Summary
We developed a novel simulation model integrating multiple data sets to project long‐term outcomes with contemporary therapy for early‐stage Hodgkin lymphoma (ESHL), namely combined modality therapy (CMT) versus chemotherapy alone (CA) via 18F‐fluorodeoxyglucose positron emission tomography response‐adaption. The model incorporated 3‐year progression‐free survival (PFS), probability of cure with/without relapse, frequency of severe late effects (LEs), and 35‐year probability of LEs. Furthermore, we generated estimates for quality‐adjusted life years (QALYs) and unadjusted survival (life years, LY) and used model projections to compare outcomes for CMT versus CA for two index patients. Patient 1: a 25‐year‐old male with favourable ESHL (stage IA); Patient 2: a 25‐year‐old female with unfavourable ESHL (stage IIB). Sensitivity analyses assessed the impact of alternative assumptions for LE probabilities. For Patient 1, CMT was superior to CA (CMT incremental gain = 0·11 QALYs, 0·21 LYs). For Patient 2, CA was superior to CMT (CA incremental gain = 0·37 QALYs, 0·92 LYs). For Patient 1, the advantage of CMT changed minimally when the proportion of severe LEs was reduced from 20% to 5% (0·15 QALYs, 0·43 LYs), whereas increasing the severity proportion for Patient 2's LEs from 20% to 80% enhanced the advantage of CA (1·1 QALYs, 6·5 LYs). Collectively, this detailed simulation model quantified the long‐term impact that varied host factors and alternative contemporary treatments have in ESHL.
Keywords: simulation modelling, Hodgkin lymphoma, decision making, health‐related quality of life, late effects of therapy
Hodgkin lymphoma (HL) is one of the most curable cancers, particularly when presenting as early‐stage disease (Evens et al, 2008; Armitage, 2010; Giulino‐Roth et al, 2015). While there is no clear consensus about overarching treatment recommendations, most approaches consist of several cycles (2–6) of multi‐agent chemotherapy with or without adjunctive involved site or involved nodal radiation therapy (ISRT or INRT, respectively) (Nachman et al, 2002; Laskar et al, 2004; Straus et al, 2004; Meyer et al, 2005, 2012; Armitage, 2010; Wolden et al, 2012; Hay et al, 2013; Percival et al, 2014; Crump et al, 2015; Giulino‐Roth et al, 2015). There remains debate, however, regarding the optimal approach to treatment, including: the number of chemotherapy cycles needed, the potential benefit of radiation therapy (RT), the use of early or interim 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) to guide treatment (i.e., response‐adapted therapy), and the role of novel therapeutics (Evens & Kostakoglu, 2014; Raemaekers et al, 2014; Radford et al, 2015; André et al, 2017).
Helping clinicians and patients assess alternative HL treatment options is challenging. First, ideal information is not available. Often, empirical data for contemporary therapies are limited to short‐term follow‐up with little available information about the risk and severity of late effects of treatment. Although long‐term follow‐up data offer insights, they are not directly relevant due to treatment changes and improvements over time. Second, the benefits and risks of different therapies depend in part on individual characteristics, such as patient age and disease involvement, among others.
Simulation modelling offers an approach for systematically and explicitly incorporating assumptions and information based on multiple data sources to explore how alternative treatments affect outcomes of interest, including survival and ‘quality‐adjusted’ survival. To illustrate this approach, we developed a model to compare two general approaches to the treatment of early stage HL (ESHL), a clinical area with an extensive literature (Nachman et al, 2002; Laskar et al, 2004; Straus et al, 2004; Meyer et al, 2005, 2012; Wolden et al, 2012; Raemaekers et al, 2014; Radford et al, 2015; André et al, 2017) that has recently been systematically reviewed (Blank et al, 2017). We projected outcomes for two 25‐year‐old patients – a male with very limited disease in the neck, and a female with more disseminated early‐stage disease, including bilateral disease of the nodal groups in the neck, chest and axillae. Our analysis shows how simulation modelling can go beyond efforts to determine which treatments are ‘best’ for the population ‘on average’ and instead, potentially identify patient‐specific preferred treatments.
Methods
Case presentations
Patient 1 is a 25‐year‐old male with ESHL (Stage IA), presenting with cervical nodal involvement. Our analysis assumes he has favourable characteristics at diagnosis (Raemaekers et al, 2014; Radford et al, 2015), including absence of B symptoms, mediastinal bulk or elevated erythrocyte sedimentation rate (ESR) (favourable).
Patient 2 is a 25‐year‐old female with ESHL (Stage IIA), presenting with right cervical, hilar and bilateral axillary disease. Her disease has an unfavourable classification, due to the number of involved nodal groups (four), although she, like Patient 1, has no evidence of B symptoms, elevated ESR or bulky disease (unfavourable).
Model structure
We developed a detailed computer simulation model to project disease natural history for paediatric and adult ESHL patients treated with chemotherapy alone (CA) or combined modality therapy (CMT) (Fig 1). The model consists of a series of health states: (i) at risk for relapse; (ii) relapse; (iii) cured without relapse; (iv) cured with relapse; (v) cured with late effects; and (vi) dead. During each model cycle (a period of 1 year in this model), simulated subjects can transition from their current health state to other health states. Whether a subject transitions to another health state depends on the transition pathway probability connecting the current and destination states. If a subject does not transition during a particular time step, that subject remains in the same health state for another cycle. We implemented the model in the C Sharp (C#) programming language 2015 (Microsoft Corporation, Redmond, WA, USA).
Figure 1.
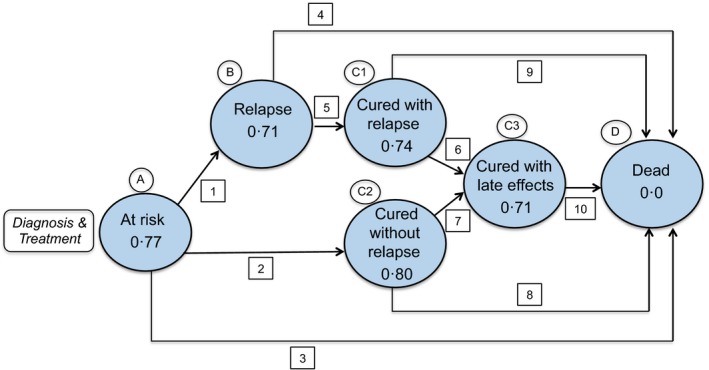
The health state transition diagram. The bubbles represent individual health states. The value within each bubble is the utility weight (or health‐related quality of life impact) of that health state. The arrows represent transition pathways between states. Scaling each year of survival by that year's utility weight (specified for each health state in the figure) and then summing the quality‐adjusted years yields quality‐adjusted survival. Please see Table 1 for values used for each transition pathway and the associated health state. *Represents range of utility weight values categorised on the presence of severe (0·67) or non‐severe (0·73) late effects.
