 diabetes, a history of severe edema, pulmonary hypertension, or heart failure. In order to enter the enrichment period, patients had to be on a stable and maximum tolerated labelled dose of an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker and a diuretic, unless medically contraindicated. The study protocol is registered with http://clinicaltrials.gov (http://www.clinicaltrials.gov [NCT01858532]). The study protocol was approved by an independent ethics committee and local and central review boards. All participants signed a consent form before the start of any study‐specific procedure.
2.2. Enrichment period
Patients who fulfilled the entry criteria and then completed a run‐in period during which renin‐angiotensin aldosterone system inhibitors and/or diuretic treatment was optimized, received 6 weeks of open label treatment with atrasentan. Baseline UACR for the enrichment period was defined as the geometric mean of all available UACR values obtained at the last visit of the run‐in period and the first enrichment visit before atrasentan administration. A minimum of 3 out of 6 scheduled samples was required to proceed. Final UACR for the enrichment period was defined as the geometric mean of all available UACR values obtained at weeks 5 and 6 of the enrichment visits. A minimum of 3 out of 6 scheduled samples was required to proceed. A chemistry panel including serum creatinine, higher baseline glycated haemoglobin (HbA1c), potassium, albumin, and hematocrit, was measured at the start and end of enrichment. Blood pressure, body weight and peripheral edema assessment were recorded at the start of enrichment and then after 1, 2, 4 and 6 weeks.
Patients who experienced weight gain of >3 kg during enrichment, and in whom the absolute serum BNP exceeded ≥300 pg/mL (300 ng/L) at the last enrichment visit, were not eligible to proceed to the double‐blind treatment period. Also, patients in whom serum creatinine increased by more than 20% and 0.5 mg/dL during enrichment were not eligible to proceed to the double‐blind treatment period. Patients with a ≥30% reduction in UACR from the beginning to the end of the enrichment period were randomized into the responder stratum, whereas those with a <30% reduction in UACR were randomized into the non‐responder stratum.3 Randomization was stratified by geographic region, baseline UACR level (≤ or >1000 mg/g), and the category of UACR reduction achieved during the enrichment period. UACR reduction categories were defined as ≥60%, ≥45% to <60%, and ≥30% to <45% for the responder population, and ≥15% to <30%, ≥0% to <15%, and <0% for the non‐responder population.
2.3. Statistical analyses
In general, baseline characteristics with continuous data were reported as mean and standard deviation (SD), characteristics with skewed distribution were reported as median and interquartile range [IQR], and categorical variables were reported as number and percentage. The absolute changes (end of enrichment – start of enrichment) for all parameters during enrichment (except for UACR and BNP) were summarized. For UACR and BNP, the absolute changes on a log scale were calculated. A transformation was then applied to summarize the per cent change from baseline. For all mean changes 95% confidence intervals (CI) are provided. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Baseline characteristics
Out of the 11 088 patients screened, 5107 entered enrichment. Of these, 4703 patients completed enrichment, while 404 did not. A total of 2648 patients were responders and were enrolled in the UACR responder stratum, while 1889 were non‐responders, and a selection of 1020 of these patients were randomized into a separate non‐responder stratum. The baseline characteristics of the 2648 responders and 1020 non‐responders are shown in Table 1. Baseline characteristics were numerically similar between responders and non‐responders. (The possible impact of small differences in baseline characteristics on renal outcomes will be tested later upon completion of the SONAR trial.) The median entry UACR level in responders was 802 mg/g (25th to 75th percentile, 450‐1466 mg/g). In non‐responders it was 920 mg/g (25th to 75th percentile, 474‐1858 mg/g). A renin‐angiotensin system inhibitor was used in 98% of patients, and more than 80% used diuretic treatment (Table 1). A history of coronary artery disease was recorded in approximately 11% of the population at the start of enrichment.
Table 1.
