ABSTRACT
Since the successful conquest of many acute, communicable (infectious) diseases through the use of vaccines and antibiotics, the currently most prevalent diseases are chronic and progressive in nature, and are all accompanied by inflammation. These diseases include neurodegenerative (e.g. Alzheimer's, Parkinson's), vascular (e.g. atherosclerosis, pre‐eclampsia, type 2 diabetes) and autoimmune (e.g. rheumatoid arthritis and multiple sclerosis) diseases that may appear to have little in common. In fact they all share significant features, in particular chronic inflammation and its attendant inflammatory cytokines. Such effects do not happen without underlying and initially ‘external’ causes, and it is of interest to seek these causes. Taking a systems approach, we argue that these causes include (i) stress‐induced iron dysregulation, and (ii) its ability to awaken dormant, non‐replicating microbes with which the host has become infected. Other external causes may be dietary. Such microbes are capable of shedding small, but functionally significant amounts of highly inflammagenic molecules such as lipopolysaccharide and lipoteichoic acid. Sequelae include significant coagulopathies, not least the recently discovered amyloidogenic clotting of blood, leading to cell death and the release of further inflammagens. The extensive evidence discussed here implies, as was found with ulcers, that almost all chronic, infectious diseases do in fact harbour a microbial component. What differs is simply the microbes and the anatomical location from and at which they exert damage. This analysis offers novel avenues for diagnosis and treatment.
Keywords: amyloid, inflammation, iron dysregulation, blood clotting, LPS, amplification
‘The great enemy of truth is very often not the lie – deliberate, contrived and dishonest – but the myth – persistent, persuasive and unrealistic. Too often we hold fast to the clichés of our forebears. We subject all facts to a prefabricated set of interpretations. We enjoy the comfort of opinion without the discomfort of thought’. John F. Kennedy, Commencement Address, Yale University, June 11 1962
‘These germs ‐ these bacilli ‐ are transparent bodies. Like glass. Like water. To make them visible you must stain them. Well, my dear Paddy, do what you will, some of them won't stain; they won't take cochineal, they won't take any methylene blue, they won't take gentian violet, they won't take any colouring matter. Consequently, though we know as scientific men that they exist, we cannot see them’. Sir Ralph Bloomfield‐Bonington. The Doctor's Dilemma. George Bernard Shaw, 1906.
I. INTRODUCTION
A very large number of chronic, degenerative diseases are accompanied by inflammation. Many of these diseases are extremely common in the modern ‘developed’ world, and include vascular (e.g. atherosclerosis, type 2 diabetes, metabolic syndrome, pre‐eclampsia, stroke), autoimmune [e.g. rheumatoid arthritis (RA), multiple sclerosis], and neurodegenerative (e.g. Alzheimer's, Parkinson's, Amyotrophic lateral sclerosis) diseases. On the face of it these diseases are quite different from each other, but in fact they share a great many hallmarks [and often comorbidities (see e.g. Agustí & Faner, 2012; Altamura & Muckenthaler, 2009; Figueira et al., 2016; Lago et al., 2011; Nanhoe‐Mahabier et al., 2009; Pretorius, Mbotwe & Kell, 2017b; Shen et al., 2016)]. As well as inflammation, these hallmarks include increased levels of inflammatory cytokines (almost a definition of inflammation), dysregulation in iron metabolism [especially the appearance of abnormal levels of ferritin in the serum (Kell & Pretorius, 2014)], and a variety of coagulopathies and haematological pathologies (abnormalities in the blood system, including its clotting properties). Many of these diseases also share other properties such as an increase in ‘insoluble’ forms of normally soluble proteins and of microparticulate material. Although they are progressive diseases, their progress is far from uniform, and they are often accompanied by fluctuating changes in physiological states (such as ‘flares’ in rheumatoid arthritis).
However, these ‘hallmarks’ are effectively physiological biomarkers; they are responses to one or more initial external stimuli, and they can and do serve as mediators for (later) manifestations of overt disease. Since effects do not happen without causes, however, the question then arises as to the identity of these external stimuli. In some cases (especially atherosclerosis and metabolic syndrome) there is evidence for a significant dietary component. However, based on a now considerable and wide‐ranging literature, we here bring together evidence that: (i) the main external stimuli are microorganisms; (ii) in contrast to what happens in conventional infectious diseases they do not proliferate unchecked, but commonly enter dormant states that make them invisible to classical microbiology; and (iii) they can be reactivated from these dormant states by the presence of ‘free’ iron (a necessary nutrient that in unliganded form is normally at low levels in the host). This reactivation releases highly potent inflammagens such as lipopolysaccharide (LPS) from Gram‐negative organisms and lipoteichoic acid (LTA) from Gram‐positives. Various sequelae, including coagulopathies, amyloid formation and cell death follow from this, and thus we argue that this general explanation – that we refer to here as the Iron Dysegulation and Dormant Microbes (IDDM) hypothesis–underpins a host of these chronic, inflammatory diseases.
As discussed previously (Kell, 2006; Kell & Knowles, 2006), a typical systems biology strategy (Alon, 2006; Klipp et al., 2005; Palsson, 2006) consists of several phases. The first is qualitative, in which we identify the main players and the main interactions among them. This is the ‘curly arrow’ version that sets out the system of interest in the form of a ‘graph’ containing nodes (players) and edges (their interactions). The nodes can be high level, e.g. processes, or lower level (e.g. individual enzymes in a network). Later steps may seek to become quantitative in the sense that we provide equations for the interactions and then seek to parameterise them (Maldonado et al., 2017). At present, we are still at the very first step or highest level, i.e. providing only the ‘curly arrow’ diagram. We are not yet even in a position to follow good practice (Le Novère et al., 2009) by discriminating the types of interaction by changing the graphical notation. Fig. 1 sets out the main steps involved, and summarises this review in the form of a ‘mind map’. Note, however, that while for convenience we have separated the various steps, some are contemporaneous, and a variety of other interactions and feedbacks are omitted for clarity of presentation. The main focus of this review is the evidence for each of the steps outlined in Fig. 1A.
Figure 1.
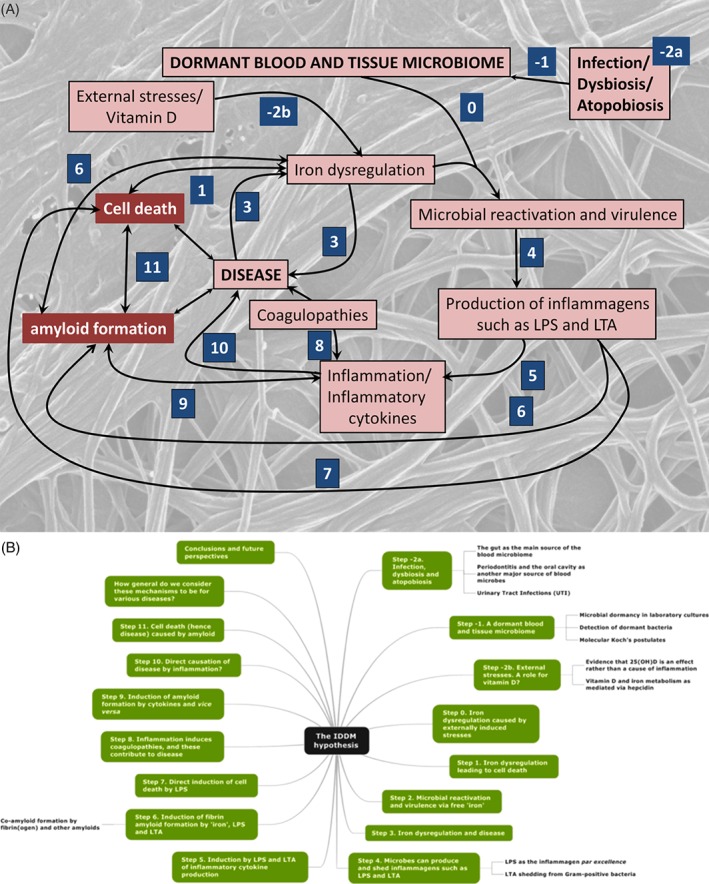
Overview of the processes involved in the Iron Dysregulation and Dormant Microbes (IDDM) hypothesis of chronic inflammatory diseases. (A) The numbered steps, starting with steps –2a and –2b, that are discussed sequentially in this review. (B) A ‘mind map’ (Buzan, 2002) of this review. LPS, lipopolysaccharide; LTA, lipoteichoic acid; 25(OH)D3, 25‐hydroxy‐D3 (vitamin D).
II. STATE –2A: INFECTION, DYSBIOSIS AND ATOPOBIOSIS
While microbiomes such as the skin microbiome (Dréno et al., 2016; Dybboe et al., 2017; Edmonds‐Wilson et al., 2015; Fitz‐Gibbon et al., 2013; Kong et al., 2017; Kong et al., 2012; Oh et al., 2016, 2013; SanMiguel & Grice, 2015; van Rensburg et al., 2015) and the gut microbiome (see Section II.1) are well known, many other sites that are widely considered sterile are in fact full of microbes (Bullman, Meyerson & Kostic, 2017; Ding & Schloss, 2014; Foster et al., 2017; Garn et al., 2016; The Human Microbiome Project Consortium, 2012; Lloyd & Marsland, 2017; Lluch et al., 2015). As well as blood, which we also discuss in detail herein, these include the respiratory system (e.g. Bassis et al., 2015; Budden et al., 2017; Dickson et al., 2017, 2016, b ; Dickson & Huffnagle, 2015; Huffnagle, Dickson & Lukacs, 2017; O'Dwyer, Dickson & Moore, 2016; Samuelson, Welsh & Shellito, 2015; Vientós‐Plotts et al., 2017, b ), neck tissue (Wang et al., 2017), breast tissue (Wang et al., 2017), and both seminal fluid (Craig et al., 2015; Hou et al., 2013; Javurek et al., 2016; Kenny & Kell, 2018; C.M. Liu et al., 2014; Mändar et al., 2015; Weng et al., 2014) and the placenta (Aagaard et al., 2014; Amarasekara et al., 2015; Antony et al., 2015; Collado et al., 2016; Pelzer et al., 2016; Prince et al., 2016; Tarazi, Agostoni & Kim, 2014; Zheng et al., 2015) (cf. Lauder et al., 2016). Indeed, probably all tissues harbour fairly considerable numbers of non‐growing microbes even under normal conditions (Bullman et al., 2017; Domingue, Turner & Schlegel, 1974; Domingue, 2010; Domingue & Woody, 1997; Gargano & Hughes, 2014; Mattman, 2001; Proal, Albert & Marshall, 2013, 2014; Proal, Lindseth & Marshall, 2017).
(1). The gut as the main source of the blood microbiome
We are surrounded by microbes, and are exposed to them constantly. In particular, the gut microbiome has attracted considerable attention, as the number of microbial cells it harbours is similar to or even greater than those in the human body – some 1013 to 1014 (Chu & Aagaard, 2016; Charbonneau et al., 2016; Foster et al., 2017; Guinane & Cotter, 2013; Mondot et al., 2013; Noecker et al., 2017; Turnbaugh et al., 2007; Walter & Ley, 2011). Recent developments include the recognition that many of the soluble metabolic products of the gut microbiome can enter the bloodstream, and hence circulate throughout the body (Dodd et al., 2017; Schroeder & Bäckhed, 2016), including to the central nervous system (CNS) where they can have profound neurological effects. This is known as the ‘gut–brain axis’ (e.g. Alonso et al., 2014; Houser & Tansey, 2017; Montiel‐Castro et al., 2013; Sandhu et al., 2017; Schroeder & Bäckhed, 2016; Sherwin, Dinan & Cryan, 2017). Large amounts of insoluble LPS are also present in the gut (∼1 g; Zaman & Zaman, 2015), and these too can pass into the bloodstream (de Punder & Pruimboom, 2015; Kell & Pretorius, 2015a; Maes, Coucke & Leunis, 2007).
Almost everything dietary, including medicines (Le Bastard et al., 2017), can affect the gut microbiome [and vice versa (Gillis et al., 2018; Koppel, Maini Rekdal & Balskus, 2017; Wilson & Nicholson, 2017)], and there is a large literature, that we do not seek to summarise (Subramanian et al., 2015), on the use of prebiotics and probiotics that are intended to modify it. There is consequently no such thing as a or the ‘normal’ gut microbiome, although certain patterns or frequencies of microbial types are seen as representing some kind of commonality (Lloyd‐Price et al., 2017), at least to the ethnic group under study. For our purposes, the main significance is that the gut microbiome is large and that it exists. ‘Dysbiosis’ is a term usually used to mean a change in the gut microbiome such that its composition differs significantly from those of the ‘normal’ (commonest) populations of interest (Olesen & Alm, 2016) and we adopt this usage herein. Unfortunately, ‘dysbiosis’ is also used, misleadingly, to refer to the appearance of gut microbes in other places; we have therefore suggested the use of the word ‘atopobiosis’ for this latter meaning [microbes in the ‘wrong’ place (Potgieter et al., 2015)].
Inevitably, some of these microbes can display atopobiosis, and enter the bloodstream from the gut (de Punder & Pruimboom, 2015; van der Meulen et al., 2016). When this influx is particularly great, it is sometimes referred to as a ‘leaky gut’ (e.g. Fasano, 2012; Kato et al., 2017; Li & Atkinson, 2015; Luettig et al., 2015; Maes, 2009; Maes et al., 2007; Mu et al., 2017; Quigley, 2016; Shukla et al., 2015; Thevaranjan et al., 2017; Wallace et al., 2014). The result of this, and of the two other main sources that we cover in Sections II.2 and III.3, is the existence of a standing crop of microbes that have entered the bloodstream (Kell, Potgieter & Pretorius, 2015; Potgieter et al., 2015). Fortunately, they do not normally lead to bacteraemia in the form of readily culturable, replicating microbes, as this could be extremely serious (Havey, Fowler & Daneman, 2011; Holland, Arnold & Fowler Jr, 2014; Versalovic et al., 2011; Wester et al., 2014).
(2). Periodontitis and the oral cavity as another major source of blood microbes
A second common origin for blood microbes is the non‐sterile oral cavity (Gargano & Hughes, 2014), whence they can enter through abrasive toothbrushing (Bhanji et al., 2002; Tomás et al., 2012) or periodontal disease. Since blood can appear in the oral cavity, there is nothing to stop the reverse process of microbial infection of the blood (Dhotre, Davane & Nagoba, 2017; Kilian et al., 2016; Koren et al., 2011) and periodontal origins represent another source of potential microbial translocation (Moon & Lee, 2016). There is considerable evidence for a significant association between periodontitis and RA (Bingham III & Moni, 2013; Cheng et al., 2018; de Smit et al., 2012; Detert et al., 2010; Konig et al., 2016; Koziel, Mydel & Potempa, 2014; Lee et al., 2015; Martinez‐Martinez et al., 2009; Mikuls et al., 2009; Monsarrat et al., 2013; Ogrendik, 2013; Potempa, Mydel & Koziel, 2017). Atherosclerosis provides another example (Chukkapalli et al., 2015; Gibson III & Genco, 2007; Kebschull, Demmer & Papapanou, 2010; Łysek et al., 2017; Mahalakshmi et al., 2017; Rangé et al., 2014; Reyes et al., 2013; Rivera et al., 2013; Teeuw et al., 2014; Toyofuku et al., 2011; Velsko et al., 2014).
(2). Urinary tract infections (UTIs)
While any location of an infection, e.g. the chest, is a potential source of microbes that could enter the bloodstream, the other main source of microbial infections of present interest is probably the urinary tract (Flores‐Mireles et al., 2015). For anatomical reasons, women are some 3.5 times more likely to suffer UTIs than are men, an infection that returns frequently because it is not completely eradicated (Blango & Mulvey, 2010; Blango et al., 2014; Ejrnæs, 2011; Hannan et al., 2012; Mysorekar & Hultgren, 2006; Pretorius et al., 2017a; Rosen et al., 2007; Schwartz et al., 2011); this brings us to the physiological state of the bacteria involved. While most would agree with the idea that certain clades of bacteria regularly enter dormant or latent states, not least Mycobacterium tuberculosis (Alnimr, 2015; Barry III et al., 2009; Chao & Rubin, 2010; Gengenbacher & Kaufmann, 2012), which can remain inactive in the lungs for decades, the idea that this may actually be the norm has not yet taken hold.
III. STEP –1: A DORMANT BLOOD AND TISSUE MICROBIOME
The chief method of classical microbiology involves plating a suitably diluted subsample from the sample of interest onto a ‘solid’ (usually agar) medium considered likely to allow their proliferation, and waiting until visible colonies are formed, the number of ‘colony‐forming units’ (CFUs) being equal to the number of ‘viable’ bacteria in the subsample. There are numerous growth media [the classic listing (Zimbro et al., 2009) runs to 700 pages], and typically rather ‘rich’ media are used. One such medium, known euphemistically as ‘chocolate’ agar, is based on blood that has been heated to 80°C to lyse erythrocytes. The concept that ‘viability’ = culturability, or the ability to replicate, is thus a cornerstone of microbiology (Postgate, 1967, 1969, 1976).
The problem with this general strategy is that not only are individual media not suitable for all organisms, but that most organisms (especially when starved) can enter physiological states in which rich media either do not support their growth or may actually kill them (and clearly it is hard to discriminate between these possibilities). However, the organisms may not be ‘dead’, as other treatments can restore them to a physiological state in which they do produce colonies on the same media. Under these circumstances we should refer to them as ‘dormant’ (Kaprelyants, Gottschal & Kell, 1993) since clearly they are not ‘dead’ – a state we take on classical semantic grounds to be irreversible. Dormancy, and any other physiological state, is then to be seen not as a property of the organism alone, but of the organism plus the test used to assess it, and thus these definitions are operational definitions (Kell et al., 1998), reflecting the ‘Schrödinger's cat' problem of quantum mechanics (Primas, 1981).
Indeed, in nature, dormancy is in fact the norm (e.g. Buerger et al., 2012; Dworkin & Shah, 2010; Jones & Lennon, 2010; Kell et al., 2015; Kell & Pretorius, 2015a; Lennon & Jones, 2011; Lewis, 2007; Potgieter et al., 2015; Rittershaus, Baek & Sassetti, 2013; Sachidanandham & Yew‐Hoong Gin, 2009; Sturm & Dworkin, 2015; G.S. Wang et al., 2015a, 2014; Wood, Knabel & Kwan, 2013). This should be seen as rather unsurprising, in that it is reasonable that organisms evolved (or were selected) such that when they ran out of essential nutrients or necessary signalling molecules, and could not replicate, they did not simply die but entered some kind of dormant state from which they might be resuscitated in better times (Mukamolova et al., 2003). In clinical microbiology, the term ‘persistence’ (Balaban et al., 2013; Cohen, Lobritz & Collins, 2013; Dehio, Berry & Bartenschlager, 2012; Fauvart, De Groote & Michiels, 2011; Gerdes & Maisonneuve, 2012; Harms, Maisonneuve & Gerdes, 2016; Holden, 2015; Kester & Fortune, 2014; Krebs, Bartel & Pannek, 2014; Lewis, 2007, 2010; Orman & Brynildsen, 2013; Shah et al., 2006; Wood et al., 2013; Y. Zhang, Yew & Barer, 2012) has come to mean operationally the same thing, i.e. a phenotypic (non‐genotypic) reversible change to an apparently non‐culturable state. In clinical settings, this is often in the presence of otherwise toxic concentrations of antibiotics, where the adoption of a dormant or ‘persistent’ state permits survival.
We note that the term ‘viable‐but‐not‐culturable’ has been used occasionally, despite the fact that this is an oxymoron if one accepts that viability = culturability. Although it is starting to be recognised that microbes said to be adopting this state may in fact be dormant (Oliver, 2010), we suggest that the term ‘viable‐but‐not‐culturable’ is avoided altogether (Kell et al., 1998).
(1). Microbial dormancy in laboratory cultures
A clear‐cut demonstration of dormancy under controlled, laboratory conditions, came from studies of Micrococcus luteus performed in the 1990s. Briefly, starvation after batch culture led to a loss of culturability to approximately 10−3 to 10−5 of the total cell count (Kaprelyants & Kell, 1992, 1993), accompanied by anticipated morphological and biochemical changes, including the conversion of most lipids to cardiolipin (Mukamolova et al., 1995). However, the cells could be resuscitated in the presence of spent culture supernatant under conditions of dilution to extinction (Kaprelyants, Mukamolova & Kell, 1994; Votyakova, Kaprelyants & Kell, 1994). The active constituent in this supernatant was a protein (Mukamolova et al., 1998) with a specific resuscitation promotion factor (Rpf) motif that is present in a wide range of actinobacteria (Mukamolova et al., 1999, 2006, 2002, b ). These features were recognised (Mukamolova et al., 2003) as an important survival strategy. The importance of the ‘dilution to extinction’ experiments was that they avoided any confounding effect of small numbers of ‘actually viable’ cells that could simply regrow and/or resuscitate others. Specifically, resuscitation of the dormant cells was enhanced considerably by an initial period of incubation in weak nutrient broth.
(2). Detection of dormant bacteria
Were the microbes that enter the blood to be capable of replicating in a medium that – like ‘chocolate’ agar – is actually quite rich in organic molecules, we would be discussing conventional, infectious diseases and bacteraemia as commonly understood, but we are not. Under normal conditions, however, either because of the innate immune system or the physiological state of the microbes, or both, normal (non‐bacteraemic) blood – as judged by classical microbiological criteria – is indeed sterile, i.e. it is not possible to detect the presence of viable bacteria in this way. To investigate whether dormant bacteria are present, we thus need culture‐independent methods, of which ultramicroscopic (e.g. Domingue et al., 1974; Domingue, 1995, 2010; Domingue & Woody, 1997; Ewald, 2002; Green, Heidger Jr & Domingue, 1974a, b ; Mattman, 2001; Potgieter et al., 2015) and molecular sequence‐based methods (Amar et al., 2011; Cherkaoui et al., 2009; Fernández‐Cruz et al., 2013; Gaibani et al., 2013; Grif et al., 2012, b ; C.L. Liu et al., 2014; Moriyama et al., 2008; NIH HMP Working Group et al., 2009; Nikkari et al., 2001; Sakka et al., 2009; Sato et al., 2014; Valencia‐Shelton & Loeffelholz, 2014; Woyke, Doud & Schulz, 2017) are by far the most common.
We also recognise that dormant bacteria can survive in white blood cells (Liehl, Zuzarte‐Luis & Mota, 2015; Miskinyte & Gordo, 2013; Miskinyte et al., 2013; Ribet & Cossart, 2015; Thwaites & Gant, 2011), and probably also in the much more prevalent red blood cells (Potgieter et al., 2015), just as can classically infectious organisms such as Bartonella spp. (Ben‐Tekaya, Gorvel & Dehio, 2013; Dehio, 2001; Eicher & Dehio, 2012; Pitassi et al., 2007; Seubert, Schulein & Dehio, 2002), Francisella tularensis (Conlan, 2011; Horzempa et al., 2011), various mycoplasmas (e.g. Groebel et al., 2009), and Streptococcus pneumoniae (Yamaguchi et al., 2013).
A large number of studies (e.g. Domingue et al., 1974; Domingue et al., 1995; Domingue & Schlegel, 1977a, b ; Domingue & Woody, 1997; Goubran et al., 2017; Mattman, 2001; Nikkari et al., 2001), reviewed previously by Amar et al. (2011), Kell & Kenny (2016), Kell et al. (2015); Kell & Pretorius (2015a) and Potgieter et al. (2015), suggests that there is indeed an authentic but dormant blood microbiome. A particularly good example comes from Damgaard et al. (2015) who reasoned that plating samples from blood bags straight onto chocolate agar exposed them to atmospheric oxygen, and that this might produce reactive oxygen species that could kill any organisms present. When instead they plated them anaerobically, the supposedly sterile blood revealed a large resident microbiome that could be cultured (and indeed sequenced). Many microbes resident in humans are as yet uncharacterised (Kowarsky et al., 2017), and evolutionary arguments support the idea that it is often better to tolerate than to fight against invading organisms (Ayres, 2016; Ayres & Schneider, 2012; Schneider & Ayres, 2008).
In particular, those recognising relationships between overt chronic, inflammatory disease and the presence of detectable microbes, can highlight that the blood and tissue microbiome is greatly enhanced in these diseases (Alonso et al., 2017; Arleevskaya et al., 2016; Berstad & Berstad, 2017; Broxmeyer, 2017a, b ; Ebringer, 2012; Ebringer & Rashid, 2009; Ebringer, Rashid & Wilson, 2010; Emery et al., 2017; Itzhaki et al., 2016; Kell & Kenny, 2016; Maheshwari & Eslick, 2015; Miklossy, 2011; Miklossy & McGeer, 2016; Pisa et al., 2017; Pretorius et al., 2017a; Pretorius, Bester & Kell, 2016a; Proal et al., 2013, 2014, 2017). We note too that while it is all too easy to dismiss such findings as ‘contaminants’, those doing so must also explain why the microbes appear at much higher levels only in the ‘disease’ samples.
(3). Molecular Koch's postulates
The Henle–Koch postulates (that microbe X causes disease Y) represent another cornerstone of infection microbiology (Autenrieth, 2016; Evans, 1976; Gradmann, 2014; Segre, 2013); they require association of the proposed pathogen with the disease and non‐association in its absence, as well as reinfection leading to renewed disease. Specifically, (i) the microorganism must be found in diseased but not in healthy individuals; (ii) the microorganism must be cultured from the diseased individual; (iii) inoculation of a healthy individual with the cultured microorganism must recapitulate the disease; and finally (iv) the microorganism must be reisolated from the inoculated, diseased individual and must match the original microorganism. Unfortunately these original concepts simply do not work in the case of dormant microbes (Antonelli & Cutler, 2016; Autenrieth, 2016; Byrd & Segre, 2016; Falkow, 1988, 2004; Fredricks & Relman, 1996; Seal et al., 2010), because it is not always possible to isolate culturable organisms from patients with the disease. In the case of Whipple's disease and the causative organism Tropheryma whipplei, a clear link between the disease and ultramicroscopically observable microbes was established (Maiwald & Relman, 2001; Relman et al., 1992) long before sequencing methods (Bentley et al., 2003) allowed the design of a medium in which the organism could be persuaded to replicate (Renesto et al., 2003). Thus, while the ideal would be the fulfilment of the original Koch's postulates, the association of specific DNA with the disease should nowadays be sufficient for the tentative identification of a causative organism even, as in the case of H. pylori and gastric ulcers (Marshall, 2001; Marshall et al., 1985, 1988), when an infectious agent was not previously suspected.
IV. STEP –2B EXTERNAL STRESSES, AND A POSSIBLE ROLE FOR VITAMIN D
By our definition, causality demands an external stimulus. External stresses can be mechanical (e.g. trauma), oxidative, pharmacological or dietary [including poisoning (Kell, 2010)] among others. We here use an example of a dietary stimulus (vitamin D3) as an illustration of the complexity of the systems under discussion.
It has been pointed out previously (e.g. Mangin, Sinha & Fincher, 2014; Proal, Albert & Marshall, 2015) that vitamin D dysregulation is a common accompaniment to chronic infection with (normally) dormant microbes. Vitamin D dysregulation typically manifests as a low serum level of calcidiol [25‐hydroxy‐D3; 25(OH)D3] and is indeed widely observed in inflammation (Table 1), although whether it is a cause or a consequence cannot of course be determined from simple co‐occurrences. The studies listed in Table 1 show associations, but not (Beveridge & Witham, 2013; Cannell, Grant & Holick, 2014; Kienreich et al., 2013) whether low vitamin D levels are a cause or an effect of inflammation (or both, under different conditions; Cannell et al., 2014), how this relates to the disease, and whether improving some aspect of vitamin D status would be a treatment option.
Table 1.