Data assumptions
Table 1 elucidates the model's detailed assumptions. We began by considering contemporary (post‐2000) randomized clinical trials that compared RT‐based CMT and treatment with chemotherapy alone for untreated ESHL with favourable features (Nachman et al, 2002; Meyer et al, 2005; Raemaekers et al, 2014; Radford et al, 2015; André et al, 2017). We did not include data from the subset of patients in earlier trials treated with extended field radiotherapy (EFRT) because this treatment is no longer standard of care. We characterized the initial advantage conferred by CMT with respect to 3‐year progression‐free survival (PFS), spread over 4 years, and extracted, where available, updated overall survival (OS), including data from the two older studies (Meyer et al, 2012; Wolden et al, 2012) and one recent study (André et al, 2017) to describe the relationship between initial treatment response (3‐year PFS) and OS.
Table 1.
Simulation model transition probability assumptions (see also Fig 1)
| Pathway(s) | Chemotherapy alone | CMT | References |
|---|---|---|---|
| A‐B [1] | 9% over 3 yearsa | 3% over 4 yearsa | Radford et al (2015) |
| A‐C2 [2] |
|
Same | b |
| A‐D [3] | Treatment‐related mortality over 3 years (0·22% per year plus background mortality rate) | Same | Meyer et al (2005); Nachman et al (2002); Radford et al (2015); Raemaekers et al (2014)Arias (2014) |
| B‐D [4] | 33% over 2 years; alternative assumption: 15% over 2 years, 10% over next 3 years | Same | Sieniawski et al (2007); Raemaekers and André, unpublished observations from H10 studyb |
| B‐C1 [5] |
|
Same | b |
| C1‐C3 [6] |
|
|
b |
| C2‐C3 [7] |
|
|
b |
| C1‐D [8] and C2‐D [9] | Background mortality rate – function of age | Same | Arias (2014) |
| C3‐D [10] |
|
Same | Levi et al (2002); Louwman et al (2001) |
CMT, combined modality therapy (chemotherapy followed by radiotherapy); SMR, standardized mortality ratio.
Mapping of Table 1 to Fig 1: A–B [1]: at risk to relapse; A‐C2 [2]: at risk to cured without relapse; A‐D [3]: at risk to dead; B‐D [4]: relapse to death; B‐C1 [5]: relapse to cured without relapse; C1‐C3 [6]: cured with relapse to cured with late effects; C2‐C3 [7]: cured without relapse to cured with late effects; C1‐D [8]: cured with relapse to dead; and C2‐D [9]: cured without relapse to dead.
3‐year probability of relapse, spread over 4 years.
Implied by the assumptions of upstream transitions. Ranges empirically estimated by research team and used in threshold sensitivity analyses.
Patients enter the model following diagnosis and completion of initial treatment. At the beginning of the simulation, we assumed that an entire cohort of 25‐year‐old subjects with a maximum lifespan of age 100 years receives treatment and starts in the ‘At‐Risk for Relapse’ state (Fig 1). While in the ‘At‐Risk for Relapse’ state, a subject can at any time ‘Relapse’ (transition pathway 1); or die due to treatment‐related or background cause (transition pathway 3). If the subject neither relapses nor dies within 4 years, he or she moves to the ‘Cured’ state (transition pathway 2).
From ‘At Risk – State A’ to ‘Relapsed – State B’ (transition pathway 1)
We estimated transition probabilities for this pathway from the 75% of subjects with negative 18FDG‐PET after three chemotherapy cycles on the RAPID (Randomised Phase III Trial to Determine the Role of FDG– PET Imaging in Clinical Stages IA/IIA Hodgkin's Disease) trial (Radford et al, 2015). We chose this trial as the basis for our estimate as it was FDG‐PET response‐adapted, limited to early‐stage patients (in particular, stages IA & IIA), and the only difference between the treatment groups was the addition of radiotherapy to the chemotherapy arm. In this example, we used the published 3‐year relapse rate of 9% for CA vs. 3% for CMT. We note, however, that our simulation model can be readily modified to reflect assumptions developed using any clinical trial that reports PFS (or similar acute surrogate survival data) findings. Because we wanted to model the effects of treatment actually given, i.e., without dilution from non‐compliance, we extracted 3‐year PFS probabilities using the ‘per protocol’ (as treated) analysis but spread these probabilities over 4 years to reflect the time period during which relapses occur (Voss et al, 2012).
From ‘At Risk – State A’ to ‘Dead – State D’ (transition pathway 3)
We assumed the 3‐year probability of death would be the total of treatment‐related annual mortality (TRM) and background, age‐based mortality. We derived the median of TRM from four recent studies (0·22% per year) (Nachman et al, 2002; Meyer et al, 2005; Raemaekers et al, 2014; Radford et al, 2015) and background mortality, which, for a 25‐year‐old is 0·28% (Arias, 2014).
From ‘At Risk – State A’ to ‘Cured without Relapse – State C2’ (transition pathway 2)
We assumed that subjects who neither die nor relapse and who therefore transition to the ‘Cured without Relapse’ state after 4 years do not subsequently relapse.
From ‘Relapsed – State B’ to ‘Dead – State D’ (transition pathway 4) or to ‘Cured following Relapse – State C1’ (transition pathway 5)
We assume that following relapse, the 5‐year mortality probability is approximately 25% (15% over the first 2 years, and 10% over the subsequent 3 years). If a relapsed subject survives for 2 years, we assume he or she is cured and moves to the ‘Cured Following Relapse’ state.
