Abstract
Background and purpose
Systematic comparisons of anterior approach (A) versus posterior approach (P) in primary total hip arthroplasty (THA) have largely focused on perioperative outcomes. In this systematic review with meta-analysis, we compared complication risk of A versus P in studies of primary THA with at least 1-year mean follow-up.
Patients and methods
We performed a systematic review of prospective and retrospective studies with at least 1-year mean follow-up that reported complications of A and P primary THA. Complications included infection, dislocation, reoperation, thromboembolic event, heterotopic ossification, wound complication, fracture, and nerve injury. Random effects meta-analysis was used for all outcomes. Complication risk was reported as rate ratio (RR) to account for differential follow-up durations; values >1 indicated higher complication risk with A and values <1 indicated lower risk with A.
Results
19 studies were included; 15 single-center comparative studies with 6,620 patients (2,278 A; 4,342 P) and 4 multicenter registries with 157,687 patients (18,735 A; 138,952 P). Median follow-up was 16 (12–64) months) with A and 18 (12–110) months with P. Anterior approach was associated with lower rate of infection (RR =0.55, p = 0.002), dislocation (RR =0.65, p = 0.03), and reoperation (RR =0.84, p < 0.001). No statistically significant differences were observed in rate of thromboembolic event (RR =0.59, p = 0.5), heterotopic ossification (RR =0.63, p = 0.1), wound complication (RR =0.93, p = 0.8), or fracture (RR =1.0, p = 0.9). There was a higher rate of patient-reported nerve injury with A (RR =2.3, p = 0.01).
Interpretation
Comparing A with P in primary THA, A was associated with lower risk of reoperation, dislocation, and infection, but higher risk of patient-reported nerve injury.
The durability of total hip arthroplasty (THA) is excellent with 10-year survivorship exceeding 90% (Hailer et al. 2015, Makela et al. 2014). All standard approaches to the hip have been shown to be safe and effective, with certain advantages and disadvantages of each approach (Mjaaland et al. 2017). While the anterior approach (A) has been increasingly used in the United States, little is known about the safety of the A relative to other common surgical approaches. Several groups (Higgins et al. 2015, Meermans et al. 2017, Putananon et al. 2018) have performed systematic reviews comparing the A with the posterior approach (P) in primary THA. However, follow-up durations of the included studies varied widely, with most studies having less than 1-year follow-up. Comparative safety evaluation of these surgical techniques over a longer period is warranted. The purpose of this systematic review with meta-analysis was to compare the complication risk of A versus P in studies with at least 1-year mean follow-up.
Methods
Literature search and data extraction
In accordance with the PRISMA guidelines, we searched MEDLINE and EMBASE for comparative studies of primary THA performed using the A or P. Therapeutic search terms consisting of THA and total hip arthroplasty were combined with the following surgical approach-specific search terms: anterior, direct, posterior, posterolateral, and Smith-Petersen. We also manually searched the Directory of Open Access Journals (DOAJ), Google Scholar, and the reference lists of included papers and relevant systematic reviews. No language or date restrictions were applied to the searches. The final search was conducted on June 30, 2017.
Study eligibility was determined by 2 independent researchers (LM, DF). Disagreements were resolved by discussion. Main inclusion criteria included comparison of A versus P in primary THA, predominant diagnosis of osteoarthritis, mean follow-up duration at least 1 year, and extractable complication data. Titles and abstracts were initially screened to exclude review articles, commentaries, letters, case reports, and obviously irrelevant studies. Full-texts of the remaining articles were retrieved and reviewed. Studies were excluded if patients received revision or bilateral THA. When multiple studies included overlapping series of patients, only the study with the largest sample size was included. Data were independently extracted from eligible peer-reviewed articles by the same 2 researchers. Data discrepancies were resolved by discussion.
