Abstract
Glucagon‐like peptide‐1 receptor agonists lower blood glucose in type 2 diabetes (T2D) partially through glucose‐dependent stimulation of insulin secretion. The aim of this study was to investigate whether beta‐cell function (as measured by HOMA2‐%B) at baseline affects the glycaemic response to dulaglutide. Dulaglutide‐treated patients from AWARD‐1, AWARD‐3 and AWARD‐6 clinical studies were categorised based on their homeostatic model assessment of beta‐cell function (HOMA2‐%B) tertiles. Changes in glycaemic measures in response to treatment with once‐weekly dulaglutide were evaluated in each HOMA2‐%B tertile. Patients with low HOMA2‐%B had higher baseline glycated haemoglobin (HbA1c), fasting and postprandial blood glucose, and longer duration of diabetes (P < .001, all) (mean low, middle and high tertiles with dulaglutide 1.5 mg: HOMAB‐2%B, 31%, 58%, 109%; HbA1c, 8.7%, 7.7%, 7.3%, respectively). At 26 weeks, the low tertile experienced larger reductions in HbA1c compared to the high tertile with dulaglutide 1.5 mg (mean; −1.55% vs. −0.98% [−16.94 vs. −10.71 mmol/mol]). Differences between low and high tertiles disappeared when adjusted for baseline HbA1c (LSM; −1.00 vs. −1.18% [−10.93 vs. −12.90 mmol/mol]). Greater decreases in fasting blood glucose and greater increases in fasting C‐peptide were observed in the low tertile. Similar increases in HOMA2‐%B were observed in all tertiles. Dulaglutide demonstrated clinically relevant HbA1c reduction irrespective of estimated baseline beta‐cell function.
Keywords: beta‐cell function, dulaglutide, GLP‐1 receptor agonist, type 2 diabetes
1. INTRODUCTION
Despite the identification of multiple defects, impaired beta‐cell function remains the main mechanism to account for the development and progression of hyperglycemia in type 2 diabetes (T2D).1 Recognition of the central pathogenic role of beta‐cell failure has relevant clinical implications. For instance, patients with lower beta‐cell function may only temporarily benefit from insulin secretagogues, which increase beta‐cell workload.2, 3 Furthermore, treatment that could exert less stress on the beta‐cell may contribute to some degree of preservation of insulin secretion over time. Moreover, therapies that reduce beta‐cell workload or induce beta‐cell rest may be most beneficial in individuals with T2D and prominent beta‐cell impairment.3, 4 In this respect, there has been growing interest in glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) as a treatment option for patients with lower beta‐cell function.
GLP‐1RAs primarily act by improving glucose homeostasis, enhancing glucose‐dependent insulin secretion, suppressing glucagon levels and slowing gastric emptying, with initial pre‐clinical observations also claiming potential beta‐cell preservation.5, 6 However, clinical data are limited. Markers for low beta‐cell function, including C‐peptide and islet autoantibodies, have been associated with reduced glycaemic response to GLP‐1RA therapy in insulin‐treated patients.7 Similarly, in an observational study, patients with a lower urinary C‐peptide creatinine ratio were associated with reduced glycaemic response to liraglutide.8 In contrast, lixisenatide improved glycaemic control irrespective of beta‐cell function, as measured by the secretory units of islets in transplantation index9 and by homeostatic model assessment for beta‐cell function, HOMA2‐%B.10 In comparison to reduced glycaemic response to GLP‐1RA therapies in patients with islet autoantibodies,7 in a condition frequently called latent autoimmune diabetes in the adult (LADA), characterised by impaired beta‐cell function—dulaglutide appears to remain effective.11 However, the effect of baseline beta‐cell function on glycaemic response to dulaglutide has not been systematically explored.
Dulaglutide, a once‐weekly GLP‐1RA approved for the treatment of T2D,12 has been evaluated in the Assessment of Weekly AdministRation of LY2189265 in Diabetes (AWARD) clinical development programme. In these clinical studies, dulaglutide significantly reduced higher baseline glycated haemoglobin (HbA1c) irrespective of age, gender, ethnicity, duration of diabetes, body mass index (BMI), body weight at baseline, or HbA1c, with a greater effect observed in patients with higher baseline HbA1c.13, 14
The aim of this study was to investigate whether beta‐cell function status at baseline measured by HOMA2‐%B affects the glycaemic response to dulaglutide. Patients with T2D enrolled in three clinical trials from the AWARD programme (AWARD‐1, AWARD‐3, and AWARD‐6) were included in the analysis.
