Abstract
Limited availability of donor organs and risk of ischemia‐reperfusion injury (IRI) seriously restrict organ transplantation. Therapeutics that can prevent or reduce IRI could potentially increase the number of transplants by increasing use of borderline organs and decreasing discards. Alpha‐1 antitrypsin (AAT) is an acute phase reactant and serine protease inhibitor that limits inflammatory tissue damage. Purified plasma–derived AAT has been well tolerated in more than 30 years of use to prevent emphysema in AAT‐deficient individuals. Accumulating evidence suggests that AAT has additional anti‐inflammatory and tissue‐protective effects including improving mitochondrial membrane stability, inhibiting apoptosis, inhibiting nuclear factor kappa B activation, modulating pro‐ vs anti‐inflammatory cytokine balance, and promoting immunologic tolerance. Cell culture and animal studies have shown that AAT limits tissue injury and promotes cell and tissue survival. AAT can promote tolerance in animal models by downregulating early inflammation and favoring induction and stabilization of regulatory T cells. The diverse intracellular and immune‐modulatory effects of AAT and its well‐established tolerability in patients suggest that it might be useful in transplantation. Clinical trials, planned and/or in progress, should help determine whether the promise of the animal and cellular studies will be fulfilled by improving outcomes in human organ transplantation.
Keywords: basic (laboratory) research/science, cell death: apoptosis, immunobiology, innate immunity, islet transplantation, lung transplantation/pulmonology, translational research/science
Short abstract
Although traditionally considered an extracellular inhibitor of serine proteases, alpha‐1‐antitrypsin also has intracellular effects that may be beneficial in transplantation, including inhibiting apoptosis and NF‐kB activation, modulating proversus anti‐inflammatory cytokine balance, and promoting tolerance.
Abbreviations
- AAT
alpha‐1‐antitrypsin
- EVLP
ex vivo lung perfusion
- IKB
inhibitor of NFKB
- IL
interleukin
- IRI
ischemia‐reperfusion injury
- NFKB
nuclear factor kappa B
- NOD
nonobese diabetic
- PiZZ
AAT‐deficient, homozygous for Z allele
- PP2A
protein phosphatase 2A
- STZ
streptozotocin
- TNF‐α
tumor necrosis factor‐α
- Tregs
regulatory T cells
1. INTRODUCTION
Despite significant advances in surgical technique and immunosuppression, challenges continue to restrict the number of transplants. Although the number of transplants performed each year in the United States is increasing, the number of people on waiting lists has nearly doubled in the past 15 years.1 Improving the viability of donor organs by reducing the damage caused by ischemia‐reperfusion injury (IRI) and activation of the innate immune system are thus key areas of ongoing research.
Alpha‐1‐antitrypsin (AAT) is a serine protease inhibitor and acute phase reactant produced mainly by the liver that limits tissue damage caused by neutrophil elastase and other extracellular enzymes.2, 3, 4 Consistent with this role, plasma concentrations of AAT can increase up to 4‐fold in response to inflammatory stimuli. Mutations in AAT can cause emphysema due to deficiency in circulating AAT, and/or liver disease likely due to a “misfolded protein response.”2, 3, 4 AAT‐deficient patients may also experience extrapulmonary pathologies, particularly panniculitis and vasculitis, attributed to overactivity of neutrophil proteases and excessive inflammation.2, 3, 4 Because of this direct clinical relevance, most studies of AAT have focused on its canonical role as an extracellular protease inhibitor, and augmentation with purified plasma–derived AAT is well established for prevention of emphysema in deficient individuals.2, 3, 4
Accumulating data, however, suggest that AAT has additional intracellular activities that may contribute to limiting inflammation and restoring homeostasis. These include stabilization of mitochondrial membranes and inhibition of caspases, which together inhibit apoptosis and increase cellular resistance to ischemia (Figure 1).4, 5, 6, 7 Inhibition of degradation of the inhibitor of NFKB (IKB), and therefore, nuclear factor kappa B (NFKB) activation, and activation of protein phosphatases alter the balance between pro‐ and anti‐inflammatory cytokines and may modulate adaptive as well as innate immunity and promote tolerance.4, 6, 7 Evidence from cellular and animal models suggests that these novel activities may be beneficial in transplantation.
Figure 1.