Definitions and outcomes
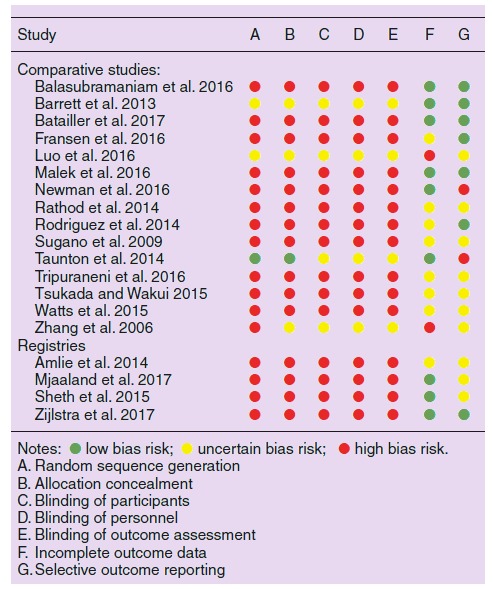
When data were reported at multiple intervals during follow-up, the final value was extracted for analysis. Complications included infection, dislocation, reoperation (for any reason), thromboembolic event, heterotopic ossification, wound complication, fracture, and nerve injury. To account for differential follow-up durations, complication data were extracted by determining the number of events and then calculating the number of person-years in each group to determine incidence rates. Risk of bias in each study was assessed with the Cochrane Collaboration tool, which included evaluations of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias (Higgins et al. 2011).
Data analysis
We assumed heterogeneous effects among studies a priori and conservatively applied a random effects model for all outcomes. Denominators were adjusted to include the number of patients or hips, as appropriate. The rate ratio (RR) was the effect size statistic of interest, which indicates the ratio of incidence rates (events per person-year) between A and P. A RR value >1 indicates higher complication incidence rate with A and a value <1 indicates lower complication incidence rate with A. For each complication, the RR and 95% confidence interval (CI) were calculated in each study and pooled among all studies. Inconsistency in complication risk among studies was quantified with the I2 statistic; values of ≤25%, 50%, and ≥75% represented low, moderate, and high inconsistency, respectively (Higgins et al. 2003). Publication bias was visually assessed with funnel plots (not shown) and quantitatively assessed using Egger’s regression test. Post hoc random effects meta-regression using the Knapp–Hartung method (Knapp and Hartung 2003) was performed to assess the possible influence of study design, median surgery year, inclusion of learning cases, and follow-up duration on complication risk. P-values were 2-sided with a significance level <0.05. Analyses were performed using Comprehensive Meta-analysis (version 3.3, Biostat, Englewood, NJ, USA).
Funding and potential conflicts of interest
This work was supported by DePuy Synthes (Raynham, MA, USA). LM received a research grant from DePuy Synthes for data analysis. JW and SB are employees of DePuy Synthes. JG, AK, and FB declare no conflict of interest in this work.
Results
Study selection
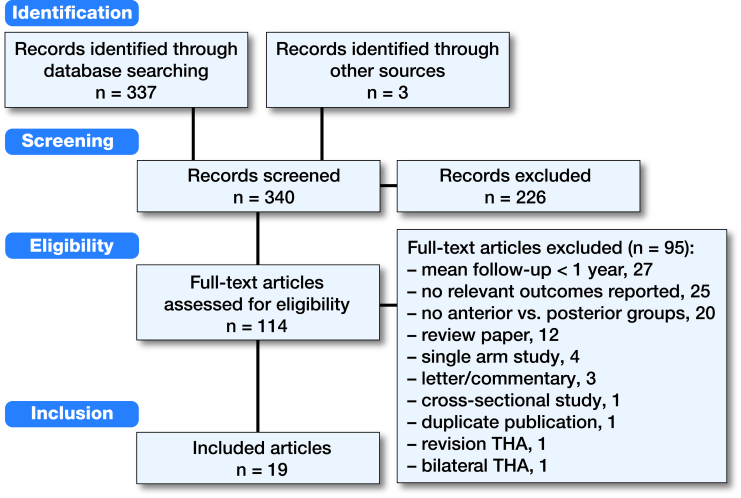
After screening 340 records for eligibility, 19 studies were included in this review, including 15 single-center comparative studies with 6,620 patients (2,278 A; 4,342 P) and 4 multicenter registries with 157,687 patients (18,735 A; 138,952 P). Primary reasons for study exclusion included mean follow-up less than 1 year (27 studies), complications not reported (25 studies), and no comparison of A with P (20 studies) (Figure).