2. MATERIAL AND METHODS
2.1. Study design
Patients from the AWARD‐1, AWARD‐3, and AWARD‐6 studies were included in the post‐hoc analysis using the available HOMA2‐%B data. In the AWARD studies, key eligibility criteria included: age ≥18 years old, T2D not optimally controlled with diet and exercise and/or at least one stable dose of oral antihyperglycaemia medication, baseline HbA1c values of ≥6.5% to 7.0% to ≤10.0%, stable weight for ≥3 months prior to screening, and a BMI of 23 to 45 kg/m2. Patients received dulaglutide in combination with metformin and pioglitazone (AWARD‐1), as monotherapy (AWARD‐3), or in combination with metformin (AWARD‐6). Individual trial results comparing dulaglutide treatment to exenatide or placebo, metformin or liraglutide are published.15, 16, 17
Data from dulaglutide‐treated patients (1.5 mg [and 0.75 mg as presented in the supporting information for this article]) in these 3 studies were pooled by dose, and the overall population was categorised into 3 groups based on baseline HOMA2‐%B (low, middle and high tertiles). Baseline characteristics and changes at week 26 in glycaemic measures were analysed. Variables analysed included HbA1c, fasting C‐peptide, fasting insulin, fasting blood glucose (FBG), self‐monitoring plasma glucose (SMPG) values including daily mean two‐hour postprandial glucose (PPG) and daily mean two‐hour excursion. SMPG excursion was calculated by subtracting the two‐hour SMPG value from the pre‐meal SMPG value. Homeostatic model assessment for beta‐cell function, HOMA2‐%B, and insulin resistance, HOMA2‐IR, indices were calculated on the basis of the updated version of the HOMA model.18, 19
2.2. Statistical analyses
Analyses were performed on the intent‐to‐treat population. Baseline characteristics for the integrated population were categorised by dulaglutide dose and HOMA2‐%B tertile. Outcome measures at 26 weeks were compared among baseline HOMA2‐%B tertiles without adjusting the other variables. In addition, the ANalysis of COVAriance (ANCOVA) model with Last Observation Carried Forward (LOCF) was used to analyse changes in HbA1c to adjust for baseline differences in HbA1c. LOCF was applied to all the efficacy endpoints at 26 weeks. The covariates of the ANCOVA model are: Baseline, Pooled Country, Treatment, Tertile, Tertile*Treatment. Data are presented as mean (unadjusted), least squares mean (LSM; adjusted for HbA1c changes) and 95% confidence intervals (CI).
3. RESULTS
Baseline characteristics for patients treated with dulaglutide 1.5 mg are presented in Table 1 (results for dulaglutide 0.75 mg are presented in Table S2). Overall, 1346 patients were included in this analysis (dulaglutide 1.5 mg, n = 817; dulaglutide 0.75 mg, n = 529). Age was similar in all HOMA2‐%B tertiles. The low tertile had the longest duration of diabetes, highest baseline HbA1c, FBG and PPG, and lowest body weight and BMI (P < .001, low vs. other tertiles).
Table 1.