Major intracellular effects of alpha‐1‐antitrypsin and how they interrelate to achieve physiologic effects.4, 5, 6, 7 IKB, inhibitor of NFKB; IL, interleukin; NFKB, nuclear factor kappa B; ROS, reactive oxygen species
In this article, we will start by reviewing the novel functions of AAT, then go on to describe the current state of research into the use of AAT in transplantation of islet cells and solid organs. AAT has also been studied for treatment of graft vs host disease following hematopoietic stem cell transplantation, but analysis of that work is beyond the focus and scope of this review.
2. NOVEL FUNCTIONS OF AAT
2.1. Mitochondrial stabilization
Hepatocytes of AAT‐deficient patients and mice display mitochondrial injury and increased autophagy, 8 suggesting that AAT may play an important role in stabilizing mitochondrial membranes. Stabilization of mitochondrial membranes and decreasing release of Ca++, cytochrome c, and other constituents may explain how AAT protects against induction of diabetes by the mitochondrial poison, streptozotocin (STZ).9, 10 This effect, and inhibition of pro‐apoptotic signaling induced by tumor necrosis factor‐α (TNF‐α),10, 11 likely contribute to stabilization of pancreatic islet β‐cells and to the prevention/treatment of diabetes reported in animal models and in some human studies.12 Preservation of islets is of obvious interest in pancreas and islet transplantation (discussed in section 3.1, Pancreatic Islet Transplantation). Marcondes et al reported that AAT alters the cellular redox state and improves mitochondrial membrane potential while also increasing expression of antioxidant enzymes such as heme oxygenase 1 (Figure 1).4, 5, 6, 7 Oxidative stress is important in inflammation and IRI, so these activities may contribute to the ability of AAT to promote cell and tissue survival and modulate inflammatory damage.10, 11, 13, 14, 15, 16, 17, 18, 19, 20 Because different types of lymphocytes, and cells in different activation states, differ in dependence on glycolysis vs oxidative phosphorylation, modulation of mitochondrial function may influence the balance between sensitization and tolerance.6
2.2. Inhibition of apoptosis
AAT has been shown to inhibit apoptosis in multiple in vitro and in vivo models.10, 11, 13, 14, 15, 16, 17, 18, 19, 20 Increased cell survival may result not only from stabilization of mitochondrial membranes, but also likely involves direct inhibition of caspases (Figure 1).4, 5, 6, 7, 10, 11, 13, 14, 15, 16, 17, 18, 19, 20 The molecular mechanism(s) of this inhibition have not been elucidated; caspases are cysteine proteases, while elastase and other canonical targets of AAT depend on serine. However, immunofluorescence studies have shown colocalization of AAT and activated caspase 3 in apoptotic but not in viable cells.11 Direct interactions between AAT and caspase 3 are also supported by co‐immunoprecipitation of AAT and caspase 3 by anti‐caspase antibodies and inhibition of enzymatic activity of purified caspase 3 by AAT in vitro.11 Furthermore, AAT has been reported to inhibit lung damage due to intratracheally administered purified caspase 3.11 AAT has been reported to inhibit caspases 1, 3, 6, 7, and 8 in different experimental systems.18, 19, 20 However, some results conflict, and it is not always clear when direct intracellular vs indirect extracellular effects are involved.18 Additional investigations and definition of the molecular mechanism(s) of caspase inhibition are clearly needed.
Interestingly, many of the presumed intracellular effects of AAT on nonserine proteases in animal and cellular models have also been shown with a small‐molecule trypsin inhibitor (ulinastatin)19 as well as an anti‐inflammatory metalloprotease inhibitor (RS113456).20 Furthermore, a recombinant AAT construct devoid of serine protease‐inhibiting activity had the same effects as native AAT on blocking cytokine gene expression and other responses to lipopolysaccharide.21 These results emphasize the need for better elucidation of the mechanisms and pathways involved.