‘Late Effects – State C3’ following ‘Cured Following Relapse – State C1’ (transition pathway 6) or ‘Cured Without Relapse – State C2’ (transition pathway 7)
Cured subjects may experience late effects (transition pathways 6 or 7), the probability of which depends on the ESHL treatment originally received and on whether the patient experienced relapse. We used Centers for Disease Control and Prevention life expectancy data to estimate mortality by age after cure (Arias, 2014) (States C1 and C2).
‘Late Effects – State C3’ – Subjects transitioning to the late effects state may experience either mild‐to‐moderate, or severe late effects. The probability that late effects will be severe depends on whether the subject experienced relapse and the salvage therapy given, and on the original ESHL treatment received. Mortality depends on late effects severity. Patients with severe late effects, such as second malignant neoplasms or severe cardiac disease, are assumed to have an elevated risk of premature death (reflected by an elevated standardized mortality ratio (SMR, Table 1), whereas we assume mortality for patients with less severe late effects matches the background mortality rate. The mortality ‘penalty’ for severe late effects was modelled using a piecewise linear shape, as suggested by Louwman et al (2001) in data on breast cancer, rather than assuming an elevated SMR of 20·3 (Levi et al, 2002) modelled linearly over the entire period of risk. After a 10‐year latency, the SMR was 15·8 for year 10‐14, linearly declining from 15·8 to 4·7 years for years 14–20 and then, remaining at 4·7 for years 20 and beyond.
Consistent with recommended practices in the field of health economics, outcomes that occur in the near term, beneficial or negative, are more salient to the decision maker than outcomes occurring in the future (Sanders et al, 2016). We implemented this concept by scaling accrued health benefits by a discount factor corresponding to when they are accrued. The discount factor is equal to: , where r is the annual discount rate (3% in our case, per recommendations) (Sanders et al, 2016) and n is the number of years following the start of the simulation when that health benefit contribution is accrued.
Outcomes
For each treatment, we estimated quality‐adjusted life expectancy (QALYs), which is survival in years, with each year scaled by a utility preference weight corresponding to that year's health state (Sox et al, 1988). In general, health state utility preference weights range from 0 to 1, with a weight of zero for the ‘Dead’ state, and a weight of 1·0 for the (hypothetical) state of ‘perfect health’. The six health states in our model (Fig 1) have utility weights ranging from zero (‘dead’ state) to as high as 0·80 (‘cured without relapse’ state). Of note, little data inform preference‐based health utilities for HL patients across these health states (Linendoll et al, 2016). Our goal was to array utility weights in a plausible gradient from severe impairment to perfect health (see below).
Areas of uncertainty and sensitivity analyses
We identified three principal areas of uncertainty in available data: (i) probability of death from relapse (transition pathway 4); (ii) probability of late effects (via transition pathways 6 or 7) and their effect on mortality (transition pathway 10); and (iii) estimation of utility weights across the six disease states.
We initially modelled the 2‐year probability of cure following relapse (transition pathway 5) from a 2007 published rate of 67% (Sieniawski et al, 2007). Given concern that this assumption may not be consistent with more contemporary data on 5‐year OS, we reduced the 2‐year 33% risk of death from relapse to approximately 25%, with a mortality risk of 15% during the first 2 years following relapse and a mortality risk of 10% over the next 3 years.
We assumed that CMT treatment elevates the probability of late effects relative to chemotherapy alone, that relapse likewise elevates the probability of late effects, and that a patient both receiving CMT and experiencing relapse would have the highest probability of late effects. Our sensitivity analysis explored the impact of altering the assumed contribution of late effects to premature mortality that has been demonstrated by others (Armstrong et al, 2009, 2016; Yeh & Diller, 2012).
We developed utility weight assumptions for each of the health states by establishing upper and lower bounds for each, and identifying likely order relationships among these weights. Patients in the ‘At‐Risk for Relapse – State A’ state experience acute treatment toxicity and uncertainty regarding relapse. We assigned this state a utility weight of 0·77 (regardless of treatment), which is the mean utility estimate among adult survivors of childhood cancer (Yeh et al, 2016) and within the range of recently published data from a study by Wu et al (2017) of 205 patients with recurrent/refractory HL undergoing salvage treatment (0·759; 95% confidence interval 0·30–0·788). We assumed that for ‘Relapse – State B’, the utility weight is 0·71, i.e., 0·06 lower than the utility weight for ‘At Risk – State A’. This decrement reflects both the toxicity associated with salvage therapy and the psychological impact of the subject's uncertain prognosis. This offset of 0·06 corresponds to the utility weight difference reported by Wu et al (2017) for salvage therapy responders and non‐responders. We assumed that the health improvement attending transition from ‘At‐Risk – State A’ to ‘Cured without Relapse – State C1’ increases utility from 0·77 to 0·80. We assume the same utility weight improvement of 0·03 (from 0·71 to 0·74) accompanies transition from ‘Relapse – State B’ to ‘Cured following Relapse – State C2’.
We assumed that the utility weight for late effects depended on severity, with a weight of 0·73 for mild‐to‐moderate effects, and a value of 0·67 for severe late effects. For these estimates, we relied on recent meta‐analyses of utilities (Peasgood et al, 2010) published in early breast cancer.
Analyses
All analyses compared the clinical benefits of CMT and CA for each of our two patient cases in terms of unadjusted survival (life years, LY) and discounted, quality‐adjusted survival (QALYs). In addition to our base case assumption analysis, we also conducted multiple sensitivity analyses to assess the impact of altering three sets of assumptions: (i) the probability of developing late effects following cure (with or without relapse), (ii) the proportion of subjects with late effects whose effects are severe (rather than mild‐to‐moderate), and (iii) the impact of severe late effects on mortality (see Table 2).
Table 2.