Baseline characteristics by HOMA2‐%B in patients treated with dulaglutide 1.5 mg
| Parameter | HOMA2‐%B tertile at baseline | ||
|---|---|---|---|
| Low (3.5%‐43.9%) (N = 293) | Middle (44.2%‐73.9%) (N = 288) | High (74.4%‐258.4%) (N = 236) | |
| HOMA2‐%B, % | 30.5 ± 9.5 | 58.1 ± 8.3 | 108.8 ± 34.5 |
| Fasting blood glucose, mmol/L | 11.2 ± 2.7 | 8.7 ± 1.8 | 7.0 ± 1.3 |
| Fasting C‐peptide, nmol/L | 0.7 ± 0.3 | 0.9 ± 0.4 | 1.3 ± 0.5 |
| Fasting serum insulin, pmol/L | 64.8 ± 41.5 | 112.2 ± 90.1 | 147.3 ± 87.8 |
| HbA1c, % | 8.7 ± 1.1 | 7.7 ± 0.8 | 7.3 ± 0.7 |
| HbA1c, mmol/mol | 71.1 ± 12.0 | 60.7 ± 8.7 | 56.5 ± 7.7 |
| Duration of diabetes, years | 8.0 ± 5.9 | 5.8 ± 5.2 | 4.8 ± 3.9 |
| Weight, kg | 90.9 ± 18.7 | 94.9 ± 18.8 | 98.2 ± 19.1 |
| BMI, kg/m2 | 31.9 ± 5.1 | 33.5 ± 5.2 | 35.1 ± 5.4 |
| HOMA2‐IR | 2.1 ± 1.2 | 2.8 ± 2.4 | 3.3 ± 1.5 |
| Daily pre‐meal mean (SMPG), mmol/L | 10.8 ± 2.7 | 8.7 ± 1.8 | 7.8 ± 1.4 |
| Daily 2‐h PP meal mean (SMPG), mmol/L | 12.5 ± 3.0 | 10.4 ± 2.1 | 9.4 ± 1.9 |
| Daily 2‐h excursion mean (SMPG), mmol/L | 1.7 ± 1.4 | 1.7 ± 1.2 | 1.6 ± 1.4 |
| Sex, female, n (%) | 134 (45.7) | 151 (52.4) | 128 (54.2) |
| Age, years | 55.6 ± 9.5 | 57.0 ± 10.0 | 55.5 ± 9.6 |
| Previous OAM treatment at screening, n (%) | |||
| No OAM | 24 (8.2) | 51 (17.7) | 36 (15.3) |
| 1 OAM | 187 (63.8) | 173 (60.1) | 132 (55.9) |
| >1 OAM | 82 (28.0) | 64 (22.2) | 68 (28.8) |
Data presented as mean ± SD or n (%).
3.1. Change in HbA1c
At 26 weeks, unadjusted reductions in HbA1c with dulaglutide 1.5 mg were significant in all tertiles (Figure 1A), with a significantly greater decrease observed in the low tertile compared to the other tertiles. When adjusted for baseline HbA1c differences (ANCOVA with LOCF analysis), HbA1c reductions among the tertiles were similar (LSM [95% CI]: −1.00% [−1.13, −0.88] [−10.93 mmol/mol {−12.35, −9.62}], −1.03% [−1.14, −0.92] [−11.26 mmol/mol {−12.46, −10.06}], −1.18% [−1.31, −1.05] [−12.90 mmol/mol {−14.32, −11.48}] for low, middle and high tertiles, respectively) (Figure 1B). Similarly, in patients treated with dulaglutide 0.75 mg, unadjusted reductions in HbA1c were significant in all tertiles, with a significantly greater decrease in the low tertile compared to the other tertiles (Figure S1A). When adjusted for baseline HbA1c differences, HbA1c reductions among the tertiles were similar (Figure S1B).
Figure 1.