Baseline characteristics in the SONAR randomized responder (≥30% decline in UACR) and non‐responder (<30% decline in UACR) population; both groups exclude patients with clinical signs of sodium retention and acute increases in serum creatinine; data are presented as mean (SD) unless otherwise noted
| Variable | Atrasentan respondersN = 2648 | Atrasentan non‐respondersN = 1020 |
|---|---|---|
| Age, years | 64.8 (8.7) | 63.7 (9.0) |
| Gender, n (%) | ||
| Male | 1965 (74.2) | 757 (74.2) |
| Female | 683 (25.8) | 263 (25.8) |
| Race, n (%) | ||
| White | 1497 (56.5) | 613 (60.1) |
| Black | 149 (5.6) | 75 (7.4) |
| Asian | 901 (34.0) | 297 (29.1) |
| Other | 101 (3.8) | 35 (3.4) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 613 (23.1) | 227 (22.3) |
| Other | 2035 (76.9) | 793 (77.7) |
| Weight, kg | 84.6 (19.3) | 86.9 (19.8) |
| Known duration of diabetes, years | 16.7 (9.1) | 15.8 (8.5) |
| Systolic blood pressure, mm Hg | 136.3 (15.0) | 135.9 (15.7) |
| Diastolic blood pressure, mm Hg | 74.9 (10.0) | 74.9 (9.8) |
| Serum albumin, g/L | 39.3 (3.5) | 38.6 (3.8) |
| Serum creatinine, μmol/L | 147.4 (42.0) | 155.6 (45.6) |
| eGFR, mL/min/1.73 m2 | 43.8 (13.7) | 41.8 (13.8) |
| Haemoglobin, g/L | 129.3 (16.9) | 129.0 (17.7) |
| HbA1c, % | 7.8 (1.5) | 7.8 (1.6) |
| Brain natriuretic peptide median (Q1‐Q3), (pg/mL) | 49.0 [26.0‐88.0] | 46.0 [26.0‐84.0] |
| Total cholesterol, mmol/L | 4.6 (1.2) | 4.6 (1.2) |
| LDL cholesterol, mmol/L | 2.7 (1.0) | 2.8 (1.0) |
| HDL cholesterol, mmol/L | 1.2 (0.4) | 1.1 (0.3) |
| Triglycerides, mmol/L | 2.5 (2.2) | 2.6 (2.0) |
| Serum potassium, mmol/L | 4.5 (0.6) | 4.5 (0.5) |
| UACR, median (Q1‐Q3), mg/g creatinine | 802 [450‐1466] | 920 [474‐1858] |
| Antihypertensives, n (%) | ||
| RAS inhibitors, n (%) | 2594 (98.0) | 998 (97.8) |
| Beta blockers, n (%) | 1103 (41.7) | 396 (38.8) |
| Calcium channel blockers, n (%) | 1526 (57.6) | 581 (57.0) |
| Diuretics, n (%) | 2186 (82.6) | 828 (81.2) |
| Loop diuretics | 1338 (50.5) | 522 (51.2) |
| Thiazides | 795 (30.0) | 275 (27.0) |
| Othera | 289 (10.9) | 115 (11.3) |
| Glucose‐lowering therapies, n (%) | ||
| Insulin glargine | 1654 (62.5) | 655 (64.2) |
| Metformin | 1009 (38.1) | 344 (33.7) |
| Sulphonylurea | 751 (28.4) | 280 (27.5) |
| Statins, n (%) | 2075 (78.4) | 809 (79.3) |
| History of coronary artery disease, n (%) | 287 (10.8) | 111 (10.9) |
| History of myocardial infarction, n (%) | 160 (6.0) | 74 (7.3) |
| History of diabetic retinopathy, n (%) | 905 (34.2) | 290 (28.4) |
Other includes chlortalidone, indapamide, mefruside, metolazone, tripamide and xipamide.