Study and patient characteristics
This review included 4 randomized controlled trials, 1 prospective nonrandomized study, 10 retrospective studies, and 4 multicenter registries. Surgeries in each group occurred during the same period in 11 studies. In 7 studies, learning curve cases comprised some or all of the A group. Median follow-up duration was 16 months (range: 12–64 months) with A and 18 months (range: 12–110 months) with P. Comparing patients treated with A versus P, baseline patient characteristics were well matched for age (median 63 years per group), female sex (median 60% versus 58%), and BMI (median 28 per group) (Table 1). The primary risks of bias were attributable to inclusion of retrospective nonrandomized studies (Table 2).
Table 1.
Study and patient characteristics
| Study | Treatment | Parallel treatment | Learning cases | Mean follow-up,months | Sample sizeb | Mean age,years | Female, % | Mean BMI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | designa | period | period | included | A | P | A | P | A | P | A | P | A | P |
| Comparative studies: | ||||||||||||||
| Balasubramaniam et al. 2016 | RN | 2006–2011 | No | Yes | 12 | 12 | 50 | 42 | 63 | 57 | 50 | 67 | 31 | 30 |
| Barrett et al. 2013 | RCT | 2010–2011 | Yes | No | 12 | 12 | 43 | 44 | 61 | 63 | 33 | 57 | 31 | 29 |
| Batailler et al. 2017 | RN | 2013–2015 | Yes | Yes | 14 | 14 | 201 | 101 | 72 | 74 | 65 | 65 | 26 | 28 |
| Fransen et al. 2016 | RN | 2012 | Yes | Yes | 12 | 12 | 45 | 38 | 64 | 63 | 67 | 63 | 25 | 28 |
| Luo et al. 2016 | RCT | 2014 | Yes | No | 14 | 14 | 52 | 52 | 62 | 64 | 67 | 58 | 23 | 24 |
| Malek et al. 2016 | RN | 2010–2014 | Yes | No | 18 | 18 | 265 | 183 | 71 | 70 | 56 | 53 | 29 | 29 |
| Newman et al. 2016 | RN | – | NR | NR | 24 | 24 | 235 | 120 | 63 | 59 | 54 | 57 | 29 | 34 |
| Rathod et al. 2014 | RN | 2007–2011 | No | No | 16 | 30 | 286 | 293 | 62 | 61 | 55 | 57 | 26 | 26 |
| Rodriguez et al. 2014 | PN | 2010 | Yes | No | 12 | 12 | 60 | 60 | 60 | 59 | 53 | 57 | 27 | 28 |
| Sugano et al. 2009 | RN | 2005–2007 | No | NR | 24 | 24 | 33 | 39 | 56 | 57 | 88 | 92 | 23 | 23 |
| Taunton et al. 2014 | RCT | 2012 | Yes | No | 12 | 12 | 27 | 27 | 62 | 66 | 56 | 52 | 28 | 29 |
| Tripuraneni et al. 2016 | RN | 2012–2015 | Yes | Yes | 14 | 13 | 66 | 66 | 60 | 60 | 61 | 61 | 28 | 28 |
| Tsukada and Wakui 2015 | RN | 2000–2009 | No | NR | 64 | 110 | 139 | 177 | 67 | 62 | 90 | 83 | 23 | 24 |
| Watts et al. 2015 | RN | 2010–2014 | NR | NR | 12 | 12 | 716 | 3,040 | 64 | 62 | 51 | 51 | 29 | 30 |
| Zhang et al. 2006 | RCT | 2002–2004 | Yes | NR | 20 | 20 | 60 | 60 | 61 | 63 | 58 | 53 | – | –c |
| Registries | ||||||||||||||
| Amlie et al. 2014 | RN | 2008–2010 | Yes | No | 24 | 30 | 421 | 421 | 67 | 66 | 69 | 64 | – | – |
| Mjaaland et al. 2017 | RN | 2008–2013 | Yes | Yes | 52 | 52 | 2,017 | 5,961 | 67 | 65 | 67 | 65 | – | – |
| Sheth et al. 2015 | RN | 2001–2011 | No | Yes | 36 | 36 | 1,851 | 31,747 | 65 | 66 | 60 | 58 | 28 | 29 |
| Zijlstra et al. 2017 | RN | 2007–2015 | No | Yes | 40 | 40 | 14,446 | 100,823 | – | – | 68 | 68 | – | – |
A = anterior approach; P = posterior approach; NR = not reported
Study design: PN = prospective nonrandomized; RCT = randomized controlled trial; RN = retrospective nonrandomized.
