Abstract
Background
Despite the growing body of evidence on growth differentiation factor 15 (GDF-15) reference values for patients with existing cardiovascular disease, limited investigation has been dedicated to characterizing the distribution and prognostic impact of GDF-15 in predominantly healthy populations. Furthermore, current cutoff values for GDF-15 fail to account for the well-documented age-dependence of circulating GDF-15.
Methods
From 810 community-dwelling older adults, we selected a group of apparently healthy participants (n = 268). From this sample, circulating GDF-15 was modeled using the generalized additive models for location scale and shape (GAMLSS) to develop age-dependent centile values. Unadjusted and adjusted Cox proportional hazards models were used to assess the association between the derived GDF-15 reference values (expressed as centiles) and all-cause mortality.
Results
Smoothed centile curves showed increasing GDF-15 with age in the apparently healthy participants. An approximately three-fold difference was observed between the 95th and 5th GDF-15 centiles across ages. In a median 8.0 years of follow-up, 97 all-cause deaths were observed in 806 participants with eligible values. In unadjusted Cox regression analyses, the hazard ratio (95% CI) for all-cause mortality per 25-unit increase in GDF-15 centile was 1.80 (1.48–2.20) and dichotomized at the 95th centile, ≥95th versus <95th, was 3.04 (1.99–4.65). Age-dependent GDF-15 centiles remained a significant predictor of all-cause mortality in all subsequent adjusted models.
Conclusions
Age-dependent GDF-15 centile values developed from a population of apparently healthy older adults are independently predictive of all-cause mortality. Therefore, GDF-15 reference values could be a useful tool for risk-stratification in a clinical setting.
ClinicalTrials.gov Identifier
Key words: All-cause mortality, GDF-15, reference values, survival analysis, protein biomarkers
Introduction
Recent scholarship has called attention to the importance of growth differentiation factor 15 (GDF-15) as a protein biomarker with valuable prognostic utility for predicting adverse outcomes in patients with cardiovascular disease (CVD). GDF-15 is a member of the transforming growth factor-beta superfamily of cytokines and is expressed in low quantities across most cell types under physiological conditions (1–3). Expression of GDF-15 is strongly induced by pro-inflammatory cytokines (1) and cellular insults such as ischemia (4), oxidative stress (5,6), and biomechanical stress to the myocardium (7). Accordingly, circulating levels of GDF-15 are characteristically elevated in patients with neoplastic disease, heart failure, and acute coronary syndrome (2,8–10). The precise mechanisms by which GDF-15 exerts its bioactivity in these conditions remains uncertain. However, evidence suggests that GDF-15 functions as an autocrine anti-inflammatory and cellular repair factor (1,4,11,12), secreted in quantities commensurate to acute and chronic tissue injury (3,13).
In both the general population and in the context of existing CVD, circulating GDF-15 is associated with a variety of risk factors and biomarkers for cardiovascular outcomes including age, blood pressure, diabetes mellitus, and N-terminal pro-brain natriuretic peptide (NT-proBNP) (2,10,14–16). Despite the growing body of evidence on GDF-15 reference values for patients with existing CVD, limited investigation has been dedicated to characterizing the distribution and prognostic impact of GDF-15 in predominantly healthy populations. Considering the mounting interest in prognostic biomarkers to support risk-stratification and clinical decision-making, the aim of the present study was two-fold: to determine reference values for the distribution of GDF-15 in a population of apparently healthy older adults and evaluate the degree to which the reference values predict all-cause mortality.
Methods
Study population
The participants were recruited from the control group of the Västmanland Myocardial Infarction Study (VaMIS; ClinicalTrials.gov Identifier: NCT01452178). A comprehensive description of the control group has been reported elsewhere (17). Briefly, patients hospitalized for acute myocardial infarction at Västmanland County Hospital, Västerås, Sweden from November 2005 to May 2011 were consecutively included in the VaMIS study. Control subjects were continuously recruited from the general population during the inclusion of the VaMIS patients and matched 1:1 by closest date of birth, same sex, and same municipality to each patient.
Data collection
All subjects underwent clinical examination, echocardiographic evaluation, and blood sampling.