2.3. NFKB activation and cytokine production
NFKB enhances transcription of many pro‐inflammatory genes, such as the cytokines TNF‐α, interleukin‐8 (IL‐8), and IL‐32. The precise mechanism(s) by which AAT inhibits NFKB activation is unclear, but likely includes dampened activation of pro‐inflammatory signaling cascades 5, 20, 21, 22, 23 as well as protection of IKB from degradation in proteasomes (Figure 1).4, 5, 6, 7, 20 Besides proinflammatory cytokines, several NFKB‐induced genes, such as Bcl‐2, promote cell survival, so most compounds that inhibit NFKB are pro‐apoptotic. In contrast, AAT may be unique in inhibiting both NFKB and apoptosis (by separate mechanisms). Increased cell survival and decreased pro‐inflammatory cytokine production are likely both beneficial in the context of organ transplantation. AAT has also been reported to activate protein phosphatase 2A (PP2A) in inflammatory leukocytes and cultured pulmonary cells.23 AAT‐deficient (PiZZ) individuals were found to have decreased PP2A activity in neutrophils and alveolar macrophages in vivo, as compared to normal controls, and PP2A activity in vivo and in vitro is increased by AAT.23 PP2A decreases activity of several phosphokinases involved in signaling cascades that increase transcription of pro‐inflammatory cytokines.23 Thus, stimulation of PP2A could contribute to inhibition of cytokine expression. AAT can also inhibit proteolytic activation of cytokine precursors, such as pro‐IL‐1, to their active forms.22
2.4. Effects on lymphocytes
It was reported more than 25 years ago that AAT bound to lymphocyte membranes, inhibited their surface proteases, and altered monocyte‐lymphocyte interactions.24 Many effects of AAT may involve inhibition of proteases produced by 1 cell that act on adjacent cells and/or inhibition of protease‐activated receptors. Together with intracellular effects, these membrane effects likely contribute to a net decrease in cytokines that favor sensitization, such as IL‐1, TNFα, IL‐6, and IL‐32, while promoting tolerance by preserving transforming growth factor‐β and increasing IL‐10 and IL‐1 receptor antagonist.4, 6, 7, 24, 25, 26, 27, 28 These effects likely contribute to the induction of regulatory T cells (Tregs) that promote allograft survival.6, 7, 9, 25, 26, 27, 28 In a mouse model, AAT induced a “cuff” of FoxP3+ Tregs around islet allografts (Figure 2).7 In both murine and human studies, AAT was reported to modulate donor‐derived dendritic cells and to increase CD4+, CD25+ FoxP3+ Tregs.6 These results suggest that a major net effect of AAT in transplantation may be to shift an inflammatory, sensitizing environment to an anti‐inflammatory, tolerance‐inducing environment.28 Initiation of these changes by AAT in the early posttransplant period could decrease the risk of delayed graft function and also promote long‐term graft acceptance. Anti‐apoptotic effects, inhibition of injurious innate immune responses and other anti‐inflammatory effects, as well as increases in Tregs all likely contribute to beneficial effects of AAT in animal models. They may also contribute to observations that AAT has beneficial effects against autoimmune diabetes in “nonobese diabetic” (NOD) mice, as well as in type 1 diabetes in humans.12, 28
Figure 2.
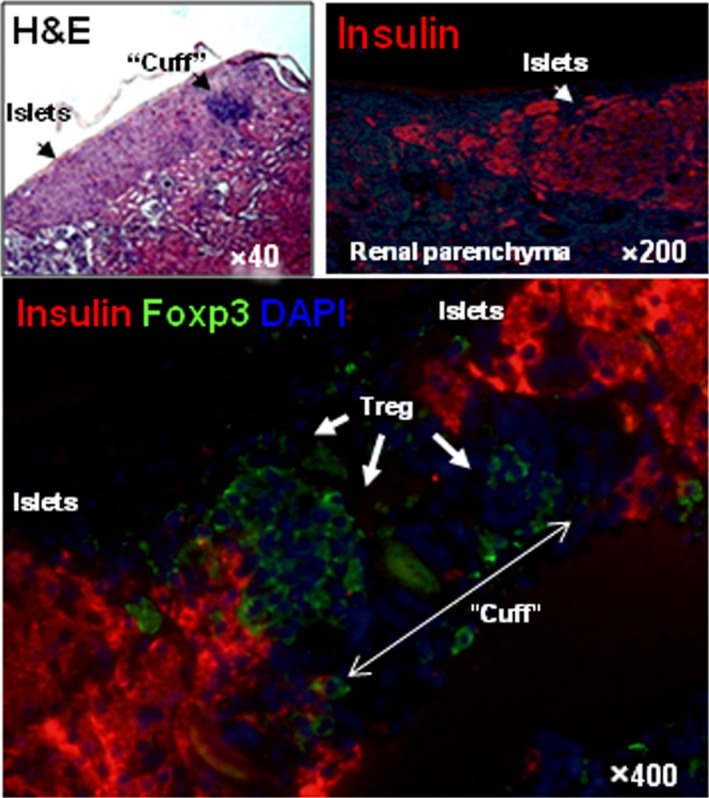
Immunohistochemistry of accepted allogeneic islet grafts. Isolated islets were grafted under the renal capsule of 6‐ to 7‐week‐old female mice, heterozygous for human AAT (background strain C57BL/6, H‐2b), rendered diabetic by a single injection of STZ, and treated with human AAT after transplantation. A representative section from a functioning 72‐day posttransplant islet is shown. Staining colors are the following: Blue, nuclei (diamidino‐2‐phenylindole [DAPI]); Red, insulin (guinea pig anti–swine insulin antibody and CY‐3‐conjugated donkey anti–guinea pig IgG); Green, Fox‐P3 (Alexa Fluor® 488 anti–mouse foxp3 antibody). Arrows show a “cuff” of FoxP3 positive (presumably) Tregs surrounding the insulin‐containing islet cells in the graft. Reproduced from Shahaf et al 7 with permission of the publisher. AAT, alpha‐1 antitrypsin; STZ, streptozotocin; Treg, regulatory T cells
