Abstract
BENEFIT and BENEFIT‐EXT were phase III studies of cytotoxic T‐cell crossmatch–negative kidney transplant recipients randomized to belatacept more intense (MI)‐based, belatacept less intense (LI)‐based, or cyclosporine‐based immunosuppression. Following study completion, presence/absence of HLA‐specific antibodies was determined centrally via solid‐phase flow cytometry screening. Stored sera from anti‐HLA–positive patients were further tested with a single‐antigen bead assay to determine antibody specificities, presence/absence of donor‐specific antibodies (DSAs), and mean fluorescent intensity (MFI) of any DSAs present. The effect of belatacept‐based and cyclosporine‐based immunosuppression on MFI was explored post hoc in patients with preexisting DSAs enrolled to BENEFIT and BENEFIT‐EXT. In BENEFIT, preexisting DSAs were detected in 4.6%, 4.9%, and 6.3% of belatacept MI‐treated, belatacept LI‐treated, and cyclosporine‐treated patients, respectively. The corresponding values in BENEFIT‐EXT were 6.0%, 5.7%, and 9.2%. In both studies, most preexisting DSAs were of class I specificity. Over the first 24 months posttransplant, a greater proportion of preexisting DSAs in belatacept‐treated versus cyclosporine‐treated patients exhibited decreases or no change in MFI. MFI decline was more apparent with belatacept MI‐based versus belatacept LI‐based immunosuppression in both studies and more pronounced in BENEFIT‐EXT versus BENEFIT. Although derived post hoc, these data suggest that belatacept‐based immunosuppression decreases preexisting DSAs more effectively than cyclosporine‐based immunosuppression.
Keywords: antibody biology, clinical research/practice, clinical trial, immunosuppressant ‐ calcineurin inhibitor: cyclosporine A (CsA), immunosuppressant ‐ fusion proteins and monoclonal antibodies: belatacept, kidney transplantation/nephrology
Short abstract
Post hoc analysis indicates that belatacept‐based immunosuppression decreases preexisting, donor‐specific HLA antibody more effectively than cyclosporine‐based immunosuppression.
Abbreviations
- AE
adverse event
- BENEFIT
Belatacept Evaluation of Nephroprotection and Efficacy as First‐Line Immunosuppression Trial
- BENEFIT‐EXT
BENEFIT‐Extended Criteria Donors Trial
- CI
confidence interval
- DSA
donor‐specific antibody
- ESRD
end‐stage renal disease
- HR
hazard ratio
- LI
less intense
- MFI
mean fluorescence intensity
- MI
more intense
- PRA
panel reactive antibody
1. INTRODUCTION
Since the presence of donor‐specific antibodies (DSAs) is associated with an increased risk of antibody‐mediated rejection and graft failure in kidney transplant recipients,1, 2 positivity for complement‐dependent lymphocytotoxic crossmatching became a contraindication to renal transplantation. However, cell‐based assays are unable to detect low DSA levels or DSAs that do not activate complement. Thus, patients who are complement‐dependent crossmatch–negative may still harbor DSAs.3 Compared with complement‐dependent T‐cell crossmatching, solid‐phase immunoassays are more sensitive,4, 5 detecting not only low class I antibody levels, but also antibodies against class II antigens and class I and class II allele‐specific antibodies.5, 6 Moreover, solid‐phase immunoassays are semi‐quantitative, permitting the categorization of DSA levels.5
The phase III Belatacept Evaluation of Nephroprotection and Efficacy as First‐Line Immunosuppression Trial (BENEFIT) and BENEFIT‐Extended Criteria Donors (BENEFIT‐EXT) studies evaluated belatacept, a selective T‐cell costimulation blocker approved worldwide to prevent kidney transplant rejection in adults.7 Kidney transplant recipients enrolled to these similarly designed studies were T‐cell lymphocytotoxic crossmatch–negative (determined locally). However, subsequent testing at a central facility of stored sera via solid‐phase flow cytometry and single‐antigen bead assay revealed that a subset of patients did possess preexisting DSAs. Importantly, the results from these prespecified exploratory analyses were not intended for use as a requirement for study entry or for patient management (ie, centers were unaware of assay results prior to transplantation). The present post hoc analyses examined the effect of belatacept‐based and cyclosporine‐based immunosuppression on MFI in the subsets of BENEFIT and BENEFIT‐EXT participants with preexisting DSAs. Efficacy and safety at 7 years (84 months) posttransplant in these patients were also evaluated.
2. METHODS
2.1. Study design
BENEFIT (NCT00256750) and BENEFIT‐EXT (NCT00114777) were 3‐year, international, partially blinded, randomized phase III studies.8, 9 Patients undergoing primary transplant with T‐cell panel reactive antibodies (PRA) ≥50% or those undergoing re‐transplantation with T‐cell PRA ≥30% were excluded, as were patients with a locally determined positive T‐cell crossmatch with the intended donor. Patients in BENEFIT received a living or standard‐criteria deceased donor kidney. Patients in BENEFIT‐EXT received an extended‐criteria donor kidney.