Although numerical differences were small in all parameters, differences between responders and non‐responders reached statistical significance (P ≤ .05) for the following characteristics: age, race, bodyweight, known duration of diabetes, serum albumin, serum creatinine, eGFR, metformin use, and history of diabetic retinopathy.
3.2. Responses in albuminuria and other cardio‐renal risk markers
A large difference in albuminuria response was observed between atrasentan responders and non‐responders. After 6 weeks of treatment with atrasentan, median UACR was reduced in responders to 401 mg/g, representing a mean change from baseline of −48.8% (95% CI, −49.8% to −47.9%). In non‐responders the median UACR after 6 weeks of treatment with atrasentan was 867 mg/g, representing a mean change from baseline of −1.2% (95% CI, −6.4% to 3.9%) (Figure 1). Among the randomized responders (N = 2648), 591 participants (22.3%) were randomized in the ≥60% UACR reduction stratum; 968 (36.6%) in the ≥45% to <60% UACR reduction stratum; and 1089 (41.1%) in the ≥30% to <45% UACR reduction stratum. Among the randomized non‐responders (N = 1020), 479 participants (47.0%) were in the ≥15% to <30% UACR reduction stratum; 286 (28.0%) in the ≥0% to <15% UACR reduction stratum; and 255 (25.0%) in the UACR >0% increase stratum.
Figure 1.
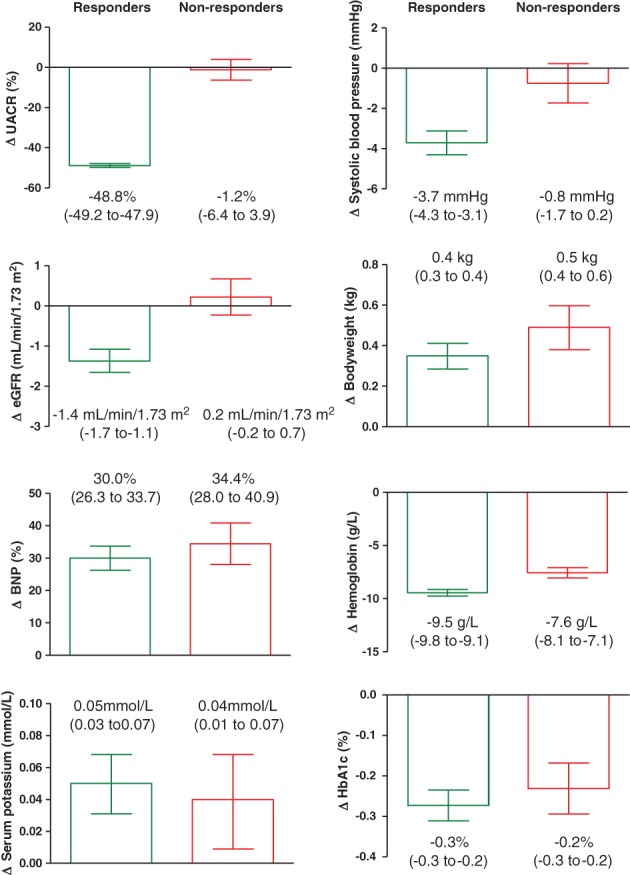
Changes in renal risk markers after 6 weeks of treatment with atrasentan. Data are presented as mean (95% CI). Numbers below each bar indicate mean (95% CI) change. Although numerical differences were small, due to the large samples, size differences between responders and non‐responders reached statistical significance (P ≤ .05) in all parameters except BNP, potassium, and Hba1c
The effects of atrasentan on all other measured cardio‐renal risk markers were similar for both responders and non‐responders (Figure 1), except for systolic blood pressure and eGFR, both of which showed a larger reduction in the responder group. While the mean change in BNP was approximately 30% in both responders and non‐responders, that change was attributed to outliers because median changes were approximately 8% in both responders and non‐responders.