Chronic, inflammatory diseases in which low vitamin D levels have been recorded
| Disease | Subtype | Comments | Reference |
|---|---|---|---|
| ‘Autoimmune’ | Review: strong inverse relationships between [25(OH)D3] and incidence of several automimmune diseases | Skaaby et al. (2015) | |
| Chronic obstructive pulmonary disease (COPD) | Clear inverse relationship between COPD and vitamin D status | Skaaby et al. (2014) | |
| Rheumatoid arthritis (RA) | Meta‐analysis of a large literature; mean [25(OH)D3] 16.5 nM lower in RA patients | Arnson et al. (2007); Lin et al., 2016) | |
| Cancer | Multiple, especially skin | Acts with vitamin D receptor (VDR) via hedgehog and ß‐catenin | Bikle (2011) |
| Skin | Role of ß‐catenin | Jiang et al. (2013) | |
| Meta‐analysis: little effect on incidence but significant effect on mortality | Keum & Giovannucci (2014) | ||
| Multiple | Epidemiological | Afzal et al. (2014b) | |
| Cardiovascular | |||
| Atherosclerosis | Detailed reviews and meta‐analyses | Kassi et al. (2013); Menezes et al. (2014) | |
| Meta‐analysis | Carvalho & Sposito (2015) | ||
| Heart failure | de Temiño et al. (2011) | ||
| Hypertension | |||
| Odds ratio (OR) = 6.13 for incident hypertension in males if [25(OH)D3] <15 ng ml−1 versus ≥ 30 ng ml−1 | Forman et al. (2007) | ||
| OR = 1.66 for incident hypertension in lowest versus highest [25(OH)D3] quartile | Forman et al. (2008) | ||
| Large meta‐analysis: 10% increase in [25(OH)D3] reduces hypertension risk by 8%; OR = 0.92 | Vimaleswaran et al. (2014) | ||
| Large meta‐analysis; risk ratio (RR) = 0.68 for highest versus lowest [25(OH)D3] category | Ke et al. (2015) | ||
| Significantly lower, including in subsequent organ damage | Pludowski et al. (2014) | ||
| OR = 13.54 for low [25(OH)D3] and risk of ischaemic stroke in hypertensives | Majumdar et al. (2015) | ||
| Myocardial infarction (MI) and cardiovascular disease | Epidemiological study; RR > 2 if [25(OH)D3] < 15 ng ml−1 (37 nM) | Giovannucci et al. (2008) | |
| Very large effects of low [25(OH)D3] on likelihood of MI and ischaemic heart disease | Brøndum‐Jacobsen et al. (2012) | ||
| Reviews | Beveridge & Witham (2013); Kienreich et al. (2013); Norman & Powell (2014) | ||
| Stroke | Review | Makariou et al. (2014) | |
| 77% of patients had insufficient vitamin D levels | Poole et al. (2006) | ||
| OR = 1.52 for ‘low’ versus ‘high’ [25(OH)D3] | Sun et al. (2012) | ||
| OR = 1.33–1.85 for ‘low’ versus ‘high’ [25(OH)D3] | Judd et al. (2016) | ||
| Poor 90‐day outcome and larger infarct volume strongly related to lower vitamin D levels | Turetsky et al. (2015) | ||
| Ischaemic only (no effect on haemorrhagic) possibly implying a role in clotting | Strong inverse relation with [25(OH)D3] | Brøndum‐Jacobsen et al. (2013) | |
| Ischaemic | [25(OH)D3] a very good predictor of favourable outcomes (OR = 1.9) | Park et al. (2015) | |
| OR = 1.6 or more for low versus high [25(OH)D3] | Chaudhuri et al. (2014) | ||
| Venous thromboembolism | 1.37 RR lowest to highest tertile for seasonally adjusted [25(OH)D3] | Brøndum‐Jacobsen et al. (2013) | |
| Metabolic | |||
| Obesity | Obesity negatively correlated with serum [25(OH)D3] | Jamal‐Allial et al. (2014) | |
| Type 2 diabetes (T2D) | Hazard ratio (HR) = 1.45 for bottom versus top quartile of [25(OH)D3] (and also raised ferritin levels in disease cohort; Forouhi et al., 2007) | Forouhi et al. (2012) | |
| 1.5 HR for bottom versus top quartile of [25(OH)D3] | Afzal et al. (2013) | ||
| 1.25 RR for a reduction of [25(OH)D3] by 25 nM, but associative and not causative | Ye et al. (2015) | ||
| Relationship with body mass index (BMI) and T2D susceptibility mediated via low vitamin D levels | Afzal et al. (2014c) | ||
| Neurodegenerative and related | |||
| Amyotrophic lateral sclerosis | No benefits from vitamin D supplements | Karam et al. (2013) | |
| Alzheimer's | OR = 0.23 for highest versus lowest quintile of vitamin D intake | Annweiler et al. (2012) | |
| HR = 2.25 for [25(OH)D3] < 25 nM and 1.53 for 25–50 nM | Littlejohns et al. (2014) | ||
| Meta‐analysis: 21% increased risk for [25(OH)D3] < 50 nM | Shen & Ji (2015) | ||
| Meta‐analyses | Banerjee et al. (2015); Lu'o'ng & Nguyên (2013) | ||
| HR = 1.25 if [25(OH)D3] < 25 nM | Afzal et al. (2014) | ||
| Cognition | Meta‐analysis | van der Schaft et al. (2013) | |
| Rates of decline in episodic memory and executive function greater in vitamin D deficiency | Miller et al. (2015) | ||
| Poorer cognitive performance if vitamin D < 10 ng ml−1 (Framingham heart study) | Karakis et al. (2016) | ||
| Cognitive scores in Minimental State Examination (MMSE) correlated with vitamin D levels | Peterson et al. (2012) | ||
| Huntington's | 89% of patients ‘deficient’ in vitamin D. Positive association between serum [25(OH)D3] levels and functional ambulation classification (FAC) scores | Chel et al. (2013) | |
| Myalgic encephalomyelitis/ chronic fatigue syndrome | Berkovitz et al. (2009); Witham et al. (2014) | ||
| Parkinson's | OR = 2.2 for [25(OH)D3] < 50 nM | Lv et al. (2014) | |
| Correlation of vitamin D levels with improved cognition and mood | Peterson et al. (2013) | ||
| Meta‐analysis | Zhao et al. (2013) |
(1). Evidence that a low 25(OH)D3 level is an effect rather than a cause of inflammation
Inflammatory cytokines can induce expression of both the vitamin D receptor (VDR) and the cytochrome P450 enzyme CYP27B1 that converts 25(OH)D3 to 1,25‐dihydroxyvitamin D3 (1,25(OH)2D3); 1,25(OH)2D3 suppresses elements of the adaptive immune system while stimulating elements of the innate immune system (Bikle, 2009). In addition (Bell, Shaw & Turner, 1984) 1,25(OH)2D3 inhibits hepatic production of 25(OH)D3, explaining how inflammation can simultaneously cause high 1,25(OH)2D3 and low 25(OH)D3 levels (Fig. 2). Obviously measuring 25(OH)D3 levels alone will be a rather poor guide to the effective vitamin D status.
Figure 2.
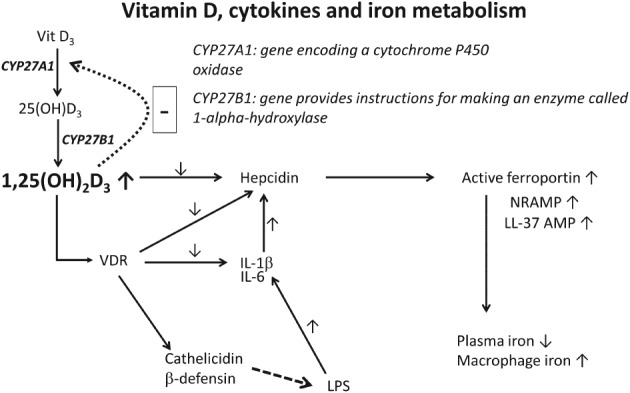
A simplified scheme showing the links between Vitamin D, cytokines and iron metabolism during chronic inflammation. 25(OH)D3, 25‐hydroxyvitamin D; 1,25(OH)2D3, calcitriol or 1,25‐dihydroxycholecalciferol; IL, interleukin; LL‐37AMP, antimicrobial peptide LL‐37; LPS, lipopolysaccharide; NRAMP, natural resistance‐associated macrophage proteins; VDR, vitamin D receptor.
Mangin et al. (2014) and Waldron et al. (2013) therefore suggested that low 25(OH)D3 concentration is a consequence of chronic inflammation rather than a cause, and that tissue bacteria could be responsible for an inflammatory disease process resulting in high 1,25(OH)2D3 and low 25(OH)D3 levels (see also Waterhouse, Perez & Albert, 2009).
One signalling role of 1,25(OH)2D3 is to activate the VDR (Carlberg & Campbell, 2013; Kongsbak et al., 2013; Schauber et al., 2007), a transcription factor that can induce the expression of over 900 genes. From an infection or innate immunity perspective, it is important that the products of these genes include antimicrobial peptides (AMPs) (Bartley, 2010a; Coussens, Martineau & Wilkinson, 2014; Fabri et al., 2011; Liu et al., 2006; Proal et al., 2014; Youssef et al., 2011) such as cathelicidin and beta defensins (Fig. 2) which are known to attack pathogens (Nnoaham & Clarke, 2008).
There is now a complex (Nama et al., 2016) and often contradictory literature (Kearns et al., 2015) regarding vitamin D supplementation. Some studies have highlighted a relationship between low 25(OH)D3 levels and Alzheimer's disease (Banerjee et al., 2015; Littlejohns et al., 2014; Lu'o'ng & Nguyên, 2013; Miller et al., 2015; Shen & Ji, 2015) (see also Table 1). A naïve view [recapitulating the now‐discredited ‘crossover theorem’ (Chance & Williams, 1955)] would suggest that vitamin D supplementation could be a solution. To date, however, there is little evidence for clinical benefits from vitamin D (Bjelakovic et al., 2014a, b ; Brøndum‐Jacobsen et al., 2015; Karam et al., 2013; Makariou et al., 2014; Newberry et al., 2014; Pilz et al., 2015; Witham et al., 2015). This may reflect different populations of individuals who respond differently to vitamin D3 supplementation (Carlberg et al., 2013; Ryynänen et al., 2014; Saksa et al., 2015), or perhaps the simultaneous presence of individuals in which the VDR responds to vitamin D as an agonist or an antagonist (Anami et al., 2014). It is known that small changes in the sequence of the VDR can have major phenotypic effects, e.g. an odds ratio (OR) for stroke of 2.97 was calculated for one particular allele (Prabhakar et al., 2015). A systems biologist will recognise that supplementation may not be the answer, and indeed there is some evidence for the opposite effect (Mangin et al., 2014; Marshall, 2008; Proal et al., 2015). Clearly we need to clarify the different roles of 25(OH)D3 and 1,25(OH)2D3, and any effects of chronic conditions on the CYP enzymes that produce them. Biomarkers [such as taurinuria (Chesney, Dabbagh & Han, 2015) for genuine vitamin D deficiency may prove useful in this work.
Finally, we recognise that signalling can be effected both by changes in the amplitude of a signal and also by changes in its frequency, as is the case for the apoptotic versus proliferative effects of nuclear factor‐kappa B (NF‐κB) (Ashall et al., 2009; Kell, 2006; Nelson et al., 2004). Vitamin D is known to have significant effects on NF‐κB (Chen et al., 2013; Szeto et al., 2007; Wu et al., 2010, b ) and VDR expression levels are partly dependent on extracellular signal‐related kinase (ERK) (Ordóñez‐Morán & Muñoz, 2009), which also oscillates (Waters et al., 2014). Vitamin D3 also regulates circadian genes (Gutierrez‐Monreal et al., 2014). Consequently, ‘oscillation‐based’ explanations of signal transduction may be relevant to the role of vitamin D in inflammation.
It is thus clear (e.g. Bartley, 2010a, b ; Mangin et al., 2014)) that there are major interactions between inflammation, infection, and vitamin D metabolism [including elements of iron and vitamin D metabolism (Zughaier et al., 2014), see below].
(2). Vitamin D and iron metabolism mediated by hepcidin
The protein hepcidin is a key regulator of mammalian iron metabolism (Ganz, 2006; Ganz & Nemeth, 2012; Michels et al., 2015; Reichert et al., 2017; Vyoral & Jiri, 2017; Zaritsky et al., 2009). As Zughaier et al. (2014) comment, 25(OH)D3 concentrations (as modified via the addition of 1,25(OH)2D but assessed by serum 25‐hydroxyvitamin D (25(OH)D)) are inversely associated with hepcidin concentrations and are positively associated with levels of haemoglobin and iron' (Carvalho et al., 2011; Icardi et al., 2013; Perlstein et al., 2011; Zaritsky et al., 2009), while hepcidin and 1,25(OH)2D3 stimulated a strong increase in levels of ferroportin 1, natural resistance associated macrophage protein 1 (NRAMP1) and LL‐37 antimicrobial peptide, which lead to a reduction in plasma iron levels (Fig. 2). The inflammatory cytokine interleukin‐6 (IL‐6) also induces hepcidin production (Chesney et al., 2015; Ganz & Nemeth, 2015; Lee et al., 2005; Nemeth et al., 2004).
Zughaier et al. (2014, p. e23) noted that ‘LPS is a major component of microbial translocation seen during chronic inflammation (Layoun & Santos, 2012; Theurl et al., 2008; Wang et al., 2009). LPS induces both hepcidin and IL‐6 expression whereas LL‐37 binds and neutralizes LPS activity (Zughaier, Shafer & Stephens, 2005)’. Increases in 1,25(OH)2D3 cause hepcidin levels to decrease, via binding of the VDR to hepcidin's promoter (Bacchetta et al., 2014, b ), and levels of IL‐1β and IL‐6 are also decreased (Fig. 2) [exacerbating the decrease in hepcidin (Ganz & Nemeth, 2015)]. Decreased hepcidin levels enhance the surface exposure of ferroportin, while associated increases in NRAMP and LL‐37 lead to potential hyperferraemia (Fig. 2). The increase in hepcidin levels via IL‐6 (Layoun & Santos, 2012; Wang et al., 2009) is partly mediated by microRNA‐155 (mi‐RNA‐155) that increases with increasing LPS levels and is inversely related to vitamin D levels (Li et al., 2014). Thus, while the process is complex, it does appear that vitamin D metabolism is intimately involved in the microbial processes that could lead to chronic, inflammatory disease.
V. STEP 0: IRON DYSREGULATION CAUSED BY EXTERNALLY INDUCED STRESSES
As any student of metabolic control analysis (Fell, 1996; Fell & Thomas, 1995; Heinrich & Rapoport, 1974; Kacser & Burns, 1973) or systems biology knows, individual metabolic steps alone rarely control the flux in biochemical networks. Thus, although we attempt to order the steps in Fig. 1A temporally, it is hard to be certain about the exact sequence of causality. Nonetheless, iron dysregulation is step 0 in our systems biology approach because of two outcomes: (i) the production of hydroxyl radicals, catalysed by ‘free’ iron that can itself lead to cell death (step 1); and (ii) the iron‐based reactivation of dormant microbes (step 2). In this section we concentrate on the first mechanism. Many reviews of general iron metabolism are available elsewhere (Kell, 2009, 2010; Kell et al., 2015; Kell & Pretorius, 2014, 2015b; Chifman et al., 2012; Mitchell & Mendes, 2013; Parmar et al., 2017).
Iron can have negative effects, as reviewed extensively elsewhere (e.g. Altamura & Muckenthaler, 2009; Anderson & Wang, 2012; Berg & Youdim, 2006; Bush & Tanzi, 2008; Castellani et al., 2012; Chifman, Laubenbacher & Torti, 2014; Collingwood & Davidson, 2014; Crichton, 2016; Crichton, Dexter & Ward, 2011; Dixon & Stockwell, 2013; Farina et al., 2013; Ganz & Nemeth, 2015; Hansen, Moen & Mandrup‐Poulsen, 2014; Jellen, Beard & Jones, 2009; Kell, 2009, 2010; Kell & Pretorius, 2014; Koskenkorva‐Frank et al., 2013; Lehmann et al., 2015; Levi & Finazzi, 2014; Mollet et al., 2016; Muhoberac & Vidal, 2013; Muller & Leavitt, 2014; Nikonorov et al., 2015; Núñez et al., 2012; Oliveira, Rocha & Fernandes, 2014; Peters, Connor & Meadowcroft, 2015; Pisano, Lombardi & Fracanzani, 2016; Rouault, 2016; Schneider, 2016; Shovlin et al., 2015, 2016; Simcox & McClain, 2013; Stankiewicz, Neema & Ceccarelli, 2014; Stephenson et al., 2014; Sullivan, 2009; Thuret, 2013; Vinchi et al., 2014; Weinreb et al., 2013; Yin et al., 2012; Zhao et al., 2012; Zhuang, Han & Yang, 2014), as well as being an essential nutrient for cell growth (cf. Posey & Gherardini, 2000). ‘Iron’ can be present as Fe2+ and Fe3+ valencies, and also has six liganding sites (four ‘equatorial’, two ‘polar’) that affect its reactivity in two linked reactions involving peroxide and superoxide (molecules that are always present in aerobic systems). The amount of ‘free’ iron varies, but Fe(III) salts are virtually insoluble at neutral pH (explaining the need for microbial siderophores, see Sections V and VII); the typical cytoplasmic levels of ‘free’ iron are in the range 1–10 µM (Hider & Kong, 2013).
Both hydrogen peroxide and superoxide are common products of the partial reduction of oxygen by mitochondria, among other sources (Kell, 2009). Hydrogen peroxide can react with free or poorly liganded Fe(II) in the Fenton reaction (Wardman & Candeias, 1996), leading to the production of very reactive and damaging hydroxyl radicals (OH•).
| (1) |
The ferric iron can then react with superoxide in the Haber–Weiss reaction (Kehrer, 2000) generating Fe(II) again, thereby effecting redox cycling:
| (2) |
In other words, catalytic quantities of unliganded or poorly liganded iron can lead to a continuing flux of hydroxyl radicals. These react in nanoseconds with almost anything, and their existence can be detected via the products of such reactions, including 8‐hydroxy‐guanine (Shin et al., 2001), 8‐hydroxy‐2′‐deoxy‐guanosine (Loft et al., 1993; Migliore et al., 2005), 4‐hydroxy‐nonenal (Ayala, Muñoz & Argüelles, 2014; Petersen & Doorn, 2004; Tsikas, 2017), various isoprostanes (Davì, Falco & Patrono, 2004; Montuschi, Barnes & Roberts II, 2007, 2004; Montuschi et al., 1998, 2000; Morrow, 2005; Schwedhelm & Boger, 2003) and malondialdehyde (Ayala et al., 2014; Del Rio, Stewart & Pellegrini, 2005; Janero, 1990; Tsikas, 2017).
This iron dysregulation can be initiated by a multitude of factors that cause cell death, which will release free iron into the bloodstream, whence it can be disseminated throughout the body (Kell & Pretorius, 2014). Such factors include mechanical damage [including trauma (Gorbunov et al., 2006, 2005, 2003; Zhang et al., 2013) and dysbiosis], nutritional stress (Schaffer, 2003, 2016), pharmacological stress (Pirmohamed et al., 2004), oxidative stress (Crichton, 2016; Kerley et al., 2018) and others (Nanba et al., 2016), many of which also involve the production of stress hormones.
VI. STEP 1: IRON DYSREGULATION LEADING TO CELL DEATH
Fenton reactions within the cell will potentially result in death via apoptosis (Lee et al., 2006; Li et al., 2016), ferroptosis (Dixon et al., 2012; Dong et al., 2015; Imai et al., 2017; Yang & Stockwell, 2016; Yu et al., 2017), and necrosis (Dong et al., 2015; Traoré & Meyer, 2007). These processes have been reviewed previously (Kell, 2009, 2010; Kell & Pretorius, 2014), but we here draw attention to the following: (i) the reducing agent ascorbic acid (vitamin C) actually becomes a pro‐oxidant when poorly liganded, e.g. with ligands such as ethylene diamine tetraacetate (EDTA) (Kell, 2009); and (ii) ferritin is an intracellular marker, so that the serum ferritin level (widely but erroneously used as a measure of iron status) is simply a sign of cell death (Kell & Pretorius, 2014). Indeed, cell death can be autocatalytic, as serum ferritin can lose its iron component (Arosio, Yokota & Drysdale, 1977; Konz et al., 2013; Nielsen et al., 2000; Watanabe et al., 2001; Yamanishi et al., 2002), such that cell death liberates free iron that, via further Fenton and Haber–Weiss reactions, can cause further cell death.
In contrast to apoptosis in nucleated cells, programmed cell death in red blood cells (RBCs) is known as eryptosis (Bissinger et al., 2013; Föller et al., 2008; Lang & Lang, 2015; E. Lang, Qadri & Lang, 2012a; Lang et al., 2010; F. Lang, Lang & Foller, 2012b; Lang & Qadri, 2012; Pretorius, du Plooy & Bester, 2016b; Qadri et al., 2011; Qadri et al., 2016; Qadri et al., 2012). It causes the release of haem from RBCs, which can eventually lead to the presence of free ‘iron’. The physiological processes taking place during eryptosis are similar to those of apoptosis, but without the involvement of the nucleus and mitochondria. Examples of eryptotic RBCs in the presence of inflammation are shown in Fig. 3A–E; Fig. 3F is an example of eryptosis induced by addition of IL‐8 to healthy whole blood.
Figure 3.
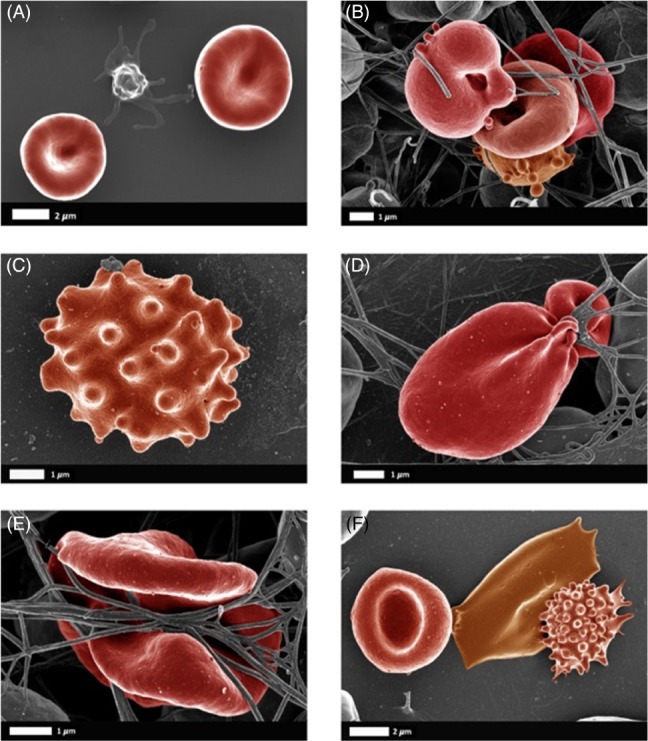
Examples of eryptotic red blood cells (RBCs) in inflammation. (A) Healthy RBCs with a platelet; (B) Type 2 diabetes (Pretorius et al., 2015); (C, D) Parkinson's disease (Pretorius et al., 2014b); (E) Rheumatoid arthritis (Olumuyiwa‐Akeredolu et al., 2017); (F) healthy whole blood exposed to interleukin‐8 (Bester & Pretorius, 2016).
VII. STEP 2: MICROBIAL REACTIVATION AND VIRULENCE VIA FREE ‘IRON’
‘Chocolate’ agar is a medium widely used for assaying bacteria via their growth, and is essentially heated blood. However, bacteria proliferate much less well in actual blood, partly due to the presence of antimicrobial components and the innate immune system but also because healthy blood in vivo normally has almost no free iron available (1–10 µM) (Armitage & Drakesmith, 2014; Chu et al., 2010; Haley & Skaar, 2012; Sivick & Mobley, 2010; Subashchandrabose & Mobley, 2015; Wessling‐Resnick, 2010). Indeed iron‐withholding (Ganz, 2009; Jurado, 1997; Nevitt, 2011; Weinberg, 2009; Weinberg & Miklossy, 2008) is a major strategy used by hosts to inhibit the growth of microbial invaders. This is often described as a ‘battle’ (Armitage & Drakesmith, 2014; Carver, 2018; Chu et al., 2010; Damron et al., 2016; Fischbach et al., 2006; Haley & Skaar, 2012; Pich & Merrell, 2013; Skaar, 2010; Stijlemans et al., 2015) or ‘struggle’ (Markel et al., 2007; Nairz et al., 2010; Reid, Anderson & Lamont, 2009) for iron between the host and invader.
In consequence, the likelihood of infection is greatly enhanced when free iron levels are raised (Boyanova, 2011; Braun, 2005; Eichhorn et al., 2006; Ishida & Johansen, 2014; Mittal et al., 2008; Nevitt, 2011; Ngok‐Ngam et al., 2009; Rodriguez & Smith, 2003; Sritharan, 2006; Su et al., 2009; Sutak et al., 2008; Vasil & Ochsner, 1999), and indeed the ‘virulence’ of microbes is strongly correlated with their expression of siderophore (iron‐binding) or iron transporter (Do, Zafar & Saier Jr, 2017; Tang & Saier Jr, 2014) genes. In addition, siderophores can act directly to induce cytokine expression (Holden et al., 2016).
An obvious corollary is that iron‐overload disorders such as hereditary haemochromatosis or the thalassaemias will result in a significantly higher susceptibility to infection (Ashrafian, 2003; Barton & Acton, 2009; Christopher, 1985; Khan, Fisher & Khakoo, 2007; Moalem, Weinberg & Percy, 2004; Muench, 1989; Weinberg, 1978, 2009). We suggest herein that it is a combination of free iron and microbial reactivation that is key to understanding chronic, inflammatory disease.
VIII. STEP 3: IRON DYSREGULATION AND DISEASE
Although we suspect that the greater significance of free iron in chronic, inflammatory diseases is via microbial activation (Fig. 1) rather than via the Fenton and Haber–Weiss reactions and oxidative stress, there is no doubt that excess iron is itself directly involved in a variety of diseases (Table 2).
Table 2.
Selected diseases in which iron dysregulation takes place
| Disease | Comments | Selected references |
|---|---|---|
| Alzheimer's disease | Likely role of iron binding to amyloid proteins | Altamura & Muckenthaler (2009); Ayton et al. (2015, 2017); Barnham & Bush (2008); Belaidi & Bush (2016); Casadesus et al. (2004); Castellani et al. (2012); Crichton (2016); Crichton et al. (2011); Gallagher et al. (2012); Gargano & Hughes (2014); Grünblatt et al. (2011); Peters et al. (2015); Pretorius et al. (2016a); Sternberg et al. (2017); Telling et al. (2017); van Duijn et al. (2017); Wood (2015) |
| Amyotrophic lateral sclerosis (Lou Gehrig's disease) | Hadzhieva et al. (2013); Ignjatović et al. (2012, 2013); Molfino et al. (2009); Oshiro et al. (2011); Sheelakumari et al. (2016); Wang et al. (2011) | |
| Atherosclerosis | Huge levels of iron in atherosclerotic plaques | Altamura & Muckenthaler (2009); Galesloot et al. (2015); Kraml (2017); Sharkey‐Toppen et al. (2014); Stadler et al. (2004); Stanley et al. (2006); Sullivan (2009); Winner III et al. (2015) |
| Type 2 diabetes, | Abundant epidemiological evidence | Altamura et al. (2017); Ambachew & Biadgo, 2017; Basuli et al. (2014); Fernández‐Cao et al., 2017; Fernández‐Real et al. (2002, 2015); Hansen et al. (2014); Huth et al. (2015); Kundu et al. (2013); Mascitelli et al. (2009); Montonen et al. (2012); Podmore et al. (2016); Simcox & McClain (2013); X. Wang et al. (2015b); Zhao et al. (2012) |
| Friedreich's ataxia | Clear mechanistic linkage via frataxin, an Fe‐S protein chaperone | (Anzovino et al. (2014); Chiang et al. (2016); Harding et al. (2016); Martelli & Puccio (2014); Richardson et al. (2010); Vaubel & Isaya (2013); Wilson (2006) |
| Oxidative DNA damage | Products of Fenton reaction | Hori et al. (2010); Mollet et al. (2016); Shaw et al. (2017); Singh & Chadha (2016); Zein et al. (2017) |
| Parkinson's disease | Dopamine makes substantia nigra especially sensitive; among the syndromes with the most evidence for iron involvement | Altamura & Muckenthaler (2009); Barnham & Bush (2008); Berg (2007); Brar et al. (2009); Costa‐Mallen et al. (2017); Crichton et al. (2011); Dusek et al. (2014); Hare et al. (2014); Lee & Andersen (2010); Maes et al. (2017); Mochizuki & Yasuda (2012); Weinreb et al. (2013) |
| Pre‐eclampsia | Considerable evidence of iron dysregulation | Entman et al. (1987); Kell (2009); Kell & Kenny (2016); Kenny & Kell (2018); Kerley et al. (2018); Rayman et al. (2002); Serdar et al. (2006); Toldi et al. (2010) |
| Rheumatoid arthritis | Considerable evidence of iron dysregulation | Baker & Ghio (2009); Dombrecht et al. (2004); Donnelly et al. (2010); Stefanova et al. (2016) |
| Stroke | Considerable evidence of iron dysregulation | Armengou & Davalos (2002); Petrova et al. (2016); Selim & Ratan (2004); Tuo et al. (2017) |
IX. STEP 4: MICROBES CAN PRODUCE AND SHED INFLAMMAGENS SUCH AS LPS AND LTA
The cell walls of Gram‐negative and Gram‐positive bacteria contain significant amounts of LPS and LTA that can become detached in response to different environmental and physiological signals (e.g. Watson et al., 1977). When shed into the host, LPS is known as endotoxin. The most extreme example of microbial shedding of inflammatory material of this type is in a condition known as the Jarisch–Herxheimer reaction (Almeida, Estanqueiro & Salgado, 2016; Belum et al., 2013; Cheung & Chee, 2009; Guerrier & D'Ortenzio, 2013; Kadam et al., 2015; Pound & May, 2005; See, Scott & Levin, 2005), which is essentially an uncontrolled cytokine storm (see Section X) caused by the rapid release of inflammagenic cell wall materials from microbes, often following bactericidal antibiotic treatment (Lepper et al., 2002).
(1). LPS as the inflammagen par excellence
The inflammagenic potency of LPS is so great that it is commonly (and ironically) even used as a model to induce symptoms more or less similar to many of the inflammatory diseases of interest. Typically this involves injecting LPS at the site of interest for such diseases. Examples of the use of endotoxin in this way include pre‐eclampsia (Cotechini et al., 2014; Faas et al., 1994; Faas et al., 2000; Lin et al., 2012; Liu et al., 2017; Rademacher, Gumaa & Scioscia, 2007; Sakawi et al., 2000; Williamson et al., 2016; Xue et al., 2015), Alzheimer's (Zhan et al., 2015, 2016), Parkinson's (Barnum & Tansey, 2010; Byler et al., 2009; Cunningham et al., 2005; He et al., 2013; Hoban et al., 2013; Hritcu & Ciobica, 2013; Hritcu et al., 2011; Liu & Bing, 2011; Miller et al., 2009; Orr, Rowe & Halliday, 2002; Santiago et al., 2010; Tufekci, Genc & Genc, 2011; Z. Zhang et al., 2012), rheumatoid arthritis (Izui, Eisenberg & Dixon, 1979; Nemeth et al., 1985), atherosclerosis (Khedoe et al., 2013), multiple sclerosis (di Penta et al., 2013; Nguyen et al., 2004), Guillain‐Barré syndrome (Prendergast & Moran, 2000), sepsis (Lewis, Seymour & Rosengart, 2016; Remick & Ward, 2005), and stroke (Becker et al., 2005; Doll et al., 2015; Shim & Wong, 2016). This far‐from‐exhaustive list illustrates well the generality of this phenomenon. In cases of stroke, infection is very common, and leads to a worse prognosis; in some cases antibiotics worsen it further (Becker et al., 2016), consistent with the view that the infecting organisms were already present, and that there is an active role of LPS shedding. We note too that some molecules such as P‐type inositol phosphate glycans can act as LPS mimics (Robillard et al., 2016). This is especially well established in pre‐eclampsia (e.g. Dawonauth et al., 2014; Kenny & Kell, 2018; Robillard et al., 2016; Scioscia et al., 2012, 2011; Williams et al., 2007) but seems to have been little investigated elsewhere.
Consistent with the above (and see de Punder & Pruimboom, 2015; Kell & Pretorius, 2015a), Table 3 lists a variety of ‘natural’ (i.e. non‐experimental) chronic inflammatory diseases for which it has been shown that steady‐state endotoxin (LPS) levels are raised and Table 4 presents examples of diseases in which raised levels of lipopolysaccharide binding protein (LBP) have been observed.
Table 3.
Diseases in which levels of lipopolysaccharide (LPS; endotoxin) are higher in patients than in matched controls
| Disease | Comments | Selected references |
|---|---|---|
| Alzheimer's disease | At sites of central nervous system (CNS) lesions | Bester et al. (2015); Poole et al. (2013); Zhan et al. (2016) |
| Amyotrophic lateral sclerosis | Zhang et al. (2009) | |
| Atherosclerosis | Kiechl et al. (2001); Ostos et al. (2002); Stoll et al. (2004) | |
| Cancer | Tumours contained high levels of bacteria and LPS | Cummins & Tangney (2013); Geller et al. (2017) |
| Type 2 diabetes | Also bound up with amylin | Andreasen et al. (2010); Cani et al. (2012); Chen et al. (2016); de Kort et al. (2011); Jayashree et al. (2014); Miklossy et al. (2008); Pussinen et al. (2011); Vergès et al. (2014) |
| Multiple sclerosis | Ballerini et al. (2017); Escribano et al. (2017) | |
| Oxidative damage | Duvigneau et al. (2008); Escribano et al. (2017); Li et al. (2016); Ozdemir et al. (2007); Ritter et al. (2006) | |
| Parkinson's disease | Chang & Li (2011); Chen et al. (2018); Forsyth et al. (2011); Girard‐Joyal & Ismail (2017); Harris et al. (2012); He et al. (2013); Hoban et al. (2013); Kelly et al. (2014); Kim et al. (2016) |
Table 4.