Sensitivity analyses and model results
| Late effect probabilitiesa | Proportion of late effects severe | Results | CMT advantage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1: with relapse | C2: no relapse | LY | LY | QALY | QALY | LY | QALY | |||||
| Patient | Chemo | CMT | Chemo | CMT | Chemo | CMT | Chemo | CMT | Chemo | CMT | Delta | Delta |
| 1·0 | 0·45 | 0·45 | 0·30 | 0·45 | 0·20 | 0·20 | 50·37 | 50·58 | 19·10 | 19·21 | 0·21 | 0·11 |
| 1·1 | 0·10 | 0·10 | 50·61 | 50·97 | 19·12 | 19·26 | 0·35 | 0·14 | ||||
| 1·2 | 0·05 | 0·05 | 50·73 | 51·16 | 19·14 | 19·29 | 0·43 | 0·15 | ||||
| 2·0 | 0·45 | 0·90 | 0·30 | 0·90 | 0·20 | 0·20 | 50·40 | 49·48 | 19·11 | 18·73 | −0·92 | −0·37 |
| 2·1 | 0·20 | 0·40 | 50·37 | 47·65 | 19·10 | 18·49 | −2·71 | −0·61 | ||||
| 2·2 | 0·20 | 0·60 | 50·37 | 45·78 | 19·10 | 18·24 | −4·59 | −0·86 | ||||
| 2·3 | 0·20 | 0·80 | 50·37 | 43·82 | 19·07 | 17·97 | −6·54 | −1·10 | ||||
Chemo, chemotherapy alone; CMT, combined modality therapy (i.e., chemotherapy followed by radiotherapy); LY, life years; QALY, quality‐adjusted life years.
35‐year late effect probabilities following 10‐year latency; Entries in BOLD differ from the base case for Patient 1 (Patient 1·0).
Results
When we considered our index patients, the model projected that CMT would confer an advantage for Patient 1 (favourable) of 0·11 QALYs (equivalent to 1·3 months of perfect health) and 0·21 unadjusted and undiscounted LYs (Patient 1·0 in Table 2). As we reduced the proportion of patients with late effects assumed to experience severe outcomes from its base case value of 20% to 5% in sensitivity analysis (Patient 1·1, 1·2 in Table 2), the relative advantage of CMT increased to 0·15 QALYS and 0·43 unadjusted, undiscounted LYs.
In contrast, for Patient 2 (unfavourable), our base case projected that CA would confer an advantage of 0·37 QALYs (equivalent to 4·4 months in perfect health) and 0·92 LYs (Patient 2·0 in Table 2). Sensitivity analysis explored the impact of increasing the assumed proportion of patients with late effects who experience severe outcomes from the base case value of 20% to as much as 80% (Patient, 2·1, 2·2, 2·3 in Table 2). These analyses showed that the alternative assumptions increased the CA advantage versus CMT to as much as 1·1 QALYs (13 months in perfect health) and 6·5 unadjusted, undiscounted LYs.
Further sensitivity analysis explored the impact of increasing patient age for each of our index patients. Specifically, we changed the base age from 25 years to 35, 45, 55, and 65 years. For Patient 1, increasing age initially increases the magnitude of the CMT benefit in both LY and QALYs, but that benefit decreases with increasing age at treatment, suggesting that there may be a “capping effect” of life expectancy. For Patient 2, the late effects ‘penalty’ is more severe than in Patient 1, but as the age at treatment increases, the penalty decreases. Indeed, at age 65 years, there is a small CMT advantage in both LY and QALY (Tables SI and SII).
Discussion
We present an analysis of a simulation model to identify the data needed to make informed treatment decisions for patients with HL, contemporaneously balancing both short‐term relapse risk and long‐term late effect risk. Treatment decisions are complicated by the lack of data on late effects associated with current/contemporary therapy (e.g., 18FDG‐PET response‐adapted therapy, the use of involved‐site RT or number of subsequent chemotherapy cycles), and by the limited data on health‐related quality of life (HRQL), as stratified by treatment.
We acknowledge that substantial research gaps remain. We utilized the analysis to help identify the data needed to make the simulation model findings ‘actionable’. Simulation results can be used to determine which areas of research are less important in the context of the present clinical question because the attendant uncertainty does not substantially influence the answers produced by our model. In short, simulation modelling may be leveraged to reproducibly incorporate available data to assist patient‐specific treatment decision‐making, and to identify the research areas that should be prioritized to ensure that confidence in the model projections is warranted.
For our model, we utilized PFS data from recent clinical trials comparing CA and CMT to help examine clinical benefit trade‐offs for two illustrative ESHL patients who presented with different disease characteristics. Specifically, we considered the impact of site and extent of disease and gender, as two illustrations of potential heterogeneity. In interpreting our findings, several factors should be considered. First, we modelled PFS based on the results of ESHL patients identified by interim FDG‐PET as having chemotherapy‐responsive disease. Second, we assumed that the probability of cure after relapse is the same for both treatments. As OS is similar across treatments at 3–5 years and at 12 years follow‐up, despite the superior short‐term PFS of CMT, suggests that the probability of cure after relapse may be higher following treatment with CA alone. Alternatively, probability of cure may also be linked to disease characteristics at diagnosis (i.e., favourable versus unfavourable), as noted in the studies reported by Raemaekers group, where in the CMT arm, only unfavourable risk patients died after relapse (Raemaekers et al, 2014; André et al, 2017) (Raemaekers, unpublished observation, 3 October 2017).
To estimate the probability of late effects with contemporary treatment for our two 25‐year‐old patients, we considered data from multiple sources. These included the Childhood Cancer Survivor Study (Armstrong et al, 2016) and the St. Jude Life Cohort (Hudson et al, 2011), to understand the changes in incidence of late effects by treatment era. To understand toxicity, we examined the study by Matasar et al (2015), reporting on the results from a single institution registry of incident HL, treated from 1975 to 2000. However, broader scale data on the morbidity and mortality associated with late effects for adult‐aged patients are largely lacking, particularly for those ESHL patients treated in the contemporary era. To assess the importance of this source of uncertainty, we explored the relationship between our projected outcomes and probabilities ranging from 0% to 90%, and made a priori assumptions about the relationship between treatments, relapse‐related treatment and the acquisition of late effects.
Previous studies have demonstrated that radiation exposure is clearly associated with late effects (Hodgson et al, 2007; Koh et al, 2007; Travis et al, 2012; Berrington de Gonzalez et al, 2013; Schaapveld et al, 2015; van Nimwegen et al, 2016). Dosimetric studies of contemporary radiotherapy for HL have shown significant reductions in normal tissue dose compared to many of the historically treated cohorts followed in studies used to quantify late effects (Maraldo et al, 2014; Zhou et al, 2016) with associated reductions in late effects (De Bruin et al, 2009). The findings reported by Schaapveld et al (2015) demonstrated that the incidence of second neoplasms did not differ by treatment era up to 2000. However, the precise long‐term burden associated with contemporary treatment of CMT or CA is not known, and hence, are a limitation of our findings.