Upon entering the enrichment period, 42 patients in the responder population and 9 patients in the non‐responder population used a sodium glucose co‐transporter 2 (SGLT2) inhibitor. The effect of atrasentan on UACR reduction was consistent and similar among those participants who used and didn't use SGLT2 inhibitors, both in the responder and non‐responder populations (Table 2).
Table 2.
Change from baseline (mean and 95% CI) in UACR in patients with and without concomitant SGLT‐2 inhibitor use during the enrichment period
| N | No SGLT2 at start of enrichment | N | SGLT2 at start of enrichment | |
|---|---|---|---|---|
| Atrasentan responders | 2603 | −48.8% (−47.8 to −49.7) | 42 | −52.3% (−56.7 to −47.8) |
| Atrasentan non‐responders | 1007 | −1.3% (−6.5 to +3.9) | 9 | +10.4% (−29.1 to +50.0) |
4. DISCUSSION
SONAR is the first clinical trial in diabetic kidney disease that employs a biomarker‐based enrichment design to better identify patients who may respond to and tolerate a new investigational drug. The completion of the enrichment phase of the SONAR trial illustrates the practical feasibility of this approach. The results show that the enrichment period has differentiated the UACR response from the UACR non‐response population. Interestingly, the baseline characteristics were similar for both populations, indicating that the UACR response to atrasentan is unlikely to be predicted by any of the usual baseline characteristics. Responses in systolic blood pressure and eGFR were slightly larger in responders compared to non‐responders, but responses in other cardio‐renal risk markers were similar between the 2 populations.
The baseline characteristics show that the SONAR trial enrolled a population at high risk of progressive decline in kidney function. In the responder population more than half of all patients had a UACR >800 mg/g, and more than half had an eGFR <42 mL/min/1.73 m2. The population was generally well treated with drugs aimed at improving important cardiovascular and renal risk factors. Almost all the patients received multiple treatments to control their blood pressure, and yet mean blood pressure remained above the recommended target, highlighting the difficulty in treating hypertension when kidney function declines. By design, the use of diuretic treatment was recommended in the protocol because endothelin receptor antagonists (ERAs) can cause sodium and fluid retention. More than 80% of all patients received such treatment, which is higher than in other trials with similar patients.6, 7 Insulin was used in more than 60% of all patients, and statins in nearly 80%.
The similarity in baseline characteristics suggests that none of the clinically used biochemical or physical parameters can be used to differentiate between atrasentan responder and non‐responder patients, and may also be unable to detect the risks of progressing to end‐stage renal or cardiovascular disease upon entry to the SONAR trial. Further examination of the stored plasma and urine samples is required to determine if novel biomarkers measured before atrasentan exposure can help in differentiating atrasentan responders from non‐responders.
Responses in most cardio‐renal risk markers were remarkably similar in the responder and non‐responder populations, except for eGFR and systolic blood pressure. It is, however, very unlikely that the small difference in blood pressure changes between responders and non‐responders explains the large separation in UACR responses. The reduction in eGFR in the responder population probably reflects a reduction in intraglomerular pressure as ERAs acutely modify renal vascular tone, and may in part explain the reduction in UACR.8, 9 Intriguingly, changes in bodyweight, BNP and haemoglobin, as proxies of sodium and fluid retention, were similar for atrasentan responders and non‐responders. These data support previous findings indicating that the sodium and fluid retention response to atrasentan is not coupled to the albuminuria‐lowering effect.10 The uncoupling of the albuminuria response from responses in other cardio‐renal risk markers is not unique to atrasentan. Previous studies with SGLT2 inhibitors and glucagon‐like peptide 1 receptor antagonists reported that the albuminuria‐lowering effect of these agents occurs regardless of responses in other cardio‐renal risk markers.11, 12, 13 Because only modest differences in blood pressure and eGFR were noted, and all other measured biochemical and physical cardio‐renal risk markers were similar for atrasentan responders and non‐responders, it is possible to speculate that any potential renoprotective effect of atrasentan is probably mediated by a reduction in UACR, although other unmeasured effects may be involved as well.