Examples of diseases in which raised lipopolysaccharide binding protein (LBP) levels have been observed
| Disease | Comments | Selected references |
|---|---|---|
| Atherosclerosis | Lepper et al. (2011, 2007); Serrano et al. (2013); see also Sallam et al. (2014) | |
| Type 2 diabetes | High‐fat diet induction and correlation with obesity | Ghanim et al. (2009); Moreno‐Navarrete et al. (2013); Sakura et al. (2017); Sun et al. (2010); Tuomi & Logomarsino (2016) |
| Multiple sclerosis | Escribano et al. (2017) | |
| Parkinson's disease | Forsyth et al. (2011); Pal et al. (2015) | |
| Rheumatoid arthritis | Kim et al. (2018); Wen et al. (2018) |
(2). LTA shedding from Gram‐positive bacteria
Gram‐positive bacteria have a cell wall structure that differs from that of Gram‐negatives both in its number of barriers and in the fact that the cell wall component equivalent to LPS is lipoteichoic acid (LTA). LTA is equivalently capable of producing an inflammatory response. In contrast to LPS, which mainly interacts with toll‐like receptor 4 (TLR4) (Balasubbramanian et al., 2017; Hoshino et al., 1999; Kell & Pretorius, 2015a; Lien et al., 2000; Poltorak et al., 1998), LTA stimulates target cells mainly by activating toll‐like receptor 2 (TLR2) (Ishii & Akira, 2004; Jiménez‐Dalmaroni, Gerswhin & Adamopoulos, 2016; Kawai & Akira, 2011; Kumar, Kawai & Akira, 2011; Kumar et al., 2013; Y. Liu et al., 2014; Mukherjee, Karmakar & Babu, 2016; Oliveira‐Nascimento, Massari & Wetzler, 2012; Schwandner et al., 1999; Underhill et al., 1999; Zähringer et al., 2008). The glycolipid anchor of LTA plays a central role, analogous to lipid A of LPS (Morath, von Aulock & Hartung, 2005).
LTA species have been rather less studied from the point of view of inflammagenesis than have LPS forms, but they clearly reside in the blood and are inflammagens (Barbero‐Becerra et al., 2011; Cinar et al., 2013; Hoogerwerf et al., 2009; Levels et al., 2003; Pirillo, Catapano & Norata, 2015). In some respects (see Section X), LTAs may be even more potent than LPS species (Pretorius et al., 2018a).
X. STEP 5: INDUCTION BY LPS AND LTA OF INFLAMMATORY CYTOKINES
The induction of inflammatory cytokines by LPS and LTA has been reviewed numerous times (e.g. (Kell & Pretorius, 2015a, 2016; Latz, Xiao & Stutz, 2013; O'Neill, Bryant & Doyle, 2009). The basic pathways (Latz et al., 2013; O'Neill et al., 2009) that lead from TLR binding to inflammatory cytokine production are shown in Figs 4 and 5 [reproduced from Kell & Pretorius (2015a) under a CC‐BY license]. They result in increased levels of circulating inflammatory cytokines and other ‘acute phase’ biomarkers, in particular IL‐1β, IL‐6, IL‐8 and tumour necrosis factor α (TNFα) (e.g. Pindjakova et al., 2017; van Rijn et al., 2016). In some cases (e.g IL‐1β), these can serve as ligands that stimulate their own synthesis (Brown et al., 2013; Small et al., 2011). A variety of small‐molecule (Donia & Fischbach, 2015) microbial products besides LPS and LTA, such as long‐ (Schirmer et al., 2016) and short‐chain (Thorburn, Macia & Mackay, 2014) fatty acids, can also lead to or modulate the formation of inflammatory cytokines. A variety of other molecules are markers of systemic inflammation; these include C‐reactive protein, serum amyloid A and fibrinogen (e.g. Bickel et al., 2002; Çetinkaya et al., 2009; Davalos & Akassoglou, 2012; De Buck et al., 2016; deRosset & Strutz, 2015; Hesselink, Aarden & Swaak, 2003; Kaptoge et al., 2012; Ridker & Silvertown, 2008; Song et al., 2006; Yildirim, Hur & Kokturk, 2013) – interestingly all correlate inversely with socioeconomic status (Jousilahti et al., 2003). The role of ferritin, another ‘acute‐phase protein’ synthesised in response to infection/inflammation, has been discussed in detail elsewhere (Kell & Pretorius, 2014).
Figure 4.
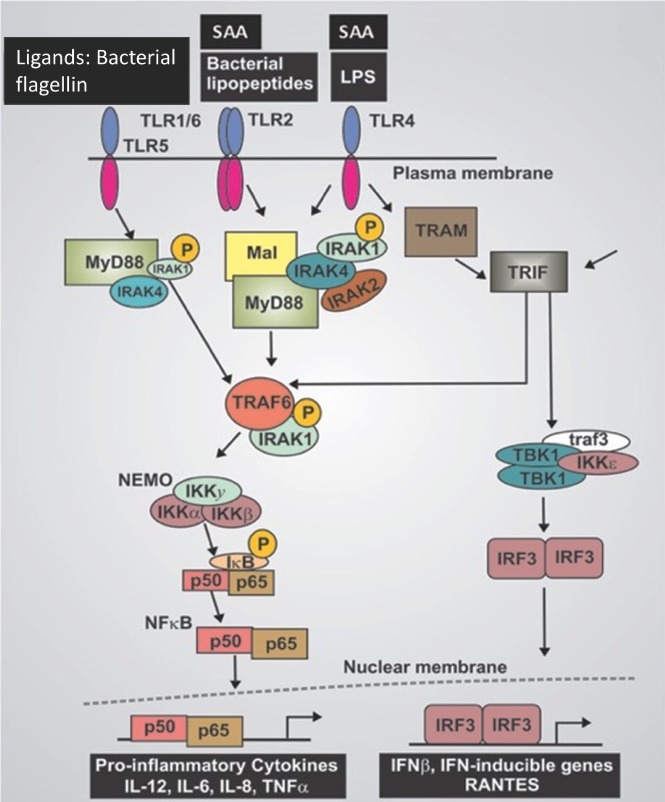
Lipopolysaccharide (LPS)‐ and serum amyloid A (SAA)‐mediated cellular production of inflammatory cytokines. Canonical pathway of LPS‐mediated release and nuclear translocation of nuclear factor‐kappa B (NF‐ κB) (based on O'Neill et al., 2009). IKK, IκB kinase complex; INF, interferon; IRF3, interferon regulatory factor 3; MyD88, myeloid differentiation primary response 88; NEMO, NF‐κB essential modulator; p50, NF‐κB subunit, p50; p65, transcription factor p65 also known as nuclear factor NF‐kappa‐B p65 subunit; RANTES, hemokine (C‐C motif) ligand 5; SAA, Serum amyloid A; TBK1, TANK‐binding kinase 1; TIRF, TIR‐domain‐containing adapter‐inducing interferon‐β; TLR, Toll‐like receptor; TRAF, TNF receptor associated factor; TRAM, TRIF‐related adaptor molecule.
Figure 5.
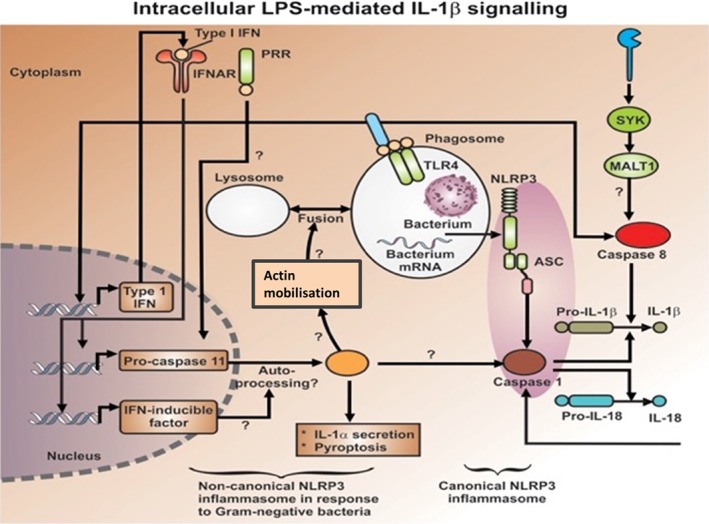
Intracellular lipopolysaccharide (LPS)‐mediated activation of caspase‐1 leading to interleukin 1β (IL‐1β) production (after Latz et al., 2013). ASC, caspase activation and recruitment domain; IL, interleukin; INF, type 1 interferon; INFAR, interferon receptor; MALT1, mucosa‐associated‐lymphoid‐tissue lymphoma‐translocation gene 1; NTLP3, nucleotide‐binding oligomerization domain‐like receptor family, pyrin domain‐containing‐3; PRR, pattern recognition receptor; SYK, spleen tyrosine kinase; TLR4, Toll‐like receptor 4.
XI. STEP 6: INDUCTION OF FIBRIN AMYLOID FORMATION BY ‘IRON’, LPS AND LTA
‘Amyloid’, more specifically an amyloid protein fibril, is defined formally (Sipe et al., 2014, p. 221) as ‘a protein that is deposited as insoluble fibrils, mainly in the extracellular spaces of organs and tissues as a result of sequential changes in protein folding that result in a condition known as amyloidosis’. As with prions (Aguzzi & Lakkaraju, 2016; Kell & Pretorius, 2017a; Prusiner, 1998; Prusiner et al., 2015), there is (or need be) no change in the primary sequence when a normally soluble protein adopts an insoluble amyloid form. Anfinsen's (1973) classical experiments had implied that the primary sequence alone can be sufficient to guide normal folding and that folding was to the state of lowest free energy. The existence of more stable conformations than those first formed upon folding implies, in contrast to this, that there is a large kinetic barrier between the most common conformation and the folded amyloid form(s) of lower free energy (Cohen & Prusiner, 1998) (Fig. 6). As many as 50 ‘amyloid’ diseases are now established (Ankarcrona et al., 2016; Buell, Dobson & Knowles, 2014; Dobson, 2013; Hung et al., 2016; Ke et al., 2017; Kholová & Niessen, 2005; Knowles, Vendruscolo & Dobson, 2014; Siakallis, Tziakouri‐Shiakalli & Georgiades, 2014), in which normally soluble proteins fold to form unusual, insoluble amyloid fibril forms and may become on‐ and off‐pathway oligomers that are particularly important for cytotoxicity (Ke et al., 2017). Their general structural hallmark is a much greater content of β‐sheets than the soluble protein, arranged perpendicular to the fibre axis (Dobson, 2001; Eisenberg & Jucker, 2012; Langkilde et al., 2015; Maji et al., 2009; Makin et al., 2005; Morris & Serpell, 2012; Serpell, 2000; Stromer & Serpell, 2005; Tsemekhman et al., 2007; Tycko & Wickner, 2013). Until recently, their insoluble and polymorphic nature made structural studies difficult (Tycko & Wickner, 2013), but recent advances in solid‐state nuclear magnetic resonance (NMR) have led to a general consensus (Colvin et al., 2016; Meier & Böckmann, 2015; Tycko, 2016; Wälti et al., 2016), at least for the major Aβ peptides. The possibility to form β‐structures in multiple ways underlies the ability of the protein to take different stable conformations (Eichner & Radford, 2011; Eisenberg & Jucker, 2012; Tycko & Wickner, 2013).
Figure 6.
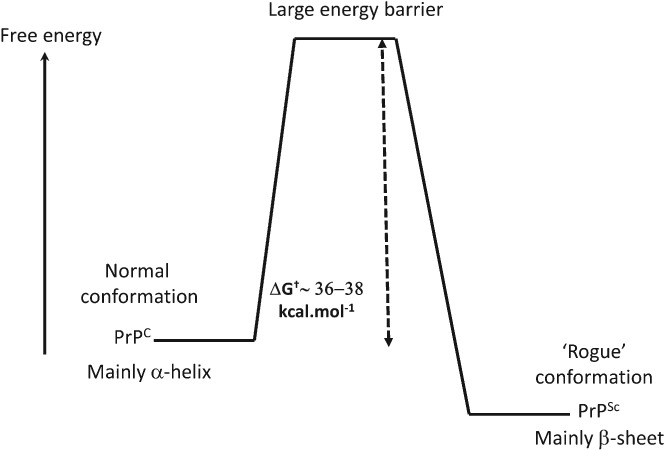
Energy barriers in prion protein formation [based on Cohen & Prusiner (1998) and Kell & Pretorius (2017a)]. Normal cell‐surface glycoprotein: PrPc; prion protein scrapie associated: PRPSC; ΔG† free energy of activation.
Even proteins not normally seen as amyloidogenic or disease‐causing can form amyloids; this is of significance in the storage of biological materials, whose shelf‐life may be shortened as a result [e.g. insulin (Nielsen et al., 2001, b , c ; Wang, 2005)]. A similar phase transition to a β‐form is involved in the action of barnacle glue (Nakano & Kamino, 2015), and bacterial inclusion bodies are largely composed of β‐amyloid (de Groot, Sabate & Ventura, 2009). Consequently, understanding this general phenomenon is also important in the field of recombinant protein production.
Blood clotting provides an interesting and novel example (Fig. 7). Scanning electron microscope (SEM) studies showed that blood or plasma clotted in the presence of unliganded iron (Lipinski & Pretorius, 2013b; Pretorius et al., 2013a, b ), formed ‘dense matted deposits’ rather than the normal spaghetti‐ or noodle‐like structures. Similar structures are seen in a variety of disease conditions (e.g. Kell & Pretorius, 2017a; Lipinski & Pretorius, 2013a, b ; Pretorius, 2011; Pretorius et al., 2011, 2016a, c , 2015, 2014a, 2017b; Pretorius & Kell, 2014; Pretorius & Oberholzer, 2009). Although a rare mutant in the fibrinogen A chain can cause the molecule to become amyloid (Benson et al., 1993; Hamidi Asl et al., 1997; Serpell et al., 2007), it was not thought that normal fibrin(ogen) would undergo this reaction. However, the observed ‘dense matted deposits’ could be stained with amyloid‐selective fluorogenic stains showing that they were in fact amyloid in nature (Kell & Pretorius, 2017a, b ; Pretorius et al., 2016c, 2017c, 2018a,b). This opens up a considerable new biology (Kell & Pretorius, 2015b). A particular feature was that this amyloidogenesis could be induced to occur by the addition of what is stoichiometrically an astonishingly low ratio of bacterial lipopolysaccharide (LPS): fibrinogen, 1:108. Figure 8A and B shows confocal micrographs of healthy (human plasma) before and after exposure to 0.4 ng l−1 LPS, followed by the addition of three fluorescent amyloid markers and thrombin. Figure 8C shows a representative clot, with added fluorescent markers, from a type 2 diabetes individual. A similar fluorescent signal to that of healthy plasma with added LPS is present.
Figure 7.
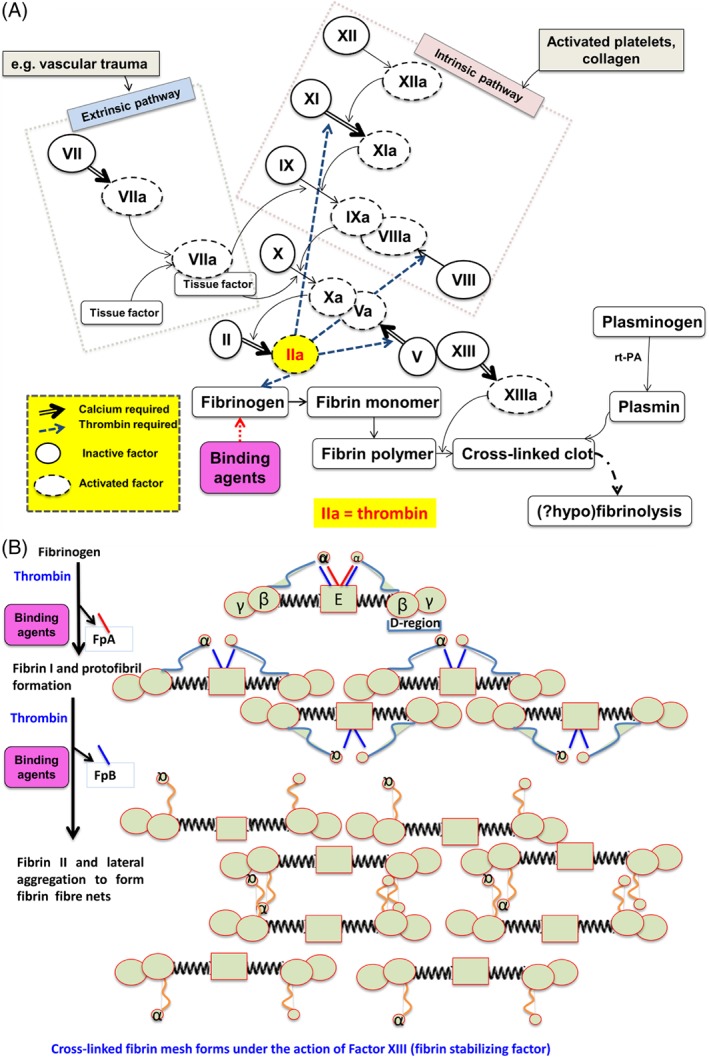
(A) The clotting cascade. Clotting can be activated by either the extrinsic or intrinsic pathway, which converge to a common pathway at factor X, and which ultimately leads to the conversion of prothrombin (factor II) to thrombin that catalyses activation and crosslinking (via factor XIII) of fibrinogen into a fibrin fibre meshwork. Rt‐PA, recombinant tissue plasminogen activator. Redrawn from Kell & Pretorius, 2015b, 2017b). (B) Conversion of soluble fibrinogen molecules to insoluble fibrin fibres during the clotting process (adapted from Kell & Pretorius, 2015b). Fibrinopeptide A and B: FpA and FpB.
Figure 8.
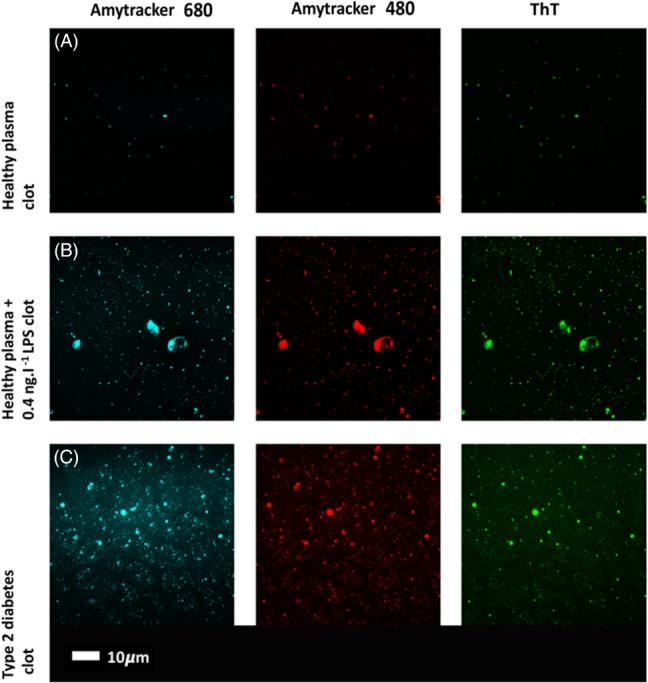
Confocal micrographs of human plasma with added fluorescent markers: Amytracker 480 (blue), Amytracker 680 (red) and Thioflavin T (ThT, green), followed by thrombin to create a fibrin clot. (A) Healthy plasma, (B) healthy plasma after exposure to 0.4 ng l−1 lipopolysaccharide (LPS) (Pretorius et al., 2016c); (C) plasma from a patient with type 2 diabetes (Pretorius et al., 2017c).
As with prions, however, thermodynamics is not an issue (the starting structures are metastable, and the adoption by one protein molecule of an unusual conformation may effectively ‘force’ other molecules of the same type to adjust their conformation. Indeed, one molecule of LPS is sufficient to change the optical properties of millions of molecules of nematic liquid crystal (Lin et al., 2011). LPS may also drive the conversion of prions into their amyloid form (Saleem et al., 2014). Finally (see Fig. 8), the amyloid structures formed from a given amyloidogenic protein (e.g. fibrinogen) can be highly heterogeneous (Annamalai et al., 2016).
(1). Co‐amyloid formation by fibrin(ogen) and other amyloids
There is considerable evidence that fibrin(ogen) can interact with other amyloid structures (Young et al., 2017). The conformation of the fibrin(ogen) involved is unknown, but we suggest that it is almost certainly amyloid as well. Recent studies (e.g. Ahn et al., 2017, 2014, 2010; Cortes‐Canteli et al., 2010, 2012; Cortes‐Canteli & Strickland, 2009; Zamolodchikov et al., 2016; Zamolodchikov & Strickland, 2012) have highlighted its interaction with Aβ peptides in Alzheimer's disease. Here, it is important to recognise that the faster kinetics of a given amyloidogenic process (such as fibrin formation) might accelerate the kinetics of a different amyloid with which it happens to interact, and that this could have important implications for the initiation of overt disease.
Serum amyloid A (SAA) is also an important and potent amyloid. SAA belongs to a family of apolipoproteins associated with high‐density lipoprotein (HDL) in plasma and is an acute‐phase protein synthesised predominantly by the liver (Eklund, Niemi & Kovanen, 2012; Hua et al., 2009; Zewinger et al., 2015). SAA modulates angiogenesis in many diseases (Lv et al., 2016) and is associated with an increase in thrombotic risk (Vitale et al., 2014). Traditionally, SAA has been considered to have a key role in the pathogenesis of amyloid A‐type amyloidosis, but it is now known to play a major role in the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis and atherosclerosis (Eklund et al., 2012). SAA has also been found within thrombus material and at sites of ruptured plaques (King, Thompson & Tannock, 2011). Interestingly, SAA expression increases markedly during bacterial infection, tissue damage, and inflammation (Lannergård et al., 2008; Li, Ooi & Heng, 2013). During acute inflammation, serum SAA levels may rise up to 1000‐fold, and under these conditions, SAA displaces apolipoprotein A‐I from HDL, thus becoming the major apolipoprotein of circulating HDL3 (Eklund et al., 2012). SAA induces the synthesis of several cytokines by binding to and activating cell‐surface receptors, including TLR2 and TLR4, formyl peptide receptor‐like 1 (FPRL1), class B scavenger receptor cluster of differentiation 36 (CD36), and the ATP receptor P2X purinoceptor 7 (P2X7). SAA also activates the inflammasome cascade, which has a key role in immune activation, and has an important role in immunomodulation (Eklund et al., 2012). The G‐coupled FPRL‐1 has been demonstrated to mediate SAA‐induced chemotaxis and cytokine release in neutrophils while TLR2 and TLR4 have been identified as novel SAA receptors mediating activities such as pro‐inflammatory cytokine expression in macrophages (Chami et al., 2015). SAA also mediates TLR2, and nitric oxide (NO) production via mitogen activated protein kinase (MAPK)/ERK signalling pathways in macrophages and TLR4SAA seems to be a ligand for the receptor for advanced glycation end products (RAGE) (Chami et al., 2015). Pro‐inflammatory and pro‐thrombotic mediators that are expressed in the presence of SAA include intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), IL‐6, IL‐8, monocyte chemotactic protein 1 (MCP‐1) and tissue factor (TF) (Chami et al., 2015). SAA can also stimulate vascular cells to express cytokines, chemokines, adhesion molecules and matrix metalloproteinases which are linked to the development of atherosclerosis (King et al., 2011).
SAA has also been detected within atherosclerotic lesions and within adipose tissue where it is hypothesised that it may play a contributory role in disease development. In the acute‐phase response, SAA is synthesised by the liver and transported primarily in association with HDL (King et al., 2011). However, there might also be localised synthesis of SAA within the vasculature or adipose tissue, where it may play a distinct role in disease development (King et al., 2011). Furthermore, SAA can be found in association with apolipoprotein B (apoB)‐containing lipoproteins, in which its biological activity may be different (King et al., 2011). Figure 4 includes a brief overview of the activities of SAA when it binds to TLR2 and TLR4.
Although very little information is available regarding the interplay between LPS and SAA, one study suggested that human hepatocytes stimulated by LPS produced SAA (Migita et al., 2004). It is well known that SAA has a pro‐thrombotic nature and upregulates a plethora of cytokines (Chami et al., 2015). It also interferes with platelet function (Lakota et al., 2011) by inhibiting platelet aggregation and modulating platelet adhesion (Sayinalp et al., 2004). Furthermore, SAA adheres to human platelets at the arginine‐glycine‐aspartic acid (RGD) adhesion motif‐ and platelet integrin αIIbβ3 receptor (also known as platelet glycoprotein GPllb‐Illa); SAA may therefore play a role in modulating platelet adhesion at vascular injury sites by sharing platelet receptors with other platelet‐adhesive proteins (Urieli‐Shoval et al., 2002). SAA consequently plays a fundamental role in creating a pro‐thrombotic environment and hypercoagulation; such an environment is the hallmark of a systemic inflammatory profile.
Many other amyloid proteins can both interact with each other and catalyse further amyloidogenesis (Liu et al., 2007; Lundmark et al., 2005; Westermark, Lundmark & Westermark, 2009), much as with prions (Kell & Pretorius, 2017a). This phenomenon is essentially what makes them possess what amount to transmissible properties (Lundmark et al., 2002; Morales, Callegari & Soto, 2015; Murakami, Ishiguro & Higuchi, 2014; Watts et al., 2014; Westermark & Westermark, 2009; Woerman et al., 2015). Given that the amyloid form of prion can catalyse its own production, there is now a developing acceptance (e.g. Kell & Pretorius, 2017a; Prusiner, 2012) that prion‐like behaviour and amyloidogenesis are simply two parts of a more general phenomenon. Another consequence is that amyloids can bind molecules such as LPS (Kumar et al., 2016).
XII. STEP 7: DIRECT INDUCTION OF CELL DEATH BY LPS
As well as its role in inducing inflammatory cytokine production, there is some evidence that LPS, albeit commonly bound to proteins that can sequester it, is itself directly cytotoxic [reviewed by Kell & Pretorius (2015a) and Williamson et al. (2016)].
XIII. STEP 8: INFLAMMATION INDUCES COAGULOPATHIES AND THESE CONTRIBUTE TO DISEASE
While we have highlighted amyloid formation as a major part of the dysregulation narrative, inflammation necessarily causes coagulopathies, if only because the concentration of fibrinogen involved (typically 1.5–4 g l−1) is associated with a variety of diseases and coagulopathies (Bickel et al., 2002; Danesh et al., 2005; Davalos & Akassoglou, 2012; Green et al., 2010; Zoccali et al., 2003).
A general feature of the blood of patients with these chronic inflammatory diseases is that it is both hypercoagulable and hypofibrinolytic (Kell & Pretorius, 2015b); clots form more easily, are stronger, and are less susceptible to proteolysis. The latter is, of course, a particular hallmark of prions (Basu et al., 2007; Saá & Cervenakova, 2015; Saleem et al., 2014; Silva et al., 2015; Woerman et al., 2018) and of amyloids generally (Rambaran & Serpell, 2008).
The kinetics of the formation of clots can be studied using thromboelastography to measure clot viscoelastic properties like clot coagulation and fibrinolysis (Pretorius et al., 2017d).
XIV. STEP 9: INDUCTION OF CYTOKINE PRODUCTION BY AMYLOID FORMATION AND VICE VERSA
There is a complex interplay (including positive feedback amplification) between inflammation, cytokine production, amyloid formation and disease (see Fig. 2). A variety of amyloid proteins can themselves induce the formation of inflammatory cytokines (e.g. Gallo et al., 2015; Meier et al., 2014; Patel et al., 2005; Spaulding et al., 2015; Westwell‐Roper et al., 2011, 2015; Westwell‐Roper, Ehses & Verchere, 2014; Yates et al., 2000) and vice versa (e.g. Schmidt et al., 2017). A simplified example of the inter‐relationship between cytokines, inflammation and visible changes to RBCs and fibrin(ogen) is shown in Fig. 9. Amyloidogenesis and eryptosis are both hallmarks of inflammation and have been associated with vascular dysfunction. However, there is a complex interaction between dysregulated inflammatory markers and the damaging effects of amyloidogenesis and inflammation, and an elementary one‐way approach to the development of inflammation versus the upregulation of inflammatory markers will be oversimplifying the complex interactions.
Figure 9.
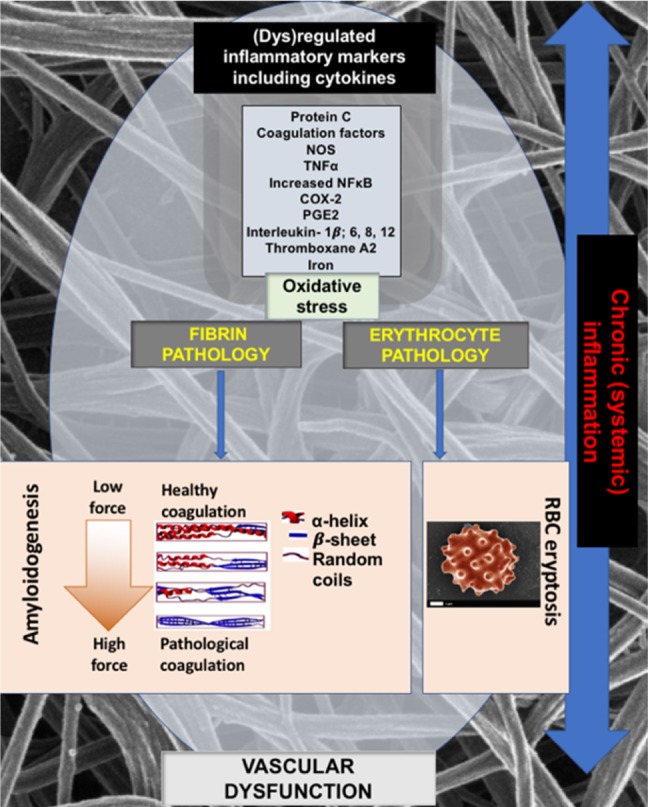
Dysregulation of inflammatory markers, including cytokines and iron, leads to oxidative stress, which in turn causes changes to both fibrin(ogen) and red blood cells (RBCs) visible as amyloidogenesis and eryptosis. Amyloidogenesis and eryptosis both leadsto inflammation but their induction is also enhanced by the presence of inflammation. COX‐2, cyclooxygenase‐2; PGE2, prostaglandin E2; NOS, nitric oxide synthase; TNFα, tumor necrosis factor alpha; thromboxane A2 is a type of thromboxane that is produced by activated platelets.