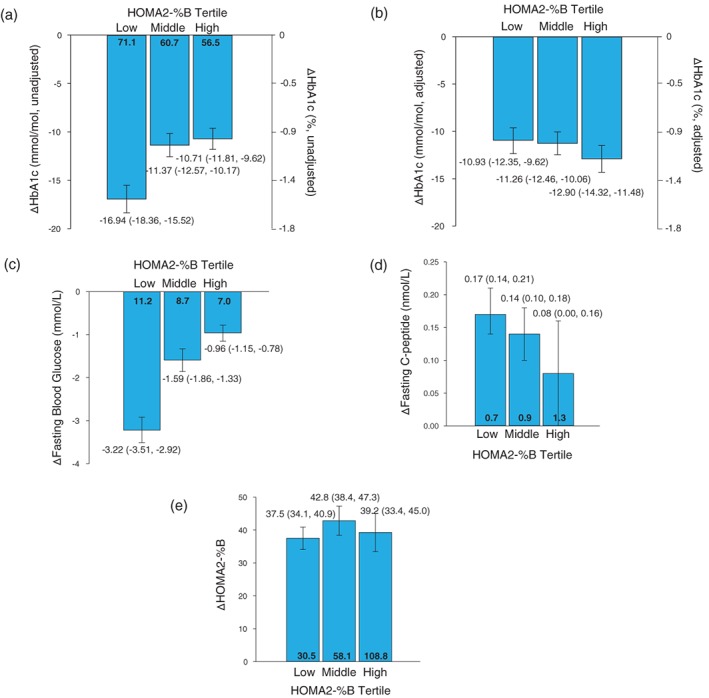
Data presented as mean (95% CI) (unadjusted) and LSM (95% CI) (adjusted). Mean baseline values are presented in bold within the bars (unadjusted). A) Change from baseline in HbA1c by HOMA2‐%B tertile (unadjusted). B) Change from baseline in HbA1c by HOMA2‐%B tertile (adjusted). C) Change from baseline in fasting blood glucose by HOMA2‐%B tertile (unadjusted). D) Change from baseline in fasting C‐peptide by HOMA2‐%B tertile (unadjusted). E) Change from baseline in HOMA2‐%B by HOMA2‐%B tertile (unadjusted)
3.2. Change in FBG, fasting C‐peptide, HOMA2‐%B, fasting serum insulin and HOMA2‐IR
At 26 weeks, reductions in FBG were significant in patients who received dulaglutide 1.5 mg in all tertiles (Figure 1C). A significantly greater decrease in FBG was observed in the low tertile compared to the other tertiles. Similar results to dulaglutide 1.5 mg were observed in patients treated with dulaglutide 0.75 mg (Figure S1C).
While the lowest baseline fasting C‐peptide levels were observed in the low HOMA2‐%B tertile (Table 1), the increase in fasting C‐peptide levels was numerically greater in patients with low baseline HOMA2‐%B at 26 weeks (Figure 1D). Similar results were observed in patients treated with dulaglutide 0.75 mg (Table S2 and Figure S1D).
At 26 weeks, significant increases in HOMA2‐%B were similar in all baseline HOMA2‐%B tertiles (mean [95% CI] increase: 37.5% [34.1, 40.9], 42.8% [38.4, 47.3] and 39.2% [33.4, 45.0] for low, middle, and high HOMA2‐%B tertiles, respectively) (Figure 1E). In patients treated with dulaglutide 0.75 mg, significant increases in HOMA2‐%B were similar in all groups (Figure S1E).
At 26 weeks, similar and significant increases in fasting serum insulin were observed in all groups, both with dulaglutide 1.5 mg (Table S1) and dulaglutide 0.75 mg (Table S3). Changes in HOMA2‐IR were not significant in any tertile treated with dulaglutide 1.5 mg or dulaglutide 0.75 mg (Tables S1 and S3, respectively).
3.3. Changes in pre‐meal glucose, 2‐hour PPG and 2‐hour SMPG
At 26 weeks, reductions in mean pre‐meal glucose and mean 2‐hour PPG were significant in patients who received dulaglutide in all tertiles. The greatest decrease was observed in patients in the low tertile for both mean pre‐meal glucose and mean two‐hour PPG (Table S1). At 26 weeks, reductions in the 2‐hour glucose excursions were significant in patients who received dulaglutide in all tertiles, with no differences observed between tertiles. Similar reductions in SMPG parameters were observed in patients treated with dulaglutide 0.75 mg (Table S3).
4. DISCUSSION
This post‐hoc analysis investigated the efficacy of once‐weekly dulaglutide by baseline beta‐cell function measured by HOMA2‐%B in patients with T2D. Overall, when adjusted for baseline HbA1c, dulaglutide treatment resulted in similar, clinically meaningful decreases in HbA1c irrespective of beta‐cell function: the glycaemic improvement achieved by dulaglutide was similar between tertiles of HOMA2‐%B.
In our cohort, more severe baseline beta‐cell impairment was associated with higher baseline HbA1c, FBG and PPG, and longer duration of diabetes. These are all characteristics of the concomitant beta‐cell function and glycaemic deterioration typically observed in the progressive course of T2D.1 In contrast, elevated insulin resistance and obesity was more common in patients with less impaired beta‐cell function, indicating that patients may have reached the glycaemic threshold of diabetes with a relatively better beta‐cell function.