Other studies have highlighted the trade‐offs when treating ESHL (Ng et al, 1999; Yeh & Diller, 2012). A decision analysis published in 1999 compared treatment with radiotherapy alone, chemotherapy alone, and CMT for ESHL and modelled quality‐adjusted life expectancy (Ng et al, 1999). The authors of this study reported that the preferred treatment was highly influenced by long‐term morbidity and HRQL estimates used in the model. A paediatric‐specific decision analysis by Yeh and Diller (2012) subsequently reported that treatment with CA extended life expectancy. This analysis used mortality risk estimates imputed from cardiac disease and secondary cancer data for childhood cancer survivors treated prior to 1986. In contrast, our analysis does not rely on data from previous treatment eras to project life expectancy and addresses the morbidity of late effects across a wide range of probabilities. Moreover, our analysis accounted for state‐specific HRQL. Quality‐adjustment of life expectancy is particularly important in ESHL due to the relatively young age of the patient population at diagnosis, the high cure rate and the impact of HRQL after cure over decades of life. The approach of quality‐adjustment using health utility weights is commonly used in the field of health economics. Some 5000 studies have reported health intervention cost‐effectiveness estimates expressed in terms of QALYs (e.g., see the Tufts Medical Center Cost Effectiveness Analysis Registry: http://www.ceaRegistry.org), and the Second US Panel on Cost Effectiveness recently recommended the use of QALYs to quantify the benefits of health interventions that influence both mortality and morbidity (Sanders et al, 2016).
We acknowledge the limitations of this study. First, because we used study level data to estimate risk of disease progression, we could not fully characterize treatment‐effect heterogeneity (Kent & Hayward, 2007). A meta‐analysis of individual patient data from randomized trials would better elucidate patient characteristics, such as age, stage and number of nodal groups involved, and more accurately characterize disease progression. Second, the model highlights the limitations in the current literature with regard to health state utility weights. In order to optimize our model, further work must be conducted to better quantify health utility weights for patients undergoing each phase of treatment and for survivors stratified by age and treatment strategy. The recent data reported from the German HL Study Group on patient preferences for health states (i.e., risk of relapse and risk of late effects), and the serial assessment of utility weights among patients with relapsed/refractory HL, as reported by Wu et al (2017), represent important first steps in this area. The ongoing Phase III trial from the Children's Oncology Group (NCT02166463) and the recently completed ECHELON‐1 trial both include preference‐based tools from which utility weights during treatment and during the ‘At Risk for Relapse’ states can be calculated. This will provide clinical researchers and clinicians with an anchoring or ‘bounding’ of utility weights against which other weights can be derived (Arnold et al, 2009).
Third, not all patients experience the same late effect risks (incidence or severity). The risk of anthracycline‐related cardiomyopathy and radiation‐related secondary malignancies varies across individuals, based on clinical factors, such as age at exposure, cumulative chemotherapy dose and radiation dose and field (Schaapveld et al, 2015; van Nimwegen et al, 2016). Susceptibility (van Leeuwen & Ng, 2016) to late effects also differs by the extent and distribution of disease, gender, genetic predisposition (Best et al, 2011; Blanco et al, 2012; Visscher et al, 2012; Sud et al, 2017), and by survivor health behaviours, such as smoking status (Castellino et al, 2011; Schaapveld et al, 2015; van Nimwegen et al, 2016). Further, data suggest that risk inferred from childhood cancer survivors may not be consistent with older age (>21 years) at exposure, arguing for further work on risk ascertainment referable to treated adults. In the current study, we illustrate heterogeneity in our two similarly aged patients by gender and disease extent. We also explored the impact of age at treatment from 25 to 65 years and found the penalty of late effects to attenuate with age (Patient 2), suggesting the importance of the relationship between exposure and risk of late effects, relative to aging. Further work using individualized data will provide important information to help clinicians and patients better understand patient‐specific risks. Nonetheless, our aggregate analysis provides useful insights about overall tendencies by providing a paradigm for decision making for ESHL, and underscores currently lacking data that are essential to capture going forward.
This model is an initial step towards developing a detailed framework to systematically integrate relevant data regarding PFS, OS and HRQL for alternative treatment choices for HL patients so that clinicians and patients can make shared decisions regarding initial therapy that is aligned with their values and preferences (acute and long‐term). The framework of this model may also be applied to other HL states (e.g., advanced‐stage disease, paediatric HL versus elderly HL, etc) as well as other cancers. Future iterations may also incorporate individual patient and tumour/biological characteristics, patient preferences, cost‐effectiveness and an overall greater range of treatments, all in order to more comprehensively inform providers and patients. Altogether, the choice of therapy for HL patients remains a complex decision. The decision model described here highlights a host of important factors to consider when assessing treatments for ESHL in the modern era and also provides the needed modelling framework to demonstrate the impact of alternative treatments on QALYs in order to ultimately assist patients and their providers in making the most optimal and individualized treatment choice.
Author contributions
SKP, MJK, JTC, DC, AME: designed research, performed research, analysed data, and wrote the paper. SMC, TOH, KMK, FGK, TJH, AJK, PJ, RMM, JR: analysed data and wrote the paper.
Conflicts of interest
The authors declare no potential conflicts of interest.
Key points
This novel simulation model provides a base framework to help evaluate treatment choices for early‐stage Hodgkin lymphoma in the modern era.
We identified additional significant gaps in data that are critically needed to optimize decision‐making in Hodgkin lymphoma.
Supporting information
Table SI. Sensitivity analysis of increasing age at treatment on model results (Index Patient #1).
Table SII. Sensitivity analysis of increasing age at treatment on model results (Index Patient #2).
Acknowledgements
The authors acknowledge Rachel Murphy‐Banks’ editorial assistance in preparing the manuscript. This project was funded in part by a research grant from the Leukemia & Lymphoma Society (Parsons/Henderson, Dual PIs).
This project was presented in an earlier version at the American Society of Hematology annual meeting, December, 2015, Orlando, FL, and at the 10th International Symposium on Hodgkin Lymphoma, October 2016, Cologne, Germany.
Contributor Information
Susan K. Parsons, Email: sparsons@tuftsmedicalcenter.org
Andrew M. Evens, Email: andrew.evens@rutgers.edu.