Participants were initially randomized (1:1:1) to receive belatacept more intense (MI)‐based, belatacept less intense (LI)‐based, or cyclosporine‐based immunosuppression for 3 years,8, 9 but a protocol amendment allowed them to continue randomized treatment beyond 3 years.10, 11 In addition to randomized treatment, patients received basiliximab induction, mycophenolate mofetil, and corticosteroids.
These studies were conducted in accordance with Good Clinical Practice guidelines and the principles outlined in the Declaration of Helsinki. The institutional review boards/ethics committees at participating centers approved the study protocols. All patients provided written informed consent.
2.2. Assessments
The presence of HLA antibodies was assessed in all randomized, transplanted patients at baseline (day 0, the day of transplant), at months 6, 12, 24, 36, 48, 60, and 84, and at the time of clinically suspected episodes of acute rejection. Antibody screening was performed centrally at Emory University using solid‐phase flow cytometry screening (FlowPRA, One Lambda, Inc., Canoga Park, CA). HLA‐A, ‐B, ‐C, ‐DRB1, ‐DRB3/4/5, ‐DQA, and ‐DQB1 antigens were identified by molecular typing methods, typically to the two‐digit (low resolution) level. HLA‐DP typing was not performed, since no study participant had HLA‐DP antibodies. Sera from patients with anti‐HLA antibodies were subsequently analyzed using Luminex single‐antigen bead assay (One Lambda, Inc.) to determine antibody class specificity, the presence/absence of DSAs, and the mean fluorescence intensity (MFI) of any DSAs present. Sera were not pretreated or diluted prior to single‐antigen bead testing. Since this was a post hoc study and because there are no established clinical thresholds for preexisting DSA MFI levels, we wanted to be conservative, considering any patient with DSA MFI levels ≥1000 as preexisting DSA‐positive. To minimize variation due to lot/technician differences, all samples—irrespective of the time at which they were drawn—were tested and analyzed at the same time. Single‐antigen testing was performed using a modified technique employing a biotin‐conjugated secondary antibody followed by phycoerythrin‐conjugated streptavidin. An MFI value of 1000 in this assay corresponds to a slightly lower MFI value than in the nonmodified (standard) assay. Details on our modified technique are described in Sullivan et al.12
The following were analyzed in each study: death or graft loss, renal function (six‐variable Modification of Diet in Renal Disease equation13), and acute rejection (central biopsy‐proven rejection that was clinically suspected for protocol‐defined reasons or clinically suspected for other reasons and treated).
2.3. Statistics
Patients were analyzed according to the intent‐to‐treat principle. Descriptive statistics were used to summarize MFI from baseline (pretransplant) to month 24 in the subsets of patients from each study with preexisting DSAs. Any DSA MFI value ≥1000 at baseline was considered positive. Time to death or graft loss was assessed in evaluable patients (patients known to be alive at month 84 or known to have died or experienced graft loss by month 84) and described using Kaplan–Meier event rates. The method of Kaplan–Meier was used to determine time to death or graft loss from randomization to month 84 in the cohorts of patients in each study who did and did not have preexisting DSAs. These cohorts were compared using hazard ratios (HRs) and 95% confidence intervals (CIs), which were calculated via Cox regression; no adjustment was made for multiplicity. Kaplan–Meier cumulative event rates were used to summarize the incidence of acute rejection. Descriptive statistics were used to summarize renal function (month 1 to month 84).
3. RESULTS
3.1. Benefit
In BENEFIT, 4.6% (10/219), 4.9% (11/226), and 6.3% (14/221) of patients randomized to belatacept MI, belatacept LI, and cyclosporine, respectively, had DSAs prior to transplant. The HLA class specificity of preexisting DSAs was similar across treatment arms, with the majority being class I (Table 1). The average baseline MFI of HLA DSAs in belatacept MI‐treated, belatacept LI‐treated, and cyclosporine‐treated patients were 10 689 (range, 1900‐24 000), 9806 (2400‐19 000), and 7014 (1166‐23 000), respectively.
Table 1.