3. EFFECTS OF AAT IN CELL AND SOLID ORGAN TRANSPLANTATION
Given the multiple effects of AAT that can improve cell survival and function, and potentially reduce immediate and long‐term inflammatory and immunologic graft injury, it seems likely that AAT could increase donor organ availability by enabling the use of organs now considered “borderline,” decreasing discard rates, and improving transplantation outcomes.
3.1. Pancreatic islet transplantation
Islet transplantation requires extraction of islets from the protease‐rich pancreatic environment. At least part of the beneficial effect of AAT in recovery of functional islets is likely related to protection of β‐cells and insulin from extracellular trypsin and other acinar‐cell proteases.29, 30 However, experimental models of diabetes also suggest that AAT protects against β‐cell loss induced by macrophage invasion, immunologic products such as TNF‐α,20, 21 and the oxidative stress that accompanies inflammation, hyperglycemia, and/or metabolic poisons.10, 31, 32 Beta cells seem particularly sensitive to poisons that bind to glucose transporters and/or cause mitochondrial damage, as evidenced by their destruction at concentrations of STZ that do not damage other cell types or organs.32 A complete discussion of AAT in diabetes is beyond the scope of this review, but data on β‐cell survival in NOD mice suggest that AAT exerts similar beneficial activities during islet recovery and transplantation. Indeed, inclusion of AAT during islet culture increases β‐cell viability, insulin content, and insulin secretion in response to glucose and other secretagogues.29, 30 When a marginal mass of islets was infused into the livers of syngeneic diabetic mice, AAT improved graft acceptance and reduced infiltration of neutrophils and macrophages into the graft, concomitant with reduced TNF‐α expression and decreased islet apoptosis.33 Multiple animal models have shown beneficial effects of AAT on islet cell survival. Three clinical trials of AAT in islet transplantation are currently listed in Clinicaltrials.gov (NCT 02947087, NCT 02464878, NCT 02713997), including the latest iteration of the “Edmonton protocol,”34 but results are not yet available.
Many islets gathered for transplantation perish from ischemia and other biologic insults that may begin in the donor, increase during recovery of the pancreas, progress through purification and culture of the islets, then continue posttransplantation until a new blood supply develops.35, 36, 37, 38 Poor survival of donor islets has imperiled clinical deployment of islet transplantation because multiple donors are required to render a single recipient diabetes‐free. An “immediate blood‐mediated inflammatory reaction” upon reperfusion of islets is mediated at least in part by increased release of the pro‐coagulant “tissue factor” and activation of extracellular protease cascades, which can be inhibited by AAT.35, 37 The massive, clinically unacceptable loss of islets due first to cell death, then to inflammation and thrombosis in the peritransplant period sensitize the host and are exacerbated by the inability to achieve tolerance. Thus, initial cellular insults and continuing immunologic damage are inextricably intertwined and diminish the probability of indefinite drug‐free islet transplant survival.