Reported as number of patients or hips.
All patients with BMI ≤27 kg/m2.
Table 2.
Cochrane risk of bias assessment
Complications
The A was associated with lower rates of infection (RR =0.55, p = 0.002 from 7 studies), dislocation (RR =0.65, p = 0.03 from 11 studies), and reoperation (RR =0.84, p < 0.001 from 16 studies). In a subgroup analysis of infection, the rate of superficial (RR =0.47, p = 0.5) and deep infection (RR =0.23, p = 0.1) remained low with A, but neither was statistically significant. When explicitly reported, the most common reasons for reoperation were aseptic loosening, dislocation, fracture, and infection in the A group and dislocation, aseptic loosening, infection, and fracture in the P group. No statistically significant differences were observed in the rate of thromboembolic event (RR =0.59, p = 0.5 from 4 studies), heterotopic ossification (RR =0.63, p = 0.1 from 4 studies), wound complication (RR =0.93, p = 0.8 from 5 studies), or fracture (RR =1.0, p = 0.9 from 10 studies). Most fracture reports were of intraoperative periprosthetic fractures; however, type and time to fracture was not consistently reported. There was a higher rate of patient-reported nerve injury with A vs. P (RR =2.3, p = 0.01 from 2 studies). Nerve injuries were described as patient-reported sensory deficit (Luo et al. 2016) or patient-reported nerve injury with no distinction between sensory and motor involvement (Amlie et al. 2014). For each complication, heterogeneity among studies was low and publication bias was not evident (Table 3).
Table 3.
Complication rates with anterior versus posterior approach in primary total hip arthroplasty
| Event rate per 100 person-years |
|||||||
|---|---|---|---|---|---|---|---|
| Outcome | Studies | A | P | Effect size Rate ratio (95% CI)a | p-value | Heterogeneity (I2), % | Publication bias (Egger’s p-value) |
| Infection | 7 | 0.2 | 0.4 | 0.55 (0.38–0.80) | 0.002 | 0 | 0.5 |
| Thromboembolic event | 4 | 0.5 | 1.1 | 0.59 (0.14–2.43) | 0.5 | 0 | 0.2 |
| Heterotopic ossification | 4 | 1.5 | 2.3 | 0.63 (0.35–1.13) | 0.1 | 0 | 0.3 |
| Dislocation | 11 | 0.2 | 0.2 | 0.65 (0.44–0.95) | 0.03 | 17 | 0.5 |
| Reoperation | 16 | 0.6 | 0.7 | 0.84 (0.75–0.93) | < 0.001 | 0 | 1.0 |
| Wound | 5 | 1.7 | 1.9 | 0.93 (0.54–1.63) | 0.8 | 0 | 0.4 |
| Fracture | 10 | 0.3 | 0.1 | 1.02 (0.75–1.38) | 0.9 | 0 | 0.2 |
| Patient-reported nerve injury | 2 | 3.0 | 1.3 | 2.31 (1.22–4.39) | 0.01 | 0 | b |
Notes: A = anterior approach; P = posterior approach.
Rate ratio >1 indicates higher complication incidence rate with anterior approach; rate ratio <1 indicates lower complication incidence rate with anterior approach.
Inadequate number of studies to calculate value.
Post hoc meta-regression
Post hoc meta-regression was performed to assess the possible influence of study design, median surgery year, inclusion of learning cases, and follow-up duration on complication risk. No covariate was statistically significantly associated with the risk of any complication. In comparative studies, there was no statistically significant difference between A vs. P in the rate of any complication. In registries, the rate of patient-reported nerve injury was higher with A while the rates of infection and reoperation were lower with A (Table 4).
Table 4.