Baseline characteristics of patients with preexisting DSAs in BENEFIT
| Belatacept MI (n = 10) | Belatacept LI (n = 11) | Cyclosporine (n = 14) | |
|---|---|---|---|
| Mean age, y (SD) | 49.6 (16.9) | 45.0 (7.2) | 43.7 (12.6) |
| Male, n (%) | 3 (30.0) | 3 (27.3) | 6 (42.9) |
| Race | |||
| White | 7 (70.0) | 6 (54.5) | 7 (50.0) |
| Black | 0 (0) | 2 (18.2) | 1 (7.1) |
| Asian | 2 (20.0) | 2 (18.2) | 1 (7.1) |
| Other | 1 (10.0) | 1 (9.1) | 5 (35.7) |
| Region | |||
| North America | 4 (40.0) | 3 (27.3) | 7 (50.0) |
| South America | 2 (20.0) | 3 (27.3) | 1 (7.1) |
| Europe | 3 (30.0) | 3 (27.3) | 4 (28.6) |
| Rest of world | 1 (10.0) | 2 (18.2) | 2 (14.3) |
| Categorized PRA, n (%) | |||
| <20% | 8 (80.0) | 8 (72.7) | 11 (78.6) |
| ≥20% | 2 (20.0) | 3 (27.3) | 2 (14.3) |
| Missinga | 0 (0) | 0 (0) | 1 (7.1) |
| Reported cause of ESRD, n (%) | |||
| Glomerulonephritis | 3 (30.0) | 4 (36.4) | 3 (21.4) |
| Diabetes | 2 (20.0) | 1 (9.1) | 3 (21.4) |
| Polycystic kidneys | 1 (10.0) | 1 (9.1) | 1 (7.1) |
| Congenital, familial, and metabolic | 1 (10.0) | 1 (9.1) | 0 (0) |
| Re‐transplant/graft failure | 0 (0) | 0 (0) | 2 (14.3) |
| Other | 3 (30.0) | 4 (36.4) | 5 (35.7) |
| T‐cell crossmatch–negative | 10 (100.0) | 11 (100.0) | 14 (100.0) |
| Preexisting DSA HLA class specificity, n (%) | |||
| Class I | 7 (70.0) | 7 (63.6) | 9 (64.3) |
| Class II | 2 (20.0) | 3 (27.3) | 4 (28.6) |
| Class I and II | 1 (10.0) | 1 (9.1) | 1 (7.1) |
| Average MFI of DSAs | |||
| Total | 10 689 | 9806 | 7014 |
| Class I‐only | 9292 | 9529 | 6605 |
| Class II‐only | 14 320 | 10 775 | 8042 |
DSA, donor‐specific antibody; ESRD, end‐stage renal disease; LI, less intense; MFI, mean fluorescence intensity; MI, more intense; PRA, panel reactive antibody; SD, standard deviation.
On the case report form, investigators had to tick off whether a patient satisfied all eligibility criteria. Although there was space on the form for investigators to provide numerical results for the highest and most recent PRA percentages, they were not required to do so. Thus, the PRA percentage is missing for some study participants.
Eighteen HLA DSAs (class I, n = 13; class II, n = 5) were detected in the 10 belatacept MI‐treated patients with preexisting DSAs. MFI values decreased or did not change for all 18 DSAs between baseline and month 24 (100.0%, 18/18) (Figure 1). Eighteen HLA DSAs (class I, n = 14; class II, n = 4) were detected in the 11 belatacept LI‐treated patients with preexisting DSAs. MFI values declined or did not change for all but one DSA (94.4%, 17/18). Twenty‐one HLA DSAs (class I, n = 14; class II, n = 7) were detected in the 14 cyclosporine‐treated patients with preexisting DSAs. The percentage of preexisting DSAs exhibiting a decline or no change in MFI was 66.7% (14/21).
Figure 1.