XV. STEP 10: DIRECT CAUSATION OF DISEASE BY INFLAMMATION?
It is hard to disentangle diseases caused or exacerbated directly by inflammation from those where the mediating agent is explicitly a cytokine. Figure 9 details the complex interactions between dysregulated inflammatory markers as the underlying cause of inflammation but simultaneously subject to inflammation as a catalytic driver of dysregulated inflammatory markers.
XVI. STEP 11: CELL DEATH (HENCE DISEASE) CAUSED BY AMYLOIDS
Induction of cell death will normally cause disease; for example, if the cells in the substantia nigra pars compacta die the patient will develop Parkinson's, and so on. A great many amyloids have been shown to be cytotoxic, and this is why they are considered in detail herein. What is less clear (Uversky, 2010), although a consensus is now emerging, is which particular class (often equivalent to size) of amyloids are particularly cytotoxic, and what causes this cytotoxicity.
The cytotoxicity of amyloids is well known (e.g. Ahmed et al., 2010; Bester et al., 2015; Hefti et al., 2013; Kayed & Lasagna‐Reeves, 2013; Liu et al., 2011; Meyer‐Luehmann et al., 2008; Minter, Taylor & Crack, 2016; Miranda et al., 2000; Rival et al., 2009; Sengupta, Nilson & Kayed, 2016). Interestingly, while larger fibrils are more easily observable microscopically, the modern view is that smaller amyloids [often invisible in conventional SEM, but see Gremer et al. (2017)] are more cytotoxic (Aitken et al., 2010; Baglioni et al., 2006; Bucciantini et al., 2002; Dobson, 2013; Fändrich, 2012; Glabe, 2006; Göransson et al., 2012; Haass & Selkoe, 2007; Janson et al., 1999; Kayed et al., 2003; Ke et al., 2017; Konarkowska et al., 2006; Meier et al., 2006; Pillay & Govender, 2013; Stefani, 2012; Trikha & Jeremic, 2013; Xue et al., 2009; Xue et al., 2010; Zhang et al., 2014). However, it would appear that almost all forms of amyloid are cytotoxic [but see Holm et al. (2007)] and that they may interconvert. Tests have not yet been performed for the recently discovered (Kell & Pretorius, 2017a; Pretorius et al., 2016c, 2018a,b) fibrin amyloid, which is considerably larger in fibre diameter than those involved in classical amyloid diseases (Kell & Pretorius, 2017a).
While multiple interactions and processes are likely to be involved, it does seem that membrane interactions are a key event in initiating cytotoxicity (Berthelot, Cullin & Lecomte, 2013; Cao & Raleigh, 2016; Caughey et al., 2009; Couthouis et al., 2010; Engel et al., 2008; Harté et al., 2014; Jang et al., 2014, 2013; Janson et al., 1999; Kegulian et al., 2015; Lee et al., 2014; Lorenzo et al., 1994; Matsuzaki, 2014; Munishkina & Fink, 2007; Okada et al., 2016; Suwalsky, Bolognin & Zatta, 2009; Ta et al., 2012; Valincius et al., 2008), mainly by apoptosis (e.g. Bram et al., 2014; Chong, Li & Maiese, 2005; Jang et al., 2004; Liu et al., 2012; Lorenzo et al., 1994; Zhang et al., 2010; Zhang et al., 2014).
XVII. HOW GENERAL DO WE CONSIDER THESE MECHANISMS TO BE FOR VARIOUS DISEASES?
The different steps considered herein are entirely generic at a broad level (microbes and their dormant states, iron dysregulation, amyloid formation), with differences only apparent at a finer scale (microbial species and the anatomical location of the various dysregulations). The conditions considered herein are all chronic inflammatory diseases, often with quite slow kinetics, and are all in effect diseases of ageing (e.g. van Beek, Kirkwood & Bassingthwaighte, 2016).
XVIII. CONCLUSIONS
(1) A systems biology strategy was used to show that chronic, inflammatory diseases have many features in common besides simple inflammation.
(2) The physiological state of most microbes in nature is neither ‘alive’ (immediately culturable on media known to support their growth) nor ‘dead’ (incapable of such replication), but dormant.
(3) The inflammatory features of chronic diseases must have external causes, and we suggest that the chief external causes are (i) inoculation by microbes that become and remain dormant, largely because they lack the free iron necessary to replicate, and (ii) traumas that induce cell death and the consequent liberation of free iron; these together are sufficient to initiate replication of the microbes.
(4) This replication is accompanied by the production and shedding of potent inflammagens such as lipopolysaccharide or lipoteichoic acid, and this continuing release explains the presence of chronic, low‐grade inflammation.
(5) Recent findings show that tiny amounts of these inflammagens can cause blood to clot into an amyloid form; such amyloid forms are also capable of inducing cell death and thereby exacerbating the release of iron.
(6) Additional to the formal literature that we have reviewed here, it seems to be commonly known that infection is in fact the proximal cause of death in Alzheimer's, Parkinson's, rheumatoid arthritis, multiple sclerosis, etc. It may, for instance, be brought on by the trauma experienced following a fall. Such infections leading to death in chronically ill patients may involve the re‐awakening of dormant bacteria rather than novel exogenous infection. This implies that therapies involving the careful use of anti‐infectives active against dormant microbes could be effective (Coates, Halls & Hu, 2011; Coates & Hu, 2006), as well as the use of nutritional iron chelators (Kell, 2009; Perron & Brumaghim, 2009; Perron et al., 2010).
(7) The role of microbes in stomach ulcers is now well established (Marshall, 2002a, b , 2003, 2006); here we add to the list of supposedly non‐communicable diseases that can be shown to have a microbial component in their aetiology.
XIX. ACKNOWLEDGEMENTS
This is paper 15 in the series ‘a dormant blood microbiome in chronic, inflammatory diseases.’ We thank the Biotechnology and Biological Sciences Research Council (grant BB/L025752/1) as well as the National Research Foundation (NRF) of South Africa for supporting this collaboration. We also thank Martin Page and Andrew Doig for critical reading of the manuscript.
XX. REFERENCES
- Aagaard, K. , Ma, J. , Antony, K. M. , Ganu, R. , Petrosino, J. & Versalovic, J. (2014). The placenta harbors a unique microbiome. Science Translational Medicine 6, 237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, S. , Bojesen, S. E. & Nordestgaard, B. G. (2013). Low 25‐hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clinical Chemistry 59, 381–391. [DOI] [PubMed] [Google Scholar]
- Afzal, S. , Bojesen, S. E. & Nordestgaard, B. G. (2014). Reduced 25‐hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimer's & Dementia 10, 296–302. [DOI] [PubMed] [Google Scholar]
- Afzal, S. , Brøndum‐Jacobsen, P. , Bojesen, S. E. & Nordestgaard, B. G. (2014b). Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. British Medical Journal 349, g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, S. , Brøndum‐Jacobsen, P. , Bojesen, S. E. & Nordestgaard, B. G. (2014c). Vitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation study. Lancet Diabetes & Endocrinology 2, 298–306. [DOI] [PubMed] [Google Scholar]
- Agustí, A. & Faner, R. (2012). Systemic inflammation and comorbidities in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 9, 43–46. [DOI] [PubMed] [Google Scholar]
- Aguzzi, A. & Lakkaraju, A. K. K. (2016). Cell biology of prions and prionoids: a status report. Trends in Cell Biology 26, 40–51. [DOI] [PubMed] [Google Scholar]
- Ahmed, M. , Davis, J. , Aucoin, D. , Sato, T. , Ahuja, S. , Aimoto, S. , Elliott, J. I. , Van Nostrand, W. E. & Smith, S. O. (2010). Structural conversion of neurotoxic amyloid‐beta1‐42 oligomers to fibrils. Nature Structural & Molecular Biology 17, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, H. J. , Chen, Z. L. , Zamolodchikov, D. , Norris, E. H. & Strickland, S. (2017). Interactions of beta‐amyloid peptide with fibrinogen and coagulation factor XII may contribute to Alzheimer's disease. Current Opinion in Hematology 24, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, H. J. , Glickman, J. F. , Poon, K. L. , Zamolodchikov, D. , Jno‐Charles, O. C. , Norris, E. H. & Strickland, S. (2014). A novel Abeta‐fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer's disease mice. Journal of Experimental Medicine 211, 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, H. J. , Zamolodchikov, D. , Cortes‐Canteli, M. , Norris, E. H. , Glickman, J. F. & Strickland, S. (2010). Alzheimer's disease peptide beta‐amyloid interacts with fibrinogen and induces its oligomerization. Proceedings of the National Academy of Sciences of the United States of America 107, 21812–21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken, J. F. , Loomes, K. M. , Scott, D. W. , Reddy, S. , Phillips, A. R. J. , Prijic, G. , Fernando, C. , Zhang, S. , Broadhurst, R. , L'Huillier, P. & Cooper, G. J. S. (2010). Tetracycline treatment retards the onset and slows the progression of diabetes in human amylin/islet amyloid polypeptide transgenic mice. Diabetes 59, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, Â. , Estanqueiro, P. & Salgado, M. (2016). The Jarisch‐Herxheimer Reaction and Brucellosis. Pediatric Infectious Disease Journal 35, 466. [DOI] [PubMed] [Google Scholar]
- Alnimr, A. M. (2015). Dormancy models for Mycobacterium tuberculosis: a minireview. Brazilian Journal of Microbiology 46, 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon, U. (2006). An Introduction To Systems Biology: Design Principles Of Biological Circuits. Chapman and Hall/CRC, London. [Google Scholar]
- Alonso, C. , Vicario, M. , Pigrau, M. , Lobo, B. & Santos, J. (2014). Intestinal barrier function and the brain‐gut axis. Advances in Experimental Medicine & Biology 817, 73–113. [DOI] [PubMed] [Google Scholar]
- Alonso, R. , Pisa, D. , Aguado, B. & Carrasco, L. (2017). Identification of fungal species in brain tissue from Alzheimer's disease by next‐generation sequencing. Journal of Alzheimers Disease 58, 55–67. [DOI] [PubMed] [Google Scholar]
- Altamura, S. , Kopf, S. , Schmidt, J. , Müdder, K. , da Silva, A. R. , Nawroth, P. & Muckenthaler, M. U. (2017). Uncoupled iron homeostasis in type 2 diabetes mellitus. Journal of Molecular Medicine 95, 1387–1398. [DOI] [PubMed] [Google Scholar]
- Altamura, S. & Muckenthaler, M. U. (2009). Iron toxicity in diseases of aging: Alzheimer's disease, Parkinson's disease and atherosclerosis. Journal of Alzheimers Disease 16, 879–895. [DOI] [PubMed] [Google Scholar]
- Amar, J. , Serino, M. , Lange, C. , Chabo, C. , Iacovoni, J. , Mondot, S. , Lepage, P. , Klopp, C. , Mariette, J. , Bouchez, O. , Perez, L. , Courtney, M. , Marre, M. , Klopp, P. , Lantieri, O. , et al. (2011). Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia 54, 3055–3061. [DOI] [PubMed] [Google Scholar]
- Amarasekara, R. , Jayasekara, R. W. , Senanayake, H. & Dissanayake, V. H. W. (2015). Microbiome of the placenta in pre‐eclampsia supports the role of bacteria in the multifactorial cause of pre‐eclampsia. Journal of Obstetrics and Gynaecology Research 41, 662–669. [DOI] [PubMed] [Google Scholar]
- Ambachew, S. & Biadgo, B. (2017). Hepcidin in iron homeostasis: diagnostic and therapeutic implications in type 2 diabetes mellitus patients. Acta Haematologica 138, 183–193. [DOI] [PubMed] [Google Scholar]
- Anami, Y. , Itoh, T. , Egawa, D. , Yoshimoto, N. & Yamamoto, K. (2014). A mixed population of antagonist and agonist binding conformers in a single crystal explains partial agonism against vitamin D receptor: active vitamin D analogues with 22R‐alkyl group. Journal of Medicinal Chemistry 57, 4351–4367. [DOI] [PubMed] [Google Scholar]
- Anderson, G. J. & Wang, F. (2012). Essential but toxic: controlling the flux of iron in the body. Clinical and Experimental Pharmacology and Physiology 39, 719–724. [DOI] [PubMed] [Google Scholar]
- Andreasen, A. S. , Pedersen‐Skovsgaard, T. , Berg, R. M. , Svendsen, K. D. , Feldt‐Rasmussen, B. , Pedersen, B. K. & Møller, K. (2010). Type 2 diabetes mellitus is associated with impaired cytokine response and adhesion molecule expression in human endotoxemia. Intensive Care Medicine 36, 1548–1555. [DOI] [PubMed] [Google Scholar]
- Anfinsen, C. B. (1973). Principles that govern the folding of protein chains. Science 181, 223–230. [DOI] [PubMed] [Google Scholar]
- Ankarcrona, M. , Winblad, B. , Monteiro, C. , Fearns, C. , Powers, E. T. , Johansson, J. , Westermark, G. T. , Presto, J. , Ericzon, B. G. & Kelly, J. W. (2016). Current and future treatment of amyloid diseases. Journal of Internal Medicine 280, 177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai, K. , Gührs, K. H. , Koehler, R. , Schmidt, M. , Michel, H. , Loos, C. , Gaffney, P. M. , Sigurdson, C. J. , Hegenbart, U. , Schönland, S. & Fändrich, M. (2016). Polymorphism of amyloid fibrils in vivo . Angewandte Chemie International Edition 55, 4822–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler, C. , Rolland, Y. , Schott, A. M. , Blain, H. , Vellas, B. , Herrmann, F. R. & Beauchet, O. (2012). Higher vitamin D dietary intake is associated with lower risk of Alzheimer's Disease: a 7‐year follow‐up. Journal of Gerontology: Series A 67, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Antonelli, G. & Cutler, S. (2016). Evolution of the Koch postulates: towards a 21st‐century understanding of microbial infection. Clinical Microbiology and Infection 22, 583–584. [DOI] [PubMed] [Google Scholar]
- Antony, K. M. , Ma, J. , Mitchell, K. B. , Racusin, D. A. , Versalovic, J. & Aagaard, K. (2015). The preterm placental microbiome varies in association with excess maternal gestational weight gain. American Journal of Obstetrics & Gynecology 212, 653 e1–653 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzovino, A. , Lane, D. J. , Huang, M. L. & Richardson, D. R. (2014). Fixing frataxin: 'ironing out' the metabolic defect in Friedreich's ataxia. British Journal of Pharmacology 171, 2174–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arleevskaya, M. I. , Kravtsova, O. A. , Lemerle, J. , Renaudineau, Y. & Tsibulkin, A. P. (2016). How rheumatoid arthritis can result from provocation of the immune system by microorganisms and viruses. Frontiers in Microbiology 7, 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengou, A. & Davalos, A. (2002). A review of the state of research into the role of iron in stroke. Journal of Nutritional Health & Aging 6, 207–208. [PubMed] [Google Scholar]
- Armitage, A. E. & Drakesmith, H. (2014). The battle for iron. Science 346, 1299–1300. [DOI] [PubMed] [Google Scholar]
- Arnson, Y. , Amital, H. & Shoenfeld, Y. (2007). Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Annals of the Rheumatic Diseases 66, 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio, P. , Yokota, M. & Drysdale, J. W. (1977). Characterization of serum ferritin in iron overload ‐ possible identity to natural apoferritin. British Journal of Haematology 36, 199–207. [DOI] [PubMed] [Google Scholar]
- Ashall, L. , Horton, C. A. , Nelson, D. E. , Paszek, P. , Ryan, S. , Sillitoe, K. , Harper, C. V. , Spiller, D. G. , Unitt, J. F. , Broomhead, D. S. , Kell, D. B. , Rand, D. , Sée, V. & White, M. R. H. (2009). Pulsatile stimulation determines timing and specificity of NFkappa‐B‐dependent transcription. Science 324, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian, H. (2003). Hepcidin: the missing link between hemochromatosis and infections. Infection & Immunity 71, 6693–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth, I. B. (2016). The microbiome in health and disease: a new role of microbes in molecular medicine. Journal of Molecular Medicine 95, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala, A. , Muñoz, M. F. & Argüelles, S. (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4‐hydroxy‐2‐nonenal. Oxidative Medicine and Cellular Longevity 2014, 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres, J. S. (2016). Cooperative microbial tolerance behaviors in host‐microbiota mutualism. Cell 165, 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres, J. S. & Schneider, D. S. (2012). Tolerance of infections. Annual Review of Immunology 30, 271–294. [DOI] [PubMed] [Google Scholar]
- Ayton, S. , Faux, N. G. , Bush, A. I. & Alzheimer's Disease Neuroimaging Initiative (2015). Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nature Communications 6, 6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton, S. , James, S. A. & Bush, A. I. (2017). Nanoscale imaging reveals big role for iron in Alzheimer's Disease. Cell Chemical Biology 24, 1192–1194. [DOI] [PubMed] [Google Scholar]
- Bacchetta, J. , Chun, R. F. , Gales, B. , Zaritsky, J. J. , Leroy, S. , Wesseling‐Perry, K. , Boregaard, N. , Rastogi, A. , Salusky, I. B. & Hewison, M. (2014). Antibacterial responses by peritoneal macrophages are enhanced following vitamin D supplementation. PLoS One 9, e116530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta, J. , Zaritsky, J. J. , Sea, J. L. , Chun, R. F. , Lisse, T. S. , Zavala, K. , Nayak, A. , Wesseling‐Perry, K. , Westerman, M. , Hollis, B. W. , Salusky, I. B. & Hewison, M. (2014). Suppression of iron‐regulatory hepcidin by vitamin D. Journal of the American Society of Nephrology 25, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni, S. , Casamenti, F. , Bucciantini, M. , Luheshi, L. M. , Taddei, N. , Chiti, F. , Dobson, C. M. & Stefani, M. (2006). Prefibrillar amyloid aggregates could be generic toxins in higher organisms. Journal of Neuroscience 26, 8160–8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J. F. & Ghio, A. J. (2009). Iron homoeostasis in rheumatic disease. Rheumatology (Oxford) 48, 1339–1344. [DOI] [PubMed] [Google Scholar]
- Balaban, N. Q. , Gerdes, K. , Lewis, K. & McKinney, J. D. (2013). A problem of persistence: still more questions than answers? Nature Reviews Microbiology 11, 587–591. [DOI] [PubMed] [Google Scholar]
- Balasubbramanian, D. , Gelston, C. A. L. , Mitchell, B. M. & Chatterjee, P. (2017). Toll‐like receptor activation, vascular endothelial function, and hypertensive disorders of pregnancy. Pharmacological Research 121, 14–21. [DOI] [PubMed] [Google Scholar]
- Ballerini, P. , Diomede, F. , Petragnani, N. , Cicchitti, S. , Merciaro, I. , Cavalcanti, M. & Trubiani, O. (2017). Conditioned medium from relapsing‐remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS‐gingivalis in THP‐1 and MO3.13 cell lines. Cytokine 96, 261–272. [DOI] [PubMed] [Google Scholar]
- Banerjee, A. , Khemka, V. K. , Ganguly, A. , Roy, D. , Ganguly, U. & Chakrabarti, S. (2015). Vitamin D and Alzheimer's Disease: neurocognition to Therapeutics. International Journal of Alzheimers Disease 2015, 192747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero‐Becerra, V. J. , Gutiérrez‐Ruiz, M. C. , Maldonado‐Bernal, C. , Téllez‐Avila, F. I. , Alfaro‐Lara, R. & Vargas‐Vorácková, F. (2011). Vigorous, but differential mononuclear cell response of cirrhotic patients to bacterial ligands. World Journal of Gastroenterology 17, 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham, K. J. & Bush, A. I. (2008). Metals in Alzheimer's and Parkinson's diseases. Current Opinion in Chemical Biology 12, 222–228. [DOI] [PubMed] [Google Scholar]
- Barnum, C. J. & Tansey, M. G. (2010). Modeling neuroinflammatory pathogenesis of Parkinson's disease. Progress in Brain Research 184, 113–132. [DOI] [PubMed] [Google Scholar]
- Barry, C. E. III , Boshoff, H. I. , Dartois, V. , Dick, T. , Ehrt, S. , Flynn, J. , Schnappinger, D. , Wilkinson, R. J. & Young, D. (2009). The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature Reviews Microbiology 7, 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley, J. (2010a). Vitamin D: emerging roles in infection and immunity. Expert Review of Anti Infective Therapy 8, 1359–1369. [DOI] [PubMed] [Google Scholar]
- Bartley, J. (2010b). Vitamin D, innate immunity and upper respiratory tract infection. Journal of Laryngology & Otology 124, 465–469. [DOI] [PubMed] [Google Scholar]
- Barton, J. C. & Acton, R. T. (2009). Hemochromatosis and Vibrio vulnificus wound infections. Journal of Clinical Gastroenterology 43, 890–893. [DOI] [PubMed] [Google Scholar]
- Bassis, C. M. , Erb‐Downward, J. R. , Dickson, R. P. , Freeman, C. M. , Schmidt, T. M. , Young, V. B. , Beck, J. M. , Curtis, J. L. & Huffnagle, G. B. (2015). Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 6, e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S. , Mohan, M. L. , Luo, X. , Kundu, B. , Kong, Q. & Singh, N. (2007). Modulation of proteinase K‐resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis. Molecular Biology of the Cell 18, 3302–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basuli, D. , Stevens, R. G. , Torti, F. M. & Torti, S. V. (2014). Epidemiological associations between iron and cardiovascular disease and diabetes. Frontiers in Pharmacology 5, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, K. J. , Kindrick, D. L. , Lester, M. P. , Shea, C. & Ye, Z. C. (2005). Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. Journal of Cerebral Blood Flow & Metabolism 25, 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, K. J. , Zierath, D. , Kunze, A. , Fecteau, L. , Lee, B. & Skerrett, S. (2016). The contribution of antibiotics, pneumonia and the immune response to stroke outcome. Journal of Neuroimmunology 295‐296, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaidi, A. A. & Bush, A. I. (2016). Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. Journal of Neurochemistry 139(Suppl. 1), 179–197. [DOI] [PubMed] [Google Scholar]
- Bell, N. H. , Shaw, S. & Turner, R. T. (1984). Evidence that 1,25‐dihydroxyvitamin D3 inhibits the hepatic production of 25‐hydroxyvitamin D in man. Journal of Clinical Investigation 74, 1540–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belum, G. R. , Belum, V. R. , Chaitanya Arudra, S. K. & Reddy, B. S. (2013). The Jarisch‐Herxheimer reaction: revisited. Travel Medicine & Infectious Disease 11, 231–237. [DOI] [PubMed] [Google Scholar]
- Benson, M. D. , Liepnieks, J. , Uemichi, T. , Wheeler, G. & Correa, R. (1993). Hereditary renal amyloidosis associated with a mutant fibrinogen alpha‐chain. Nature Genetics 3, 252–255. [DOI] [PubMed] [Google Scholar]
- Ben‐Tekaya, H. , Gorvel, J. P. & Dehio, C. (2013). Bartonella and Brucella ‐‐ weapons and strategies for stealth attack. Cold Spring Harbor Perspectives in Medicine 3, a010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley, S. D. , Maiwald, M. , Murphy, L. D. , Pallen, M. J. , Yeats, C. A. , Dover, L. G. , Norbertczak, H. T. , Besra, G. S. , Quail, M. A. , Harris, D. E. , von Herbay, A. , Goble, A. , Rutter, S. , Squares, R. , Squares, S. , Barrell, B. G. , Parkhill, J. & Relman, D. A. (2003). Sequencing and analysis of the genome of the Whipple's disease bacterium Tropheryma whipplei. Lancet 361, 637–644. [DOI] [PubMed] [Google Scholar]
- Berg, D. (2007). Disturbance of iron metabolism as a contributing factor to SN hyperechogenicity in Parkinson's disease: implications for idiopathic and monogenetic forms. Neurochemical Research 32, 1646–1654. [DOI] [PubMed] [Google Scholar]
- Berg, D. & Youdim, M. B. H. (2006). Role of iron in neurodegenerative disorders. Topics in Magnetic Resonance Imaging 17, 5–17. [DOI] [PubMed] [Google Scholar]
- Berkovitz, S. , Ambler, G. , Jenkins, M. & Thurgood, S. (2009). Serum 25‐hydroxy vitamin D levels in chronic fatigue syndrome: a retrospective survey. International Journal for Vitamin and Nutrition Research 79, 250–254. [DOI] [PubMed] [Google Scholar]
- Berstad, K. & Berstad, J. E. R. (2017). Parkinson's disease; the hibernating spore hypothesis. Medical Hypotheses 104, 48–53. [DOI] [PubMed] [Google Scholar]
- Berthelot, K. , Cullin, C. & Lecomte, S. (2013). What does make an amyloid toxic: morphology, structure or interaction with membrane? Biochimie 95, 12–19. [DOI] [PubMed] [Google Scholar]
- Bester, J. & Pretorius, E. (2016). Effects of IL‐1beta, IL‐6 and IL‐8 on erythrocytes, platelets and clot viscoelasticity. Scientific Reports 6, 32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester, J. , Soma, P. , Kell, D. B. & Pretorius, E. (2015). Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer‐type dementia, and the possible role of bacterial lipopolysaccharides (LPS). Oncotarget Gerontology 6, 35284–35303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge, L. A. & Witham, M. D. (2013). Vitamin D and the cardiovascular system. Osteoporosis International 24, 2167–2180. [DOI] [PubMed] [Google Scholar]
- Bhanji, S. , Williams, B. , Sheller, B. , Elwood, T. & Mancl, L. (2002). Transient bacteremia induced by toothbrushing a comparison of the Sonicare toothbrush with a conventional toothbrush. Pediatric Dental Journal 24, 295–299. [PubMed] [Google Scholar]
- Bickel, C. , Rupprecht, H. J. , Blankenberg, S. , Espiniola‐Klein, C. , Schlitt, A. , Rippin, G. , Hafner, G. , Treude, R. , Othman, H. , Hofmann, K. P. , Meyer, J. & AtheroGene Investigators (2002). Relation of markers of inflammation (C‐reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long‐term mortality in patients with angiographically proven coronary artery disease. American Journal of Cardiology 89, 901–908. [DOI] [PubMed] [Google Scholar]
- Bikle, D. D. (2009). Vitamin D and immune function: understanding common pathways. Current Osteoporosis Reports 7, 58–63. [DOI] [PubMed] [Google Scholar]
- Bikle, D. D. (2011). The vitamin D receptor: a tumor suppressor in skin. Discovery Medicine 11, 7–17. [PMC free article] [PubMed] [Google Scholar]
- Bingham, C. O. III & Moni, M. (2013). Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Current Opinion in Rheumatology 25, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissinger, R. , Modicano, P. , Frauenfeld, L. , Lang, E. , Jacobi, J. , Faggio, C. & Lang, F. (2013). Estramustine‐induced suicidal erythrocyte death. Cellular Physiology and Biochemistry 32, 1426–1436. [DOI] [PubMed] [Google Scholar]
- Bjelakovic, G. , Gluud, L. L. , Nikolova, D. , Whitfield, K. , Krstic, G. , Wetterslev, J. & Gluud, C. (2014a). Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 6, CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic, G. , Gluud, L. L. , Nikolova, D. , Whitfield, K. , Wetterslev, J. , Simonetti, R. G. , Bjelakovic, M. & Gluud, C. (2014b). Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 1, CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blango, M. G. & Mulvey, M. A. (2010). Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrobial Agents and Chemotherapy 54, 1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blango, M. G. , Ott, E. M. , Erman, A. , Veranic, P. & Mulvey, M. A. (2014). Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One 9, e93327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanova, L. (2011). Role of Helicobacter pylori virulence factors for iron acquisition from gastric epithelial cells of the host and impact on bacterial colonization. Future Microbiology 6, 843–846. [DOI] [PubMed] [Google Scholar]
- Bram, Y. , Frydman‐Marom, A. , Yanai, I. , Gilead, S. , Shaltiel‐Karyo, R. , Amdursky, N. & Gazit, E. (2014). Apoptosis induced by islet amyloid polypeptide soluble oligomers is neutralized by diabetes‐associated specific antibodies. Scientific Reports 4, 4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar, S. , Henderson, D. , Schenck, J. & Zimmerman, E. A. (2009). Iron accumulation in the substantia nigra of patients with Alzheimer disease and parkinsonism. Archives of Neurology 66, 371–374. [DOI] [PubMed] [Google Scholar]
- Braun, V. (2005). Bacterial iron transport related to virulence. Contributions to Microbiology 12, 210–233. [DOI] [PubMed] [Google Scholar]
- Brøndum‐Jacobsen, P. , Benn, M. , Afzal, S. & Nordestgaard, B. G. (2015). No evidence that genetically reduced 25‐hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. International Journal of Epidemiology 44, 651–661. [DOI] [PubMed] [Google Scholar]
- Brøndum‐Jacobsen, P. , Benn, M. , Jensen, G. B. & Nordestgaard, B. G. (2012). 25‐hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population‐based study and meta‐analyses of 18 and 17 studies. Arteriosclerosis, Thrombosis & Vascular Biology 32, 2794–2802. [DOI] [PubMed] [Google Scholar]
- Brøndum‐Jacobsen, P. , Benn, M. , Tybjærg‐Hansen, A. & Nordestgaard, B. G. (2013). 25‐Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. Journal of Thrombosis and Haemostasis 11, 423–431. [DOI] [PubMed] [Google Scholar]
- Brøndum‐Jacobsen, P. , Nordestgaard, B. G. , Schnohr, P. & Benn, M. (2013). 25‐hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta‐analysis. Annals of Neurology 73, 38–47. [DOI] [PubMed] [Google Scholar]
- Brown, G. T. , Narayanan, P. , Li, W. , Silverstein, R. L. & McIntyre, T. M. (2013). Lipopolysaccharide stimulates platelets through an IL‐1beta autocrine loop. Journal of Immunology 191, 5196–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer, L. (2017a). Dr. Oskar Fischer's Curious Little Alzheimer's Germ. Scientia Ricerca 1, 160–178. [Google Scholar]
- Broxmeyer, L. (2017b). What James Parkinson really thought was behind Parkinson's Disease. Scientia Ricerca 1, 103–119. [Google Scholar]
- Bucciantini, M. , Giannoni, E. , Chiti, F. , Baroni, F. , Formigli, L. , Zurdo, J. , Taddei, N. , Ramponi, G. , Dobson, C. M. & Stefani, M. (2002). Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507–511. [DOI] [PubMed] [Google Scholar]