Subgroup analysis of study design on complication rates with anterior versus posterior approach in primary total hip arthroplasty
| Comparative studies |
Registries |
||||
|---|---|---|---|---|---|
| Outcome | Studies | Rate ratio (95% CI)a | Studies | Rate ratio (95% CI)a | p-valueb |
| Infection | 6 | 0.66 (0.16–2.7) | 1 | 0.55 (0.37–0.80) | 0.8 |
| Thromboembolic event | 4 | 0.59 (0.14–2.4) | 0 | – | – |
| Heterotopic ossification | 3 | 0.58 (0.30–1.2) | 1 | 0.81 (0.24–2.7) | 0.6 |
| Dislocation | 8 | 0.55 (0.17–1.8) | 3 | 0.74 (0.39–1.4) | 0.7 |
| Reoperation | 12 | 1.03 (0.60–1.8) | 4 | 0.83 (0.72–0.95) | 0.5 |
| Wound | 5 | 0.93 (0.54–1.6) | 0 | – | – |
| Fracture | 9 | 1.7 (0.79–3.7) | 1 | 0.93 (0.66–1.3) | 0.2 |
| Patient-reported nerve injury | 1 | 5.0 (0.24–104) | 1 | 2.2 (1.2–4.3) | 0.6 |
Rate ratio >1 indicates higher complication incidence rate with anterior approach; RR <1 indicates lower complication incidence rate with anterior approach.
Comparison of rate ratio in comparative studies versus registries, derived from Knapp– Hartung random effects meta-regression model.
Discussion
We conducted a systematic review and meta-analysis of comparative studies of A versus P primary THA with at least 1-year mean follow-up. An anterior approach was associated with a lower risk of reoperation, dislocation, and infection, but higher risk of patient-reported nerve injury. No difference was seen in the rate of thromboembolic event, heterotopic ossification, wound complication, or fracture. While heterogeneity or publication bias was not evident for any outcome, the possibility of such influences cannot be ruled out given the small number of studies reporting each complication.
A criticism of the A in primary THA is the presence of a learning curve, during which complication rates may be elevated. In an analysis of over 5,000 THA procedures, 50 or more A procedures were required to overcome the learning curve (de Steiger et al. 2015). In a single-surgeon experience with the first 500 A cases, the most dramatic reduction in complication rates occurred after the first 100 cases (Hartford and Bellino 2017). We identified no substantial influence of learning case inclusion on complication rates in meta-regression although this analysis was limited since it was not possible to determine the percentage of the entire sample comprising learning cases.
We identified a higher rate of patient-reported nerve injury with A. In the study of Amlie et al. (2014), nerve injury was self-reported in 5.9% of A patients at 24 months follow-up and 3.3% of P patients at 30 months follow-up; however, there was no distinction between sensory or motor involvement. In another comparative study (Luo et al. 2016), sensory deficit was 3.8% with A and 0% with P at 14 months’ follow-up. While comparative nerve injury data were limited to these 2 studies, a high incidence of sensory deficit with A has been reported in other studies (Bhargava et al. 2010, Goulding et al. 2010). This is primarily attributable to likely iatrogenic injury of the lateral cutaneous femoral nerve. Despite the higher patient-reported nerve injury rate with A, long-term functional limitations or higher reoperation rates are unlikely with these events based on the findings from other studies (Bhargava et al. 2010, Goulding et al. 2010).
In a meta-analysis comparing A and P (Higgins et al. 2015), there were no group differences in risk of intraoperative fracture and lower risk of dislocation with A. More recently, a systematic review compared anterior, posterior, and lateral approaches in primary THA (Meermans et al. 2017). In that review, complications were not systematically evaluated although the authors concluded that there were similar rates of complications between surgical approaches. In a network meta-analysis of randomized controlled trials (Putananon et al. 2018), complication risk was reported to be lower with P vs. A (1.0% vs. 1.4%); however, specific complications were not described. Among these reviews, follow-up duration varied considerably and was generally less than 1 year. Key differences in our meta-analysis are inclusion of only those studies with mean follow-up of at least 1 year, reporting of multiple specific complications, and statistical adjustment to account for differential follow-up periods among studies.
Several aspects of our meta-analysis are novel including the longest duration follow-up of any A versus P review and a comprehensive assessment of complication rates. There are also several limitations. First, despite the longest mean follow-up of any review on this topic, it must be acknowledged that data derived from 16 (A) to 18 (P) months median follow-up must be considered preliminary. Further, while the RR statistic allows for group comparison of event rates on a common scale (per person-year), event rates that are non-constant with respect to time may complicate interpretation of these results. Second, while osteoarthritis was the predominant diagnosis in each study, reporting of THA indications was inconsistent and may have confounded outcomes. Third, due to the small number of studies reporting certain complications, some complication estimates reported in this review may change with the addition of data by future studies. Further, the influence of study design on complication rates should be interpreted cautiously given the small number of studies for subgroup comparisons. Fourth, complication reporting was generally inconsistent among studies. Adherence to standardized complication reporting guidelines would greatly improve data transparency and consistency in the THA literature. Fifth, no conclusions regarding complication risk with anterolateral or lateral approaches in THA may be derived from this review. Finally, 14 of 19 included studies were retrospective in nature, which are inherently prone to bias.