Dulaglutide treatment resulted in greater decreases in HbA1c in patients with low baseline HOMA2‐%B and high baseline HbA1c concentration. This was anticipated since greater reductions in HbA1c are typically observed in patients with higher baseline HbA1c.20 When adjusting for baseline HbA1c, these differences disappeared and the overall treatment response to dulaglutide in terms of glycaemic control was similar between all HOMA2‐%B tertiles.
HOMA2‐%B increased to a similar extent in all tertiles, indicating that beta‐cell function improved regardless of baseline beta‐cell function. Higher absolute, and even greater relative, increases in fasting C‐peptide from baseline were observed in patients with low HOMA2‐%B. At baseline, these patients also had markedly lower C‐peptide concentration, and a proportionally larger increase with dulaglutide therapy. Beta‐cell function doubled in this group due to the simultaneous drop in fasting glucose concentration and increased fasting plasma insulin. It was notable that while the relative increase from baseline in HOMA2‐%B was greater in the low tertile, the actual HOMA2‐%B achieved at 26 weeks was still lower compared to the high tertile.
Similar improvements in beta‐cell function with dulaglutide were also reported under fasting conditions, as well as after a standardised test meal.21 While the mechanism of improved beta‐cell function with GLP‐1RAs is not fully understood, their stimulation of insulin biosynthesis, excretion, and improvements in glucose sensitivity of beta‐cells, may play an important role.22
Overall, the glycaemic response to dulaglutide is maintained regardless of differences in baseline beta‐cell function. Conflicting results have been reported as to whether or not beta‐cell function affects the glycaemic response to GLP‐1RAs. A recent study which also used HOMA2‐%B measurements showed similar reductions in HbA1c with once‐daily lixisenatide in patients with low HOMA2‐%B as those with less deteriorated beta‐cell function,10 suggesting the presence of sufficient beta‐cell function that could still respond to stimulation by GLP‐1RAs. Nonetheless, the absolute residual beta‐cell capacity may be key in determining the response to GLP‐1RAs. In the Predicting Response to Incretin Based Agents (PRIBA) study, reduced glycaemic responses to liraglutide, exenatide twice daily, and exenatide once weekly, were previously observed in insulin‐treated patients with low beta‐cell function and severe insulin deficiency.7 Obviously, those patients in the PRIBA study had considerably higher baseline HbA1c (9.7%), BMI (39.7 kg/m2), lower beta‐cell function, severe insulin deficiency (i.e. fasting C‐peptide ≤0.25 nmol/L), and were frequently on insulin treatment, in comparison to the patients in the current study. Indeed, patients with a longer duration of diabetes, on higher insulin doses, and with a lower beta‐cell function, were less likely to switch from insulin to exenatide therapy.23 In contrast to the effects with the GLP‐1RAs examined in the PRIBA study, dulaglutide efficacy was maintained with a longer duration of diabetes,14 or in the presence of the islet autoantibodies characteristic of LADA.24 Consistent to this, is a study showing a significantly less marked glycaemic response to liraglutide was associated with low postprandial urinary C‐peptide creatinine ratio.8 In summary, therapeutic benefits can still be achieved if the remaining beta‐cell function is sufficient to support the effects of GLP‐1RAs. GLP‐1RAs may also exert favourable effects through direct or indirect insulin‐induced suppression of glucagon,21 which may account for persistent efficacy even in advanced stages of the disease.
One limitation of this study is the post‐hoc nature of the analysis and the lack of a placebo or active comparator. HOMA2‐%B indicates beta‐cell function only in fasting conditions and is a weak indicator for dynamic beta‐cell capabilities. Despite the relatively large sample size, the duration of the studies in the current analysis was limited to 6 months, which may not represent the effects of long‐term use of dulaglutide. Finally, it is worth noting that even the lowest tertile retained sufficient beta‐cell function irrespective of long duration of diabetes, which may not reflect the disease characteristics typically observed in the general population of T2D with similar duration. Therefore, studies with special patient populations with even more severe beta‐cell dysfunction are needed to explore this further.