References
- André, M.P.E. , Girinsky, T. , Federico, M. , Reman, O. , Fortpied, C. , Gotti, M. , Casasnovas, O. , Brice, P. , van der Maazen, R. , Re, A. , Edeline, V. , Ferme, C. , van Imhoff, G. , Merli, F. , Bouabdallah, R. , Sebban, C. , Specht, L. , Stamatoullas, A. , Delarue, R. , Fiaccadori, V. , Bellei, M. , Raveloarivahy, T. , Versari, A. , Hutchings, M. , Meignan, M. & Raemaekers, J. (2017) Early positron emission tomography response‐adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology, 35, 1786–1794. [DOI] [PubMed] [Google Scholar]
- Arias, E. (2014) United States life tables, 2010. National Vital Statistics Reports, 63, 1–63. [PubMed] [Google Scholar]
- Armitage, J.O. (2010) Early‐stage Hodgkin's lymphoma. New England Journal of Medicine, 363, 653–662. [DOI] [PubMed] [Google Scholar]
- Armstrong, G.T. , Liu, Q. , Yasui, Y. , Huang, S. , Ness, K.K. , Leisenring, W. , Hudson, M.M. , Donaldson, S.S. , King, A.A. , Stovall, M. , Krull, K.R. , Robison, L.L. & Packer, R.J. (2009) Long‐term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. Journal of the National Cancer Institute, 101, 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, G.T. , Chen, Y. , Yasui, Y. , Leisenring, W. , Gibson, T.M. , Mertens, A.C. , Stovall, M. , Oeffinger, K.C. , Bhatia, S. , Krull, K.R. , Nathan, P.C. , Neglia, J.P. , Green, D.M. , Hudson, M.M. & Robison, L.L. (2016) Reduction in late mortality among 5‐year survivors of childhood cancer. New England Journal of Medicine, 374, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, D. , Girling, A. , Stevens, A. & Lilford, R. (2009) Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ, 339, b2688. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez, A. , Gilbert, E. , Curtis, R. , Inskip, P. , Kleinerman, R. , Morton, L. , Rajaraman, P. & Little, M.P. (2013) Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose‐response relationship. International Journal of Radiation Oncology, Biology, Physics, 86, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best, T. , Li, D. , Skol, A.D. , Kirchhoff, T. , Jackson, S.A. , Yasui, Y. , Bhatia, S. , Strong, L.C. , Domchek, S.M. , Nathanson, K.L. , Olopade, O.I. , Huang, R.S. , Mack, T.M. , Conti, D.V. , Offit, K. , Cozen, W. , Robison, L.L. & Onel, K. (2011) Variants at 6q21 implicate PRDM1 in the etiology of therapy‐induced second malignancies after Hodgkin's lymphoma. Nature Medicine, 17, 941–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, J.G. , Sun, C.L. , Landier, W. , Chen, L. , Esparza‐Duran, D. , Leisenring, W. , Mays, A. , Friedman, D.L. , Ginsberg, J.P. , Hudson, M.M. , Neglia, J.P. , Oeffinger, K.C. , Ritchey, A.K. , Villaluna, D. , Relling, M.V. & Bhatia, S. (2012) Anthracycline‐related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes – a report from the Children's Oncology Group. Journal of Clinical Oncology, 30, 1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, O. , von Tresckow, B. , Monsef, I. , Specht, L. , Engert, A. & Skoetz, N. (2017) Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma. Cochrane Database Systematic Review, 4, CD007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino, S.M. , Geiger, A.M. , Mertens, A.C. , Leisenring, W.M. , Tooze, J.A. , Goodman, P. , Stovall, M. , Robison, L.L. & Hudson, M.M. (2011) Morbidity and mortality in long‐term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood, 117, 1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump, M. , Herst, J. , Baldassarre, F. , Sussman, J. , MacEachern, J. , Hodgson, D. & Cheung, M.C. (2015) Evidence‐based focused review of the role of radiation therapy in the treatment of early‐stage Hodgkin lymphoma. Blood, 125, 1708–1716. [DOI] [PubMed] [Google Scholar]
- De Bruin, M.L. , Sparidans, J. , van't Veer, M.B. , Noordijk, E.M. , Louwman, M.W. , Zijlstra, J.M. , van den, B.H. , Russell, N.S. , Broeks, A. , Baaijens, M.H. , Aleman, B.M. & van Leeuwen, F.E. (2009) Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. Journal of Clinical Oncology, 27, 4239–4246. [DOI] [PubMed] [Google Scholar]
- Evens, A.M. & Kostakoglu, L. (2014) The role of FDG‐PET in defining prognosis of Hodgkin lymphoma for early‐stage disease. Blood, 124, 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens, A.M. , Hutchings, M. & Diehl, V. (2008) Treatment of Hodgkin lymphoma: the past, present, and future. Nature Clinical Practice: Oncology, 5, 543–556. [DOI] [PubMed] [Google Scholar]
- Giulino‐Roth, L. , Keller, F.G. , Hodgson, D.C. & Kelly, K.M. (2015) Current approaches in the management of low risk Hodgkin lymphoma in children and adolescents. British Journal of Haematology, 169, 647–660. [DOI] [PubMed] [Google Scholar]
- Hay, A.E. , Klimm, B. , Chen, B.E. , Goergen, H. , Shepherd, L.E. , Fuchs, M. , Gospodarowicz, M.K. , Borchmann, P. , Connors, J.M. , Markova, J. , Crump, M. , Lohri, A. , Winter, J.N. , Dorken, B. , Pearcey, R.G. , Diehl, V. , Horning, S.J. , Eich, H.T. , Engert, A. , Meyer, R.M. & Conducted by the NCIC Clinical Trials Group (Canada) and German Hodgkin Study Group (GHSG) . (2013) An individual patient‐data comparison of combined modality therapy and ABVD alone for patients with limited‐stage Hodgkin lymphoma. Annals of Oncology, 24, 3065–3069. [DOI] [PubMed] [Google Scholar]
- Hodgson, D.C. , Koh, E.S. , Tran, T.H. , Heydarian, M. , Tsang, R. , Pintilie, M. , Xu, T. , Huang, L. , Sachs, R.K. & Brenner, D.J. (2007) Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer, 110, 2576–2586. [DOI] [PubMed] [Google Scholar]