At the baseline examination, demographics and medical history were collected. Patient-reported diagnoses of myocardial infarction (MI), transient ischemic attack (TIA)/stroke, and diabetes mellitus were confirmed from medical records. History of heart failure, cancer, hypertension, and medication use were self-reported. By the use of echocardiography, left ventricular systolic dysfunction was defined as having a left ventricular ejection fraction (LVEF) of <45% and significant valvular disease as having moderate to severe stenosis or regurgitation in the aortic or mitral valves. Peripheral artery disease was determined present if the participant had an ankle–brachial index (ABI) value <0.91 or >1.39 (18). Non-sinus rhythm was determined by electrocardiography. Other major electrocardiographic (ECG) changes were based on the Minnesota code (MC) and determined present if any of the following conditions were met: Q wave (MC 1.1–1.2), ST segment depression (MC 4.1–4.2), T wave change (MC 5.1–5.2), A-V conduction defect (MC 1.1–1.2), ventricular conduction defect (MC 1.1–1.2), or left ventricular hypertrophy (S wave amplitude in lead V1 + R wave amplitude in V5 or V6 > 3.5 mV combined with MC 4.1–4.2 or 5.1–5.2 in lead V5 or V6). Blood pressure was measured manually and is reported as the mean of two measurements on the left arm after five minutes of rest with the subject in a seated position. Information on smoking status and leisure-time physical activity was self-reported.
Blood sampling procedure
Blood samples were taken in 5 mL lithium heparin-coated vacuum tubes and centrifuged at 2000g for 10 min (Becton Dickinson and Co.) or 2200g for 10 min (Vacuette, Greiner Bio-One). Plasma was reallocated to 5 mL plastic tubes and frozen at −70° Celsius within two hours. Plasma samples remained stored at −70° Celsius. Before analysis, the samples were thawed at room temperature, mixed and centrifuged at 3470g at 4° Celsius for 15 min, and robotically aliquoted into a microtiter plate by the Tecan Freedom Evolyzer system.
Group selection
From the control group of the VaMIS study (n = 855), we excluded individuals with missing values for circulating GDF-15 (n = 39), missing values for group selection (n = 5), and extreme measurements of GDF-15 reflected by standard measurements and secondly by the Olink Proximity Extension Assay chip (Olink Bioscience) with otherwise standard demographics and medical history (n = 1). To characterize the distribution of GDF-15 in older adults of apparently good health, subjects with a history of MI (n = 32), TIA/stroke (n = 47), heart failure (n = 21), hypertension (n = 375), LVEF <45% (n = 20), significant valvular disease (n = 11), peripheral artery disease (n = 70), angina pectoris (n = 56), non-sinus rhythm (n = 37), major ECG changes (n = 134), cancer (n = 77), diabetes (n = 75), body mass index >35 (n = 21), and those taking regular cardiovascular medication (n = 399) were designated to the ‘unhealthy’ group (n = 542), and the remaining subjects were designated to the ‘apparently healthy’ group (n = 268), resulting in a total of 810 participants included in the present study. All participants provided written informed consent. The Ethics Committee of Uppsala, Sweden approved the study (Protocol number: 2005:169).
Biochemistry
Plasma concentrations of GDF-15 were measured using a commercially available sandwich immunoassay using monoclonal antibodies and biotin-streptavidin separation (Elecys GDF-15 and Cobas e411; Roche Diagnostics, Germany). LDL cholesterol was estimated using Friedewald’s formula.
End-point and follow-up
The primary end-point was all-cause mortality. All-cause mortality was selected as the end-point to account for the frequent uncertainty in attributing the cause of death in older adults as well as to extend previous lines of investigation between GDF-15 and all-cause mortality. Study participants were followed until 9 March 2017, with a median (1st to 3rd quartile) follow-up of 8.0 (6.8–9.4) years.
Statistical analysis
Data are presented as median (interquartile range) for continuous variables and number (percentage) for categorical variables, as indicated. Differences in baseline characteristics and GDF-15 between healthy and unhealthy participants were tested using Mann–Whitney U and chi-square tests. Clinical and biochemical quantities stratified by GDF-15 quartiles were compared using Kruskal–Wallis and chi-square tests. Spearman rank correlation was used to evaluate the relationship between GDF-15 and continuous clinical and biochemical variables that reached statistical significance across GDF-15 quartiles. Reference values presented as centile curves were generated to describe GDF-15 values as a function of age using the generalized additive models for location scale and shape (GAMLSS). This relationship was modeled using the lognormal distribution. Ten-fold cross-validation was used to validate the GAMLSS model, with −2 × log(likelihood) of the fitted GAMLSS model calculated as the performance measure.