In conclusion, our findings indicate that treatment with once‐weekly dulaglutide improves glycaemic control regardless of baseline beta‐cell function, suggesting it can be a valid treatment option for patients with T2D at different stages of severity. It is also noted that some level of residual beta‐cell function is necessary to maintain the glycaemic efficacy of GLP‐1RAs.
Conflict of interest
C. M. serves, or has served, on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, Bristol‐Myers Squibb, AstraZeneca, Pfizer, Janssen Pharmaceuticals, Boehringer Ingelheim, Hanmi Pharmaceuticals, Roche Diagnostics, Medtronic, Mannkind, Intrexon, Dianax and UCB, and serves, or has served, on the speakers bureau for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Boehringer Ingelheim, Astra Zeneca and Novartis. KU Leuven has received research support for C. M. from Medtronic, Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Roche Diagnostics, Abbott, Intrexon and Novartis. S. D. P. has received honoraria for attending meetings, consultancy fees, speaker fees, and/or travel grants for Astra Zeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Merck Sharp and Dohme Ltd., Novartis Pharmaceuticals, Novo Nordisk, Novartis Pharmaceuticals, Sanofi, Laboratoires Servier, and Takeda Pharmaceuticals. F. B., V. T. T., I. P., N. J., A. H. and L. ‐E. G. ‐P. are employees and shareholders of Eli Lilly and Company. C. A. K. is an employee of Eli Lilly and Company.
Author contributions
N. J. was responsible for the statistical considerations in the analysis. L‐E. G‐P. is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the design of this post‐hoc analysis and participated in critical reviewing and interpreting the data for the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supporting information
Figure S1. Change in efficacy parameters at 26 weeks with dulaglutide 0.75 mg by baseline HOMA2‐%B. Data presented as mean (95% CI) [unadjusted] and LSM (95% CI) [adjusted]. Mean baseline values are presented in bold within the bars [unadjusted].
Table S1. Change from baseline in efficacy parameters by HOMA2‐%B in patients receiving dulaglutide 1.5 mg.
Table S2. Baseline characteristics by HOMA2‐%B in patients receiving dulaglutide 0.75 mg.
Table S3. Change from baseline in efficacy parameters by HOMA2‐%B in patients receiving dulaglutide 0.75 mg.
Mathieu C, Del Prato S, Botros FT, et al. Effect of once weekly dulaglutide by baseline beta‐cell function in people with type 2 diabetes in the AWARD programme. Diabetes Obes Metab. 2018;20:2023–2028. 10.1111/dom.13313
Funding information This work is sponsored by Eli Lilly and Company
Partial data from this study was previously presented at the American Diabetes Association 76th Annual Scientific Sessions, held in New Orleans, Louisiana, from June 10–14, 2016.
REFERENCES
- 1. Kahn SE. The relative contributions of insulin resistance and beta‐cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3‐19. [DOI] [PubMed] [Google Scholar]
- 2. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427‐2443. [DOI] [PubMed] [Google Scholar]
- 3. Saisho Y. β‐cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6:109‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Raalte DH, Verchere CB. Improving glycaemic control in type 2 diabetes: stimulate insulin secretion or provide beta‐cell rest? Diabetes Obes Metab. 2017;19:1205‐1213. [DOI] [PubMed] [Google Scholar]
- 5. Madsbad S. Review of head‐to‐head comparisons of glucagon‐like peptide‐1 receptor agonists. Diabetes Obes Metab. 2016;18:317‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Raalte DH, Bunck MC, Smits MM, et al. Exenatide improves β‐cell function up to 3 years of treatment in patients with type 2 diabetes: a randomised controlled trial. Eur J Endocrinol. 2016;175:345‐352. [DOI] [PubMed] [Google Scholar]
- 7. Jones AG, McDonald TJ, Shields BM, et al. Markers of β‐cell failure predict poor glycemic response to GLP‐1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39:250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thong KY, McDonald TJ, Hattersley AT, et al. The association between postprandial urinary C‐peptide creatinine ratio and the treatment response to liraglutide: a multi‐centre observational study. Diab Med. 2014;31:403‐411. [DOI] [PubMed] [Google Scholar]
- 9. Yabe D, Ambos A, Cariou B, et al. Efficacy of lixisenatide in patients with type 2 diabetes: a post hoc analysis of patients with diverse β‐cell function in the GetGoal‐M and GetGoal‐S trials. J Diabetes Complications. 2016;30:1385‐1392. [DOI] [PubMed] [Google Scholar]
- 10. Bonadonna RC, Blonde L, Antsiferov M, et al. Lixisenatide as add‐on treatment among patients with different β‐cell function levels as assessed by HOMA‐β index. Diabetes Metab Res Rev. 2017;33:e2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paolo Pozzilli RDL, Peters A, Buzzetti R, et al. Reduction of A1c with dulaglutide in type 2 diabetes (T2D) patients positive or negative for GAD antibodies (GADA): a post hoc analysis of AWARD ‐2, ‐4 and ‐5. Diabetes. 2017;66(suppl 1):A229‐A398. [Google Scholar]
- 12.Trulicity [Prescribing Information]. Indianapolis, IN: Lilly USA, LLC; 2017 http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed November 13, 2017.