- Budden, K. F. , Gellatly, S. L. , Wood, D. L. , Cooper, M. A. , Morrison, M. , Hugenholtz, P. & Hansbro, P. M. (2017). Emerging pathogenic links between microbiota and the gut‐lung axis. Nature Reviews Microbiology 15, 55–63. [DOI] [PubMed] [Google Scholar]
- Buell, A. K. , Dobson, C. M. & Knowles, T. P. J. (2014). The physical chemistry of the amyloid phenomenon: thermodynamics and kinetics of filamentous protein aggregation. Essays in Biochemistry 56, 11–39. [DOI] [PubMed] [Google Scholar]
- Buerger, S. , Spoering, A. , Gavrish, E. , Leslin, C. , Ling, L. & Epstein, S. S. (2012). Microbial scout hypothesis, stochastic exit from dormancy, and the nature of slow growers. Applied and Environmental Microbiology 78, 3221–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullman, S. , Meyerson, M. & Kostic, A. D. (2017). Emerging concepts and technologies for the discovery of microorganisms involved in human disease. Annual Review of Pathology 12, 217–244. [DOI] [PubMed] [Google Scholar]
- Bush, A. I. & Tanzi, R. E. (2008). Therapeutics for Alzheimer's disease based on the metal hypothesis. Neurotherapeutics 5, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzan, T. (2002). How to Mind Map. Thorsons, London. [Google Scholar]
- Byler, S. L. , Boehm, G. W. , Karp, J. D. , Kohman, R. A. , Tarr, A. J. , Schallert, T. & Barth, T. M. (2009). Systemic lipopolysaccharide plus MPTP as a model of dopamine loss and gait instability in C57Bl/6J mice. Behavioural Brain Research 198, 434–439. [DOI] [PubMed] [Google Scholar]
- Byrd, A. L. & Segre, J. A. (2016). Adapting Koch's postulates. Science 351, 224–226. [DOI] [PubMed] [Google Scholar]
- Cani, P. D. , Osto, M. , Geurts, L. & Everard, A. (2012). Involvement of gut microbiota in the development of low‐grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell, J. J. , Grant, W. B. & Holick, M. F. (2014). Vitamin D and inflammation. Dermato‐Endocrinology 6, e983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, P. & Raleigh, D. P. (2016). In vitro studies of membrane permeability induced by amyloidogenic polypeptides using large unilamellar vesicles. Methods in Molecular Biology 1345, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg, C. & Campbell, M. J. (2013). Vitamin D receptor signaling mechanisms: integrated actions of a well‐defined transcription factor. Steroids 78, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg, C. , Seuter, S. , de Mello, V. D. F. , Schwab, U. , Voutilainen, S. , Pulkki, K. , Nurmi, T. , Virtanen, J. , Tuomainen, T. P. & Uusitupa, M. (2013). Primary vitamin D target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS One 8, e71042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, C. , Isakova, T. , Collerone, G. , Olbina, G. , Wolf, M. , Westerman, M. & Gutiérrez, O. M. (2011). Hepcidin and disordered mineral metabolism in chronic kidney disease. Clinical Nephrology 76, 90–98. [DOI] [PubMed] [Google Scholar]
- Carvalho, L. S. F. & Sposito, A. C. (2015). Vitamin D for the prevention of cardiovascular disease: are we ready for that? Atherosclerosis 241, 729–740. [DOI] [PubMed] [Google Scholar]
- Carver, P. L. (2018). The battle for iron between humans and microbes. Current Medicinal Chemistry 18, 25–36. [DOI] [PubMed] [Google Scholar]
- Casadesus, G. , Smith, M. A. , Zhu, X. , Aliev, G. , Cash, A. D. , Honda, K. , Petersen, R. B. & Perry, G. (2004). Alzheimer disease: evidence for a central pathogenic role of iron‐mediated reactive oxygen species. Journal of Alzheimers Disease 6, 165–169. [DOI] [PubMed] [Google Scholar]
- Castellani, R. J. , Moreira, P. I. , Perry, G. & Zhu, X. (2012). The role of iron as a mediator of oxidative stress in Alzheimer disease. BioFactors 38, 133–138. [DOI] [PubMed] [Google Scholar]
- Caughey, B. , Baron, G. S. , Chesebro, B. & Jeffrey, M. (2009). Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annual Review of Biochemistry 78, 177–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çetinkaya, M. , Özkan, H. , Köksal, N. , Çelebi, S. & Hacımustafaoğlu, M. (2009). Comparison of serum amyloid A concentrations with those of C‐reactive protein and procalcitonin in diagnosis and follow‐up of neonatal sepsis in premature infants. Journal of Perinatology 29, 225–231. [DOI] [PubMed] [Google Scholar]
- Chami, B. , Barrie, N. , Cai, X. , Wang, X. , Paul, M. , Morton‐Chandra, R. , Sharland, A. , Dennis, J. M. , Freedman, S. B. & Witting, P. K. (2015). Serum amyloid A receptor blockade and incorporation into high‐density lipoprotein modulates its pro‐inflammatory and pro‐thrombotic activities on vascular endothelial cells. International Journal of Molecular Sciences 16, 11101–11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance, B. & Williams, G. R. (1955). Respiratory enzymes in oxidative phosphorylation. III The steady state. Journal of Biological Chemistry 217, 409–427. [PubMed] [Google Scholar]
- Chang, S. & Li, L. (2011). Metabolic endotoxemia: a novel concept in chronic disease pathology. Journal of Medical Science 31, 191–209. [Google Scholar]
- Chao, M. C. & Rubin, E. J. (2010). Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annual Review of Microbiology 64, 293–311. [DOI] [PubMed] [Google Scholar]
- Charbonneau, M. R. , Blanton, L. V. , DiGiulio, D. B. , Relman, D. A. , Lebrilla, C. B. , Mills, D. A. & Gordon, J. I. (2016). A microbial perspective of human developmental biology. Nature 535, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, J. R. , Mridula, K. R. , Alladi, S. , Anamika, A. , Umamahesh, M. , Balaraju, B. , Swath, A. & Bandaru, V. S. (2014). Serum 25‐hydroxyvitamin D deficiency in ischemic stroke and subtypes in Indian patients. Journal of Stroke 16, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chel, V. G. , Ooms, M. E. , van der Bent, J. , Veldkamp, F. , Roos, R. A. , Achterberg, W. P. & Lips, P. (2013). High prevalence of vitamin D deficiency and insufficiency in patients with manifest Huntington disease: an explorative study. Dermato‐Endocrinology 5, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Liu, J. , Jiang, L. , Ran, X. , He, D. , Li, Y. , Huang, B. , Wang, W. & Fu, S. (2018). Galangin reduces the loss of dopaminergic neurons in an LPS‐evoked model of Parkinson's disease in rats. International Journal of Molecular Sciences 19, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. L. , Lu, W. S. & Li, Y. S. (2016). Berberine ameliorates type 2 diabetes via modulation of Bifidobacterium species, tumor necrosis factor‐alpha, and lipopolysaccharide. International Journal of Clinical and Experimental Medicine 9, 9365–9372. [Google Scholar]
- Chen, Y. , Zhang, J. , Ge, X. , Du, J. , Deb, D. K. & Li, Y. C. (2013). Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. Journal of Biological Chemistry 288, 19450–19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Meade, J. , Mankia, K. , Emery, P. & Devine, D. A. (2018). Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis. Best Practice & Research: Clinical Rheumatology 31, 19–30. [DOI] [PubMed] [Google Scholar]
- Cherkaoui, A. , Emonet, S. , Ceroni, D. , Candolfi, B. , Hibbs, J. , Francois, P. & Schrenzel, J. (2009). Development and validation of a modified broad‐range 16S rDNA PCR for diagnostic purposes in clinical microbiology. Journal of Microbiological Methods 79, 227–231. [DOI] [PubMed] [Google Scholar]
- Chesney, R. W. , Dabbagh, S. & Han, X. (2015). Newer insights into the taurinuria of vitamin D deficiency: a review. Advances in Experimental Medicine & Biology 803, 651–664. [DOI] [PubMed] [Google Scholar]
- Cheung, C. M. & Chee, S. P. (2009). Jarisch‐Herxheimer reaction: paradoxical worsening of tuberculosis chorioretinitis following initiation of antituberculous therapy. Eye (London, England) 23, 1472–1473. [DOI] [PubMed] [Google Scholar]
- Chiang, S. , Kovacevic, Z. , Sahni, S. , Lane, D. J. , Merlot, A. M. , Kalinowski, D. S. , Huang, M. L. & Richardson, D. R. (2016). Frataxin and the molecular mechanism of mitochondrial iron‐loading in Friedreich's ataxia. Clinical Science 130, 853–870. [DOI] [PubMed] [Google Scholar]
- Chifman, J. , Kniss, A. , Neupane, P. , Williams, I. , Leung, B. , Deng, Z. , Mendes, P. , Hower, V. , Torti, F. M. , Akman, S. A. , Torti, S. V. & Laubenbacher, R. (2012). The core control system of intracellular iron homeostasis: a mathematical model. Journal of Theoretical Biology 300, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifman, J. , Laubenbacher, R. & Torti, S. V. (2014). A systems biology approach to iron metabolism. Advances in Experimental Medicine & Biology 844, 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, Z. Z. , Li, F. & Maiese, K. (2005). Erythropoietin requires NF‐kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta‐amyloid toxicity. Current Neurovascular Research 2, 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher, G. W. (1985). Escherichia coli bacteremia, meningitis, and hemochromatosis. Archives of Internal Medicine 145, 1908. [PubMed] [Google Scholar]
- Chu, B. C. , Garcia‐Herrero, A. , Johanson, T. H. , Krewulak, K. D. , Lau, C. K. , Peacock, R. S. , Slavinskaya, Z. & Vogel, H. J. (2010). Siderophore uptake in bacteria and the battle for iron with the host; a bird's eye view. Biometals 23, 601–611. [DOI] [PubMed] [Google Scholar]
- Chu, D. M. & Aagaard, K. M. (2016). Microbiome: eating for trillions. Nature 532, 316–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli, S. S. , Velsko, I. M. , Rivera‐Kweh, M. F. , Zheng, D. , Lucas, A. R. & Kesavalu, L. (2015). Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoEnull mice. PLoS One 10, e0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar, M. U. , Islam, M. A. , Pröll, M. , Kocamis, H. , Tholen, E. , Tesfaye, D. , Looft, C. , Schellander, K. & Uddin, M. J. (2013). Evaluation of suitable reference genes for gene expression studies in porcine PBMCs in response to LPS and LTA. BMC Research Notes 6, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates, A. R. , Halls, G. & Hu, Y. (2011). Novel classes of antibiotics or more of the same? British Journal of Pharmacology 163, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates, A. R. M. & Hu, Y. (2006). New strategies for antibacterial drug design: targeting non‐multiplying latent bacteria. Drugs in R&D 7, 133–151. [DOI] [PubMed] [Google Scholar]
- Cohen, F. E. & Prusiner, S. B. (1998). Pathologic conformations of prion proteins. Annual Review of Biochemistry 67, 793–819. [DOI] [PubMed] [Google Scholar]
- Cohen, N. R. , Lobritz, M. A. & Collins, J. J. (2013). Microbial persistence and the road to drug resistance. Cell Host & Microbe 13, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado, M. C. , Rautava, S. , Aakko, J. , Isolauri, E. & Salminen, S. (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports 6, 23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingwood, J. F. & Davidson, M. R. (2014). The role of iron in neurodegenerative disorders: insights and opportunities with synchrotron light. Frontiers in Pharmacology 5, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin, M. T. , Silvers, R. , Ni, Q. Z. , Can, T. V. , Sergeyev, I. , Rosay, M. , Donovan, K. J. , Michael, B. , Wall, J. , Linse, S. & Griffin, R. G. (2016). Atomic resolution structure of monomorphic Abeta42 amyloid fibrils. Journal of the American Chemical Society 138, 9663–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan, J. W. (2011). Francisella tularensis: a red‐blooded pathogen. Journal of Infectious Diseases 204, 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes‐Canteli, M. , Paul, J. , Norris, E. H. , Bronstein, R. , Ahn, H. J. , Zamolodchikov, D. , Bhuvanendran, S. , Fenz, K. M. & Strickland, S. (2010). Fibrinogen and beta‐amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron 66, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes‐Canteli, M. & Strickland, S. (2009). Fibrinogen, a possible key player in Alzheimer's disease. Journal of Thrombosis and Haemostasis 7, 146–150. [DOI] [PubMed] [Google Scholar]
- Cortes‐Canteli, M. , Zamolodchikov, D. , Ahn, H. J. , Strickland, S. & Norris, E. H. (2012). Fibrinogen and altered hemostasis in Alzheimer's Disease. Journal of Alzheimers Disease 32, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa‐Mallen, P. , Gatenby, C. , Friend, S. , Maravilla, K. R. , Hu, S. C. , Cain, K. C. , Agarwal, P. & Anzai, Y. (2017). Brain iron concentrations in regions of interest and relation with serum iron levels in Parkinson disease. Journal of the Neurological Sciences 378, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotechini, T. , Komisarenko, M. , Sperou, A. , Macdonald‐Goodfellow, S. , Adams, M. A. & Graham, C. H. (2014). Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. Journal of Experimental Medicine 211, 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens, A. K. , Martineau, A. R. & Wilkinson, R. J. (2014). Anti‐Inflammatory and Antimicrobial actions of vitamin D in combating TB/HIV. Scientifica 2014, 903680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couthouis, J. , Marchal, C. , D'Angelo, F. , Berthelot, K. & Cullin, C. (2010). The toxicity of an "artificial" amyloid is related to how it interacts with membranes. Prion 4, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, L. B. , Peck, J. D. , Xu, J. , Sankaranarayanan, K. , Warinner, C. , Hansen, K. R. , Anderson, M. & Lewis, C. M. (2015). Characterizing the semen microbiome and associations with semen parameters: the chasm study. Fertility and Sterility 104, E66–E66. [Google Scholar]
- Crichton, R. R. (2016). Iron Metabolism ‐ From Molecular Mechanisms to Clinical Consequences, Fourth Edition (). John Wiley, Chichester. [Google Scholar]
- Crichton, R. R. , Dexter, D. T. & Ward, R. J. (2011). Brain iron metabolism and its perturbation in neurological diseases. Journal of Neural Transmission 118, 301–314. [DOI] [PubMed] [Google Scholar]
- Cummins, J. & Tangney, M. (2013). Bacteria and tumours: causative agents or opportunistic inhabitants? Infectious Agents and Cancer 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C. , Wilcockson, D. C. , Campion, S. , Lunnon, K. & Perry, V. H. (2005). Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. Journal of Neuroscience 25, 9275–9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard, C. , Magnussen, K. , Enevold, C. , Nilsson, M. , Tolker‐Nielsen, T. , Holmstrup, P. & Nielsen, C. H. (2015). Viable bacteria associated with red blood cells and plasma in freshly drawn blood donations. PLoS One 10, e0120826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron, F. H. , Oglesby‐Sherrouse, A. G. , Wilks, A. & Barbier, M. (2016). Dual‐seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia. Scientific Reports 6, 39172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh, J. , Lewington, S. , Thompson, S. G. , Lowe, G. D. , Collins, R. , Kostis, J. B. , Wilson, A. C. , Folsom, A. R. , Wu, K. , Benderly, M. , Goldbourt, U. , Willeit, J. , Kiechl, S. , Yarnell, J. W. , Sweetnam, P. M. , et al. (2005). Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta‐analysis. JAMA 294, 1799–1809. [DOI] [PubMed] [Google Scholar]
- Davalos, D. & Akassoglou, K. (2012). Fibrinogen as a key regulator of inflammation in disease. Seminars in Immunopathology 34, 43–62. [DOI] [PubMed] [Google Scholar]
- Davì, G. , Falco, A. & Patrono, C. (2004). Determinants of F2‐isoprostane biosynthesis and inhibition in man. Chemistry and Physics of Lipids 128, 149–163. [DOI] [PubMed] [Google Scholar]
- Dawonauth, L. , Rademacher, L. , L'Omelette, A. D. , Jankee, S. , Lee Kwai Yan, M. Y. , Jeeawoody, R. B. & Rademacher, T. W. (2014). Urinary inositol phosphoglycan‐P type: near patient test to detect preeclampsia prior to clinical onset of the disease. A study on 416 pregnant Mauritian women. Journal of Reproductive Immunology 101‐102, 148–152. [DOI] [PubMed] [Google Scholar]
- De Buck, M. , Gouwy, M. , Wang, J. M. , Van Snick, J. , Proost, P. , Struyf, S. & Van Damme, J. (2016). The cytokine‐serum amyloid A‐chemokine network. Cytokine & Growth Factor Reviews 30, 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, N. S. , Sabate, R. & Ventura, S. (2009). Amyloids in bacterial inclusion bodies. Trends in Biochemical Sciences 34, 408–416. [DOI] [PubMed] [Google Scholar]
- de Kort, S. , Keszthelyi, D. & Masclee, A. A. (2011). Leaky gut and diabetes mellitus: what is the link? Obesity Reviews 12, 449–458. [DOI] [PubMed] [Google Scholar]
- de Punder, K. & Pruimboom, L. (2015). Stress induces endotoxemia and low‐grade inflammation by increasing barrier permeability. Frontiers in Immunology 6, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smit, M. , Westra, J. , Vissink, A. , Doornbos‐van der Meer, B. , Brouwer, E. & van Winkelhoff, A. J. (2012). Periodontitis in established rheumatoid arthritis patients: a cross‐sectional clinical, microbiological and serological study. Arthritis Research & Therapy 14, R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Temiño, Á. R. , Gil, J. , Perez, T. , Gonzalez, M. , Pineda, M. , Dueñas‐Laita, A. & Pérez‐Castrillón, J. L. (2011). Association between vitamin D deficiency and heart failure in the elderly. International Journal of Cardiology 152, 407–408. [DOI] [PubMed] [Google Scholar]
- Dehio, C. (2001). Bartonella interactions with endothelial cells and erythrocytes. Trends in Microbiology 9, 279–285. [DOI] [PubMed] [Google Scholar]
- Dehio, C. , Berry, C. & Bartenschlager, R. (2012). Persistent intracellular pathogens. FEMS Microbiology Reviews 36, 513. [DOI] [PubMed] [Google Scholar]
- Del Rio, D. , Stewart, A. J. & Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases 15, 316–328. [DOI] [PubMed] [Google Scholar]
- deRosset, L. & Strutz, K. L. (2015). Developmental origins of chronic inflammation: a review of the relationship between birth weight and C‐reactive protein. Annals of Epidemiology 25, 539–543. [DOI] [PubMed] [Google Scholar]
- Detert, J. , Pischon, N. , Burmester, G. R. & Buttgereit, F. (2010). The association between rheumatoid arthritis and periodontal disease. Arthritis Research & Therapy 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhotre, S. V. , Davane, M. S. & Nagoba, B. S. (2017). Periodontitis, bacteremia and infective endocarditis: a review study. Archives of Pediatric Infectious Diseases 5, e41067. [Google Scholar]
- di Penta, A. , Moreno, B. , Reix, S. , Fernandez‐Diez, B. , Villanueva, M. , Errea, O. , Escala, N. , Vandenbroeck, K. , Comella, J. X. & Villoslada, P. (2013). Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One 8, e54722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, R. P. , Erb‐Downward, J. R. , Freeman, C. M. , McCloskey, L. , Falkowski, N. R. , Huffnagle, G. B. & Curtis, J. L. (2017). Bacterial topography of the healthy human lower respiratory tract. MBio 8, e02287‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, R. P. , Erb‐Downward, J. R. , Martinez, F. J. & Huffnagle, G. B. (2016). The Microbiome and the Respiratory Tract. Annual Review of Physiology 78, 481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, R. P. & Huffnagle, G. B. (2015). The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathogens 11, e1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, R. P. , Singer, B. H. , Newstead, M. W. , Falkowski, N. R. , Erb‐Downward, J. R. , Standiford, T. J. & Huffnagle, G. B. (2016). Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nature Microbiology 1, 16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, T. & Schloss, P. D. (2014). Dynamics and associations of microbial community types across the human body. Nature 509, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S. J. , Lemberg, K. M. , Lamprecht, M. R. , Skouta, R. , Zaitsev, E. M. , Gleason, C. E. , Patel, D. N. , Bauer, A. J. , Cantley, A. M. , Yang, W. S. , Morrison, B. III & Stockwell, B. R. (2012). Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S. J. & Stockwell, B. R. (2013). The role of iron and reactive oxygen species in cell death. Nature Chemical Biology 10, 9–17. [DOI] [PubMed] [Google Scholar]
- Do, J. , Zafar, H. & Saier, M. H. Jr. (2017). Comparative genomics of transport proteins in probiotic and pathogenic Escherichia coli and Salmonella enterica strains. Microbial Pathogenesis 107, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C. M. (2001). The structural basis of protein folding and its links with human disease. Philosophical Transactions of the Royal Society of London B: Biological Sciences 356, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C. M. (2013). The amyloid phenomenon and its significance In Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties (ed. Otzen D. E.), pp. 1–19. Wiley‐VCH, Weinheim. [Google Scholar]
- Dodd, D. , Spitzer, M. H. , Van Treuren, W. , Merrill, B. D. , Hryckowian, A. J. , Higginbottom, S. K. , Le, A. , Cowan, T. M. , Nolan, G. P. , Fischbach, M. A. & Sonnenburg, J. L. (2017). A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll, D. N. , Engler‐Chiurazzi, E. B. , Lewis, S. E. , Hu, H. , Kerr, A. E. , Ren, X. & Simpkins, J. W. (2015). Lipopolysaccharide exacerbates infarct size and results in worsened post‐stroke behavioral outcomes. Behavioral and Brain Functions 11, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrecht, E. J. , Cos, P. , Vanden Berghe, D. , Van Offel, J. F. , Schuerwegh, A. J. , Bridts, C. H. , Stevens, W. J. & De Clerck, L. S. (2004). Selective in vitro antioxidant properties of bisphosphonates. Biochemical and Biophysical Research Communications 314, 675–680. [DOI] [PubMed] [Google Scholar]
- Domingue, G. , Turner, B. & Schlegel, J. U. (1974). Cell‐wall deficient bacterial variants in kidney tissue. Detection by immunofluorescence. Urology 3, 288–292. [DOI] [PubMed] [Google Scholar]
- Domingue, G. J. (1995). Electron dense cytoplasmic particles and chronic infection ‐ a bacterial pleomorphy hypothesis. Endocytobiosis . Cell Research 11, 19–40. [Google Scholar]
- Domingue, G. J. (2010). Demystifying pleomorphic forms in persistence and expression of disease: are they bacteria, and is peptidoglycan the solution? Discovery Medicine 10, 234–246. [PubMed] [Google Scholar]
- Domingue, G. J. , Ghoniem, G. M. , Bost, K. L. , Fermin, C. & Human, L. G. (1995). Dormant microbes in interstitial cystitis. The Journal of Urology 153, 1321–1326. [PubMed] [Google Scholar]
- Domingue, G. J. & Schlegel, J. U. (1977a). Novel bacterial structures in human blood. II. Bacterial variants as etiologic agents in idiopathic hematuria. Transactions of the American Association of Genito‐Urinary Surgeons 69, 61–64. [PubMed] [Google Scholar]
- Domingue, G. J. & Schlegel, J. U. (1977b). Novel bacterial structures in human blood: cultural isolation. Infection & Immunity 15, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingue, G. J. & Woody, H. B. (1997). Bacterial persistence and expression of disease. Clinical Microbiology Reviews 10, 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, T. , Liao, D. , Liu, X. & Lei, X. (2015). Using small molecules to dissect non‐apoptotic programmed cell death: necroptosis, ferroptosis, and pyroptosis. Chembiochem 16, 2557–2561. [DOI] [PubMed] [Google Scholar]
- Donia, M. S. & Fischbach, M. A. (2015). Small molecules from the human microbiota. Science 349, 1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly, S. C. , Joshi, N. G. , Thorburn, D. , Cooke, A. , Reid, G. , Neilson, M. , Capell, H. & Stanley, A. J. (2010). Prevalence of genetic haemochromatosis and iron overload in patients attending rheumatology and joint replacement clinics. Scottish Medical Journal 55, 14–16. [DOI] [PubMed] [Google Scholar]
- Dréno, B. , Araviiskaia, E. , Berardesca, E. , Gontijo, G. , Sanchez Viera, M. , Xiang, L. F. , Martin, R. & Bieber, T. (2016). Microbiome in healthy skin, update for dermatologists. Journal of the European Academy of Dermatology and Venereology 30, 2038–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek, P. , Roos, P. M. , Litwin, T. , Schneider, S. A. , Flaten, T. P. & Aaseth, J. (2014). The neurotoxicity of iron, copper and manganese in Parkinson's and Wilson's diseases. Journal of Trace Elements in Medicine & Biology 31, 193–203. [DOI] [PubMed] [Google Scholar]
- Duvigneau, J. C. , Piskernik, C. , Haindl, S. , Kloesch, B. , Hartl, R. T. , Huttemann, M. , Lee, I. , Ebel, T. , Moldzio, R. , Gemeiner, M. , Redl, H. & Kozlov, A. V. (2008). A novel endotoxin‐induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron‐mediated mitochondrial dysfunction. Laboratory Investigation 88, 70–77. [DOI] [PubMed] [Google Scholar]
- Dworkin, J. & Shah, I. M. (2010). Exit from dormancy in microbial organisms. Nature Reviews Microbiology 8, 890–896. [DOI] [PubMed] [Google Scholar]
- Dybboe, R. , Bandier, J. , Skov, L. , Engstrand, L. & Johansen, J. D. (2017). The role of the skin microbiome in atopic dermatitis: a systematic review. British Journal of Dermatology 177, 1272–1278. [DOI] [PubMed] [Google Scholar]
- Ebringer, A. (2012). Rheumatoid Arthritis and Proteus. Springer, London. [Google Scholar]
- Ebringer, A. & Rashid, T. (2009). Rheumatoid arthritis is caused by Proteus: the molecular mimicry theory and Karl Popper. Frontiers in Bioscience 1, 577–586. [DOI] [PubMed] [Google Scholar]
- Ebringer, A. , Rashid, T. & Wilson, C. (2010). Rheumatoid arthritis, Proteus, anti‐CCP antibodies and Karl Popper. Autoimmunity Reviews 9, 216–223. [DOI] [PubMed] [Google Scholar]
- Edmonds‐Wilson, S. L. , Nurinova, N. I. , Zapka, C. A. , Fierer, N. & Wilson, M. (2015). Review of human hand microbiome research. Journal of Dermatological Science 80, 3–12. [DOI] [PubMed] [Google Scholar]
- Eicher, S. C. & Dehio, C. (2012). Bartonella entry mechanisms into mammalian host cells. Cellular Microbiology 14, 1166–1173. [DOI] [PubMed] [Google Scholar]
- Eichhorn, H. , Lessing, F. , Winterberg, B. , Schirawski, J. , Kämper, J. , Müller, P. & Kahmann, R. (2006). A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis . Plant Cell 18, 3332–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner, T. & Radford, S. E. (2011). A diversity of assembly mechanisms of a generic amyloid fold. Molecular Cell 43, 8–18. [DOI] [PubMed] [Google Scholar]
- Eisenberg, D. & Jucker, M. (2012). The amyloid state of proteins in human diseases. Cell 148, 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejrnæs, K. (2011). Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli . Danish Medical Bulletin 58, B4187. [PubMed] [Google Scholar]
- Eklund, K. K. , Niemi, K. & Kovanen, P. T. (2012). Immune functions of serum amyloid A. Critical Reviews in Immunology 32, 335–348. [DOI] [PubMed] [Google Scholar]
- Emery, D. C. , Shoemark, D. K. , Batstone, T. E. , Waterfall, C. M. , Coghill, J. A. , Cerajewska, T. L. , Davies, M. , West, N. X. & Allen, S. J. (2017). 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer's post‐mortem brain. Frontiers in Aging Neuroscience 9, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, M. F. M. , Khemtémourian, L. , Kleijer, C. C. , Meeldijk, H. J. D. , Jacobs, J. , Verkleij, A. J. , de Kruijff, B. , Killian, J. A. & Hoppener, J. W. M. (2008). Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proceedings of the National Academy of Sciences of the United States of America 105, 6033–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman, S. S. , Kambam, J. R. , Bradley, C. A. & Cousar, J. B. (1987). Increased levels of carboxyhemoglobin and serum iron as an indicator of increased red cell turnover in preeclampsia. American Journal of Obstetrics & Gynecology 156, 1169–1173. [DOI] [PubMed] [Google Scholar]
- Escribano, B. M. , Medina‐Fernandez, F. J. , Aguilar‐Luque, M. , Aguera, E. , Feijoo, M. , Garcia‐Maceira, F. I. , Lillo, R. , Vieyra‐Reyes, P. , Giraldo, A. I. , Luque, E. , Drucker‐Colin, R. & Tunez, I. (2017). Lipopolysaccharide binding protein and oxidative stress in a multiple sclerosis model. Neurotherapeutics 14, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, A. S. (1976). Causation and disease: the Henle‐Koch postulates revisited. Yale Journal of Biology and Medicine 49, 175–195. [PMC free article] [PubMed] [Google Scholar]
- Ewald, P. W. (2002). Plague Time: The New Germ Theory of Disease. Anchor Books, New York. [Google Scholar]
- Faas, M. M. , Schuiling, G. A. , Baller, J. F. , Visscher, C. A. & Bakker, W. W. (1994). A new animal model for human preeclampsia: ultra‐low‐dose endotoxin infusion in pregnant rats. American Journal of Obstetrics & Gynecology 171, 158–164. [DOI] [PubMed] [Google Scholar]
- Faas, M. M. , Schuiling, G. A. , Linton, E. A. , Sargent, I. L. & Redman, C. W. G. (2000). Activation of peripheral leukocytes in rat pregnancy and experimental preeclampsia. American Journal of Obstetrics & Gynecology 182, 351–357. [DOI] [PubMed] [Google Scholar]
- Fabri, M. , Stenger, S. , Shin, D. M. , Yuk, J. M. , Liu, P. T. , Realegeno, S. , Lee, H. M. , Krutzik, S. R. , Schenk, M. , Sieling, P. A. , Teles, R. , Montoya, D. , Iyer, S. S. , Bruns, H. , Lewinsohn, D. M. , Hollis, B. W. , et al. (2011). Vitamin D is required for IFN‐gamma‐mediated antimicrobial activity of human macrophages. Science Translational Medicine 3, 104ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow, S. (1988). Molecular Koch's postulates applied to microbial pathogenicity. Reviews of Infectious Diseases 10(Suppl. 2), S274–S276. [DOI] [PubMed] [Google Scholar]