AAT has exhibited immediate posttransplant cytoprotective effects upon mouse islet transplants, even with syngeneic donors.33 More importantly, in an autologous cynomolgus islet model, insidious loss of β‐cells was prevented by short‐term AAT treatment during the early posttransplant period.38 While loss of islets and frank diabetes were observed in untreated islet autograft recipients by 180 days posttransplant, AAT‐treated recipients had normally functioning grafts for more than 4 years posttransplant.38
3.2. Lung transplantation
Lungs are among the least utilized donor organs because of damage before and/or during recovery, storage, and transport. The transplant procedure itself invariably includes ischemia and reperfusion, which add additional injury. Protease exposure, inflammation and cell death, as well as damage to vascular endothelium and activation of complement and coagulation cascades are major contributors to lung tissue injury and primary graft dysfunction.
To understand the potential beneficial effects of AAT, Gao et al first used a human lung epithelial cell–culture (BEAS‐2B) model of cold ischemia by reducing the temperature of the cells to 4°C and substituting nonserum‐containing preservation solution (Perfadex®) for the standard culture media, which contains 10% fetal calf serum. Reperfusion was then simulated by switching back to serum‐containing medium at 37°C. Compared to controls, addition of human AAT significantly reduced cell mortality in a dose‐dependent manner, paralleled by decreased caspase‐3 activation, and decreased production of IL‐6 and IL‐8.15 In rat models of in situ lung ischemia/reperfusion and lung transplantation after cold preservation of the donor organ, AAT reduced lung injury and improved function.15 Encouraged by these results, the same group then conducted a preclinical trial with a pig lung transplant model, in which donor lungs had been preserved at 4°C for 24 hours. Human AAT (240 mg/kg) or albumin (as a control) were given to the porcine recipients 30 minutes before reperfusion. At 4 hours postoperatively, AAT‐treated recipients had significant improved lung compliance, decreased wet/dry ratio, decreased permeability index, and improved exchange of both O2 and CO2. These effects were accompanied by stabilization of IKB and caspase‐1 in lung homogenates, decreased apoptosis and caspase 3 in tissue sections, and decreased pro‐inflammatory cytokines in plasma (significant for IL‐6 and interferon‐γ). Plasma neutrophil elastase and circulating thrombin‐antithrombin complexes were also decreased.39 A phase 2 clinical trial of intravenous AAT to prevent primary graft dysfunction in lung transplant is under development at the University of Toronto.
Ex vivo lung perfusion (EVLP) has been introduced to permit objective evaluation of donor lungs before transplantation and reconditioning to improve the function of the organs when necessary. Optimizing ex vivo perfusion conditions may thus increase donor lung acceptability. Lin et al tested whether addition of AAT (3 mg/mL) to the perfusate improves donor lung function. They stored pig donor lungs at 4°C for 24 hours, then performed EVLP at 37°C for 12 hours. Compared with donor pig lungs that received perfusate alone, those that received AAT during EVLP had decreased cell death, decreased leakage of Ca++ into the perfusate, improved gas exchange, decreased pulmonary vascular resistance, increased lung compliance, decreased edema, and reduced expression of inflammatory cytokines ex vivo.40 A preclinical trial of AAT to repair seriously damaged human donor lungs declined from clinical use is currently ongoing.
3.3. Liver
Unlike islets and lung, studies of AAT in liver transplant models have been limited. However, consistent with data in other organs, administration of AAT in several acute mouse models protected hepatocytes against apoptosis, decreased activity of caspase 1, 8, and 3 (as assessed in homogenates), and improved survival of the animals.18, 19 Although no data are available on AAT in human liver transplantation per se, use of a low molecular weight trypsin inhibitor in a mouse liver ex vivo perfusion and transplantation model improved graft survival with effects similar to those reported for ATT (ie, inhibition of apoptosis, decreased IL‐6, and increased IL‐10), suggesting that further exploration of the mechanisms of action and potential of AAT for liver transplantation is also warranted.18, 19
3.4. Kidney
Although data are limited, it seems likely that AAT could also be beneficial in kidney transplantation. Models of renal IRI reiterate the anti‐apoptotic, anti‐inflammatory, and immune protective functions of AAT.13, 41 In rat single kidney allo‐transplantation, administration of AAT‐primed recipient‐strain dendritic cells reduced serum pro‐inflammatory cytokines, increased circulating IL‐10, increased CD4+, increased Foxp3+ Tregs, and reduced leukocyte infiltration.42 Effects of AAT on the donor organ per se were not reported.