During the recruitment for the SONAR trial, SGLT2 inhibitors were already being used by a few patients. Based on the positive results of recent and ongoing trials with these agents in patients with T2D, SGLT2 inhibitors may become part of the standard therapeutic management to delay progression of renal disease in patients with T2D. This study's discovery that the albuminuria‐lowering effects of atrasentan are similar regardless of concomitant SGLT2 use suggests that ERAs could complement SGLT2 inhibitors in delaying progressive renal function decline. A drug–drug interaction study is required to test this hypothesis.
The large UACR reduction during the enrichment phase had important implications for the predefined design of the SONAR trial. Throughout the ongoing follow‐up period of the SONAR trial, the steering committee and AbbVie regularly reviewed the event rate for the primary renal outcomes, and they observed a much lower event rate than had originally been expected. If this was not due to a large treatment effect, then this lower than expected event rate could reduce the predefined power of the trial. Although it was possible that the carefully selected enrolled population had a genuinely very low renal risk, this explanation appeared improbable, considering that the baseline and response characteristics of the responder population were similar to those of the non‐responder population. On the other hand, the large UACR reduction observed in the responder population supports the possibility of a large renoprotective effect.
The lower than expected event rate meant that, in order to collect the planned 425 primary endpoints, a very long follow‐up period would be needed, and that was not feasible. Consequently, the sponsor made a decision not to continue with the ongoing follow‐ups, and the clinical trial sites were notified accordingly in late 2017. At the point when the trial was stopped, more than 121 projected renal events were set to be accumulated, resulting in more than 90% power to detect a hazard ratio of 0.55, and more than 80% power to detect a hazard ratio of 0.60. While these effect sizes are larger than originally planned (the trial had been designed to detect a hazard ratio of 0.73), both the CANVAS and EMPAREG trials observed risk reduction of a similar magnitude, despite reductions in albuminuria that were of a lesser magnitude than those observed among the responder population in SONAR.14, 15 The results of the SONAR trial are expected to be available in 2018.
In conclusion, the SONAR trial enrolled 3668 patients with T2D mellitus and kidney disease who are at high risk of progressive renal dysfunction and cardiovascular events. The enrichment period successfully identified an atrasentan UACR responder population which could not be separated from non‐responders by a standard panel of biochemical or physical characteristics. This selection of patients showing a profound UACR reduction without clinical signs of sodium and fluid retention supports the possibility of a large atrasentan effect on renal outcomes of clinical and regulatory importance. The final results of the SONAR trial will deliver definitive evidence of the long‐term efficacy and safety of atrasentan in this enriched population.
ACKNOWLEDGEMENTS
The sponsor, AbbVie Inc., and the authors thank the participants in the clinical trial and all study investigators for their contributions.
Conflict of interest
H. J. L. H. is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Janssen, and Merck. He has a policy of all honoraria being paid to his employer.
D. d. Z. is a consultant for, and received honoraria (to employer) from AbbVie, Astellas, Bayer, Boehringer Ingelheim, Novo Nordisk, Fresenius, Janssen, and Mitsubishi Tanabe.