- Falkow, S. (2004). Molecular Koch's postulates applied to bacterial pathogenicity ‐ a personal recollection 15 years later. Nature Reviews Microbiology 2, 67–72. [DOI] [PubMed] [Google Scholar]
- Fändrich, M. (2012). Oligomeric intermediates in amyloid formation: structure determination and mechanisms of toxicity. Journal of Molecular Biology 421, 427–440. [DOI] [PubMed] [Google Scholar]
- Farina, M. , Avila, D. S. , da Rocha, J. B. & Aschner, M. (2013). Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochemistry International 62, 575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano, A. (2012). Leaky gut and autoimmune diseases. Clinical Reviews in Allergy & Immunology 42, 71–78. [DOI] [PubMed] [Google Scholar]
- Fauvart, M. , De Groote, V. N. & Michiels, J. (2011). Role of persister cells in chronic infections: clinical relevance and perspectives on anti‐persister therapies. Journal of Medical Microbiology 60, 699–709. [DOI] [PubMed] [Google Scholar]
- Fell, D. A. (1996). Understanding the Control of Metabolism. Portland Press, London. [Google Scholar]
- Fell, D. A. & Thomas, S. (1995). Physiological control of metabolic flux: the requirement for multisite modulation. Biochemical Journal 311, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Cao, J. C. , Aranda, N. , Ribot, B. , Tous, M. & Arija, V. (2017). Elevated iron status and risk of gestational diabetes mellitus: a systematic review and meta‐analysis. Maternal & Child Nutrition 13 10.1111/mcn.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Cruz, A. , Marin, M. , Kestler, M. , Alcala, L. , Rodriguez‐Créixems, M. & Bouza, E. (2013). The value of combining blood culture and SeptiFast data for predicting complicated bloodstream infections caused by Gram‐positive bacteria or Candida species. Journal of Clinical Microbiology 51, 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Real, J. M. , López‐Bermejo, A. & Ricart, W. (2002). Cross‐talk between iron metabolism and diabetes. Diabetes 51, 2348–2354. [DOI] [PubMed] [Google Scholar]
- Fernández‐Real, J. M. , McClain, D. & Manco, M. (2015). Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 38, 2169–2176. [DOI] [PubMed] [Google Scholar]
- Figueira, I. , Fernandes, A. , Mladenovic Djordjevic, A. , Lopez‐Contreras, A. , Henriques, C.M. , Selman, C. , Ferreiro, E. , Gonos, E.S. , Trejo, J.L. , Misra, J. , Rasmussen, L.J. , Xapelli, S. , Ellam, T. & Bellantuono, I. (2016). Interventions for age‐related diseases: Shifting the paradigm. Mech Ageing Dev 160, 69–92. [DOI] [PubMed] [Google Scholar]
- Fischbach, M. A. , Lin, H. N. , Liu, D. R. & Walsh, C. T. (2006). How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nature Chemical Biology 2, 132–138. [DOI] [PubMed] [Google Scholar]
- Fitz‐Gibbon, S. , Tomida, S. , Chiu, B. H. , Nguyen, L. , Du, C. , Liu, M. , Elashoff, D. , Erfe, M. C. , Loncaric, A. , Kim, J. , Modlin, R. L. , Miller, J. F. , Sodergren, E. , Craft, N. , Weinstock, G. M. & Li, H. (2013). Propionibacterium acnes strain populations in the human skin microbiome associated with acne. Journal of Investigative Dermatology 133, 2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Mireles, A. L. , Walker, J. N. , Caparon, M. & Hultgren, S. J. (2015). Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature Reviews Microbiology 13, 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föller, M. , Geiger, C. , Mahmud, H. , Nicolay, J. & Lang, F. (2008). Stimulation of suicidal erythrocyte death by amantadine. European Journal of Pharmacology 581, 13–18. [DOI] [PubMed] [Google Scholar]
- Forman, J. P. , Curhan, G. C. & Taylor, E. N. (2008). Plasma 25‐hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 52, 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman, J. P. , Giovannucci, E. , Holmes, M. D. , Bischoff‐Ferrari, H. A. , Tworoger, S. S. , Willett, W. C. & Curhan, G. C. (2007). Plasma 25‐hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Forouhi, N. G. , Harding, A. H. , Allison, M. , Sandhu, M. S. , Welch, A. , Luben, R. , Bingham, S. , Khaw, K. T. & Wareham, N. J. (2007). Elevated serum ferritin levels predict new‐onset type 2 diabetes: results from the EPIC‐Norfolk prospective study. Diabetologia 50, 949–956. [DOI] [PubMed] [Google Scholar]
- Forouhi, N. G. , Ye, Z. , Rickard, A. P. , Khaw, K. T. , Luben, R. , Langenberg, C. & Wareham, N. J. (2012). Circulating 25‐hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)‐Norfolk cohort and updated meta‐analysis of prospective studies. Diabetologia 55, 2173–2182. [DOI] [PubMed] [Google Scholar]
- Forsyth, C. B. , Shannon, K. M. , Kordower, J. H. , Voigt, R. M. , Shaikh, M. , Jaglin, J. A. , Estes, J. D. , Dodiya, H. B. & Keshavarzian, A. (2011). Increased intestinal permeability correlates with sigmoid mucosa alpha‐synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One 6, e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, K. R. , Schluter, J. , Coyte, K. Z. & Rakoff‐Nahoum, S. (2017). The evolution of the host microbiome as an ecosystem on a leash. Nature 548, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks, D. N. & Relman, D. A. (1996). Sequence‐based identification of microbial pathogens ‐ a reconsideration of Koch's postulates. Clinical Microbiology Reviews 9, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaibani, P. , Mariconti, M. , Bua, G. , Bonora, S. , Sassera, D. , Landini, M. P. , Mulatto, P. , Novati, S. , Bandi, C. & Sambri, V. (2013). Development of a broad‐range 23S rDNA real‐time PCR assay for the detection and quantification of pathogenic bacteria in human whole blood and plasma specimens. BioMed Research International 2013, 264651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galesloot, T. E. , Janss, L. L. , Burgess, S. , Kiemeney, L. A. L. M. , Heijer, M. d. , Graaf, J. d. , Holewijn, S. , Benyamin, B. , Whitfield, J. B. , Swinkels, D. W. & Vermeulen, S. H. (2015). Iron and hepcidin as risk factors in atherosclerosis: what do the genes say? BMC Genetics 16, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, J. J. , Finnegan, M. E. , Grehan, B. , Dobson, J. , Collingwood, J. F. & Lynch, M. A. (2012). Modest amyloid deposition is associated with iron dysregulation, microglial activation, and oxidative stress. Journal of Alzheimers Disease 28, 147–161. [DOI] [PubMed] [Google Scholar]
- Gallo, P. M. , Rapsinski, G. J. , Wilson, R. P. , Oppong, G. O. , Sriram, U. , Goulian, M. , Buttaro, B. , Caricchio, R. , Gallucci, S. & Tükel, Ç. (2015). Amyloid‐DNA composites of bacterial biofilms stimulate autoimmunity. Immunity 42, 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz, T. (2006). Hepcidin‐‐a peptide hormone at the interface of innate immunity and iron metabolism. Current Topics in Microbiology and Immunology 306, 183–198. [DOI] [PubMed] [Google Scholar]
- Ganz, T. (2009). Iron in innate immunity: starve the invaders. Current Opinion in Immunology 21, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz, T. & Nemeth, E. (2012). Hepcidin and iron homeostasis. Biochimica et Biophysica Acta 1823, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz, T. & Nemeth, E. (2015). Iron homeostasis in host defence and inflammation. Nature Reviews Immunology 15, 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano, L. M. & Hughes, J. M. (2014). Microbial origins of chronic diseases. Annual Review of Public Health 35, 65–82. [DOI] [PubMed] [Google Scholar]
- Garn, H. , Bahn, S. , Baune, B. T. , Binder, E. B. , Bisgaard, H. , Chatila, T. A. , Chavakis, T. , Culmsee, C. , Dannlowski, U. , Gay, S. , Gern, J. , Haahtela, T. , Kircher, T. , Müller‐Ladner, U. , Neurath, M. F. , et al. (2016). Current concepts in chronic inflammatory diseases: interactions between microbes, cellular metabolism, and inflammation. Journal of Allergy and Clinical Immunology 138, 47–56. [DOI] [PubMed] [Google Scholar]
- Geller, L. T. , Barzily‐Rokni, M. , Danino, T. , Jonas, O. H. , Shental, N. , Nejman, D. , Gavert, N. , Zwang, Y. , Cooper, Z. A. , Shee, K. , Thaiss, C. A. , Reuben, A. , Livny, J. , Avraham, R. , Frederick, D. T. , et al. (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher, M. & Kaufmann, S. H. E. (2012). Mycobacterium tuberculosis: success through dormancy. FEMS Microbiology Reviews 36, 514–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, K. & Maisonneuve, E. (2012). Bacterial persistence and toxin‐antitoxin loci. Annual Review of Microbiology 66, 103–123. [DOI] [PubMed] [Google Scholar]
- Ghanim, H. , Abuaysheh, S. , Sia, C. L. , Korzeniewski, K. , Chaudhuri, A. , Fernandez‐Real, J. M. & Dandona, P. (2009). Increase in plasma endotoxin concentrations and the expression of Toll‐like receptors and suppressor of cytokine signaling‐3 in mononuclear cells after a high‐fat, high‐carbohydrate meal: implications for insulin resistance. Diabetes Care 32, 2281–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, F. C. III & Genco, C. A. (2007). Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: disparate diseases with commonalities in pathogenesis through TLRs. Current Pharmaceutical Design 13, 3665–3675. [DOI] [PubMed] [Google Scholar]
- Gillis, C. C. , Hughes, E. R. , Spiga, L. , Winter, M. G. , Zhu, W. , Furtado de Carvalho, T. , Chanin, R. B. , Behrendt, C. L. , Hooper, L. V. , Santos, R. L. & Winter, S. E. (2018). Dysbiosis‐associated change in host metabolism generates lactate to support Salmonella growth. Cell Host & Microbe 23, 54–64 e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci, E. , Liu, Y. , Hollis, B. W. & Rimm, E. B. (2008). 25‐hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Archives of Internal Medicine 168, 1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard‐Joyal, O. & Ismail, N. (2017). Effect of LPS treatment on tyrosine hydroxylase expression and Parkinson‐like behaviors. Hormones and Behaviour 89, 1–12. [DOI] [PubMed] [Google Scholar]
- Glabe, C. G. (2006). Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiology of Aging 27, 570–575. [DOI] [PubMed] [Google Scholar]
- Göransson, A. L. , Nilsson, K. P. R. , Kågedal, K. & Brorsson, A. C. (2012). Identification of distinct physiochemical properties of toxic prefibrillar species formed by Abeta peptide variants. Biochemical and Biophysical Research Communications 420, 895–900. [DOI] [PubMed] [Google Scholar]
- Gorbunov, N. V. , Asher, L. V. , Ayyagari, V. & Atkins, J. L. (2006). Inflammatory leukocytes and iron turnover in experimental hemorrhagic lung trauma. Experimental and Molecular Pathology 80, 11–25. [DOI] [PubMed] [Google Scholar]
- Gorbunov, N. V. , McFaul, S. J. , Januszkiewicz, A. & Atkins, J. L. (2005). Pro‐inflammatory alterations and status of blood plasma iron in a model of blast‐induced lung trauma. International Journal of Immunopathology and Pharmacology 18, 547–556. [DOI] [PubMed] [Google Scholar]
- Gorbunov, N. V. , Nath, J. , Parker, J. M. & Zaucha, G. M. (2003). Electron paramagnetic resonance analysis of transferrin‐bound iron in animal models of blunt trauma. Journal of Trauma and Acute Care Surgery 54, 574–583. [DOI] [PubMed] [Google Scholar]
- Goubran, H. , Seghatchian, J. , Radosevic, J. , Ragab, G. & Burnouf, T. (2017). The microbiome and transfusion in cancer patients. Transfusion and Apheresis Science 56, 330–335. [DOI] [PubMed] [Google Scholar]
- Gradmann, C. (2014). A spirit of scientific rigour: Koch's postulates in twentieth‐century medicine. Microbes & Infection 16, 885–892. [DOI] [PubMed] [Google Scholar]
- Green, D. , Chan, C. , Kang, J. , Liu, K. , Schreiner, P. , Jenny, N. S. & Tracy, R. P. (2010). Longitudinal assessment of fibrinogen in relation to subclinical cardiovascular disease: the CARDIA study. Journal of Thrombosis and Haemostasis 8, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. T. , Heidger, P. M. Jr. & Domingue, G. (1974a). Demonstration of the phenomena of microbial persistence and reversion with bacterial L‐forms in human embryonic kidney cells. Infection & Immunity 10, 889–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. T. , Heidger, P. M. Jr. & Domingue, G. (1974b). Proposed reproductive cycle for a relatively stable L‐phase variant of Streptococcus faecalis . Infection & Immunity 10, 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremer, L. , Scholzel, D. , Schenk, C. , Reinartz, E. , Labahn, J. , Ravelli, R. B. G. , Tusche, M. , Lopez‐Iglesias, C. , Hoyer, W. , Heise, H. , Willbold, D. & Schröder, G. F. (2017). Fibril structure of amyloid‐beta(1‐42) by cryo‐electron microscopy. Science 358, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grif, K. , Fille, M. , Wurzner, R. , Weiss, G. , Lorenz, I. , Gruber, G. , Eschertzhuber, S. , Nachbaur, D. , Lass‐Flörl, C. & Orth, D. (2012). Rapid detection of bloodstream pathogens by real‐time PCR in patients with sepsis. Wiener Klinische Wochenschrift 124, 266–270. [DOI] [PubMed] [Google Scholar]
- Grif, K. , Heller, I. , Prodinger, W. M. , Lechleitner, K. , Lass‐Florl, C. & Orth, D. (2012). Improvement of detection of bacterial pathogens in normally sterile body sites with a focus on orthopedic samples by use of a commercial 16S rRNA broad‐range PCR and sequence analysis. Journal of Clinical Microbiology 50, 2250–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groebel, K. , Hoelzle, K. , Wittenbrink, M. M. , Ziegler, U. & Hoelzle, L. E. (2009). Mycoplasma suis invades porcine erythrocytes. Infection & Immunity 77, 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünblatt, E. , Bartl, J. & Riederer, P. (2011). The link between iron, metabolic syndrome, and Alzheimer's disease. Journal of Neural Transmission 118, 371–379. [DOI] [PubMed] [Google Scholar]
- Guerrier, G. & D'Ortenzio, E. (2013). The Jarisch‐Herxheimer reaction in leptospirosis: a systematic review. PLoS One 8, e59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinane, C. M. & Cotter, P. D. (2013). Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therapeutic Advances in Gastroenterology 6, 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez‐Monreal, M. A. , Cuevas‐Diaz Duran, R. , Moreno‐Cuevas, J. E. & Scott, S. P. (2014). A role for 1alpha,25‐dihydroxyvitamin D3 in the expression of circadian genes. Journal of Biological Rhythms 29, 384–388. [DOI] [PubMed] [Google Scholar]
- Haass, C. & Selkoe, D. J. (2007). Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta‐peptide. Nature Reviews Molecular Cell Biology 8, 101–112. [DOI] [PubMed] [Google Scholar]
- Hadzhieva, M. , Kirches, E. , Wilisch‐Neumann, A. , Pachow, D. , Wallesch, M. , Schoenfeld, P. , Paege, I. , Vielhaber, S. , Petri, S. , Keilhoff, G. & Mawrin, C. (2013). Dysregulation of iron protein expression in the G93A model of amyotrophic lateral sclerosis. Neuroscience 230, 94–101. [DOI] [PubMed] [Google Scholar]
- Haley, K. P. & Skaar, E. P. (2012). A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes & Infection 14, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi Asl, L. , Liepnieks, J. J. , Uemichi, T. , Rebibou, J. M. , Justrabo, E. , Droz, D. , Mousson, C. , Chalopin, J. M. , Benson, M. D. , Delpech, M. & Grateau, G. (1997). Renal amyloidosis with a frame shift mutation in fibrinogen aalpha‐chain gene producing a novel amyloid protein. Blood 90, 4799–4805. [PubMed] [Google Scholar]
- Hannan, T. J. , Totsika, M. , Mansfield, K. J. , Moore, K. H. , Schembri, M. A. & Hultgren, S. J. (2012). Host‐pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiology Reviews 36, 616–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, J. B. , Moen, I. W. & Mandrup‐Poulsen, T. (2014). Iron: the hard player in diabetes pathophysiology. Acta Physiologica 210, 717–732. [DOI] [PubMed] [Google Scholar]
- Harding, I. H. , Raniga, P. , Delatycki, M. B. , Stagnitti, M. R. , Corben, L. A. , Storey, E. , Georgiou‐Karistianis, N. & Egan, G. F. (2016). Tissue atrophy and elevated iron concentration in the extrapyramidal motor system in Friedreich ataxia: the IMAGE‐FRDA study. Journal of Neurology, Neurosurgery, and Psychiatry 87, 1261–1263. [DOI] [PubMed] [Google Scholar]
- Hare, D. J. , Lei, P. , Ayton, S. , Roberts, B. R. , Grimm, R. , George, J. L. , Bishop, D. P. , Beavis, A. D. , Donovan, S. J. , McColl, G. , Volitakis, I. , Masters, C. L. , Adlard, P. A. , Cherny, R. A. , Bush, A. I. , et al. (2014). An iron–dopamine index predicts risk of parkinsonian neurodegeneration in the substantia nigra pars compacta. Chemical Science 5, 2160–2169. 10.1039/c3sc53461h. [DOI] [Google Scholar]
- Harms, A. , Maisonneuve, E. & Gerdes, K. (2016). Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354, 1390 (aaf4268). [DOI] [PubMed] [Google Scholar]
- Harris, M. A. , Tsui, J. K. , Marion, S. A. , Shen, H. & Teschke, K. (2012). Association of Parkinson's disease with infections and occupational exposure to possible vectors. Movement Disorders 27, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Harté, E. , Maalouli, N. , Shalabney, A. , Texier, E. , Berthelot, K. , Lecomte, S. & Alves, I. D. (2014). Probing the kinetics of lipid membrane formation and the interaction of a nontoxic and a toxic amyloid with plasmon waveguide resonance. Chemical Communications 50, 4168–4171. [DOI] [PubMed] [Google Scholar]
- Havey, T. C. , Fowler, R. A. & Daneman, N. (2011). Duration of antibiotic therapy for bacteremia: a systematic review and meta‐analysis. Critical Care 15, R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q. , Yu, W. , Wu, J. , Chen, C. , Lou, Z. , Zhang, Q. , Zhao, J. , Wang, J. & Xiao, B. (2013). Intranasal LPS‐mediated Parkinson's model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS One 8, e78418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti, F. , Goure, W. F. , Jerecic, J. , Iverson, K. S. , Walicke, P. A. & Krafft, G. A. (2013). The case for soluble Abeta oligomers as a drug target in Alzheimer's disease. Trends in Pharmacological Science 34, 261–266. [DOI] [PubMed] [Google Scholar]
- Heinrich, R. & Rapoport, T. A. (1974). A linear steady‐state treatment of enzymatic chains. General properties, control and effector strength. European Journal of Biochemistry 42, 89–95. [DOI] [PubMed] [Google Scholar]
- Hesselink, D. A. , Aarden, L. A. & Swaak, A. J. G. (2003). Profiles of the acute‐phase reactants C‐reactive protein and ferritin related to the disease course of patients with systemic lupus erythematosus. Scandinavian Journal of Rheumatology 32, 151–155. [DOI] [PubMed] [Google Scholar]
- Hider, R. C. & Kong, X. (2013). Iron speciation in the cytosol: an overview. Dalton Transactions 42, 3220–3229. [DOI] [PubMed] [Google Scholar]
- Hoban, D. B. , Connaughton, E. , Connaughton, C. , Hogan, G. , Thornton, C. , Mulcahy, P. , Moloney, T. C. & Dowd, E. (2013). Further characterisation of the LPS model of Parkinson's disease: a comparison of intra‐nigral and intra‐striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain, Behavior, and Immunity 27, 91–100. [DOI] [PubMed] [Google Scholar]
- Holden, D. W. (2015). Persisters unmasked. Science 347, 30–32. [DOI] [PubMed] [Google Scholar]
- Holden, V. I. , Breen, P. , Houle, S. , Dozois, C. M. & Bachman, M. A. (2016). Klebsiella pneumoniae siderophores induce inflammation, bacterial dissemination, and HIF‐1alpha stabilization during pneumonia. mBio 7, e01397‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, T. L. , Arnold, C. & Fowler, V. G. Jr. (2014). Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 312, 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, N. K. , Jespersen, S. K. , Thomassen, L. V. , Wolff, T. Y. , Sehgal, P. , Thomsen, L. A. , Christiansen, G. , Andersen, C. B. , Knudsen, A. D. & Otzen, D. E. (2007). Aggregation and fibrillation of bovine serum albumin. Biochimica et Biophysica Acta 1774, 1128–1138. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf, J. J. , de Vos, A. F. , Levi, M. , Bresser, P. , van der Zee, J. S. , Draing, C. , von Aulock, S. & van der Poll, T. (2009). Activation of coagulation and inhibition of fibrinolysis in the human lung on bronchial instillation of lipoteichoic acid and lipopolysaccharide. Critical Care Medicine 37, 619–625. [DOI] [PubMed] [Google Scholar]
- Hori, A. , Mizoue, T. , Kasai, H. , Kawai, K. , Matsushita, Y. , Nanri, A. , Sato, M. & Ohta, M. (2010). Body iron store as a predictor of oxidative DNA damage in healthy men and women. Cancer Science 101, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzempa, J. , O'Dee, D. M. , Stolz, D. B. , Franks, J. M. , Clay, D. & Nau, G. J. (2011). Invasion of erythrocytes by Francisella tularensis . Journal of Infectious Diseases 204, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, K. , Takeuchi, O. , Kawai, T. , Sanjo, H. , Ogawa, T. , Takeda, Y. , Takeda, K. & Akira, S. (1999). Toll‐like receptor 4 (TLR4)‐deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. Journal of Immunology 162, 3749–3752. [PubMed] [Google Scholar]
- Hou, D. , Zhou, X. , Zhong, X. , Settles, M. L. , Herring, J. , Wang, L. , Abdo, Z. , Forney, L. J. & Xu, C. (2013). Microbiota of the seminal fluid from healthy and infertile men. Fertility and Sterility 100, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser, M. C. & Tansey, M. G. (2017). The gut‐brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinson's Disease 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hritcu, L. & Ciobica, A. (2013). Intranigral lipopolysaccharide administration induced behavioral deficits and oxidative stress damage in laboratory rats: relevance for Parkinson's disease. Behavioural Brain Research 253, 25–31. [DOI] [PubMed] [Google Scholar]
- Hritcu, L. , Ciobica, A. , Stefan, M. , Mihasan, M. , Palamiuc, L. & Nabeshima, T. (2011). Spatial memory deficits and oxidative stress damage following exposure to lipopolysaccharide in a rodent model of Parkinson's disease. Neuroscience Research 71, 35–43. [DOI] [PubMed] [Google Scholar]
- Hua, S. , Song, C. , Geczy, C. L. , Freedman, S. B. & Witting, P. K. (2009). A role for acute‐phase serum amyloid A and high‐density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis. Redox Report 14, 187–196. [DOI] [PubMed] [Google Scholar]
- Huffnagle, G. B. , Dickson, R. P. & Lukacs, N. W. (2017). The respiratory tract microbiome and lung inflammation: a two‐way street. Mucosal Immunology 10, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, A. S. M. , Liang, Y. , Chow, T. C. H. , Tang, H. C. , Wu, S. L. Y. , Wai, M. S. M. & Yew, D. T. (2016). Mutated tau, amyloid and neuroinflammation in Alzheimer disease‐A brief review. Progress in Histochemistry and Cytochemistry 51, 1–8. [DOI] [PubMed] [Google Scholar]
- Huth, C. , Beuerle, S. , Zierer, A. , Heier, M. , Herder, C. , Kaiser, T. , Koenig, W. , Kronenberg, F. , Oexle, K. , Rathmann, W. , Roden, M. , Schwab, S. , Seissler, J. , Stockl, D. , Meisinger, C. , Peters, A. & Thorand, B. (2015). Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. European Journal of Endocrinology 173, 643–653. [DOI] [PubMed] [Google Scholar]
- Icardi, A. , Paoletti, E. , De Nicola, L. , Mazzaferro, S. , Russo, R. & Cozzolino, M. (2013). Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrology Dialysis Transplantation 28, 1672–1679. [DOI] [PubMed] [Google Scholar]
- Ignjatović, A. , Stević, Z. , Lavrnić, D. , Nikolić‐Kokić, A. , Blagojević, D. , Spasić, M. & Spasojević, I. (2012). Inappropriately chelated iron in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Amyotrophic Lateral Sclerosis 13, 357–362. [DOI] [PubMed] [Google Scholar]
- Ignjatović, A. , Stević, Z. , Lavrnić, S. , Daković, M. & Bačić, G. (2013). Brain iron MRI: a biomarker for amyotrophic lateral sclerosis. Journal of Magnetic Resonance Imaging 38, 1472–1479. [DOI] [PubMed] [Google Scholar]
- Imai, H. , Matsuoka, M. , Kumagai, T. , Sakamoto, T. & Koumura, T. (2017). Lipid peroxidation‐dependent cell death regulated by GPx4 and ferroptosis. Current Topics in Microbiology and Immunology 403, 143–170. [DOI] [PubMed] [Google Scholar]
- Ishida, J. H. & Johansen, K. L. (2014). Iron and infection in hemodialysis patients. Seminars in Dialysis 27, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, K. J. & Akira, S. (2004). Toll‐like receptors and sepsis. Current Infectious Disease Reports 6, 361–366. [DOI] [PubMed] [Google Scholar]
- Itzhaki, R. F. , Lathe, R. , Balin, B. J. , Ball, M. J. , Braak, H. , Bearer, E. L. , Bullido, M. J. , Carter, C. , Clerici, M. , Cosby, S. L. , Del Tredici, K. , Field, H. , Fulop, T. , Grassi, C. , Griffin, W. S. T. , et al. (2016). Microbes and Alzheimer's disease. Journal of Alzheimers Disease 51, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui, S. , Eisenberg, R. A. & Dixon, F. J. (1979). IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. Journal of Immunology 122, 2096–2102. [PubMed] [Google Scholar]
- Jamal‐Allial, A. , Griffith, J. L. & Tucker, K. L. (2014). The longitudinal association of vitamin D serum concentrations & adiposity phenotype. Journal of Steroid Biochemistry and Molecular Biology 144(Pt. A), 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero, D. R. (1990). Malondialdehyde and thiobarbituric acid reactivity as diagnostic indexes of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine 9, 515–540. [DOI] [PubMed] [Google Scholar]
- Jang, H. , Arce, F. T. , Ramachandran, S. , Kagan, B. L. , Lal, R. & Nussinov, R. (2014). Disordered amyloidogenic peptides may insert into the membrane and assemble into common cyclic structural motifs. Chemical Society Reviews 43, 6750–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, H. , Connelly, L. , Arce, F. T. , Ramachandran, S. , Kagan, B. L. , Lal, R. & Nussinov, R. (2013). Mechanisms for the insertion of toxic, fibril‐like beta‐amyloid oligomers into the membrane. Journal of Chemical Theory and Computation 9, 822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J. H. , Aruoma, O. I. , Jen, L. S. , Chung, H. Y. & Surh, Y. J. (2004). Ergothioneine rescues PC12 cells from beta‐amyloid‐induced apoptotic death. Free Radical Biology and Medicine 36, 288–299. [DOI] [PubMed] [Google Scholar]
- Janson, J. , Ashley, R. H. , Harrison, D. , McIntyre, S. & Butler, P. C. (1999). The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate‐sized toxic amyloid particles. Diabetes 48, 491–498. [DOI] [PubMed] [Google Scholar]
- Javurek, A. B. , Spollen, W. G. , Ali, A. M. M. , Johnson, S. A. , Lubahn, D. B. , Bivens, N. J. , Bromert, K. H. , Ellersieck, M. R. , Givan, S. A. & Rosenfeld, C. S. (2016). Discovery of a novel seminal fluid microbiome and influence of estrogen receptor alpha genetic status. Scientific Reports 6, 23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayashree, B. , Bibin, Y. S. , Prabhu, D. , Shanthirani, C. S. , Gokulakrishnan, K. , Lakshmi, B. S. , Mohan, V. & Balasubramanyam, M. (2014). Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Molecular and Cellular Biochemistry 388, 203–210. [DOI] [PubMed] [Google Scholar]
- Jellen, L. C. , Beard, J. L. & Jones, B. C. (2009). Systems genetics analysis of iron regulation in the brain. Biochimie 91, 1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. J. , Teichert, A. E. , Fong, F. , Oda, Y. & Bikle, D. D. (2013). 1alpha,25(OH)2‐dihydroxyvitamin D3/VDR protects the skin from UVB‐induced tumor formation by interacting with the beta‐catenin pathway. Journal of Steroid Biochemistry and Molecular Biology 136, 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez‐Dalmaroni, M. J. , Gerswhin, M. E. & Adamopoulos, I. E. (2016). The critical role of toll‐like receptors‐‐from microbial recognition to autoimmunity: a comprehensive review. Autoimmunity Reviews 15, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. E. & Lennon, J. T. (2010). Dormancy contributes to the maintenance of microbial diversity. Proceedings of the National Academy of Sciences of the United States of America 107, 5881–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousilahti, P. , Salomaa, V. , Rasi, V. , Vahtera, E. & Palosuo, T. (2003). Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. Journal of Epidemiology and Community Health 57, 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd, S. E. , Morgan, C. J. , Panwar, B. , Howard, V. J. , Wadley, V. G. , Jenny, N. S. , Kissela, B. M. & Gutiérrez, O. M. (2016). Vitamin D deficiency and incident stroke risk in community‐living black and white adults. International Journal of Stroke 11, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado, R. L. (1997). Iron, infections, and anemia of inflammation. Clinical Infectious Diseases 25, 888–895. [DOI] [PubMed] [Google Scholar]
- Kacser, H. & Burns, J. A. (1973). The control of flux In Rate Control of Biological Processes. Symposium of the Society for Experimental Biology Vol 27 (ed. Davies D. D.), pp. 65–104. Cambridge University Press, Cambridge. [PubMed] [Google Scholar]
- Kadam, P. , Gregory, N. A. , Zelger, B. & Carlson, J. A. (2015). Delayed onset of the Jarisch‐Herxheimer reaction in doxycycline‐treated disease: a case report and review of its histopathology and implications for pathogenesis. American Journal of Dermatopathology 37, e68–e74. [DOI] [PubMed] [Google Scholar]
- Kaprelyants, A. S. , Gottschal, J. C. & Kell, D. B. (1993). Dormancy in non‐sporulating bacteria. FEMS Microbiology Reviews 10, 271–286. [DOI] [PubMed] [Google Scholar]
- Kaprelyants, A. S. & Kell, D. B. (1992). Rapid assessment of bacterial viability and vitality using rhodamine 123 and flow cytometry. Journal of Applied Bacteriology 72, 410–422. [Google Scholar]