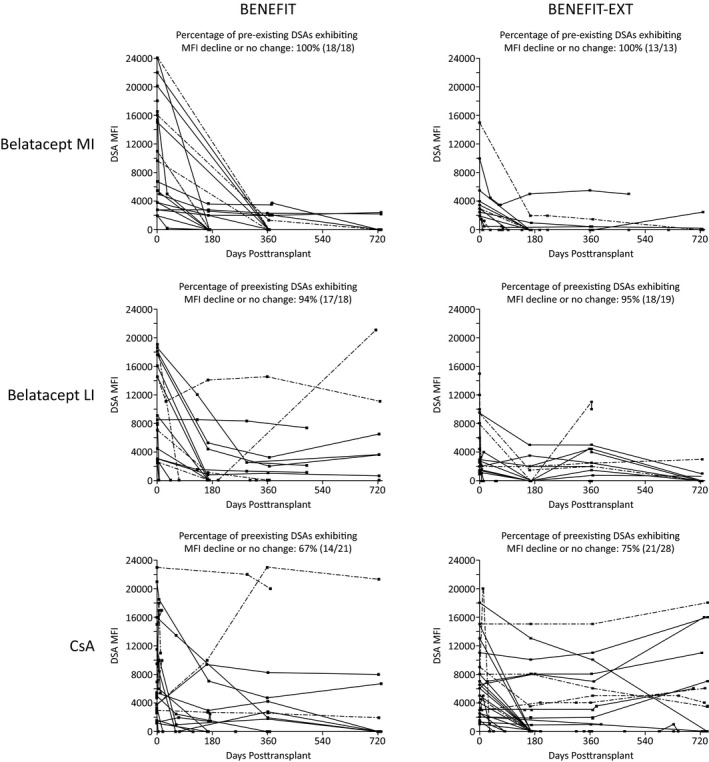
MFI between baseline and month 24 in the subsets of patients in BENEFIT and BENEFIT‐EXT with preexisting DSAs. Dashed lines denote MHC class II preexisting DSAs. CsA, cyclosporine; DSA, donor‐specific antibody; LI, less intense; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; MI, more intense
At month 84, 100.0% (10/10) of patients with preexisting DSAs randomized to belatacept MI, 72.7% (8/11) of those with preexisting DSAs randomized to belatacept LI, and 57.1% (8/14) of patients with preexisting DSAs randomized to cyclosporine were evaluated for death or graft loss. Kaplan–Meier estimated rates of death or graft loss at month 84 for belatacept MI‐treated, belatacept LI‐treated, and cyclosporine‐treated patients were 10.0%, 31.8%, and 29.1%, respectively. Across the three treatment arms, 35 patients had preexisting DSAs and 631 did not. The Kaplan–Meier cumulative event rate for death or graft loss at month 84 among patients with preexisting DSAs was 23.1%; the corresponding value for patients without preexisting DSAs was 15.2%. The HR for the comparison of patients with versus without preexisting DSAs was 1.47 (95% CI 0.68‐3.18; P = .33) (Figure 2A).
Figure 2.
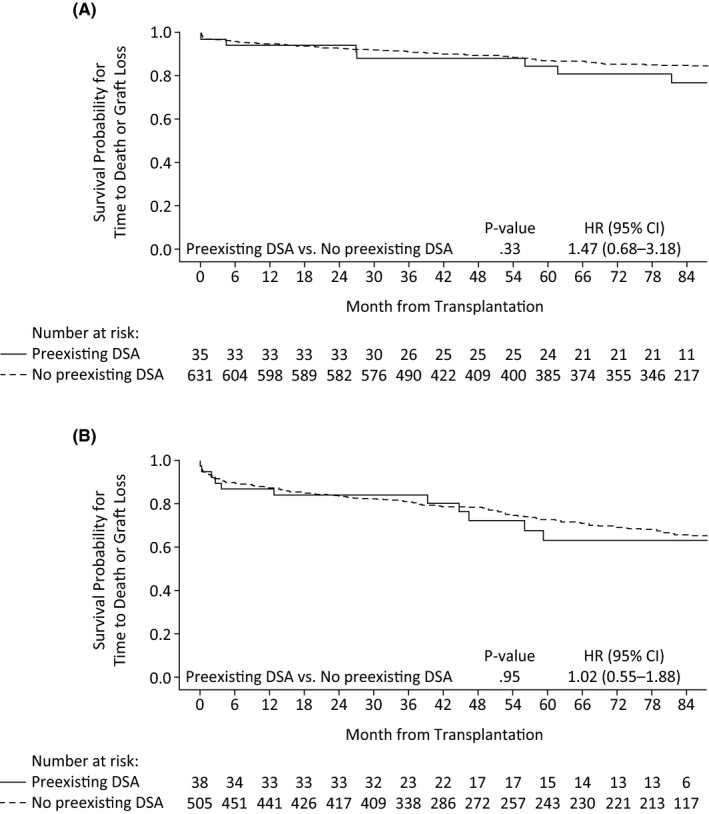
Kaplan–Meier curve for time to death or graft loss in patients with or without DSAs in (A) BENEFIT and (B) BENEFIT‐EXT. DSA, donor‐specific antibody; LI, less intense; MI, more intense
In the subset of patients with preexisting DSAs, mean estimated GFR (as observed) between month 3 and month 84 remained essentially stable for those randomized to belatacept MI, increased for those randomized to belatacept LI, and decreased for those randomized to cyclosporine (Figure 3A). Mean estimated GFR (standard deviation) for belatacept MI at months 12, 36, 60, and 84 was 69.1 (22.8), 74.6 (23.9), 66.5 (21.9), and 64.2 (20.7) mL/min per 1.73 m2, respectively. The corresponding values for belatacept LI were 66.0 (21.7), 76.4 (23.4), 98.5 (31.8), and 88.5 (18.2) mL/min per 1.73 m2. Mean estimated GFR for cyclosporine at months 12, 36, 60, and 84 was 58.6 (27.1), 56.1 (20.5), 47.7 (17.3), and 50.2 (9.3) mL/min per 1.73 m2, respectively.
Figure 3.