Cox proportional hazards models were used to assess the association between GDF-15 reference values and all-cause mortality. GDF-15 centile values corresponding to each participant’s age and GDF-15 values were calculated based on the centile curves; these values entered adjusted and unadjusted models as a continuous variable. Adjusted model 1 was adjusted for age and sex. For a subsequent adjusted model, best subset selection using a golden section search algorithm (BeSS package version 1.0.3) was used to determine the optimal subset of parameters among the following variables: body mass index, smoking status, systolic blood pressure, NT-proBNP, and cystatin C. The model that yielded the smallest Akaike information criterion (AIC) included the following explanatory variables: smoking status, NT-proBNP, and cystatin C. In Adjusted model 2, these covariates were included with GDF-15, age, and sex. Crude and adjusted Cox regression models were also calculated using GDF-15 centiles dichotomized at the 95th centile. A separate best subset selection with the dichotomized GDF-15 centile variable yielded an identical set of covariates. Age was included in adjusted models using a restricted cubic spline with three knots. NT-proBNP was log-transformed (logNT-proBNP) in adjusted models. The proportional hazard assumption was verified for all explanatory variables by inspection of scaled Schoenfeld residual plots and for the entire model by a test of independence between the scaled Schoenfeld residuals and time. Inspection of Martingale residual plots was used to verify the linearity assumption. Additionally, the Kaplan–Meier estimator was used to estimate the survival function of all-cause mortality by GDF-15 centile dichotomized at the 95th centile; differences in survival curves were compared using the log-rank test.
All statistical analyses were performed using R 3.4.3 (R Foundation for Statistical Computing, 2017, Vienna, Austria; http://www.r-project.org). Two-sided P values <0.05 were considered to be statistically significant.
Results
Baseline characteristics
Apparently healthy participants were younger, had a lower proportion of overweight (BMI 25–29.9) and obese (BMI ≥ 30) individuals, included fewer current or previous smokers, and engaged in more strenuous leisure-time physical activity compared with unhealthy participants (Table 1). Significant differences in systolic blood pressure and blood lipid profiles were also observed between the apparently healthy and unhealthy groups; healthy participants had higher total and LDL cholesterol compared with unhealthy participants. No significant differences in sex or diastolic blood pressure were observed between the apparently healthy and unhealthy groups. The distribution of GDF-15 was significantly different between apparently healthy and unhealthy participants (P < 0.0001; Supplementary Figure 1).
Table 1.
Baseline characteristics of study participants.
| Variablea | All controls (n = 810) | Apparently healthy (n = 268) | Unhealthy (n = 542) | P valueb |
|---|---|---|---|---|
| Age (years) | 68 (14) | 63.5 (14) | 70 (13) | <0.0001 |
| Sex | 0.5947 | |||
| Female | 238 (29.4) | 75 (28) | 163 (30.1) | |
| Male | 572 (70.6) | 193 (72) | 379 (69.9) | |
| Body mass index (kg/m2) | <0.0001 | |||
| <25 | 293 (36.2) | 127 (47.4) | 166 (30.6) | |
| 25–29.9 | 386 (47.7) | 113 (42.2) | 273 (50.4) | |
| ≥30 | 131 (16.2) | 28 (10.4) | 103 (19) | |
| Systolic blood pressure (mmHg) | 145 (28) | 140 (24.3) | 147.8 (26.5) | <0.0001 |
| Diastolic blood pressure (mmHg) | 80 (13.8) | 80 (11) | 80 (16) | 0.1108 |
| Smoking status | 0.0303 | |||
| Never smoked | 348 (43) | 130 (48.5) | 218 (40.2) | |
| Current or previous smoker | 462 (57) | 138 (51.5) | 324 (59.8) | |
| Physical activity, leisure-time | <0.0001 | |||
| Low to mild | 514 (63.5) | 138 (51.5) | 376 (69.4) | |
| Moderate to strenuous | 296 (36.5) | 130 (48.5) | 166 (30.6) | |
| Biochemical analyses | ||||
| Triglycerides (mmol/L) | 1.2 (0.7) | 1.1 (0.6) | 1.2 (0.8) | 0.0021 |
| Total cholesterol (mmol/L) | 5.6 (1.7) | 5.9 (1.3) | 5.4 (1.9) | <0.0001 |
| HDL cholesterol (mmol/L) | 1.28 (0.54) | 1.35 (0.55) | 1.25 (0.52) | 0.0038 |
| LDL cholesterol (mmol/L) | 3.6 (1.6) | 3.9 (1.1) | 3.4 (1.6) | <0.0001 |
| Fasting plasma glucose (mmol/L) | 5.9 (1.2) | 5.6 (0.9) | 6 (1.3) | <0.0001 |
Continuous variables are presented as median (interquartile range). Categorical variables are presented as number (percentage).
Mann–Whitney U test for continuous variables. Chi-square test for categorical variables.