- Kaprelyants, A. S. & Kell, D. B. (1993). Dormancy in stationary‐phase cultures of Micrococcus luteus: flow cytometric analysis of starvation and resuscitation. Applied and Environmental Microbiology 59, 3187–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprelyants, A. S. , Mukamolova, G. V. & Kell, D. B. (1994). Estimation of dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell‐free spent medium at high dilution. FEMS Microbiology Letters 115, 347–352. [Google Scholar]
- Kaptoge, S. , Di Angelantonio, E. , Pennells, L. , Wood, A. M. , White, I. R. , Gao, P. , Walker, M. , Thompson, A. , Sarwar, N. , Caslake, M. , Butterworth, A. S. , Amouyel, P. , Assmann, G. , Bakker, S. J. , Barr, E. L. , et al. (2012). C‐reactive protein, fibrinogen, and cardiovascular disease prediction. New England Journal of Medicine 367, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakis, I. , Pase, M. P. , Beiser, A. , Booth, S. L. , Jacques, P. F. , Rogers, G. , DeCarli, C. , Vasan, R. S. , Wang, T. J. , Himali, J. J. , Annweiler, C. & Seshadri, S. (2016). Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham Heart Study. Journal of Alzheimer's Disease 51, 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam, C. , Barrett, M. J. , Imperato, T. , MacGowan, D. J. L. & Scelsa, S. (2013). Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. Journal of Clinical Neuroscience 20, 1550–1553. [DOI] [PubMed] [Google Scholar]
- Kassi, E. , Adamopoulos, C. , Basdra, E. K. & Papavassiliou, A. G. (2013). Role of vitamin D in atherosclerosis. Circulation 128, 2517–2531. [DOI] [PubMed] [Google Scholar]
- Kato, T. , Honda, Y. , Kurita, Y. , Iwasaki, A. , Sato, T. , Kessoku, T. , Uchiyama, S. , Ogawa, Y. , Ohkubo, H. , Higurashi, T. , Yamanaka, T. , Usuda, H. , Wada, K. & Nakajima, A. (2017). Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: a prospective randomized pilot study in healthy volunteers. PLoS One 12, e0175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, T. & Akira, S. (2011). Toll‐like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. [DOI] [PubMed] [Google Scholar]
- Kayed, R. , Head, E. , Thompson, J. L. , McIntire, T. M. , Milton, S. C. , Cotman, C. W. & Glabe, C. G. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. [DOI] [PubMed] [Google Scholar]
- Kayed, R. & Lasagna‐Reeves, C. A. (2013). Molecular mechanisms of amyloid oligomers toxicity. Journal of Alzheimers Disease 33(Suppl. 1), S67–S78. [DOI] [PubMed] [Google Scholar]
- Ke, L. , Mason, R. S. , Kariuki, M. , Mpofu, E. & Brock, K. E. (2015). Vitamin D status and hypertension: a review. Integrated Blood Pressure Control 8, 13–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke, P. C. , Sani, M. A. , Ding, F. , Kakinen, A. , Javed, I. , Separovic, F. , Davis, T. P. & Mezzenga, R. (2017). Implications of peptide assemblies in amyloid diseases. Chemical Society Reviews 46, 6492–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns, M. D. , Alvarez, J. A. , Seidel, N. & Tangpricha, V. (2015). Impact of vitamin D on infectious disease. American Journal of Medical Sciences 349, 245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull, M. , Demmer, R. T. & Papapanou, P. N. (2010). "Gum bug, leave my heart alone!"‐‐epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. Journal of Dental Research 89, 879–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegulian, N. C. , Sankhagowit, S. , Apostolidou, M. , Jayasinghe, S. A. , Malmstadt, N. , Butler, P. C. & Langen, R. (2015). Membrane curvature‐sensing and curvature‐inducing activity of islet amyloid polypeptide and its implications for membrane disruption. Journal of Biological Chemistry 290, 25782–25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer, J. P. (2000). The Haber‐Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. (2006). Metabolomics, modelling and machine learning in systems biology: towards an understanding of the languages of cells. The 2005 Theodor Bücher lecture. FEBS Journal 273, 873–894. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. (2009). Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Medical Genomics 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell, D. B. (2010). Towards a unifying, systems biology understanding of large‐scale cellular death and destruction caused by poorly liganded iron: Parkinson's, Huntington's, Alzheimer's, prions, bactericides, chemical toxicology and others as examples. Archives of Toxicology 577, 825–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell, D. B. , Kaprelyants, A. S. , Weichart, D. H. , Harwood, C. L. & Barer, M. R. (1998). Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Van Leeuwenhoek 73, 169–187. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. & Kenny, L. C. (2016). A dormant microbial component in the development of pre‐eclampsia. Frontiers in Medicine: Obstetrics and Gynecology 3, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell, D. B. & Knowles, J. D. (2006). The role of modeling in systems biology In System Modeling in Cellular Biology: From Concepts to Nuts and Bolts (eds Szallasi Z., Stelling J. and Periwal V.), pp. 3–18. MIT Press, Cambridge. [Google Scholar]
- Kell, D. B. , Potgieter, M. & Pretorius, E. (2015). Individuality, phenotypic differentiation, dormancy and ‘persistence’ in culturable bacterial systems: commonalities shared by environmental, laboratory, and clinical microbiology. F1000Research 4, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell, D. B. & Pretorius, E. (2014). Serum ferritin is an important disease marker, and is mainly a leakage product from damaged cells. Metallomics 6, 748–773. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. & Pretorius, E. (2015a). On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS‐induced cell death. Integrative Biology 7, 1339–1377. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. & Pretorius, E. (2015b). The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integrative Biology 7, 24–52. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. & Pretorius, E. (2016). To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? bioRxiv preprint. BioRxiv, 057851. 10.1101/057851. [DOI] [PMC free article] [PubMed]
- Kell, D. B. & Pretorius, E. (2017a). Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progress in Biophysics & Molecular Biology 123, 16–41. [DOI] [PubMed] [Google Scholar]
- Kell, D. B. & Pretorius, E. (2017b). To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? Seminars in Thrombosis and Hemostasis, in press. 10.1055/s-0037-1604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, L. P. , Carvey, P. M. , Keshavarzian, A. , Shannon, K. M. , Shaikh, M. , Bakay, R. A. & Kordower, J. H. (2014). Progression of intestinal permeability changes and alpha‐synuclein expression in a mouse model of Parkinson's disease. Movement Disorders 29, 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, L. C. & Kell, D. B. (2018). Immunological tolerance, pregnancy and pre‐eclampsia: the roles of semen microbes and the father, p. 198796 Obstetrics and Gynecology, Frontiers in Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley, R. N. , McCarthy, C. , Kell, D. B. & Kenny, L. C. (2018). The potential therapeutic effects of ergothioneine in pre‐eclampsia. Free Radical Biology and Medicine 117, 145–157. [DOI] [PubMed] [Google Scholar]
- Kester, J. C. & Fortune, S. M. (2014). Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Critical Reviews in Biochemistry and Molecular Biology 49, 91–101. [DOI] [PubMed] [Google Scholar]
- Keum, N. & Giovannucci, E. (2014). Vitamin D supplements and cancer incidence and mortality: a meta‐analysis. British Journal of Cancer 111, 976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, F. A. , Fisher, M. A. & Khakoo, R. A. (2007). Association of hemochromatosis with infectious diseases: expanding spectrum. International Journal of Infectious Diseases 11, 482–487. [DOI] [PubMed] [Google Scholar]
- Khedoe, P. P. S. J. , Wong, M. C. , Wagenaar, G. T. M. , Plomp, J. J. , van Eck, M. , Havekes, L. M. , Rensen, P. C. N. , Hiemstra, P. S. & Berbée, J. F. B. (2013). The effect of PPE‐induced emphysema and chronic LPS‐induced pulmonary inflammation on atherosclerosis development in APOE*3‐LEIDEN mice. PLoS One 8, e80196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholová, I. & Niessen, H. W. M. (2005). Amyloid in the cardiovascular system: a review. Journal of Clinical Pathology 58, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechl, S. , Egger, G. , Mayr, M. , Wiedermann, C. J. , Bonora, E. , Oberhollenzer, F. , Muggeo, M. , Xu, Q. , Wick, G. , Poewe, W. & Willeit, J. (2001). Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation 103, 1064–1070. [DOI] [PubMed] [Google Scholar]
- Kienreich, K. , Tomaschitz, A. , Verheyen, N. , Pieber, T. , Gaksch, M. , Grübler, M. R. & Pilz, S. (2013). Vitamin D and cardiovascular disease. Nutrients 5, 3005–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian, M. , Chapple, I. L. C. , Hannig, M. , Marsh, P. D. , Meuric, V. , Pedersen, A. M. L. , Tonetti, M. S. , Wade, W. G. & Zaura, E. (2016). The oral microbiome ‐ an update for oral healthcare professionals. British Dental Journal 221, 657–666. [DOI] [PubMed] [Google Scholar]
- Kim, C. , Lv, G. , Lee, J. S. , Jung, B. C. , Masuda‐Suzukake, M. , Hong, C. S. , Valera, E. , Lee, H. J. , Paik, S. R. , Hasegawa, M. , Masliah, E. , Eliezer, D. & Lee, S. J. (2016). Exposure to bacterial endotoxin generates a distinct strain of alpha‐synuclein fibril. Scientific Reports 6, 30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. , Mun, S. , Lee, J. , Park, A. , Seok, A. , Chun, Y. T. & Kang, H. G. (2018). Proteomics analysis reveals differential pattern of widespread protein expression and novel role of histidine‐rich glycoprotein and lipopolysaccharide‐binding protein in rheumatoid arthritis. International Journal of Biological Macromolecules 109, 704–710. [DOI] [PubMed] [Google Scholar]
- King, V. L. , Thompson, J. & Tannock, L. R. (2011). Serum amyloid A in atherosclerosis. Current Opinions in Lipidology 22, 302–307. [DOI] [PubMed] [Google Scholar]
- Klipp, E. , Herwig, R. , Kowald, A. , Wierling, C. & Lehrach, H. (2005). Systems Biology in Practice: Concepts, Implementation and Clinical Application. Wiley/VCH, Berlin. [Google Scholar]
- Knowles, T. P. J. , Vendruscolo, M. & Dobson, C. M. (2014). The amyloid state and its association with protein misfolding diseases. Nature Reviews Molecular Cell Biology 15, 384–396. [DOI] [PubMed] [Google Scholar]
- Konarkowska, B. , Aitken, J. F. , Kistler, J. , Zhang, S. & Cooper, G. J. S. (2006). The aggregation potential of human amylin determines its cytotoxicity towards islet beta‐cells. FEBS Journal 273, 3614–3624. [DOI] [PubMed] [Google Scholar]
- Kong, H. H. , Andersson, B. , Clavel, T. , Common, J. E. , Jackson, S. A. , Olson, N. D. , Segre, J. A. & Traidl‐Hoffmann, C. (2017). Performing skin microbiome research: a method to the madness. Journal of Investigative Dermatology 137, 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, H. H. , Oh, J. , Deming, C. , Conlan, S. , Grice, E. A. , Beatson, M. A. , Nomicos, E. , Polley, E. C. , Komarow, H. D. , Program, N. C. S. , Murray, P. R. , Turner, M. L. & Segre, J. A. (2012). Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Research 22, 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsbak, M. , Levring, T. B. , Geisler, C. & Rode von Essen, M. (2013). The vitamin D receptor and T cell function. Frontiers in Immunology 4, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig, M. F. , Abusleme, L. , Reinholdt, J. , Palmer, R. J. , Teles, R. P. , Sampson, K. , Rosen, A. , Nigrovic, P. A. , Sokolove, J. , Giles, J. T. , Moutsopoulos, N. M. & Andrade, F. (2016). Aggregatibacter actinomycetemcomitans‐induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Science Translational Medicine 8, 369ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konz, T. , Añón Alvarez, E. , Montes‐Bayon, M. & Sanz‐Medel, A. (2013). Antibody labeling and elemental mass spectrometry (inductively coupled plasma‐mass spectrometry) using isotope dilution for highly sensitive ferritin determination and iron‐ferritin ratio measurements. Analytical Chemistry 85, 8334–8340. [DOI] [PubMed] [Google Scholar]
- Koppel, N. , Maini Rekdal, V. & Balskus, E. P. (2017). Chemical transformation of xenobiotics by the human gut microbiota. Science 356, 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren, O. , Spor, A. , Felin, J. , Fåk, F. , Stombaugh, J. , Tremaroli, V. , Behre, C. J. , Knight, R. , Fagerberg, B. , Ley, R. E. & Bäckhed, F. (2011). Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America 108(Suppl. 1), 4592–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskenkorva‐Frank, T. S. , Weiss, G. , Koppenol, W. H. & Burckhardt, S. (2013). The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radical Biology and Medicine 65, 1174–1194. [DOI] [PubMed] [Google Scholar]
- Kowarsky, M. , Camunas, J. , Kertesz, M. , Vlaminck, I. D. , Koh, W. , Pan, W. , Martin, L. , Neff, N. , Okamoto, J. , Wong, R. , Kharbanda, S. , El‐Sayed, Y. , Blumenfeld, Y. , Stevenson, D. K. , Shaw, G. , Wolfe, N. D. & Quake, S. R. (2017). Humans are colonized by many uncharacterized and highly divergent microbes. BioRxiv, 113746. 10.1101/113746. [DOI] [PMC free article] [PubMed]
- Koziel, J. , Mydel, P. & Potempa, J. (2014). The link between periodontal disease and rheumatoid arthritis: an updated review. Current Rheumatology Reports 16, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraml, P. (2017). The role of iron in the pathogenesis of atherosclerosis. Physiological Research 66, S55–S67. [DOI] [PubMed] [Google Scholar]
- Krebs, J. , Bartel, P. & Pannek, J. (2014). Bacterial persistence in the prostate after antibiotic treatment of chronic bacterial prostatitis in men with spinal cord injury. Urology 83, 515–520. [DOI] [PubMed] [Google Scholar]
- Kumar, D. K. V. , Choi, S. H. , Washicosky, K. J. , Eimer, W. A. , Tucker, S. , Ghofrani, J. , Lefkowitz, A. , McColl, G. , Goldstein, L. E. , Tanzi, R. E. & Moir, R. D. (2016). Amyloid‐beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Science Translational Medicine 8, 340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, H. , Kawai, T. & Akira, S. (2011). Pathogen recognition by the innate immune system. International Reviews of Immunology 30, 16–34. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Ingle, H. , Prasad, D. V. & Kumar, H. (2013). Recognition of bacterial infection by innate immune sensors. Critical Reviews in Microbiology 39, 229–246. [DOI] [PubMed] [Google Scholar]
- Kundu, D. , Roy, A. , Mandal, T. , Bandyopadhyay, U. , Ghosh, E. & Ray, D. (2013). Relation of iron stores to oxidative stress in type 2 diabetes. Nigerian Journal of Clinical Practice 16, 100–103. [DOI] [PubMed] [Google Scholar]
- Lago, F. , Gómez, R. , Conde, J. , Scotece, M. , Gómez‐Reino, J. J. & Gualillo, O. (2011). Cardiometabolic comorbidities and rheumatic diseases: focus on the role of fat mass and adipokines. Arthritis Care & Research (Hoboken) 63, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Lakota, K. , Resnik, N. , Mrak‐Poljšak, K. , Sodin‐Šemrl, S. & Veranič, P. (2011). Colocalization of serum amyloid a with microtubules in human coronary artery endothelial cells. Journal of Biomedicine and Biotechnology 2011, 528276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, E. & Lang, F. (2015). Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Seminars in Cell & Developmental Biology 39, 35–42. [DOI] [PubMed] [Google Scholar]
- Lang, E. , Qadri, S. M. & Lang, F. (2012a). Killing me softly ‐ Suicidal erythrocyte death. International Journal of Biochemistry & Cell Biology 44, 1236–1243. [DOI] [PubMed] [Google Scholar]
- Lang, F. , Lang, E. & Foller, M. (2012b). Physiology and pathophysiology of eryptosis. Transfusion Medicine and Hemotherapy 39, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, F. , Gulbins, E. , Lang, P. A. , Zappulla, D. & Foller, M. (2010). Ceramide in suicidal death of erythrocytes. Cellular Physiology & Biochemistry 26, 21–28. [DOI] [PubMed] [Google Scholar]
- Lang, F. & Qadri, S. M. (2012). Mechanisms and significance of eryptosis, the suicidal death of erythrocytes. Blood Purification 33, 125–130. [DOI] [PubMed] [Google Scholar]
- Langkilde, A. E. , Morris, K. L. , Serpell, L. C. , Svergun, D. I. & Vestergaard, B. (2015). The architecture of amyloid‐like peptide fibrils revealed by X‐ray scattering, diffraction and electron microscopy. Acta Crystallographica Section D 71, 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannergård, A. , Larsson, A. , Friman, G. & Ewald, U. (2008). Human serum amyloid A (SAA) and high sensitive C‐reactive protein (hsCRP) in preterm newborn infants with nosocomial infections. Acta Paediatrica 97, 1061–1065. [DOI] [PubMed] [Google Scholar]
- Latz, E. , Xiao, T. S. & Stutz, A. (2013). Activation and regulation of the inflammasomes. Nature Reviews Immunology 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder, A. P. , Roche, A. M. , Sherrill‐Mix, S. , Bailey, A. , Laughlin, A. L. , Bittinger, K. , Leite, R. , Elovitz, M. A. , Parry, S. & Bushman, F. D. (2016). Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layoun, A. & Santos, M. M. (2012). Bacterial cell wall constituents induce hepcidin expression in macrophages through MyD88 signaling. Inflammation 35, 1500–1506. [DOI] [PubMed] [Google Scholar]
- Le Bastard, Q. , Al‐Ghalith, G. A. , Gregoire, M. , Chapelet, G. , Javaudin, F. , Dailly, E. , Batard, E. , Knights, D. & Montassier, E. (2017). Systematic review: human gut dysbiosis induced by non‐antibiotic prescription medications. Alimentary Pharmacology and Therapeutics 47, 442–345. [DOI] [PubMed] [Google Scholar]
- Le Novère, N. , Hucka, M. , Mi, H. , Moodie, S. , Schreiber, F. , Sorokin, A. , Demir, E. , Wegner, K. , Aladjem, M. , Wimalaratne, S. M. , Bergman, F. T. , Gauges, R. , Ghazal, P. , Hideya, K. , Li, L. , et al. (2009). The systems biology graphical notation. Nature Biotechnology 27, 735–741. [DOI] [PubMed] [Google Scholar]
- Lee, D. W. & Andersen, J. K. (2010). Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? Journal of Neurochemistry 112, 332–339. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Gillman, A. L. , Jang, H. , Ramachandran, S. , Kagan, B. L. , Nussinov, R. & Teran Arce, F. (2014). Role of the fast kinetics of pyroglutamate‐modified amyloid‐beta oligomers in membrane binding and membrane permeability. Biochemistry 53, 4704–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. C. , Son, Y. O. , Choi, K. C. & Jang, Y. S. (2006). Hydrogen peroxide induces apoptosis of BJAB cells due to formation of hydroxyl radicals via intracellular iron‐mediated Fenton chemistry in glucose oxidase‐mediated oxidative stress. Molecules and Cells 22, 21–29. [PubMed] [Google Scholar]
- Lee, J. Y. , Choi, I. A. , Kim, J. H. , Kim, K. H. , Lee, E. Y. , Lee, E. B. , Lee, Y. M. & Song, Y. W. (2015). Association between anti‐Porphyromonas gingivalis or anti‐alpha‐enolase antibody and severity of periodontitis or rheumatoid arthritis (RA) disease activity in RA. BMC Musculoskeletal Disorders 16, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. , Peng, H. , Gelbart, T. , Wang, L. & Beutler, E. (2005). Regulation of hepcidin transcription by interleukin‐1 and interleukin‐6. Proceedings of the National Academy of Sciences of the United States of America 102, 1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, C. , Islam, S. , Jarosch, S. , Zhou, J. , Hoskin, D. , Greenshields, A. , Al‐Banna, N. , Sharawy, N. , Sczcesniak, A. , Kelly, M. , Wafa, K. , Cheliak, W. & Holbein, B. (2015). The utility of iron chelators in the management of inflammatory disorders. Mediators of Inflammation 2015, 516740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon, J. T. & Jones, S. E. (2011). Microbial seed banks: the ecological and evolutionary implications of dormancy. Nature Reviews Microbiology 9, 119–130. [DOI] [PubMed] [Google Scholar]
- Lepper, P. M. , Held, T. K. , Schneider, E. M. , Bölke, E. , Gerlach, H. & Trautmann, M. (2002). Clinical implications of antibiotic‐induced endotoxin release in septic shock. Intensive Care Medicine 28, 824–833. [DOI] [PubMed] [Google Scholar]
- Lepper, P. M. , Kleber, M. E. , Grammer, T. B. , Hoffmann, K. , Dietz, S. , Winkelmann, B. R. , Boehm, B. O. & März, W. (2011). Lipopolysaccharide‐binding protein (LBP) is associated with total and cardiovascular mortality in individuals with or without stable coronary artery disease‐‐results from the Ludwigshafen Risk and Cardiovascular Health Study (LURIC). Atherosclerosis 219, 291–297. [DOI] [PubMed] [Google Scholar]
- Lepper, P. M. , Schumann, C. , Triantafilou, K. , Rasche, F. M. , Schuster, T. , Frank, H. , Schneider, E. M. , Triantafilou, M. & von Eynatten, M. (2007). Association of lipopolysaccharide‐binding protein and coronary artery disease in men. Journal of the American College of Cardiology 50, 25–31. [DOI] [PubMed] [Google Scholar]
- Levels, J. H. M. , Abraham, P. R. , van Barreveld, E. P. , Meijers, J. C. M. & van Deventer, S. J. G. (2003). Distribution and kinetics of lipoprotein‐bound lipoteichoic acid. Infection & Immunity 71, 3280–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, S. & Finazzi, D. (2014). Neurodegeneration with brain iron accumulation: update on pathogenic mechanisms. Frontiers in Pharmacology 5, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, A. J. , Seymour, C. W. & Rosengart, M. R. (2016). Current murine models of sepsis. Surgical Infections 17, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, K. (2007). Persister cells, dormancy and infectious disease. Nature Reviews Microbiology 5, 48–56. [DOI] [PubMed] [Google Scholar]
- Lewis, K. (2010). Persister cells. Annual Review of Microbiology 64, 357–372. [DOI] [PubMed] [Google Scholar]
- Li, C. , Ma, D. , Chen, M. , Zhang, L. , Zhang, L. , Zhang, J. , Qu, X. & Wang, C. (2016). Ulinastatin attenuates LPS‐induced human endothelial cells oxidative damage through suppressing JNK/c‐Jun signaling pathway. Biochemical and Biophysical Research Communications 474, 572–578. [DOI] [PubMed] [Google Scholar]
- Li, H. , Ooi, S. Q. & Heng, C. K. (2013). The role of NF‐kB in SAA‐induced peroxisome proliferator‐activated receptor g activation. Atherosclerosis 227, 72–78. [DOI] [PubMed] [Google Scholar]
- Li, S. W. , Liu, C. M. , Guo, J. , Marcondes, A. M. , Deeg, J. , Li, X. & Guan, F. (2016). Iron overload induced by ferric ammonium citrate triggers reactive oxygen species‐mediated apoptosis via both extrinsic and intrinsic pathways in human hepatic cells. Human & Experimental Toxicology 35, 598–607. [DOI] [PubMed] [Google Scholar]
- Li, X. & Atkinson, M. A. (2015). The role for gut permeability in the pathogenesis of type 1 diabetes ‐ a solid or leaky concept? Pediatric Diabetes 16, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. C. , Chen, Y. , Liu, W. & Thadhani, R. (2014). MicroRNA‐mediated mechanism of vitamin D regulation of innate immune response. Journal of Steroid Biochemistry and Molecular Biology 144(Pt. A), 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehl, P. , Zuzarte‐Luis, V. & Mota, M. M. (2015). Unveiling the pathogen behind the vacuole. Nature Reviews Microbiology 13, 589–598. [DOI] [PubMed] [Google Scholar]
- Lien, E. , Means, T. K. , Heine, H. , Yoshimura, A. , Kusumoto, S. , Fukase, K. , Fenton, M. J. , Oikawa, M. , Qureshi, N. , Monks, B. , Finberg, R. W. , Ingalls, R. R. & Golenbock, D. T. (2000). Toll‐like receptor 4 imparts ligand‐specific recognition of bacterial lipopolysaccharide. Journal of Clinical Investigation 105, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. , Zeng, P. , Xu, Z. Y. , Ye, D. Y. , Yu, X. F. , Wang, N. , Tang, J. , Zhou, Y. & Huang, Y. P. (2012). Treatment of Lipoxin A4 and its analogue on low‐dose endotoxin induced preeclampsia in rat and possible mechanisms. Reproductive Toxicology 34, 677–685. [DOI] [PubMed] [Google Scholar]
- Lin, I. H. , Miller, D. S. , Bertics, P. J. , Murphy, C. J. , de Pablo, J. J. & Abbott, N. L. (2011). Endotoxin‐induced structural transformations in liquid crystalline droplets. Science 332, 1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. , Liu, J. , Davies, M. L. & Chen, W. (2016). Serum vitamin D level and rheumatoid arthritis disease activity: review and meta‐analysis. PLoS One 11, e0146351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski, B. & Pretorius, E. (2013a). Iron‐induced fibrin in cardiovascular disease. Current Neurovascular Research 10, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski, B. & Pretorius, E. (2013b). The role of iron‐induced fibrin in the pathogenesis of Alzheimer's disease and the protective role of magnesium. Frontiers in Human Neuroscience 7, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohns, T. J. , Henley, W. E. , Lang, I. A. , Annweiler, C. , Beauchet, O. , Chaves, P. H. , Fried, L. , Kestenbaum, B. R. , Kuller, L. H. , Langa, K. M. , Lopez, O. L. , Kos, K. , Soni, M. & Llewellyn, D. J. (2014). Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B. , Moloney, A. , Meehan, S. , Morris, K. , Thomas, S. E. , Serpell, L. C. , Hider, R. , Marciniak, S. J. , Lomas, D. A. & Crowther, D. C. (2011). Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. Journal of Biological Chemistry 286, 4248–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. L. , Ai, H. W. , Wang, W. P. , Chen, L. , Hu, H. B. , Ye, T. , Zhu, X. H. , Wang, F. , Liao, Y. L. , Wang, Y. , Ou, G. , Xu, L. , Sun, M. , Jian, C. , Chen, Z. J. , Li, L. , Zhang, B. , Tian, L. , Wang, B. , Yan, S. & Sun, Z. Y. (2014). Comparison of 16S rRNA gene PCR and blood culture for diagnosis of neonatal sepsis. Archives de Pédiatrie 21, 162–169. [DOI] [PubMed] [Google Scholar]
- Liu, C. M. , Osborne, B. J. W. , Hungate, B. A. , Shahabi, K. , Huibner, S. , Lester, R. , Dwan, M. G. , Kovacs, C. , Contente‐Cuomo, T. L. , Benko, E. , Aziz, M. , Price, L. B. & Kaul, R. (2014). The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathogens 10, e1004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. & Bing, G. (2011). Lipopolysaccharide animal models for Parkinson's disease. Parkinsons Disease 2011, 327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. T. , Stenger, S. , Li, H. , Wenzel, L. , Tan, B. H. , Krutzik, S. R. , Ochoa, M. T. , Schauber, J. , Wu, K. , Meinken, C. , Kamen, D. L. , Wagner, M. , Bals, R. , Steinmeyer, A. , Zugel, U. , Gallo, R. L. , et al. (2006). Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science 311, 1770–1773. [DOI] [PubMed] [Google Scholar]
- Liu, R. , Wu, C. X. , Zhou, D. , Yang, F. , Tian, S. , Zhang, L. , Zhang, T. T. & Du, G. H. (2012). Pinocembrin protects against beta‐amyloid‐induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)‐independent signaling pathways and regulating mitochondrion‐mediated apoptosis. BMC Medicine 10, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Cui, D. , Hoshii, Y. , Kawano, H. , Une, Y. , Gondo, T. & Ishihara, T. (2007). Induction of murine AA amyloidosis by various homogeneous amyloid fibrils and amyloid‐like synthetic peptides. Scandinavian Journal of Immunology 66, 495–500. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Yang, J. , Bao, J. , Li, X. , Ye, A. , Zhang, G. & Liu, H. (2017). Activation of the cholinergic anti‐inflammatory pathway by nicotine ameliorates lipopolysaccharide‐induced preeclampsia‐like symptoms in pregnant rats. Placenta 49, 23–32. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Yin, H. , Zhao, M. & Lu, Q. (2014). TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clinical Reviews in Allergy & Immunology 47, 136–147. [DOI] [PubMed] [Google Scholar]
- Lloyd, C. M. & Marsland, B. J. (2017). Lung homeostasis: influence of age, microbes, and the immune system. Immunity 46, 549–561. [DOI] [PubMed] [Google Scholar]
- Lloyd‐Price, J. , Mahurkar, A. , Rahnavard, G. , Crabtree, J. , Orvis, J. , Hall, A. B. , Brady, A. , Creasy, H. H. , McCracken, C. , Giglio, M. G. , McDonald, D. , Franzosa, E. A. , Knight, R. , White, O. & Huttenhower, C. (2017). Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 550, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluch, J. , Servant, F. , Païssé, S. , Valle, C. , Valière, S. , Kuchly, C. , Vilchez, G. , Donnadieu, C. , Courtney, M. , Burcelin, R. , Amar, J. , Bouchez, O. & Lelouvier, B. (2015). The characterization of novel tissue microbiota using an optimized 16S metagenomic sequencing pipeline. PLoS One 10, e0142334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft, S. , Fischer‐Nielsen, A. , Jeding, I. B. , Vistisen, K. & Poulsen, H. E. (1993). 8‐Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. Journal of Toxicology and Environmental Health 40, 391–404. [DOI] [PubMed] [Google Scholar]
- Lorenzo, A. , Razzaboni, B. , Weir, G. C. & Yankner, B. A. (1994). Pancreatic islet cell toxicity of amylin associated with type‐2 diabetes mellitus. Nature 368, 756–760. [DOI] [PubMed] [Google Scholar]