3.5. Other organs
Only scant information on the effects of AAT in transplantation of other organs (eg, cornea, heart, intestine, and composite tissues) is available, but some interesting findings have emerged. In animal models of myocardial ischemia/reperfusion and infarction, administration of AAT reduced infarction area, improved left ventricular function, and reduced cellular apoptosis, caspase activation, and leukocyte infiltration.16 Analogous beneficial effects have been described in a growing number of other models of IRI in situ and in additional transplant models. It seems almost certain that inhibition of multiple types of proteases, not just elastase, are involved. AAT also has lipid‐binding and anti‐oxidant activities43 and may directly or indirectly inhibit other signaling cascades not discussed here. Identification of the mechanisms and development of biomarkers for clinical efficacy could hasten application of AAT and/or development of more efficient synthetic molecules.
4. SAFETY OF AAT IN HUMANS
Purified, plasma‐derived AAT augmentation therapy for AAT deficiency has been licensed in the United States for nearly 30 years. Long‐term intravenous use of AAT, usually at a dose of 60 mg/kg per week, is well tolerated in AAT‐deficient individuals without reports of neutralizing antibodies.2, 3 In several studies, deficient individuals randomized to treatment with AAT reported fewer adverse events than those receiving placebo (reviewed in references 2 and 3). AAT treatment of cystic fibrosis patients by both inhaled and intravenous routes also appears to be safe and well tolerated despite their chronic infections with Pseudomonas aeruginosa and other bacteria.44 In early clinical trials for type‐1 diabetes, and in small human trials for other conditions, neither acute nor cumulative adverse effects attributable to AAT have emerged.
5. FUTURE DIRECTIONS
Despite advances in organ preservation and immunosuppression, donor graft supply and acceptability continue to limit transplantation. AAT has long been known to protect tissues by inhibiting proteases. Accumulating evidence suggests that additional effects of AAT may favorably impact cell survival and tissue integrity. Inhibiting immediate, innate responses and modulating pro‐ vs anti‐inflammatory cytokines may reduce early injury and also shift the local environment towards a protective, immunotolerant state.
Recognition of putative intracellular effects of AAT, in addition to its canonical role as an extracellular serine protease inhibitor, have led to a broad range of preclinical studies that suggest the possibility of multiple beneficial effects. These potentially include improving the donor organ by administration even before retrieval and/or during ex vivo perfusion. Studies of addition of AAT to transport solutions and/or during cold stasis have not yet been reported. Intraoperative administration may ameliorate IRI and immediate inflammatory responses. Postoperatively, AAT may help prevent immunologic sensitization and rejection without increasing the risk of infection or other toxicities of current immunosuppressives. Multiple commercially available formulations of AAT have been used for augmentation in deficient patients for more than 30 years, with minimal adverse consequences. Several human trials of AAT in transplantation have been launched and/or are under development. This review is intended to summarize their rationale and illustrate the potential of AAT to improve transplant outcomes.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. M Berger and M Uknis are employees of CSL Behring and own stock. It should be noted that CSL Behring manufactures and markets human plasma‐derived AAT. M Liu has received research grant funding from CSL Behring. M Koulmanda has received research funding from Baxter (now Shire), which also manufactures and markets human plasma–derived AAT.
ACKNOWLEDGMENTS
Technical and editorial assistance was provided by Mary Beth Moncrief, PhD, and Jillian Gee, PhD, Synchrony Medical Communications, LLC, West Chester, PA, under the direction of the authors. Funding for this support was provided by CSL Behring, King of Prussia, PA.
Berger M, Liu M, Uknis ME, Koulmanda M. Alpha‐1‐antitrypsin in cell and organ transplantation. Am J Transplant. 2018;18:1589–1595. 10.1111/ajt.14756
REFERENCES
- 1. Organ Procurement and Transplantation Network . National data. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. 2017. Accessed March 24, 2017.