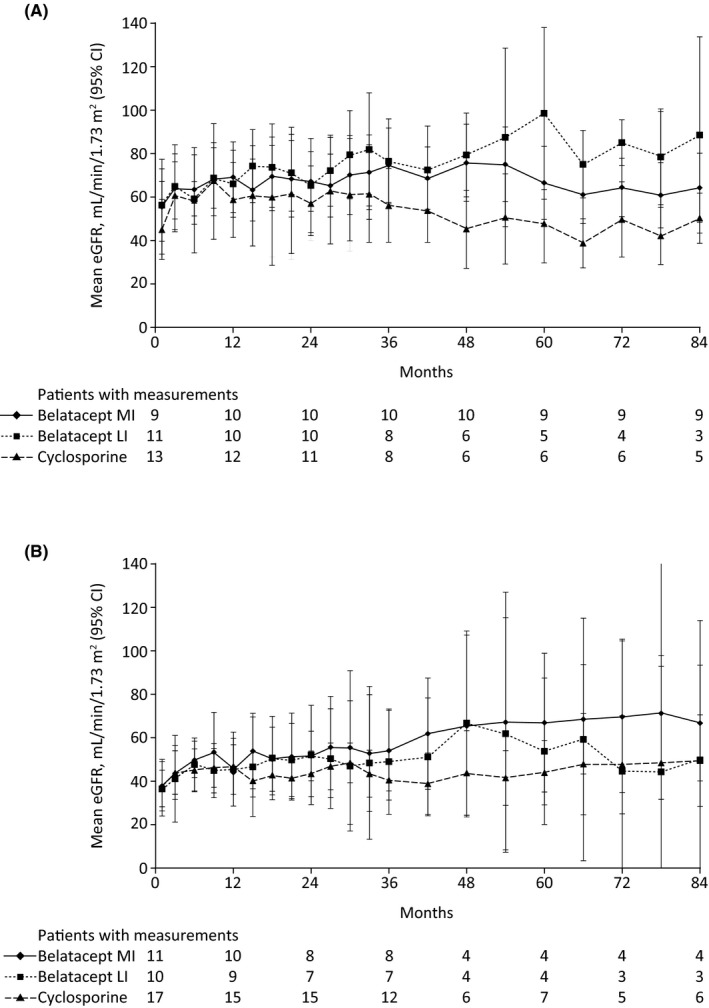
Mean estimated GFR (observed) in the subsets of patients with preexisting DSAs in (A) BENEFIT and (B) BENEFIT‐EXT. CI, confidence interval; DSA, donor‐specific antibodies; eGFR, estimated GFR; LI, less intense; MI, more intense
Two belatacept MI‐treated, two belatacept LI‐treated, and one cyclosporine‐treated patient with preexisting DSAs experienced acute rejection; all rejection events occurred by 12 months posttransplant. The Kaplan–Meier cumulative event rates of acute rejection at month 84 for preexisting DSA‐positive patients randomized to belatacept MI, belatacept LI, and cyclosporine were 20.0%, 18.2%, and 10.0%, respectively.
3.2. Benefit‐ext
In BENEFIT‐EXT, 6.0% (11/184), 5.7% (10/175), and 9.2% (17/184) of patients randomized to belatacept MI, belatacept LI, and cyclosporine, respectively, had DSAs prior to transplant. The HLA class specificity of preexisting DSAs was similar across treatment arms, with the majority being class I (Table 2). Compared with the other two treatment arms, proportionally more patients with preexisting DSAs assigned to belatacept MI had baseline PRA <20% and proportionally fewer patients with preexisting DSAs assigned to cyclosporine were male (Table 2). The average baseline MFI of HLA DSAs in belatacept MI‐treated, belatacept LI‐treated, and cyclosporine‐treated patients were 4454 (range, 1900‐15 000), 5495 (900‐15 000), and 6204 (1000‐18 000), respectively.
Table 2.