- 13. Davidson JA, Manghi FP, Yu M, Linetzky B, Landó LF. Efficacy and safety of dulaglutide in hispanic/latino patients with type 2 diabetes in the AWARD clinical program. Endocr Pract. 2016;22:1406‐1414. [DOI] [PubMed] [Google Scholar]
- 14. Gallwitz B, Dagogo‐Jack S, Thieu V, et al. Effect of once‐weekly dulaglutide on HbA1c and fasting blood glucose in patient subpopulations by gender, duration of diabetes, and baseline HbA1c. Diabetes Obes Metab. 2018;20:409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 16. Umpierrez G, Povedano ST, Manghi FP, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37:2168‐2176. [DOI] [PubMed] [Google Scholar]
- 17. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added on to pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159‐2167. [DOI] [PubMed] [Google Scholar]
- 18. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191‐2192. [DOI] [PubMed] [Google Scholar]
- 19. University of Oxford . HOMA calculator. https://www.dtu.ox.ac.uk/homacalculator/. Accessed May 11, 2015.
- 20. DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose‐lowering therapies: a meta‐analysis of randomized clinical trials. Diabet Med. 2010;27:309‐317. [DOI] [PubMed] [Google Scholar]
- 21. Mari A, Del Prato S, Ludvik B, et al. Differential effects of once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide and metformin on pancreatic β‐cell and insulin sensitivity during a standardized test meal in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:834‐839. [DOI] [PubMed] [Google Scholar]
- 22. Lee YS, Jun HS. Anti‐diabetic actions of glucagon‐like peptide‐1 on pancreatic beta‐cells. Metabolism. 2014;63:9‐19. [DOI] [PubMed] [Google Scholar]
- 23. Davis SN, Johns D, Xu H, Northrup JH, Brodows RG. Exploring the substitution of exenatide for insulin in patients with type 2 diabetes treated with insulin in combination with oral antidiabetes agents. Diabetes Care. 2007;30:2767‐2772. [DOI] [PubMed] [Google Scholar]
- 24. Pozzilli P, Leslie RD, Peters AL, et al. Dulaglutide treatment results in effective glycemic control in Latent Autoimmune Diabetes in Adults (LADA): a post‐hoc analysis of the AWARD‐2, ‐4, and ‐5 trials. Diabetes Obes Metab. 2018. 10.1111/dom.13237 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Change in efficacy parameters at 26 weeks with dulaglutide 0.75 mg by baseline HOMA2‐%B. Data presented as mean (95% CI) [unadjusted] and LSM (95% CI) [adjusted]. Mean baseline values are presented in bold within the bars [unadjusted].
Table S1. Change from baseline in efficacy parameters by HOMA2‐%B in patients receiving dulaglutide 1.5 mg.
Table S2. Baseline characteristics by HOMA2‐%B in patients receiving dulaglutide 0.75 mg.
Table S3. Change from baseline in efficacy parameters by HOMA2‐%B in patients receiving dulaglutide 0.75 mg.