Clinical and biochemical associations with circulating GDF-15
To examine within-group relationships between GDF-15 and associated clinical and biochemical quantities, quartiles of GDF-15 were generated for both the apparently healthy and unhealthy groups. Among apparently healthy participants, increasing GDF-15 quartile was associated with age (P < 0.0001), systolic blood pressure (P = 0.0059), NT-proBNP (P < 0.0001), and cystatin C (P < 0.0001). No significant associations were found between increasing GDF-15 quartile and BMI, sex, smoking status, diastolic blood pressure, or C-reactive protein (CRP) (Table 2; Supplementary Figure 2).
Table 2.
Clinical and biochemical factors by quartiles of GDF-15 in apparently healthy participants.
| GDF-15 quartile (ng/L) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Variablea | Total | (400–654) | (656–897) | (897–1154) | (1159–3950) | P valueb |
| Number | 268 | 67 | 67 | 67 | 67 | |
| Demographics | ||||||
| Age (years) | 63.5 (14) | 55 (10.5) | 63 (12) | 67 (10) | 70 (12) | <0.0001 |
| Body mass index (kg/m2) | 25.2 (4) | 25.6 (3.7) | 24.8 (3.7) | 25.4 (3.4) | 25 (5.1) | 0.6698 |
| Sex, female | 75 (28) | 13 (19.4) | 20 (29.9) | 23 (34.3) | 19 (28.4) | 0.2717 |
| Medical history | ||||||
| Current or previous smoker | 138 (51.5) | 35 (52.2) | 31 (46.3) | 36 (53.7) | 36 (53.7) | 0.7974 |
| Systolic blood pressure (mmHg) | 140 (24.3) | 131 (21.3) | 141.5 (20.5) | 143 (23) | 144 (31) | 0.0059 |
| Diastolic blood pressure (mmHg) | 80 (11) | 80 (11.3) | 80 (11) | 79 (9) | 80 (10) | 0.6224 |
| Biochemical analyses | ||||||
| NT-proBNP (ng/L) | 57 (68.25) | 44 (45) | 51 (54) | 57 (69.5) | 89 (126) | <0.0001 |
| CRP (mg/L) | 1.2 (1.51) | 1.1 (1.13) | 1.1 (1.25) | 1.2 (1.25) | 1.5 (2.02) | 0.1011 |
| Cystatin C (mg/L) | 1.04 (0.19) | 1 (0.15) | 0.98 (0.2) | 1.06 (0.15) | 1.15 (0.23) | <0.0001 |
Continuous variables are presented as median (interquartile range). Categorical variables are presented as number (percentage).
Kruskal–Wallis test for continuous variables. Chi-square test for categorical variables.
CRP = C-reactive protein; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Among unhealthy participants, increasing GDF-15 quartile was associated with age (P < 0.0001), diastolic blood pressure (P = 0.0012), NT-proBNP (P < 0.0001), CRP (P = 0.0025), and cystatin C (P < 0.0001). Furthermore, increasing GDF-15 quartile was associated with the majority of clinical exclusion criteria used to select the apparently healthy participants. No significant associations were observed between increasing GDF-15 quartile and BMI, sex, smoking status, or systolic blood pressure (Table 3; Supplementary Figure 3).
Table 3.
Clinical and biochemical factors by quartiles of GDF-15 in unhealthy participants.
| GDF-15 quartile (ng/L) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Variablea | Total | (417–854) | (855–1186) | (1187–1627) | (1632–11063) | P valueb |
| Number | 542 | 136 | 135 | 136 | 135 | |
| Demographics | ||||||
| Age (years) | 70 (13) | 61.5 (12) | 69 (13) | 72 (10) | 75 (8) | <0.0001 |