- Hudson, M.M. , Ness, K.K. , Nolan, V.G. , Armstrong, G.T. , Green, D.M. , Morris, E.B. , Spunt, S.L. , Metzger, M.L. , Krull, K.R. , Klosky, J.L. , Srivastava, D.K. & Robison, L.L. (2011) Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatric Blood & Cancer, 56, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, D.M. & Hayward, R.A. (2007) Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA, 298, 1209–1212. [DOI] [PubMed] [Google Scholar]
- Koh, E.S. , Tran, T.H. , Heydarian, M. , Sachs, R.K. , Tsang, R.W. , Brenner, D.J. , Pintilie, M. , Xu, T. , Chung, J. , Paul, N. & Hodgson, D.C. (2007) A comparison of mantle versus involved‐field radiotherapy for Hodgkin's lymphoma: reduction in normal tissue dose and second cancer risk. Radiation Oncology (London, England), 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskar, S. , Gupta, T. , Vimal, S. , Muckaden, M.A. , Saikia, T.K. , Pai, S.K. , Naresh, K.N. & Dinshaw, K.A. (2004) Consolidation radiation after complete remission in Hodgkin's disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? Journal of Clinical Oncology, 22, 62–68. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, F.E. & Ng, A.K. (2016) Long‐term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematology: The Education Program of the American Society of Hematology, 2016, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, F. , Randimbison, L. , Te, V.C. & La Vecchia, C. (2002) Long‐term mortality of women with a diagnosis of breast cancer. Oncology, 63, 266–269. [DOI] [PubMed] [Google Scholar]
- Linendoll, N. , Saunders, T. , Burns, R. , Nyce, J.D. , Wendell, K.B. , Evens, A.M. & Parsons, S.K. (2016) Health‐related quality of life in Hodgkin lymphoma: a systematic review. Health and Quality of Life Outcomes, 14, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwman, W.J. , Klokman, W.J. & Coebergh, J.W. (2001) Excess mortality from breast cancer 20 years after diagnosis when life expectancy is normal. British Journal of Cancer, 84, 700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldo, M.V. , Jorgensen, M. , Brodin, N.P. , Aznar, M.C. , Vogelius, I.R. , Petersen, P.M. , Berthelsen, A.K. , Christensen, C.B. , Hjalgrim, L.L. & Specht, L. (2014) The impact of involved node, involved field and mantle field radiotherapy on estimated radiation doses and risk of late effects for pediatric patients with Hodgkin lymphoma. Pediatric Blood & Cancer, 61, 717–722. [DOI] [PubMed] [Google Scholar]
- Matasar, M.J. , Ford, J.S. , Riedel, E.R. , Salz, T. , Oeffinger, K.C. & Straus, D.J. (2015) Late morbidity and mortality in patients with Hodgkin's lymphoma treated during adulthood. Journal of the National Cancer Institute, 107, djv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, R.M. , Gospodarowicz, M.K. , Connors, J.M. , Pearcey, R.G. , Bezjak, A. , Wells, W.A. , Burns, B.F. , Winter, J.N. , Horning, S.J. , Dar, A.R. , Djurfeldt, M.S. , Ding, K. , Shepherd, L.E. & National Cancer Institute of Canada Clinical Trials Group & Eastern Cooperative Oncology Group . (2005) Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited‐stage Hodgkin's lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. Journal of Clinical Oncology, 23, 4634–4642. [DOI] [PubMed] [Google Scholar]
- Meyer, R.M. , Gospodarowicz, M.K. , Connors, J.M. , Pearcey, R.G. , Wells, W.A. , Winter, J.N. , Horning, S.J. , Dar, A.R. , Shustik, C. , Stewart, D.A. , Crump, M. , Djurfeldt, M.S. , Chen, B.E. & Shepherd, L.E. (2012) ABVD alone versus radiation‐based therapy in limited‐stage Hodgkin's lymphoma. New England Journal of Medicine, 366, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman, J.B. , Sposto, R. , Herzog, P. , Gilchrist, G.S. , Wolden, S.L. , Thomson, J. , Kadin, M.E. , Pattengale, P. , Davis, P.C. , Hutchinson, R.J. & White, K. (2002) Randomized comparison of low‐dose involved‐field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. Journal of Clinical Oncology, 20, 3765–3771. [DOI] [PubMed] [Google Scholar]
- Ng, A.K. , Weeks, J.C. , Mauch, P.M. & Kuntz, K.M. (1999) Decision analysis on alternative treatment strategies for favorable‐prognosis, early‐stage Hodgkin's disease. Journal of Clinical Oncology, 17, 3577–3585. [DOI] [PubMed] [Google Scholar]
- van Nimwegen, F.A. , Schaapveld, M. , Cutter, D.J. , Janus, C.P. , Krol, A.D. , Hauptmann, M. , Kooijman, K. , Roesink, J. , van der Maazen, R. , Darby, S.C. , Aleman, B.M. & van Leeuwen, F.E. (2016) Radiation dose‐response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. Journal of Clinical Oncology, 34, 235–243. [DOI] [PubMed] [Google Scholar]
- Peasgood, T. , Ward, S.E. & Brazier, J. (2010) Health‐state utility values in breast cancer. Expert Review of Pharmacoeconomics & Outcomes Research, 10, 553–566. [DOI] [PubMed] [Google Scholar]
- Percival, M.E. , Hoppe, R.T. & Advani, R.H. (2014) Bulky mediastinal classical Hodgkin lymphoma in young women. Oncology (Williston Park, N.Y.), 28, 253–256, 258–260, C253. [PubMed] [Google Scholar]
- Radford, J. , Illidge, T. , Counsell, N. , Hancock, B. , Pettengell, R. , Johnson, P. , Wimperis, J. , Culligan, D. , Popova, B. , Smith, P. , McMillan, A. , Brownell, A. , Kruger, A. , Lister, A. , Hoskin, P. , O'Doherty, M. & Barrington, S. (2015) Results of a trial of PET‐directed therapy for early‐stage Hodgkin's lymphoma. New England Journal of Medicine, 372, 1598–1607. [DOI] [PubMed] [Google Scholar]