- 2. de Serres F, Blanco I. Role of alpha‐1 antitrypsin in human health and disease. J Intern Med. 2014;276:311‐335. [DOI] [PubMed] [Google Scholar]
- 3. Silverman EK, Sandhaus RA. Clinical practice. Alpha1‐antitrypsin deficiency. N Engl J Med. 2009;360:2749‐2757. [DOI] [PubMed] [Google Scholar]
- 4. Ehlers MR. Immune‐modulating effects of alpha‐1 antitrypsin. Biol Chem. 2014;395:1187‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou X, Shapiro L, Fellingham G, et al. HIV replication in CD4 + T lymphocytes in the presence and absence of follicular dendritic cells: inhibition of replication mediated by alpha‐1‐antitrypsin through altered IκBα ubiquitination. J Immunol. 2011;186:3148‐3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcondes AM, Karoopongse E, Lesnikova M, et al. α‐1‐Antitrypsin (AAT)‐modified donor cells suppress GVHD but enhance the GVL effect: a role for mitochondrial bioenergetics. Blood. 2014;124:2881‐2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahaf G, Moser H, Ozeri E, et al. α‐1‐antitrypsin gene delivery reduces inflammation, increases T‐regulatory cell population size and prevents islet allograft rejection. Mol Med. 2011;17:1000‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teckman JH, An JK, Blomenkamp K, et al. Mitochondrial autophagy and injury in the liver in α 1‐antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286:G851‐G862. [DOI] [PubMed] [Google Scholar]
- 9. Lewis EC, Shapiro L, Bowers OJ, et al. α1‐antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102:12153‐12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang B, Lu Y, Campbell‐Thompson M, et al. α1‐antitrypsin protects β‐cells from apoptosis. Diabetes. 2007;56:1316‐1323. [DOI] [PubMed] [Google Scholar]
- 11. Petrache I, Fijalkowska I, Medler TR, et al. α‐1 antitrypsin inhibits caspase‐3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleixo‐Lima G, Ventura H, Medini M, et al. Mechanistic evidence in support of alpha1‐antitrypsin as a therapeutic approach for type 1 diabetes. J Diabetes Sci Technol. 2014;8:1193‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daemen MA, Heemskerk VH, van't Veer C, et al. Functional protection by acute phase proteins alpha(1)‐acid glycoprotein and alpha(1)‐antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 2000;102:1420‐1426. [DOI] [PubMed] [Google Scholar]
- 14. Feng Y, Hu L, Xu Q, et al. Cytoprotective role of alpha‐1 antitrypsin in vascular endothelial cell under hypoxia/reoxygenation condition. J Cardiovasc Pharmacol. 2015;66:96‐107. [DOI] [PubMed] [Google Scholar]
- 15. Gao W, Zhao J, Kim H, et al. α1‐Antitrypsin inhibits ischemia reperfusion‐induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33:309‐315. [DOI] [PubMed] [Google Scholar]
- 16. Toldo S, Seropian IM, Mezzaroma E, et al. Alpha‐1 antitrypsin inhibits caspase‐1 and protects from acute myocardial ischemia‐reperfusion injury. J Mol Cell Cardiol. 2011;51:244‐251. [DOI] [PubMed] [Google Scholar]
- 17. Serban KA, Petrache I. Alpha‐1 antitrypsin and lung cell apoptosis. Ann Am Thorac Soc. 2016;13:S146‐S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jedicke N, Struever N, Aggrawal N, et al. alpha‐1‐antitrypsin inhibits acute liver failure in mice. Hepatology. 2014;59:2299‐2308. [DOI] [PubMed] [Google Scholar]
- 19. Guan L, Liu H, Fu P, et al. The protective effects of trypsin inhibitor on hepatic ischemia‐reperfusion injury and liver graft survival. Oxid Med Cell Longev. 2016;2016:1429835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Churg A, Dai J, Zay K, et al. Alpha‐1‐antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti‐inflammatory effects. Lab Invest. 2001;81:12. [DOI] [PubMed] [Google Scholar]
- 21. Jonigk D, Al‐Omari M, Maegel L, et al. Anti‐inflammatory and immunomodulatory properties of α1‐antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA. 2013;110:15007‐15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, He Y, Abraham B, et al. Cytosolic, autocrine alpha‐1 proteinase inhibitor (A1PI) inhibits caspase‐1 and blocks IL‐1β dependent cytokine release in monocytes. PLoS ONE. 2012;7:e51078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geraghty P, Eden E, Pillai M, et al. α1‐Antitrypsin activates protein phosphatase 2A to counter lung inflammatory responses. Am J Respir Crit Care Med. 2014;190:1229‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bata J, Revillard JP. Interaction between alpha 1 antitrypsin and lymphocyte surface proteases: immunoregulatory effects. Agents Actions. 1981;11:614‐616. [DOI] [PubMed] [Google Scholar]