| BMI (kg/m2) | 26.9 (5) | 26.9 (4.6) | 26.7 (5) | 26.8 (4.8) | 27.1 (5.6) | 0.8573 |
| Sex, female | 163 (30.1) | 44 (32.4) | 49 (36.3) | 36 (26.5) | 34 (25.2) | 0.1580 |
| Medical history | ||||||
| Current or previous smoker | 324 (59.8) | 74 (54.4) | 79 (58.5) | 90 (66.2) | 81 (60) | 0.2576 |
| Systolic blood pressure (mmHg) | 147.8 (26.5) | 144.5 (25.9) | 148 (26) | 149 (28.3) | 149 (30) | 0.2184 |
| Diastolic blood pressure (mmHg) | 80 (16) | 84 (14) | 80 (12.5) | 80 (17.3) | 79.5 (14.8) | 0.0012 |
| Hypertension | 375 (69.2) | 90 (66.2) | 94 (69.6) | 100 (73.5) | 91 (67.4) | 0.5736 |
| Diabetes mellitus | 75 (13.8) | 4 (2.9) | 9 (6.7) | 17 (12.5) | 45 (33.3) | <0.0001 |
| Non-sinus rhythm | 37 (6.8) | 4 (2.9) | 7 (5.2) | 9 (6.6) | 17 (12.6) | 0.0125 |
| Major ECG changes | 134 (25.1) | 28 (20.9) | 25 (19.2) | 38 (27.9) | 43 (32.3) | 0.0478 |
| Angina pectoris | 56 (10.3) | 8 (5.9) | 12 (8.9) | 14 (10.3) | 22 (16.3) | 0.0385 |
| Myocardial Infarction | 32 (5.9) | 0 (0) | 10 (7.4) | 12 (8.8) | 10 (7.4) | 0.0084 |
| Heart failure | 21 (3.9) | 0 (0) | 2 (1.5) | 8 (5.9) | 11 (8.1) | 0.0013 |
| LVEF <45% | 20 (3.7) | 1 (0.7) | 4 (3) | 7 (5.2) | 8 (6) | 0.0967 |
| Significant valvular disease | 11 (2) | 2 (1.5) | 4 (3) | 3 (2.2) | 2 (1.5) | 0.7939 |
| TIA/stroke | 47 (8.7) | 4 (2.9) | 11 (8.1) | 13 (9.6) | 19 (14.1) | 0.0129 |
| Peripheral artery disease | 70 (12.9) | 12 (8.8) | 11 (8.1) | 17 (12.5) | 30 (22.2) | 0.0017 |
| Cancer | 77 (14.2) | 9 (6.6) | 18 (13.3) | 24 (17.6) | 26 (19.4) | 0.0130 |
| Biochemical analyses | ||||||
| NT-proBNP (ng/L) | 104.5 (189.5) | 62.5 (68) | 108 (139.5) | 145 (237.8) | 167 (428.5) | <0.0001 |
| CRP (mg/L) | 1.55 (2.23) | 1.25 (1.53) | 1.4 (1.75) | 1.8 (2.53) | 1.8 (2.97) | 0.0025 |
| Cystatin C (mg/L) | 1.12 (0.24) | 1.03 (0.18) | 1.09 (0.19) | 1.17 (0.23) | 1.23 (0.34) | <0.0001 |
Continuous variables are presented as median (interquartile range). Categorical variables are presented as number (percentage).
Kruskal–Wallis test for continuous variables. Chi-square test for categorical variables.
CRP: C-reactive protein; ECG: electrocardiographic; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-brain natriuretic peptide; TIA: transient ischemic attack.
Age-dependent reference values for GDF-15 in apparently healthy older adults
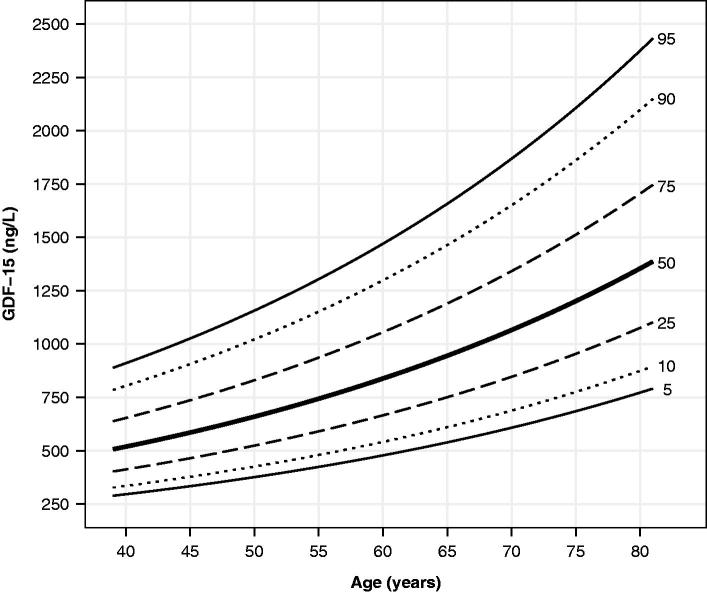
Reference values of GDF-15 by age for healthy participants are displayed as centile curves in Figure 1 (corresponding tabulated values are presented in Supplementary Table 1). The range of expected values broadened with age and centile magnitude. Expected values at the 50th percentile differed more than two-fold between the oldest and youngest healthy participants. Moreover, there was an approximately three-fold difference between the 95th and 5th centiles observed across ages. Ten-fold cross-validation of the GAMLSS model that generated the age-dependent GDF-15 centiles showed a high degree of similarity in model performance. Namely, the −2 × log(likeli-hood) value of the model trained on all 268 observations was 3832.7, and the cross-validated −2 × log(likelihood) was 3837.0.