D. K. consults for AbbVie and has received grant support from the US National Institutes of Health (NIH). H.‐H. P. has equity in Merck and Novo Nordisk and has received consulting and lecture fees from AstraZeneca, Abbott, Novartis, and Reata. D. W. K. declares the following relationships: consultant for AbbVie, Relypsa, Corvia Medical, and Bayer; research grant funding from Novartis, Bayer, and St. Luke's Hospital of Kansas City; and stock ownership in Gilead Sciences. R. C. R. is a member of the Steering Committee of SONAR and has received honoraria from AbbVie, AstraZeneca, and Boehringer Ingelheim, and has lectured for Amgen, Takeda, AstraZeneca, and Roche. F. F. H. is a consultant for, and received honoraria from AbbVie and AstraZeneca. G. B. is a consultant for Bayer, Merck, KBP, Relypsa, Janssen, and AbbVie. G. B. is principal investigator of the FIDELIO trial (Bayer), and serves on steering committees for the CREDENCE (Janssen) and SONAR trials (AbbVie). H. M. is a consultant for AbbVie and Teijin, receives speaker honoraria from Astellas, Boehringer Ingelheim, MSD, and Tanabe Mitsubishi. J. M. declares the following relationships: consultant for AbbVie, Amgen, AstraZeneca, BMS, Cardiorentis, Dalcor, GlaxoSmithKline, Roche Pharmaceuticals, Merck, Novartis, Pfizer, Theracos, Oxford University/Bayer, and Kings College London/Vifor‐Fresenius Pharma. Honoraria are paid to his employer, Glasgow University. S. T. is a member of the steering committee of SONAR and has received consultant fees from AbbVie and Bayer, and has received honoraria from Servier and Valeant. V. P. serves on steering committees for trials funded by AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer; and serves on advisory boards and/or has spoken at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol‐Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae. He has a policy of all honoraria being paid to his employer. J. J. B., J. D., D. L. A., T. T. Y. and M. W. are employees of AbbVie and may own stock or stock options.
Author contributions
H. J. L. H. and D. d. Z. wrote the draft of this report. T. Y. performed statistical analyses. All the authors contributed to interpretation and critical revision of the publication. D. d. Z. takes full responsibility for this report.
Heerspink HJL, Andress DL, Bakris G, et al. Baseline characteristics and enrichment results from the SONAR trial. Diabetes Obes Metab. 2018;20:1829–1835. 10.1111/dom.13315
Funding information This study was supported by AbbVie Inc.
REFERENCES
- 1. de Zeeuw D, Heerspink HJ. Unmet need in diabetic nephropathy: failed drugs or trials? Lancet Diabetes Endocrinol. 2016;4:638‐640. [DOI] [PubMed] [Google Scholar]
- 2. de Zeeuw D, Heerspink HJL, Jardine M, Perkovic V. Renal trials in diabetes need a platform: time for a global approach? Lancet Diabetes Endocrinol. 2017. [Epub ahead of print]. 10.1016/S2213-8587(17)30263-2. [DOI] [PubMed] [Google Scholar]
- 3. Heerspink HJL, Andress DL, Bakris G, et al. Rationale and protocol of the Study of Diabetic Nephropathy with Atrasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018. 10.1111/dom.13245. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohan DE, Pritchett Y, Molitch M, et al. Addition of atrasentan to renin‐angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22:763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parving HH, Brenner BM, McMurray JJ, et al. Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio‐Renal Endpoints (ALTITUDE). J Renin Angiotensin Aldosterone Syst. 2012;13:387‐393. [DOI] [PubMed] [Google Scholar]
- 7. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892‐1903. [DOI] [PubMed] [Google Scholar]
- 8. Dhaun N, MacIntyre IM, Kerr D, et al. Selective endothelin‐A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57:772‐779. [DOI] [PubMed] [Google Scholar]
- 9. Dhaun N, Macintyre IM, Melville V, et al. Blood pressure‐independent reduction in proteinuria and arterial stiffness after acute endothelin‐A receptor antagonism in chronic kidney disease. Hypertension. 2009;54:113‐119. [DOI] [PubMed] [Google Scholar]
- 10. Kohan D, Heerspink HJL, Coll B, et al. Atrasentan‐associated fluid retention in patients with diabetic nephropathy: predictive markers and impact on albuminuria reduction. Clin J Am Soc Nephrol. 2015;10:1568‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heerspink HJ, Johnsson E, Gause‐Nilsson I, et al. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin‐angiotensin blockers. Diabetes Obes Metab. 2016;18:590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, HJL H. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12:751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin‐to‐creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA‐REG OUTCOME randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2017;5:610‐621. [DOI] [PubMed] [Google Scholar]
- 14. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 15. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]