- 25. Marcondes AM, Li X, Tabellini L, et al. Inhibition of IL‐32 activation by alpha‐1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood. 2011;118:5031‐5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abecassis A, Schuster R, Shahaf G, et al. α1‐antitrypsin increases interleukin‐1 receptor antagonist production during pancreatic islet graft transplantation. Cell Mol Immunol. 2014;11:377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis EC, Mizrahi M, Toledano M, et al. α1‐Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A. 2008;105:16236‐16241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koulmanda M, Bhasin M, Hoffman L, et al. Curative and β cell regenerative effects of α1‐antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci USA. 2008;105:16242‐16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loganathan G, Dawra RK, Pugazhenthi S, et al. Insulin degradation by acinar cell proteases creates a dysfunctional environment for human islets before/after transplantation: benefits of alpha‐1 antitrypsin treatment. Transplantation. 2011;92:1222‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimoda M, Noguchi H, Fujita Y, et al. Improvement of porcine islet isolation by inhibition of trypsin activity during pancreas preservation and digestion using α1‐antitrypsin. Cell Transplant. 2012;21:465‐471. [DOI] [PubMed] [Google Scholar]
- 31. Kalis M, Kumar R, Janciauskiene S, et al. α 1‐antitrypsin enhances insulin secretion and prevents cytokine‐mediated apoptosis in pancreatic β‐cells. Islets. 2010;2:185‐189. [DOI] [PubMed] [Google Scholar]
- 32. Eleazu C, Eleazu K, Chukwuma S, et al. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koulmanda M, Bhasin M, Fan Z, et al. Alpha 1‐antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci USA. 2012;109:15443‐15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aralast NP in inslet transplant, NCT02520076. https://www.clinicaltrials.gov/ct2/show/NCT02520076?term=islet%2C+edmonton&cond=Diabetes&draw=3&rank=22. Accessed December 6, 2017.
- 35. Bennet W, Sundberg B, Groth CG, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implication for clinical intraportal islet transplantation? Diabetes. 1999;48:14. [DOI] [PubMed] [Google Scholar]
- 36. Goto M, Johansson U, Eich TM, et al. Key factors for human islet isolation and clinical transplantation. Transplant Proc. 2005;37:1315‐1316. [DOI] [PubMed] [Google Scholar]
- 37. Wang J, Sun Z, Gou W, et al. α‐1 antitrypsin enhances islet engraftment by suppression of instant blood‐mediated inflammatory reaction. Diabetes. 2017;66:970‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koulmanda M, Sampathkumar RS, Bhasin M, et al. Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant. 2014;14:1543‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iskender I, Sakamoto J, Nakajima D, et al. Human alpha1‐antitrypsin improves early post‐transplant lung function: pre‐clinical studies in a pig lung transplant model. J Heart Lung Transplant. 2016;35:913‐921. [DOI] [PubMed] [Google Scholar]
- 40. Lin H, Chen M, Tian F, et al. Alpha‐1 antitrypsin improves function of porcine donor lungs during ex vivo lung perfusion [published online ahead of print on October 2, 2017]. J Heart Lung Transplant. 10.1016/j.healun.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 41. Maicas N, van der Vlag J, Bublitz J, et al. Human alpha‐1‐antitrypsin (hAAT) therapy reduces renal dysfunction and acute tubular necrosis in a murine model of bilateral kidney ischemia‐reperfusion injury. PLoS ONE. 2017;12:e0168981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen G, Li J, Chen L, et al. α1‐antitrypsin‐primed tolerogenic dendritic cells prolong allograft kidney transplants survival in rats. Int Immunopharmacol. 2016;31:216‐221. [DOI] [PubMed] [Google Scholar]
- 43. Frenzel E, Wrenger S, Brugger B, et al. Alpha‐1‐antitrypsin combines with plasma fatty acids and induces angiopoietin‐like protein 4 expression. J Immunol. 2015;195:3605‐3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaggar A, Chen J, Chmiel JF, et al. Inhaled alpha‐proteinase inhibitor therapy in patients with cystic fibrosis. J Cyst Fibros. 2016;15:227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]