In summary, comparing A with P in primary THA, A was associated with a lower rate of reoperation, dislocation, and infection, but a higher rate of patient-reported nerve injury.
Conception and design: LM, SB. Data collection: LM. Data analysis: LM. Writing the article: LM. Critical revision of the article: LM, JG, AK, FB, JW, SB
The authors would like to thank David Fay, PhD for assistance with literature review.
Acta thanks Johan Kärrholm and other anonymous reviewers for help with peer review of this study
PRISMA study flow diagram.
References
- Amlie E, Havelin L I, Furnes O, Baste V, Nordsletten L, Hovik O, Dimmen S.. Worse patient-reported outcome after lateral approach than after anterior and posterolateral approach in primary hip arthroplasty. A cross-sectional questionnaire study of 1,476 patients 1–3 years after surgery. Acta Orthop 2014; 85(5): 463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam U, Dowsey M, Ma F, Dunin A, Choong P.. Functional and clinical outcomes 244 following anterior hip replacement: a 5-year comparative study versus posterior approach. ANZ J Surg 2016; 86 (7-8): 589–93. [DOI] [PubMed] [Google Scholar]
- Barrett W P, Turner S E, Leopold J P.. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty 2013; 28(9): 1634–8. [DOI] [PubMed] [Google Scholar]
- Batailler C, Fary C, Batailler P, Servien E, Neyret P, Lustig S.. Total hip arthroplasty using direct anterior approach and dual mobility cup: safe and efficient strategy against post-operative dislocation. Int Orthop 2017; 41 (3): 499–506. [DOI] [PubMed] [Google Scholar]
- Bhargava T, Goytia R N, Jones L C, Hungerford M W.. Lateral femoral cutaneous nerve impairment after direct anterior approach for total hip arthroplasty. Orthopedics 2010; 33(7): 472. [DOI] [PubMed] [Google Scholar]
- de Steiger R N, Lorimer M, Solomon M.. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 2015; 473(12): 3860–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen B, Hoozemans M, Vos S.. Direct anterior approach versus posterolateral approach in total hip arthroplasty: one surgeon, two approaches. Acta Orthop Belg 2016; 82 (2): 240–8. [PubMed] [Google Scholar]
- Goulding K, Beaule P E, Kim P R, Fazekas A.. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res 2010; 468(9): 2397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailer N P, Lazarinis S, Makela K T, Eskelinen A, Fenstad A M, Hallan G, Havelin L, Overgaard S, Pedersen A B, Mehnert F, Karrholm J.. Hydroxyapatite coating does not improve uncemented stem survival after total hip arthroplasty! Acta Orthop 2015; 86(1): 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford J M, Bellino M J.. The learning curve for the direct anterior approach for total hip arthroplasty: a single surgeon’s first 500 cases. Hip Int 2017; 27(5): 483–8. [DOI] [PubMed] [Google Scholar]
- Higgins B T, Barlow D R, Heagerty N E, Lin T J.. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty 2015; 30(3): 419–34. [DOI] [PubMed] [Google Scholar]
- Higgins J P, Altman D G, Gotzsche P C, Juni P, Moher D, Oxman A D, Savovic J, Schulz K F, Weeks L, Sterne J A, Cochrane Bias Methods G, Cochrane Statistical Methods G.. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson S G, Deeks J J, Altman D G.. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp G, Hartung J.. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22(17): 2693–710. [DOI] [PubMed] [Google Scholar]
- Luo Z, Chen M, Shangxi F, Hu F, Ni Z, Cheng P, Ji X, Wu K, Zhang X.. [Comparison of clinical efficacy of total hip arthroplasty and lateral posterior approach for total hip arthroplasty]. Chinese J Med Sci 2016; 96: 2807–12. [DOI] [PubMed] [Google Scholar]