- Raemaekers, J.M. , André, M.P. , Federico, M. , Girinsky, T. , Oumedaly, R. , Brusamolino, E. , Brice, P. , Ferme, C. , van der Maazen, R. , Gotti, M. , Bouabdallah, R. , Sebban, C.J. , Lievens, Y. , Re, A. , Stamatoullas, A. , Morschhauser, F. , Lugtenburg, P.J. , Abruzzese, E. , Olivier, P. , Casasnovas, R.O. , van, I.G. , Raveloarivahy, T. , Bellei, M. , van der Borght, T. , Bardet, S. , Versari, A. , Hutchings, M. , Meignan, M. & Fortpied, C. (2014) Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology, 32, 1188–1194. [DOI] [PubMed] [Google Scholar]
- Sanders, G.D. , Neumann, P.J. , Basu, A. , Brock, D.W. , Feeny, D. , Krahn, M. , Kuntz, K.M. , Meltzer, D.O. , Owens, D.K. , Prosser, L.A. , Salomon, J.A. , Sculpher, M.J. , Trikalinos, T.A. , Russell, L.B. , Siegel, J.E. & Ganiats, T.G. (2016) Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: second panel on cost‐effectiveness in health and medicine. JAMA, 316, 1093–1103. [DOI] [PubMed] [Google Scholar]
- Schaapveld, M. , Aleman, B.M. , van Eggermond, A.M. , Janus, C.P. , Krol, A.D. , van der Maazen, R.W. , Roesink, J. , Raemaekers, J.M. , de Boer, J.P. , Zijlstra, J.M. , van Imhoff, G.W. , Petersen, E.J. , Poortmans, P.M. , Beijert, M. , Lybeert, M.L. , Mulder, I. , Visser, O. , Louwman, M.W. , Krul, I.M. , Lugtenburg, P.J. & van Leeuwen, F.E. (2015) Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. New England Journal of Medicine, 373, 2499–2511. [DOI] [PubMed] [Google Scholar]
- Sieniawski, M. , Franklin, J. , Nogova, L. , Glossmann, J.P. , Schober, T. , Nisters‐Backes, H. , Diehl, V. & Josting, A. (2007) Outcome of patients experiencing progression or relapse after primary treatment with two cycles of chemotherapy and radiotherapy for early‐stage favorable Hodgkin's lymphoma. Journal of Clinical Oncology, 25, 2000–2005. [DOI] [PubMed] [Google Scholar]
- Sox, H.C. , Blatt, M.A. , Higgins, M.C. & Marton, K.I. (1988) Medical Decision Making. Butterworths, London. [Google Scholar]
- Straus, D.J. , Portlock, C.S. , Qin, J. , Myers, J. , Zelenetz, A.D. , Moskowitz, C. , Noy, A. , Goy, A. & Yahalom, J. (2004) Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood, 104, 3483–3489. [DOI] [PubMed] [Google Scholar]
- Sud, A. , Thomsen, H. , Sundquist, K. , Houlston, R.S. & Hemminki, K. (2017) Risk of second cancer in Hodgkin lymphoma survivors and influence of family history. Journal of Clinical Oncology, 35, 1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis, L.B. , Ng, A.K. , Allan, J.M. , Pui, C.H. , Kennedy, A.R. , Xu, X.G. , Purdy, J.A. , Applegate, K. , Yahalom, J. , Constine, L.S. , Gilbert, E.S. & Boice, J.D. Jr (2012) Second malignant neoplasms and cardiovascular disease following radiotherapy. Journal of the National Cancer Institute, 104, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher, H. , Ross, C.J. , Rassekh, S.R. , Barhdadi, A. , Dube, M.P. , Al‐Saloos, H. , Sandor, G.S. , Caron, H.N. , van Dalen, E.C. , Kremer, L.C. , van der Pal, H.J. , Brown, A.M. , Rogers, P.C. , Phillips, M.S. , Rieder, M.J. , Carleton, B.C. , Hayden, M.R. & Canadian Pharmacogenomics Network for Drug Safety Consortium . (2012) Pharmacogenomic prediction of anthracycline‐induced cardiotoxicity in children. Journal of Clinical Oncology, 30, 1422–1428. [DOI] [PubMed] [Google Scholar]
- Voss, S.D. , Chen, L. , Constine, L.S. , Chauvenet, A. , Fitzgerald, T.J. , Kaste, S.C. , Slovis, T. & Schwartz, C.L. (2012) Surveillance computed tomography imaging and detection of relapse in intermediate‐ and advanced‐stage pediatric Hodgkin's lymphoma: a report from the Children's Oncology Group. Journal of Clinical Oncology, 30, 2635–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolden, S.L. , Chen, L. , Kelly, K.M. , Herzog, P. , Gilchrist, G.S. , Thomson, J. , Sposto, R. , Kadin, M.E. , Hutchinson, R.J. & Nachman, J. (2012) Long‐term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma – a report from the Children's Oncology Group. Journal of Clinical Oncology, 30, 3174–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, E. , Liao, J & Balakumaran, A. (2017) A trial‐based EuroQol EQ‐5D health utility analysis in patients with classical Hodgkin lymphoma. J Clin Oncol, 35, (suppl: abstr e19011). [Google Scholar]
- Yeh, J.M. & Diller, L. (2012) Pediatric Hodgkin lymphoma: trade‐offs between short‐ and long‐term mortality risks. Blood, 120, 2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, J.M. , Hanmer, J. , Ward, Z.J. , Leisenring, W.M. , Armstrong, G.T. , Hudson, M.M. , Stovall, M. , Robison, L.L. , Oeffinger, K.C. & Diller, L. (2016) Chronic conditions and utility‐based health‐related quality of life in adult childhood cancer survivors. Journal of the National Cancer Institute, 108, djw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, R. , Ng, A. , Constine, L.S. , Stovall, M. , Armstrong, G.T. , Neglia, J.P. , Friedman, D.L. , Kelly, K. , FitzGerald, T.J. & Hodgson, D.C. (2016) A comparative evaluation of normal tissue doses for patients receiving radiation therapy for Hodgkin lymphoma on the Childhood Cancer Survivor Study and Recent Children's Oncology Group Trials. International Journal of Radiation Oncology, Biology, Physics, 95, 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Sensitivity analysis of increasing age at treatment on model results (Index Patient #1).
Table SII. Sensitivity analysis of increasing age at treatment on model results (Index Patient #2).