Baseline characteristics of patients with preexisting DSAs in BENEFIT‐EXT
| Belatacept MI (n = 11) | Belatacept LI (n = 10) | Cyclosporine (n = 17) | |
|---|---|---|---|
| Mean age, y (SD) | 54.7 (12.6) | 53.8 (14.2) | 57.7 (11.9) |
| Male, n (%) | 6 (54.5) | 5 (50.0) | 5 (29.4) |
| Race | |||
| White | 8 (72.7) | 9 (90.0) | 14 (82.4) |
| Black | 0 (0) | 0 (0) | 2 (11.8) |
| Asian | 0 (0) | 0 (0) | 0 (0) |
| Other | 3 (27.3) | 1 (10.0) | 1 (5.9) |
| Region | |||
| North America | 2 (18.2) | 1 (10.0) | 7 (41.2) |
| South America | 4 (36.4) | 3 (30.0) | 4 (23.5) |
| Europe | 5 (45.5) | 6 (60.0) | 6 (35.3) |
| Rest of world | 0 (0) | 0 (0) | 0 (0) |
| Categorized PRA, n (%) | |||
| <20% | 11 (100.0) | 8 (80.0) | 14 (82.4) |
| ≥20% | 0 (0) | 1 (10.0) | 2 (11.8) |
| Missinga | 0 (0) | 1 (10.0) | 1 (5.9) |
| Reported cause of ESRD, n (%) | |||
| Glomerulonephritis | 1 (9.1) | 3 (30.0) | 0 (0) |
| Diabetes | 2 (18.2) | 0 (0) | 4 (23.5) |
| Polycystic kidneys | 3 (27.3) | 1 (10.0) | 3 (17.6) |
| Congenital, familial, and metabolic | 1 (9.1) | 0 (0) | 0 (0) |
| Hypertensive nephrosclerosis | 0 (0) | 3 (30.0) | 3 (17.6) |
| Renovascular and other | 0 (0) | 1 (10.0) | 0 (0) |
| Tubular and interstitial diseases | 1 (9.1) | 1 (10.0) | 2 (11.8) |
| Other | 3 (27.3) | 1 (10.0) | 5 (29.4) |
| T‐cell crossmatch–negative | 11 (100.0) | 10 (100.0) | 17 (100.0) |
| Preexisting DSA HLA class specificity, n (%) | |||
| Class I | 8 (72.7) | 7 (70.0) | 12 (70.6) |
| Class II | 2 (18.2) | 2 (20.0) | 2 (11.8) |
| Class I and II | 1 (9.1) | 1 (10.0) | 3 (17.6) |
| Average MFI of DSAs | |||
| Total | 4454 | 5495 | 6204 |
| Class I‐only | 4000 | 4494 | 5877 |
| Class II‐only | 5475 | 10 833 | 7400 |
DSA, donor‐specific antibody; ESRD, end‐stage renal disease; LI, less intense; MFI, mean fluorescence intensity; MI, more intense; PRA, panel reactive antibody; SD, standard deviation.
On the case report form, investigators had to tick off whether a patient satisfied all eligibility criteria. Although there was space on the form for investigators to provide numerical results for the highest and most recent PRA percentages, they were not required to do so. Thus, the PRA percentage is missing for some study participants.
Thirteen HLA DSAs (class I, n = 9; class II, n = 4) were detected in the 11 belatacept MI‐treated patients with preexisting DSAs. MFI values decreased or did not change for all 13 DSAs between baseline and month 24 (100.0%, 13/13) (Figure 1). Nineteen HLA DSAs (class I, n = 16; class II, n = 3) were detected in the 10 belatacept LI‐treated patients with preexisting DSAs. MFI values declined or did not change for all but one DSA (94.7%, 18/19). Twenty‐eight HLA DSAs (class I, n = 22; class II, n = 6) were detected in the 17 cyclosporine‐treated patients with preexisting DSAs. The percentage of preexisting DSAs exhibiting a decline or no change in MFI was 75.0% (21/28).
At month 84, 72.7% (8/11) of patients with preexisting DSAs randomized to belatacept MI, 70.0% (7/10) of those with preexisting DSAs randomized to belatacept LI, and 52.9% (9/17) of patients with preexisting DSAs randomized to cyclosporine were evaluated for death or graft loss. Kaplan–Meier estimated rates of death or graft loss at month 84 for belatacept MI‐treated, belatacept LI‐treated, and cyclosporine‐treated patients were 41.8%, 46.7%, and 27.4%, respectively. Across the three treatment arms, 38 patients had preexisting DSAs and 505 did not. The Kaplan–Meier cumulative event rate for death or graft loss at month 84 among patients with preexisting DSAs was 36.7%; the corresponding value for patients without preexisting DSAs was 34.7%. The HR for the comparison of patients with vs without preexisting DSAs was 1.02 (95% CI 0.55‐1.88; P = .95) (Figure 2B).
Among patients with preexisting DSAs, mean estimated GFR (as observed) between month 3 and month 84 tended to increase for all three regimens, but the increase was most pronounced for the belatacept MI‐based regimen (Figure 3B). Mean estimated GFR (standard deviation) for belatacept MI at months 12, 36, 60, and 84 was 45.1 (15.9), 53.9 (22.2), 66.8 (20.1), and 66.6 (16.8) mL/min per 1.73 m2, respectively. The corresponding values for belatacept LI were 45.3 (22.2), 48.8 (26.3), 53.6 (21.2), and 49.6 (25.9) mL/min per 1.73 m2. Mean estimated GFR for cyclosporine at months 12, 36, 60, and 84 was 46.7 (23.2), 40.3 (14.4), 43.7 (16.1), and 49.3 (20.1) mL/min per 1.73 m2, respectively.