- Makela K T, Matilainen M, Pulkkinen P, Fenstad A M, Havelin L I, Engesaeter L, Furnes O, Overgaard S, Pedersen A B, Karrholm J, Malchau H, Garellick G, Ranstam J, Eskelinen A.. Countrywise results of total hip replacement: an analysis of 438,733 hips based on the Nordic Arthroplasty Register Association database. Acta Orthop 2014; 85(2): 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek I A, Royce G, Bhatti S U, Whittaker J P, Phillips S P, Wilson I R, et al. A comparison 287 between the direct anterior and posterior approaches for total hip arthroplasty: the role of an ‘Enhanced Recovery’ pathway. Bone Joint J 2016; 98-B (6): 754–60. [DOI] [PubMed] [Google Scholar]
- Meermans G, Konan S, Das R, Volpin A, Haddad F S.. The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J 2017; 99-B(6): 732–40. [DOI] [PubMed] [Google Scholar]
- Mjaaland K E, Svenningsen S, Fenstad A M, Havelin L I, Furnes O, Nordsletten L.. Implant survival after minimally invasive anterior or anterolateral vs. conventional posterior or direct lateral approach: an analysis of 21,860 total hip arthroplasties from the Norwegian Arthroplasty Register (2008 to 2013). J Bone Joint Surg Am 2017; 99(10): 840–7. [DOI] [PubMed] [Google Scholar]
- Newman E A, Holst D C, Bracey D N, Russell G B, Lang J E.. Incidence of heterotopic ossification in direct anterior vs posterior approach to total hip arthroplasty: a 298 retrospective radiographic review. Int Orthop 2016; 40 (9): 1967–73. [DOI] [PubMed] [Google Scholar]
- Putananon C, Tuchinda H, Arirachakaran A, Wongsak S, Narinsorasak T, Kongtharvonskul J.. Comparison of direct anterior, lateral, posterior and posterior-2 approaches in total hip arthroplasty: network meta-analysis. Eur J Orthop Surg Traumatol 2018; 28(2): 255–67. [DOI] [PubMed] [Google Scholar]
- Rathod P A, Bhalla S, Deshmukh A J, Rodriguez J A.. Does fluoroscopy with anterior hip arthroplasty decrease acetabular cup variability compared with a nonguided posterior approach? Clin Orthop Relat Res 2014; 472 (6): 1877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J A, Deshmukh A J, Rathod P A, Greiz M L, Deshmane P P, Hepinstall M S, et al. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res 2014; 472 (2): 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth D, Cafri G, Inacio M C, Paxton E W, Namba R S.. Anterior and anterolateral approaches for THA are associated with lower dislocation risk without higher revision risk. Clin Orthop Relat Res 2015; 473 (11): 3401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano N, Takao M, Sakai T, Nishii T, Miki H, Nakamura N.. Comparison of mini-incision total hip arthroplasty through an anterior approach and a posterior approach using navigation. Orthop Clin North Am 2009; 40 (3): 365–70. [DOI] [PubMed] [Google Scholar]
- Taunton M J, Mason J B, Odum S M, Springer B D.. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty 2014; 29 (9 Suppl): 169–72. [DOI] [PubMed] [Google Scholar]
- Tripuraneni K R, Munson N R, Archibeck M J, Carothers J T.. Acetabular abduction and dislocations in direct anterior vs posterior total hip arthroplasty: a retrospective, matched cohort study. J Arthroplasty 2016; 31(10): 2299–302. [DOI] [PubMed] [Google Scholar]
- Tsukada S, Wakui M.. Lower dislocation rate following total hip arthroplasty via direct anterior approach than via posterior approach: five-year-average follow-up results. Open Orthop J 2015; 9: 157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C D, Houdek M T, Wagner E R, Sculco P K, Chalmers B P, Taunton M J.. High risk of wound complications following direct anterior total hip arthroplasty in obese patients. J Arthroplasty 2015; 30 (12): 2296–8. [DOI] [PubMed] [Google Scholar]
- Zhang X L, Wang Q, Jiang Y, Zeng B F.. [Minimally invasive total hip arthroplasty with anterior incision]. Zhonghua Wai Ke Za Zhi 2006; 44 (8): 512–5. [PubMed] [Google Scholar]
- Zijlstra W P, De Hartog B, Van Steenbergen L N, Scheurs B W, Nelissen R.. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty. Acta Orthop 2017; 88(4): 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]