Figure 1.
Smoothed centile curves showing circulating GDF-15 concentration by age in apparently healthy participants (n = 286).
Prognostic impact of GDF-15 reference values
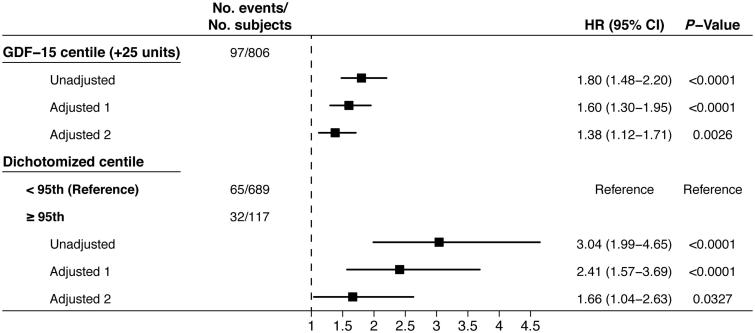
A total of 97 all-cause deaths were observed during follow-up in 806 participants with complete values for adjusted survival analysis. To evaluate the prognostic impact of GDF-15 reference values established from a population of apparently healthy older adults, age-dependent GDF-15 centile values were used to predict all-cause mortality (Figure 2). In unadjusted Cox regression analysis, GDF-15 centile values were strongly associated with risk of death (per 25 unit increase; HR, 1.80; 95% CI, 1.48–2.20; P < 0.0001). GDF-15 centile values remained strongly predictive of all-cause mortality when adjusted for age and sex (per 25 unit increase; HR, 1.60; 95% CI, 1.30–1.95; P < 0.0001) and further adjusted for age, sex, smoking status, logNT-proBNP, and cystatin C (per 25 unit increase; HR, 1.38; 95% CI, 1.12–1.70; P = 0.0026).
Figure 2.
Uni- and multivariable Cox regression models by continuous and dichotomized age-dependent GDF-15 centile and all-cause mortality. Adjusted model 1 is adjusted for age and sex. Adjusted model 2 is adjusted for age, sex, smoking status, logNT-proBNP, and cystatin C.
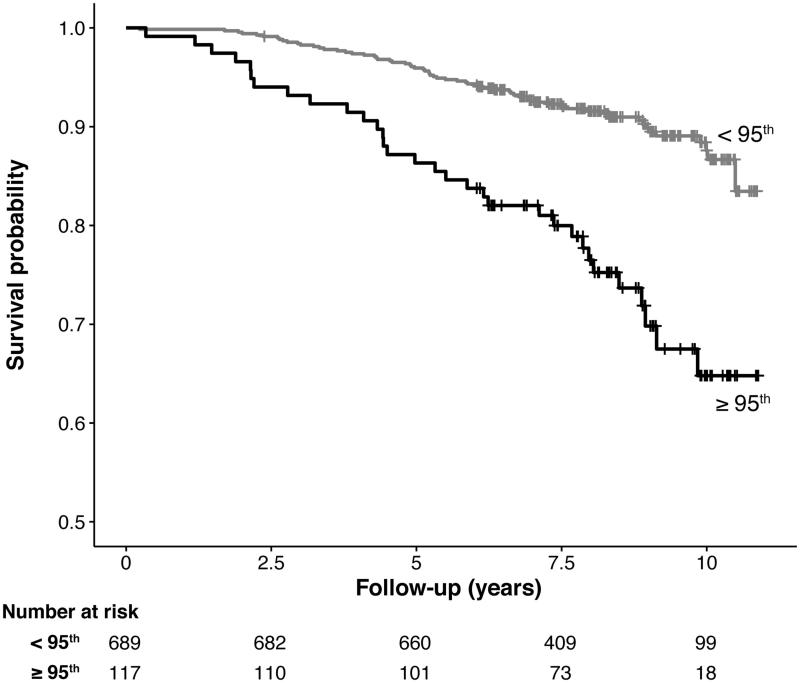
Additionally, GDF-15 centile values were dichotomized at the 95th centile to model an upper limit for the reference values. Kaplan–Meier analysis yielded a highly significant difference in the survival curves between the dichotomized groups (log-rank chi-square = 29.4, P < 0.0001) (Figure 3). Subsequent Cox regression analysis yielded a consistently strong association between GDF-15 centile values greater than or equal to 95 and all-cause mortality. Individuals at or above the 95th centile had a significantly higher risk of death in unadjusted analysis (HR, 3.04; 95% CI, 1.99–4.65; P < 0.0001), and this association remained significant when adjusted for age and sex (HR, 2.41; 95% CI, 1.57–3.69; P < 0.0001) and adjusted for age, sex, smoking status, logNT-proBNP, and cystatin C (HR, 1.66; 95% CI, 1.04–2.63; P = 0.0327) (Figure 2).