Two belatacept MI‐treated, four belatacept LI‐treated, and three cyclosporine‐treated patients with preexisting DSAs experienced acute rejection. The Kaplan–Meier cumulative event rates of acute rejection at month 84 for preexisting DSA‐positive patients randomized to belatacept MI, belatacept LI, and cyclosporine were 25.0%, 40.0%, and 19.1%, respectively.
4. DISCUSSION
When the BENEFIT and BENEFIT‐EXT studies were undertaken, it was standard practice to assess donor‐recipient histocompatibility using only cell‐based cytotoxicity assays. When retrospectively examining stored patient sera using solid‐phase assays, which are more sensitive than T‐cell lymphocytotoxic crossmatching, we discovered that 5.3% of BENEFIT participants and 7.0% of BENEFIT‐EXT participants had DSAs prior to transplant. If it were not for the technological limitations of the time, these patients would have been excluded from BENEFIT and BENEFIT‐EXT. Although some of these patients may not be transplanted based on today's clinical practice, the results of these post hoc analyses showed that belatacept‐based immunosuppression was generally effective and well‐tolerated in patients who prior to transplant were T‐cell lymphocytotoxic crossmatch–negative, but DSA‐positive via solid‐phase testing—at least for the levels of DSAs detected in the present analysis. Moreover, belatacept‐based versus cyclosporine‐based immunosuppression was found to result in a greater proportion of preexisting DSAs exhibiting a MFI decline, which may translate to improved clinical outcomes. However, we cannot discount the possibility that concomitant treatment with mycophenolate mofetil may have contributed to the observed decline in DSA MFI. Moreover, as part of the eligibility requirements, patients only had to be T‐cell crossmatch‐negative. Thus, some study participants may have been B‐cell crossmatch‐positive. It is possible that clinical management of patients who were B‐cell crossmatch‐positive may have also affected DSA MFI levels.
The effect of belatacept‐based treatment on DSA MFI levels was more pronounced in BENEFIT‐EXT, where patients received extended‐criteria donor kidneys, than in BENEFIT, where patients received living or standard‐criteria deceased donor kidneys. This may have been due to the generally lower DSA MFI level at baseline in BENEFIT‐EXT than in BENEFIT. However, in both studies, the decline in the level of circulating DSAs (as measured by MFI) was more apparent with belatacept MI than with belatacept LI, the approved regimen. Since antibody testing was protocol‐specified to occur every 6 months during the first year posttransplant and every 12 months thereafter, the precise timing of the decline in DSA MFI is unknown.
In pooled analyses of time to death or graft loss in patients with and without DSAs prior to transplant, the presence of preexisting DSAs did not impact graft survival over 84 months in either BENEFIT or BENEFIT‐EXT; Kaplan–Meier estimated rates of death or graft loss at month 84 were similar in the subgroups of patients with and without preexisting DSAs. In addition to being non–complement‐fixing (and thus escaping detection via cytotoxic T‐cell crossmatching), it is possible that these preexisting DSAs were also “less pathogenic.” Unfortunately, due to the small sample sizes in both BENEFIT and BENEFIT‐EXT, it was not possible to examine the effects of individual patients’ treatment on either time to death or time to graft loss in patients with preexisting DSAs.
Renal function over 7 years of follow‐up was either stable or improved in belatacept‐treated patients with preexisting DSAs. In the subset of BENEFIT participants with preexisting DSAs, mean estimated GFR values at month 84 were 64.2‐88.5 mL/min per 1.73 m2 for belatacept‐based immunosuppression and 50.2 mL/min per 1.73 m2 for cyclosporine‐based immunosuppression, which compares favorably with estimated GFR values calculated at 7 years posttransplant in the overall patient population (belatacept, 70.4‐72.1 mL/min per 1.73 m2; cyclosporine, 44.9 mL/min per 1.73 m2).14 Estimated GFR values at month 84 in the subset of BENEFIT‐EXT participants with preexisting DSAs were 49.6‐66.6 mL/min per 1.73 m2 for those receiving belatacept and 49.3 mL/min per 1.73 m2 for those receiving cyclosporine. The corresponding values in the overall BENEFIT‐EXT study population were 53.9‐54.2 and 35.3 mL/min per 1.73 m2.15
In BENEFIT, the Kaplan–Meier cumulative event rate of acute rejection at month 84 in the subset of belatacept‐treated patients with preexisting DSAs was comparable to that reported in the overall study population (18.2‐20.0% vs. 18.3‐24.4%, respectively); similar results were seen for cyclosporine (10.0% vs. 11.4%, respectively).14 The cumulative event rates of acute rejection in the subsets of patients with preexisting DSAs randomized to belatacept MI (25.0%) and cyclosporine (19.1%) in BENEFIT‐EXT were similar to the rates seen in the overall study population (belatacept MI, 21.1%; cyclosporine, 17.3%). However, the rate of acute rejection was numerically higher in the subset of BENEFIT‐EXT participants with preexisting DSAs randomized to belatacept LI compared with the overall population of belatacept LI‐treated patients at 7 years posttransplant (40.0% vs. 19.5%).15 These discrepant results could be attributable to the limited sample size.