Figure 3.
Kaplan–Meier survival curve for all study participants stratified by GDF-15 centile value dichotomized at the 95th centile. Log-rank chi-square = 29.4, P < 0.0001.
Discussion
In the present study, we determined age-dependent reference values for circulating GDF-15 in a population of apparently healthy older adults. Moreover, we found that GDF-15 reference values were strongly predictive of all-cause mortality when treated as continuous or categorical measures. GDF-15 centiles remained an independent predictor of all-cause mortality when adjusted for age, sex, smoking status, logNT-proBNP, and cystatin C. To our knowledge, this is the first study to provide continuous reference values for GDF-15 in apparently healthy older adults and demonstrate a robust association between reference values and risk of all-cause mortality.
Circulating GDF-15 is well-known to increase with age. Therefore, it is important to develop age-dependence reference limits to realize the clinical implementation of GDF-15 measurements. Such reference limits should be developed from a community-dwelling, and ideally healthy, population. Previous studies have reported levels of circulating GDF-15 in community-dwelling (16,19,20) and apparently healthy (2) populations. However, to our knowledge, no in-depth characterization of the GDF-15 distribution in these studies has been conducted beyond conventional summary statistics, and no age-dependent summaries have been reported. A 2007 study by Kempf and colleagues (2) proposed a GDF-15 concentration of 1200 ng/L as the upper reference limit for elderly individuals. Despite the widespread use of this cutoff value in subsequent studies, we submit that this approach obscures potentially valuable age-dependent fluctuations in GDF-15. Alternatively, we provide age-dependent reference values derived from an evidently healthy population to establish a framework for risk-stratification studies.
In both the apparently healthy and unhealthy groups of our community-based study population, we found associations between GDF-15 quartile and age, blood pressure, NT-proBNP, and cystatin C. Additionally, GDF-15 was associated with a variety of chronic diseases and cardiovascular pathologies among the unhealthy participants. These findings are in agreement with consistent reports of association between GDF-15 and numerous risk factors and biomarkers for CVD (3). From a mechanistic perspective, these broad associations fail to shed light on the precise physiology of GDF-15 but are perhaps informative nonetheless. It has been speculated that GDF-15 is best characterized as a generalized marker for oxidative stress, aging, and/or cellular damage (3,9).
This classification is supported by consistent findings that GDF-15 is associated with both disease-specific outcomes and all-cause mortality in the setting of various conditions, including cancer (21,22), stable coronary heart disease (14), atrial fibrillation (23,24), and acute coronary syndrome (10,25). While the majority of investigation between GDF-15 and all-cause mortality has indeed been conducted in the setting of existing CVD, the community-based Rancho Bernardo (16) and Framingham Heart (19) studies found associations between GDF-15 and all-cause mortality comparable to the present study. To that end, our findings corroborate previous work identifying GDF-15 as an independent risk factor for all-cause mortality.
Strengths
There are several strengths of the present study. First, we selected a group of apparently healthy older adults without major disease history, vascular and cardiac pathologies, or regular medication use for cardiovascular disease. Given these selection criteria, the subsequent GDF-15 reference values derived from this group reflect a relatively high standard of health against which other populations can be compared. The concentrations of circulating GDF-15 detected in our study are similar to other community-based studies (2,16,19,26). However, GDF-15 is often reported to not be normally distributed, thereby limiting comparisons across studies using conventional summary statistics. Therefore, we utilized a GAMLSS modeling approach, which has been used by the World Health Organization to develop its child growth standards (27), to provide a more precise characterization of the GDF-15 distribution in our population by establishing age-dependent centile values. Additional strengths of our study include a relatively long follow-up time and one centralized location for plasma storage and analysis of GDF-15.
Limitations
Several limitations of this study should be mentioned. First, several important diagnoses (hypertension, heart failure, cancer) used to select the ‘apparently healthy’ group were only available as self-reported data, thereby introducing the possibility of recall bias in group selection. Additionally, the relatively small number of all-cause deaths observed during follow-up potentially reduces the power of our survival analysis. Lastly, the study population is composed entirely of Caucasian older adults, thereby limiting the generalizability of our results to other populations of more diverse age or ethnic composition.
Conclusions
In conclusion, age-dependent reference values for circulating GDF-15 were developed from a population of apparently healthy older adults. GDF-15 levels expressed as age-dependent centiles were strongly associated with all-cause mortality in a community-based sample of older adults. Therefore, GDF-15 reference values could be a useful tool for risk-stratification in a clinical setting.
Acknowledgements
The authors acknowledge the support of the Fulbright US Student program.
Disclosure statement
The authors report no conflicts of interest.
Funding
The study was supported by Sparbanksstiftelsen Nya [Grants 552, 693, 0932, and 2297]; the County of Västmanland; and the Swedish Medical Association. Roche Diagnostics, Switzerland, provided the GDF-15 assay. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Notes on contributors
Steven Doerstling is a Fulbright US Student grantee and research assistant at the Centre for Clinical Research, Uppsala University, Västerås, Sweden.
Pär Hedberg is an Associate Professor at the Department of Clinical Physiology, Västmanland County Hospital, and the Centre for Clinical Research, Uppsala University, Västerås, Sweden.
John Ohrvik, PhD, is Professor in Biostatistics at the Centre for Clinical Research, Uppsala University, Sweden.
Jerzy Leppert, MD, PhD, is Professor in Family Medicine at Uppsala University, Senior Researcher at the Centre for Clinical Research, Västerås, Sweden.
Egil Henriksen, deceased 13 March 2018. He was an Associate Professor at the Department of Clinical Physiology, Västmanland County Hospital, Västerås, Sweden.
References
- 1.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–91. [DOI] [PubMed] [Google Scholar]
- 3.Wollert KC, Kempf T, Wallentin L.. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–51. [DOI] [PubMed] [Google Scholar]
- 4.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–60. [DOI] [PubMed] [Google Scholar]
- 5.Bonaterra GA, Zugel S, Thogersen J, Walter SA, Haberkorn U, Strelau J, et al. Growth differentiation factor-15 deficiency inhibits atherosclerosis progression by regulating interleukin-6-dependent inflammatory response to vascular injury. J Am Heart Assoc. 2012;1:e002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–33. [DOI] [PubMed] [Google Scholar]
- 7.Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, et al. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51:309–18. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–50. [PubMed] [Google Scholar]
- 9.Corre J, Hebraud B, Bourin P.. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;2:946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2011;31:203–10. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–50. [DOI] [PubMed] [Google Scholar]
- 12.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–8. [DOI] [PubMed] [Google Scholar]
- 13.Eggers KM, Kempf T, Lind L, Sundstrom J, Wallentin L, Wollert KC, et al. Relations of growth-differentiation factor-15 to biomarkers reflecting vascular pathologies in a population-based sample of elderly subjects. Scand J Clin Lab Invest. 2012;72:45–51. [DOI] [PubMed] [Google Scholar]
- 14.Hagstrom E, Held C, Stewart RA, Aylward PE, Budaj A, Cannon CP, et al. Growth differentiation factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin Chem. 2017;63:325–33. [DOI] [PubMed] [Google Scholar]
- 15.Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007;116:1540–8. [DOI] [PubMed] [Google Scholar]
- 16.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E.. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123:2101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedberg P, Hammar C, Selmeryd J, Viklund J, Leppert J, Hellberg A, et al. Left ventricular systolic dysfunction in outpatients with peripheral atherosclerotic vascular disease: prevalence and association with location of arterial disease. Eur J Heart Fail. 2014;16:625–32. [DOI] [PubMed] [Google Scholar]
- 18.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell. 2010;9:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallin U, Glimelius B, Jirstrom K, Darmanis S, Nong RY, Ponten F, et al. Growth differentiation factor 15: a prognostic marker for recurrence in colorectal cancer. Br J Cancer. 2011;104:1619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corre J, Labat E, Espagnolle N, Hebraud B, Avet-Loiseau H, Roussel M, et al. Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 2012;72:1395–406. [DOI] [PubMed] [Google Scholar]
- 23.Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847–58. [DOI] [PubMed] [Google Scholar]
- 24.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, et al. Growth-differentiation factor 15 and risk of major bleeding in atrial fibrillation: insights from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Am Heart J. 2017;190:94–103. [DOI] [PubMed] [Google Scholar]
- 25.Skau E, Henriksen E, Wagner P, Hedberg P, Siegbahn A, Leppert J.. GDF-15 and TRAIL-R2 are powerful predictors of long-term mortality in patients with acute myocardial infarction. Eur J Prev Cardiol. 2017;24:1576–83. [DOI] [PubMed] [Google Scholar]
- 26.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur Heart J. 2009;30:2346–53. [DOI] [PubMed] [Google Scholar]
- 27.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]