These analyses are limited by the fact that they were conducted post hoc. As a consequence, the numbers of patients analyzed were small, and MFI was not available at all time points for all individuals. In addition, patients were not randomized according to preexisting DSA status. Moreover, patient sera were only subjected to single‐antigen bead testing if the results of the solid‐phase flow cytometry screening assay were positive. Because solid‐phase antibody screening may be less sensitive than single‐antigen bead testing, it is possible that patients with weak preexisting DSAs may have gone undetected. Although they should be interpreted with caution, these preliminary findings suggest that belatacept‐based relative to cyclosporine‐based immunosuppression may better control the persistence of preexisting DSAs posttransplant, as determined by MFI. It is intriguing to speculate that belatacept may have the potential to reduce HLA antibodies in sensitized individuals awaiting a kidney transplant, but additional studies are needed to test this hypothesis.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose. M. Roberts, M. Polinsky, and L. Yang are salaried employees of and own stock in Bristol‐Myers Squibb. R. Townsend and H.‐U. Meier‐Kriesche were salaried employees of Bristol‐Myers Squibb at the time that these analyses were undertaken and still own stock in Bristol‐Myers Squibb. C. P. Larsen has received grants from Bristol‐Myers Squibb. R. Bray and H. Gebel have no conflicts of interest to disclose.
Supporting information
ACKNOWLEDGMENTS
The BENEFIT and BENEFIT‐EXT studies were sponsored by Bristol‐Myers Squibb. Support for third‐party writing assistance for this manuscript was provided by Tiffany DeSimone, PhD, of CodonMedical, an Ashfield Company, part of UDG Healthcare plc, and was funded by Bristol‐Myers Squibb.
Bray RA, Gebel HM, Townsend R, et al. Posttransplant reduction in preexisting donor‐specific antibody levels after belatacept‐ versus cyclosporine‐based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT‐EXT. Am J Transplant. 2018;18:1774–1782. 10.1111/ajt.14738
R. Townsend and H.‐U. Meier‐Kriesche were employees of Bristol‐Myers Squibb at the time that these analyses were undertaken.
References
- 1. Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735‐739. [DOI] [PubMed] [Google Scholar]
- 2. Mohan S, Palanisamy A, Tsapepas D, et al. Donor‐specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061‐2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mulley WR, Kanellis J. Understanding crossmatch testing in organ transplantation: a case‐based guide for the general nephrologist. Nephrology. 2011;16:125‐133. [DOI] [PubMed] [Google Scholar]
- 4. Pei R, Lee J, Chen T, Rojo S, Terasaki PI. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol. 1999;60:1293‐1302. [DOI] [PubMed] [Google Scholar]
- 5. Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non‐HLA antibodies in transplantation. Transplantation. 2013;95:19‐47. [DOI] [PubMed] [Google Scholar]
- 6. Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43‐49. [DOI] [PubMed] [Google Scholar]
- 7. Bristol‐Myers Squibb. Belatacept (NULOJIX) Prescribing Information. Princeton, NJ: Bristol‐Myers Squibb Company; 2016. [Google Scholar]
- 8. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535‐546. [DOI] [PubMed] [Google Scholar]
- 9. Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT‐EXT study). Am J Transplant. 2010;10:547‐557. [DOI] [PubMed] [Google Scholar]
- 10. Rostaing L, Vincenti F, Grinyó J, et al. Long‐term belatacept exposure maintains efficacy and safety at 5 years: results from the long‐term extension of the BENEFIT study. Am J Transplant. 2013;13:2875‐2883. [DOI] [PubMed] [Google Scholar]
- 11. Charpentier B, Medina Pestana JO, Del C Rial M, et al. Long‐term exposure to belatacept in recipients of extended criteria donor kidneys. Am J Transplant. 2013;13:2884‐2891. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan HC, Gebel HM, Bray RA. Understanding solid‐phase HLA antibody assays and the value of MFI. Hum Immunol. 2017;78:471‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461‐470. [DOI] [PubMed] [Google Scholar]
- 14. Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med. 2016;374:333‐343. [DOI] [PubMed] [Google Scholar]
- 15. Durrbach A, Pestana JM, Florman S, et al. Long‐term outcomes in belatacept‐ versus cyclosporine‐treated recipients of extended criteria donor kidneys: final results from BENEFIT‐EXT, a phase III randomized study. Am J Transplant. 2016;16:3192‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