- Lu'o'ng, K. V. Q. & Nguyên, L. T. H. (2013). The role of vitamin D in Alzheimer's disease: possible genetic and cell signaling mechanisms. American Journal of Alzheimer's Disease & Other Dementias 28, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luettig, J. , Rosenthal, R. , Barmeyer, C. & Schulzke, J. D. (2015). Claudin‐2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 3, e977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark, K. , Westermark, G. T. , Nystrom, S. , Murphy, C. L. , Solomon, A. & Westermark, P. (2002). Transmissibility of systemic amyloidosis by a prion‐like mechanism. Proceedings of the National Academy of Sciences of the United States of America 99, 6979–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark, K. , Westermark, G. T. , Olsen, A. & Westermark, P. (2005). Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: cross‐seeding as a disease mechanism. Proceedings of the National Academy of Sciences of the United States of America 102, 6098–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, M. , Xia, Y. F. , Li, B. , Liu, H. , Pan, J. Y. , Li, B. B. , Zhang, C. & An, F. S. (2016). Serum amyloid A stimulates vascular endothelial growth factor receptor 2 expression and angiogenesis. Journal of Physiology and Biochemistry 72, 71–81. [DOI] [PubMed] [Google Scholar]
- Lv, Z. , Qi, H. , Wang, L. , Fan, X. , Han, F. , Wang, H. & Bi, S. (2014). Vitamin D status and Parkinson's disease: a systematic review and meta‐analysis. Neurological Sciences 35, 1723–1730. [DOI] [PubMed] [Google Scholar]
- Łysek, R. P. , Szafraniec, K. , Polak, M. , Jankowski, P. , Micek, A. , Wolfshaut‐Wolak, R. , Czarnecka, D. , Potempa, J. & Pająk, A. (2017). Relationship between past myocardial infarction, periodontal disease and Porphyromonas gingivalis serum antibodies: a case‐control study. Cardiology Journal 10.5603/CJ.a2017.0015. [DOI] [PubMed] [Google Scholar]
- Maes, M. (2009). Leaky gut in chronic fatigue syndrome: a review. Activitas Nervosa Superior Rediviva 51, 21–28. [Google Scholar]
- Maes, M. , Coucke, F. & Leunis, J. C. (2007). Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuroendocrinology Letters 28, 739–744. [PubMed] [Google Scholar]
- Maes, M. , De Farias, C. C. , Bonifacio, K. L. , Matsumoto, A. K. , Bortolasci, C. C. , Nogueira, A. S. , Brinholi, F. F. , Morimoto, H. K. , de Melo, L. B. , Moreira, E. G. & Barbosa, D. S. (2017). Parkinson's disease is accompanied by intertwined alterations in iron metabolism and activated immune‐inflammatory and oxidative stress pathways. CNS and Neurological Disorders ‐ Drug Targets 16, 484–491. [DOI] [PubMed] [Google Scholar]
- Mahalakshmi, K. , Krishnan, P. , Krishna Baba, M. G. , Dhivyapriya, V. & Arumugam, S. B. (2017). "Association of periodontopathic anaerobic bacterial co‐occurrence to atherosclerosis" ‐ A cross‐sectional study. Anaerobe 44, 66–72. [DOI] [PubMed] [Google Scholar]
- Maheshwari, P. & Eslick, G. D. (2015). Bacterial infection and Alzheimer's disease: a meta‐analysis. Journal of Alzheimers Disease 43, 957–966. [DOI] [PubMed] [Google Scholar]
- Maiwald, M. & Relman, D. A. (2001). Whipple's disease and Tropheryma whippelii: secrets slowly revealed. Clinical Infectious Diseases 32, 457–463. [DOI] [PubMed] [Google Scholar]
- Maji, S. K. , Wang, L. , Greenwald, J. & Riek, R. (2009). Structure‐activity relationship of amyloid fibrils. FEBS Letters 583, 2610–2617. [DOI] [PubMed] [Google Scholar]
- Majumdar, V. , Prabhakar, P. , Kulkarni, G. B. & Christopher, R. (2015). Vitamin D status, hypertension and ischemic stroke: a clinical perspective. Journal of Human Hypertension 29, 669–674. [DOI] [PubMed] [Google Scholar]
- Makariou, S. E. , Michel, P. , Tzoufi, M. S. , Challa, A. & Milionis, H. J. (2014). Vitamin D and stroke: promise for prevention and better outcome. Current Vascular Pharmacology 12, 117–124. [DOI] [PubMed] [Google Scholar]
- Makin, O. S. , Atkins, E. , Sikorski, P. , Johansson, J. & Serpell, L. C. (2005). Molecular basis for amyloid fibril formation and stability. Proceedings of the National Academy of Sciences of the United States of America 102, 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, E. M. , Leoncikas, V. , Fisher, C. P. , Moore, J. B. , Plant, N. J. & Kierzek, A. M. (2017). Integration of genome scale metabolic networks and gene regulation of metabolic enzymes with physiologically based pharmacokinetics. CPT: Pharmacometrics & Systems Pharmacology 6, 732–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mändar, R. , Punab, M. , Borovkova, N. , Lapp, E. , Kiiker, R. , Korrovits, P. , Metspalu, A. , Krjutškov, K. , Nõlvak, H. , Preem, J. K. , Oopkaup, K. , Salumets, A. & Truu, J. (2015). Complementary seminovaginal microbiome in couples. Research in Microbiology 166, 440–447. [DOI] [PubMed] [Google Scholar]
- Mangin, M. , Sinha, R. & Fincher, K. (2014). Inflammation and vitamin D: the infection connection. Inflammation Research 63, 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markel, T. A. , Crisostomo, P. R. , Wang, M. , Herring, C. M. , Meldrum, K. K. , Lillemoe, K. D. & Meldrum, D. R. (2007). The struggle for iron: gastrointestinal microbes modulate the host immune response during infection. Journal of Leukocyte Biology 81, 393–400. [DOI] [PubMed] [Google Scholar]
- Marshall, B. (2003). Helicobacter pylori: past, present and future. Keio . Journal of Medicine 52, 80–85. [DOI] [PubMed] [Google Scholar]
- Marshall, B. (2006). Helicobacter connections. ChemMedChem 1, 783–802. [DOI] [PubMed] [Google Scholar]
- Marshall, B. J. (2001). One hundred years of discovery and rediscovery of Helicobacter pylori and its association with peptic ulcer disease In Helicobacter Pylori: Physiology and Genetics (eds Mobley H. L. T., Mendz G. L. and Hazell S. L.), pp. 19–24. ASM Press, Washington, DC. [PubMed] [Google Scholar]
- Marshall, B. J. (2002a). Helicobacter pylori: 20 years on. Clinical Medicine 2, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, B. J. (2002b). Helicobacter Pioneers: Firsthand Accounts from the Scientists Who Discovered Helicobacters. Blackwell, Melbourne. [Google Scholar]
- Marshall, B. J. , Armstrong, J. A. , McGechie, D. B. & Glancy, R. J. (1985). Attempt to fulfil Koch's postulates for pyloric Campylobacter . Medical Journal of Australia 142, 436–439. [DOI] [PubMed] [Google Scholar]
- Marshall, B. J. , Goodwin, C. S. , Warren, J. R. , Murray, R. , Blincow, E. D. , Blackbourn, S. J. , Phillips, M. , Waters, T. E. & Sanderson, C. R. (1988). Prospective double‐blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori . Lancet 2, 1437–1442. [DOI] [PubMed] [Google Scholar]
- Marshall, T. G. (2008). Vitamin D discovery outpaces FDA decision making. BioEssays 30, 173–182. [DOI] [PubMed] [Google Scholar]
- Martelli, A. & Puccio, H. (2014). Dysregulation of cellular iron metabolism in Friedreich ataxia: from primary iron‐sulfur cluster deficit to mitochondrial iron accumulation. Frontiers in Pharmacology 5, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Martinez, R. E. , Abud‐Mendoza, C. , Patiño‐Marin, N. , Rizo‐Rodríguez, J. C. , Little, J. W. & Loyola‐Rodríguez, J. P. (2009). Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. Journal of Clinical Periodontology 36, 1004–1010. [DOI] [PubMed] [Google Scholar]
- Mascitelli, L. , Pezzetta, F. & Goldstein, M. R. (2009). Iron, type 2 diabetes mellitus, and Alzheimer's disease. Cellular and Molecular Life Sciences 66, 2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki, K. (2014). How do membranes initiate Alzheimer's Disease? Formation of toxic amyloid fibrils by the amyloid beta‐protein on ganglioside clusters. Accounts of Chemical Research 47, 2397–2404. [DOI] [PubMed] [Google Scholar]
- Mattman, L. (2001). Cell Wall Deficient Forms: Stealth Pathogens, Third Edition (). CRC Press, Boca Raton. [Google Scholar]
- Meier, B. H. & Böckmann, A. (2015). The structure of fibrils from 'misfolded' proteins. Current Opinion in Structural Biology 30, 43–49. [DOI] [PubMed] [Google Scholar]
- Meier, D. T. , Morcos, M. , Samarasekera, T. , Zraika, S. , Hull, R. L. & Kahn, S. E. (2014). Islet amyloid formation is an important determinant for inducing islet inflammation in high‐fat‐fed human IAPP transgenic mice. Diabetologia 57, 1884–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, J. J. , Kayed, R. , Lin, C. Y. , Gurlo, T. , Haataja, L. , Jayasinghe, S. , Langen, R. , Glabe, C. G. & Butler, P. C. (2006). Inhibition of human IAPP fibril formation does not prevent beta‐cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. American Journal of Physiology ‐ Endocrinology and Metabolism 291, E1317–E1324. [DOI] [PubMed] [Google Scholar]
- Menezes, A. R. , Lamb, M. C. , Lavie, C. J. & DiNicolantonio, J. J. (2014). Vitamin D and atherosclerosis. Current Opinion in Cardiology 29, 571–577. [DOI] [PubMed] [Google Scholar]
- Meyer‐Luehmann, M. , Spires‐Jones, T. L. , Prada, C. , Garcia‐Alloza, M. , de Calignon, A. , Rozkalne, A. , Koenigsknecht‐Talboo, J. , Holtzman, D. M. , Bacskai, B. J. & Hyman, B. T. (2008). Rapid appearance and local toxicity of amyloid‐beta plaques in a mouse model of Alzheimer's disease. Nature 451, 720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels, K. , Nemeth, E. , Ganz, T. & Mehrad, B. (2015). Hepcidin and host defense against infectious diseases. PLoS Pathogens 11, e1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita, K. , Abiru, S. , Nakamura, M. , Komori, A. , Yoshida, Y. , Yokoyama, T. , Daikoku, M. , Ueki, T. , Takii, Y. , Yano, K. , Yastuhashi, H. , Eguchi, K. & Ishibashi, H. (2004). Lipopolysaccharide signaling induces serum amyloid A (SAA) synthesis in human hepatocytes in vitro. FEBS Letters 569, 235–239. [DOI] [PubMed] [Google Scholar]
- Migliore, L. , Fontana, I. , Colognato, R. , Coppede, F. , Siciliano, G. & Murri, L. (2005). Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer's disease and in other neurodegenerative diseases. Neurobiology of Aging 26, 587–595. [DOI] [PubMed] [Google Scholar]
- Miklossy, J. (2011). Emerging roles of pathogens in Alzheimer disease. Expert Reviews in Molecular Medicine 13, e30. [DOI] [PubMed] [Google Scholar]
- Miklossy, J. , Martins, R. , Darbinian, N. , Khalili, K. & McGeer, P. L. (2008). Type 2 diabetes: local inflammation and direct effect of bacterial toxic components. Open Pathology Journal 2, 86–95. [Google Scholar]
- Miklossy, J. & McGeer, P. L. (2016). Common mechanisms involved in Alzheimer's disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation. Aging (Albany NY) 8, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuls, T. R. , Payne, J. B. , Reinhardt, R. A. , Thiele, G. M. , Maziarz, E. , Cannella, A. C. , Holers, V. M. , Kuhn, K. A. & O'Dell, J. R. (2009). Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. International Immunopharmacology 9, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. W. , Harvey, D. J. , Beckett, L. A. , Green, R. , Farias, S. T. , Reed, B. R. , Olichney, J. M. , Mungas, D. M. & DeCarli, C. (2015). Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurology 72, 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. L. , James‐Kracke, M. , Sun, G. Y. & Sun, A. Y. (2009). Oxidative and inflammatory pathways in Parkinson's disease. Neurochemical Research 34, 55–65. [DOI] [PubMed] [Google Scholar]
- Minter, M. R. , Taylor, J. M. & Crack, P. J. (2016). The contribution of neuroinflammation to amyloid toxicity in Alzheimer's disease. Journal of Neurochemistry 136, 457–474. [DOI] [PubMed] [Google Scholar]
- Miranda, S. , Opazo, C. , Larrondo, L. F. , Munoz, F. J. , Ruiz, F. , Leighton, F. & Inestrosa, N. C. (2000). The role of oxidative stress in the toxicity induced by amyloid beta‐peptide in Alzheimer's disease. Progress in Neurobiology 62, 633–648. [DOI] [PubMed] [Google Scholar]
- Miskinyte, M. & Gordo, I. (2013). Increased survival of antibiotic‐resistant Escherichia coli inside macrophages. Antimicrobial Agents and Chemotherapy 57, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskinyte, M. , Sousa, A. , Ramiro, R. S. , de Sousa, J. A. , Kotlinowski, J. , Caramalho, I. , Magalhães, S. , Soares, M. P. & Gordo, I. (2013). The genetic basis of Escherichia coli pathoadaptation to macrophages. PLoS Pathogens 9, e1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, S. & Mendes, P. (2013). A computational model of liver iron metabolism. PLoS Computational Biology 9, e1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, R. , Sharma, S. , Chhibber, S. & Harjai, K. (2008). Iron dictates the virulence of Pseudomonas aeruginosa in urinary tract infections. Journal of Biomedical Science 15, 731–741. [DOI] [PubMed] [Google Scholar]
- Moalem, S. , Weinberg, E. D. & Percy, M. E. (2004). Hemochromatosis and the enigma of misplaced iron: implications for infectious disease and survival. Biometals 17, 135–139. [DOI] [PubMed] [Google Scholar]
- Mochizuki, H. & Yasuda, T. (2012). Iron accumulation in Parkinson's disease. Journal of Neural Transmission 119, 1511–1514. [DOI] [PubMed] [Google Scholar]
- Molfino, A. , Kushta, I. , Tommasi, V. , Fanelli, F. R. & Muscaritoli, M. (2009). Amyotrophic lateral sclerosis, enteral nutrition and the risk of iron overload. Journal of Neurology 256, 1015–1016. [DOI] [PubMed] [Google Scholar]
- Mollet, I. G. , Patel, D. , Govani, F. S. , Giess, A. , Paschalaki, K. , Periyasamy, M. , Lidington, E. C. , Mason, J. C. , Jones, M. D. , Game, L. , Ali, S. & Shovlin, C. L. (2016). Low dose iron treatments induce a DNA damage response in human endothelial cells within minutes. PLoS One 11, e0147990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondot, S. , de Wouters, T. , Doré, J. & Lepage, P. (2013). The human gut microbiome and its dysfunctions. Digestive Diseases 31, 278–285. [DOI] [PubMed] [Google Scholar]
- Monsarrat, P. , Vergnes, J. N. , Cantagrel, A. , Algans, N. , Cousty, S. , Kémoun, P. , Bertrand, C. , Arrivé, E. , Bou, C. , Sédarat, C. , Schaeverbeke, T. , Nabet, C. & Sixou, M. (2013). Effect of periodontal treatment on the clinical parameters of patients with rheumatoid arthritis: study protocol of the randomized, controlled ESPERA trial. Trials 14, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel‐Castro, A. J. , González‐Cervantes, R. M. , Bravo‐Ruiseco, G. & Pacheco‐López, G. (2013). The microbiota‐gut‐brain axis: neurobehavioral correlates, health and sociality. Frontiers in Integrative Neuroscience 7, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montonen, J. , Boeing, H. , Steffen, A. , Lehmann, R. , Fritsche, A. , Joost, H. G. , Schulze, M. B. & Pischon, T. (2012). Body iron stores and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Potsdam study. Diabetologia 55, 2613–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi, P. , Barnes, P. & Roberts, L. J. II (2007). Insights into oxidative stress: the isoprostanes. Current Medicinal Chemistry 14, 703–717. [DOI] [PubMed] [Google Scholar]
- Montuschi, P. , Barnes, P. J. & Roberts, L. J. II. (2004). Isoprostanes: markers and mediators of oxidative stress. FASEB Journal 18, 1791–1800. [DOI] [PubMed] [Google Scholar]
- Montuschi, P. , Ciabattoni, G. , Paredi, P. , Pantelidis, P. , du Bois, R. M. , Kharitonov, S. A. & Barnes, P. J. (1998). 8‐Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. American Journal of Respiratory and Critical Care Medicine 158, 1524–1527. [DOI] [PubMed] [Google Scholar]
- Montuschi, P. , Collins, J. V. , Ciabattoni, G. , Lazzeri, N. , Corradi, M. , Kharitonov, S. A. & Barnes, P. J. (2000). Exhaled 8‐isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. American Journal of Respiratory and Critical Care Medicine 162, 1175–1177. [DOI] [PubMed] [Google Scholar]
- Moon, J. H. & Lee, J. H. (2016). Probing the diversity of healthy oral microbiome with bioinformatics approaches. BMB Reports 49, 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, R. , Callegari, K. & Soto, C. (2015). Prion‐like features of misfolded Abeta and tau aggregates. Virus Research 207, 106–112. [DOI] [PubMed] [Google Scholar]
- Morath, S. , von Aulock, S. & Hartung, T. (2005). Structure/function relationships of lipoteichoic acids. Journal of Endotoxin Research 11, 348–356. [DOI] [PubMed] [Google Scholar]
- Moreno‐Navarrete, J. M. , Escoté, X. , Ortega, F. , Serino, M. , Campbell, M. , Michalski, M. C. , Laville, M. , Xifra, G. , Luche, E. , Domingo, P. , Sabater, M. , Pardo, G. , Waget, A. , Salvador, J. , Giralt, M. , et al. (2013). A role for adipocyte‐derived lipopolysaccharide‐binding protein in inflammation‐ and obesity‐associated adipose tissue dysfunction. Diabetologia 56, 2524–2537. [DOI] [PubMed] [Google Scholar]
- Moriyama, K. , Ando, C. , Tashiro, K. , Kuhara, S. , Okamura, S. , Nakano, S. , Takagi, Y. , Miki, T. , Nakashima, Y. & Hirakawa, H. (2008). Polymerase chain reaction detection of bacterial 16S rRNA gene in human blood. Microbiology and Immunology 52, 375–382. [DOI] [PubMed] [Google Scholar]
- Morris, K. L. & Serpell, L. C. (2012). X‐ray fibre diffraction studies of amyloid fibrils. Methods in Molecular Biology 849, 121–135. [DOI] [PubMed] [Google Scholar]
- Morrow, J. D. (2005). Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arteriosclerosis, Thrombosis & Vascular Biology 25, 279–286. [DOI] [PubMed] [Google Scholar]
- Mu, Q. , Kirby, J. , Reilly, C. M. & Luo, X. M. (2017). Leaky gut as a danger signal for autoimmune diseases. Frontiers in Immunology 8, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench, K. H. (1989). Hemochromatosis and infection: alcohol and iron, oysters and sepsis. American Journal of Medicine 87, 40N–43N. [PubMed] [Google Scholar]
- Muhoberac, B. B. & Vidal, R. (2013). Abnormal iron homeostasis and neurodegeneration. Frontiers in Aging Neuroscience 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Kaprelyants, A. S. , Kell, D. B. & Young, M. (2003). Adoption of the transiently non‐culturable state ‐ a bacterial survival strategy? Advances in Microbial Physiology 47, 65–129. [DOI] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Kaprelyants, A. S. , Young, D. I. , Young, M. & Kell, D. B. (1998). A bacterial cytokine. Proceedings of the National Academy of Sciences of the United States of America 95, 8916–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Kormer, S. S. , Kell, D. B. & Kaprelyants, A. S. (1999). Stimulation of the multiplication of Micrococcus luteus by an autocrine growth factor. Archives of Microbiology 172, 9–14. [DOI] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Murzin, A. G. , Salina, E. G. , Demina, G. R. , Kell, D. B. , Kaprelyants, A. S. & Young, M. (2006). Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation. Molecular Microbiology 59, 84–98. [DOI] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Turapov, O. A. , Kazarian, K. , Telkov, M. , Kaprelyants, A. S. , Kell, D. B. & Young, M. (2002). The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Molecular Microbiology 46, 611–621. [DOI] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Turapov, O. A. , Young, D. I. , Kaprelyants, A. S. , Kell, D. B. & Young, M. (2002). A family of autocrine growth factors in Mycobacterium tuberculosis . Molecular Microbiology 46, 623–635. [DOI] [PubMed] [Google Scholar]
- Mukamolova, G. V. , Yanopolskaya, N. D. , Votyakova, T. V. , Popov, V. I. , Kaprelyants, A. S. & Kell, D. B. (1995). Biochemical changes accompanying the long‐term starvation of Micrococcus luteus cells in spent growth medium. Archives of Microbiology 163, 373–379. [Google Scholar]
- Mukherjee, S. , Karmakar, S. & Babu, S. P. (2016). TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Brazilian Journal of Infectious Diseases 20, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, M. & Leavitt, B. R. (2014). Iron dysregulation in Huntington's disease. Journal of Neurochemistry 130, 328–350. [DOI] [PubMed] [Google Scholar]
- Munishkina, L. A. & Fink, A. L. (2007). Fluorescence as a method to reveal structures and membrane‐interactions of amyloidogenic proteins. Biochimica et Biophysica Acta 1768, 1862–1885. [DOI] [PubMed] [Google Scholar]
- Murakami, T. , Ishiguro, N. & Higuchi, K. (2014). Transmission of systemic AA amyloidosis in animals. Veterinary Pathology 51, 363–371. [DOI] [PubMed] [Google Scholar]
- Mysorekar, I. U. & Hultgren, S. J. (2006). Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proceedings of the National Academy of Sciences of the United States of America 103, 14170–14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz, M. , Schroll, A. , Sonnweber, T. & Weiss, G. (2010). The struggle for iron ‐ a metal at the host‐pathogen interface. Cellular Microbiology 12, 1691–1702. [DOI] [PubMed] [Google Scholar]
- Nakano, M. & Kamino, K. (2015). Amyloid‐like conformation and interaction for the self‐assembly in barnacle underwater cement. Biochemistry 54, 826–835. [DOI] [PubMed] [Google Scholar]
- Nama, N. , Menon, K. , Iliriani, K. , Pojsupap, S. , Sampson, M. , O'Hearn, K. , Zhou, L. L. , McIntyre, L. , Fergusson, D. & McNally, J. D. (2016). A systematic review of pediatric clinical trials of high dose vitamin D. PeerJ 4, e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba, S. , Ikeda, F. , Baba, N. , Takaguchi, K. , Senoh, T. , Nagano, T. , Seki, H. , Takeuchi, Y. , Moritou, Y. , Yasunaka, T. , Ohnishi, H. , Miyake, Y. , Takaki, A. , Nouso, K. , Iwasaki, Y. & Yamamoto, K. (2016). Association of hepatic oxidative stress and iron dysregulation with HCC development after interferon therapy in chronic hepatitis C. Journal of Clinical Pathology 69, 226–233. [DOI] [PubMed] [Google Scholar]
- Nanhoe‐Mahabier, W. , de Laat, K. F. , Visser, J. E. , Zijlmans, J. , de Leeuw, F. E. & Bloem, B. R. (2009). Parkinson disease and comorbid cerebrovascular disease. Nature Reviews Neurology 5, 533–541. [DOI] [PubMed] [Google Scholar]
- Nelson, D. E. , Ihekwaba, A. E. C. , Elliott, M. , Gibney, C. A. , Foreman, B. E. , Nelson, G. , See, V. , Horton, C. A. , Spiller, D. G. , Edwards, S. W. , McDowell, H. P. , Unitt, J. F. , Sullivan, E. , Grimley, R. , Benson, N. , et al. (2004). Oscillations in NF‐kB signalling control the dynamics of gene expression. Science 306, 704–708. [DOI] [PubMed] [Google Scholar]
- Nemeth, E. , Rivera, S. , Gabayan, V. , Keller, C. , Taudorf, S. , Pedersen, B. K. & Ganz, T. (2004). IL‐6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. Journal of Clinical Investigation 113, 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth, K. , Falus, A. , Elekes, E. , Bohm, U. & Meretey, K. (1985). Induction of human rheumatoid factor and other autoantibodies by bacterial lipopolysaccharide. Acta Microbiologica et Immunologica Hungarica 32, 249–258. [PubMed] [Google Scholar]
- Nevitt, T. (2011). War‐Fe‐re: iron at the core of fungal virulence and host immunity. Biometals 24, 547–558. [DOI] [PubMed] [Google Scholar]
- Newberry, S. J. , Chung, M. , Shekelle, P. G. , Booth, M. S. , Liu, J. L. , Maher, A. R. , Motala, A. , Cui, M. , Perry, T. , Shanman, R. & Balk, E. M. (2014). Vitamin D and Calcium: A Systematic Review of Health Outcomes (Update). Agency for Healthcare Research and Quality, Rockville. [DOI] [PubMed] [Google Scholar]
- Ngok‐Ngam, P. , Ruangkiattikul, N. , Mahavihakanont, A. , Virgem, S. S. , Sukchawalit, R. & Mongkolsuk, S. (2009). Roles of Agrobacterium tumefaciens RirA in iron regulation, oxidative stress response, and virulence. Journal of Bacteriology 191, 2083–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, M. D. , D'Aigle, T. , Gowing, G. , Julien, J. P. & Rivest, S. (2004). Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. Journal of Neuroscience 24, 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, L. , Frokjaer, S. , Brange, J. , Uversky, V. N. & Fink, A. L. (2001). Probing the mechanism of insulin fibril formation with insulin mutants. Biochemistry 40, 8397–8409. [DOI] [PubMed] [Google Scholar]
- Nielsen, L. , Frokjaer, S. , Carpenter, J. F. & Brange, J. (2001). Studies of the structure of insulin fibrils by Fourier transform infrared (FTIR) spectroscopy and electron microscopy. Journal of Pharmacological Sciences 90, 29–37. [DOI] [PubMed] [Google Scholar]
- Nielsen, L. , Khurana, R. , Coats, A. , Frokjaer, S. , Brange, J. , Vyas, S. , Uversky, V. N. & Fink, A. L. (2001). Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry 40, 6036–6046. [DOI] [PubMed] [Google Scholar]
- Nielsen, P. , Günther, U. , Dürken, M. , Fischer, R. & Düllmann, J. (2000). Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. Journal of Laboratory and Clinical Medicine 135, 413–418. [DOI] [PubMed] [Google Scholar]
- NIH HMP Working Group , Peterson, J. , Garges, S. , Giovanni, M. , McInnes, P. , Wang, L. , Schloss, J. A. , Bonazzi, V. , McEwen, J. E. , Wetterstrand, K. A. , Deal, C. , Baker, C. C. , Di Francesco, V. , Howcroft, T. K. , Karp, R. W. , et al. (2009). The NIH human microbiome project. Genome Research 19, 2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkari, S. , McLaughlin, I. J. , Bi, W. , Dodge, D. E. & Relman, D. A. (2001). Does blood of healthy subjects contain bacterial ribosomal DNA? Journal of Clinical Microbiology 39, 1956–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonorov, A. A. , Skalnaya, M. G. , Tinkov, A. A. & Skalny, A. V. (2015). Mutual interaction between iron homeostasis and obesity pathogenesis. Journal of Trace Elements in Medicine & Biology 30, 207–214. [DOI] [PubMed] [Google Scholar]
- Nnoaham, K. E. & Clarke, A. (2008). Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. International Journal of Epidemiology 37, 113–119. [DOI] [PubMed] [Google Scholar]
- Noecker, C. , McNally, C. P. , Eng, A. & Borenstein, E. (2017). High‐resolution characterization of the human microbiome. Translational Research 179, 7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, P. E. & Powell, J. T. (2014). Vitamin D and cardiovascular disease. Circulation Research 114, 379–393. [DOI] [PubMed] [Google Scholar]
- Núñez, M. T. , Urrutia, P. , Mena, N. , Aguirre, P. , Tapia, V. & Salazar, J. (2012). Iron toxicity in neurodegeneration. Biometals 25, 761–776. [DOI] [PubMed] [Google Scholar]
- O'Dwyer, D. N. , Dickson, R. P. & Moore, B. B. (2016). The lung microbiome, immunity, and the pathogenesis of chronic lung disease. Journal of Immunology 196, 4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrendik, M. (2013). Rheumatoid arthritis is an autoimmune disease caused by periodontal pathogens. International Journal of General Medicine 6, 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J. , Byrd, A. L. , Park, M. , Program, N. C. S. , Kong, H. H. & Segre, J. A. (2016). Temporal Stability of the Human Skin Microbiome. Cell 165, 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J. , Freeman, A. F. , Program, N. C. S. , Park, M. , Sokolic, R. , Candotti, F. , Holland, S. M. , Segre, J. A. & Kong, H. H. (2013). The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Research 23, 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, A. K. , Teranishi, K. , Isas, J. M. , Bedrood, S. , Chow, R. H. & Langen, R. (2016). Diabetic risk factors promote islet amyloid polypeptide misfolding by a common, membrane‐mediated mechanism. Scientific Reports 6, 31094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen, S. W. & Alm, E. J. (2016). Dysbiosis is not an answer. Nature Microbiology 1, 16228. [DOI] [PubMed] [Google Scholar]
- Oliveira, F. , Rocha, S. & Fernandes, R. (2014). Iron metabolism: from health to disease. Journal of Clinical Laboratory Analysis 28, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira‐Nascimento, L. , Massari, P. & Wetzler, L. M. (2012). The Role of TLR2 in Infection and Immunity. Frontiers in Immunology 3, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. D. (2010). Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiology Reviews 34, 415–425. [DOI] [PubMed] [Google Scholar]
- Olumuyiwa‐Akeredolu, O. O. , Soma, P. , Buys, A. V. , Debusho, L. K. & Pretorius, E. (2017). Characterizing pathology in erythrocytes using morphological and biophysical membrane properties: relation to impaired hemorheology and cardiovascular function in rheumatoid arthritis. Biochimica et Biophysica Acta 1859, 2381–2391. [DOI] [PubMed] [Google Scholar]
- O'Neill, L. A. J. , Bryant, C. E. & Doyle, S. L. (2009). Therapeutic targeting of toll‐like receptors for infectious and inflammatory diseases and cancer. Pharmacological Reviews 61, 177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordóñez‐Morán, P. & Muñoz, A. (2009). Nuclear receptors: genomic and non‐genomic effects converge. Cell Cycle 8, 1675–1680. [DOI] [PubMed] [Google Scholar]
- Orman, M. A. & Brynildsen, M. P. (2013). Establishment of a method to rapidly assay bacterial persister metabolism. Antimicrobial Agents and Chemotherapy 57, 4398–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, C. F. , Rowe, D. B. & Halliday, G. M. (2002). An inflammatory review of Parkinson's disease. Progress in Neurobiology 68, 325–340. [DOI] [PubMed] [Google Scholar]
- Oshiro, S. , Morioka, M. S. & Kikuchi, M. (2011). Dysregulation of iron metabolism in Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Advances in Pharmacological Sciences 2011, 378278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostos, M. A. , Recalde, D. , Zakin, M. M. & Scott‐Algara, D. (2002). Implication of natural killer T cells in atherosclerosis development during a LPS‐induced chronic inflammation. FEBS Letters 519, 23–29. [DOI] [PubMed] [Google Scholar]
- Ozdemir, D. , Uysal, N. , Tugyan, K. , Gonenc, S. , Acikgoz, O. , Aksu, I. & Ozkan, H. (2007). The effect of melatonin on endotoxemia‐induced intestinal apoptosis and oxidative stress in infant rats. Intensive Care Medicine 33, 511–516. [DOI] [PubMed] [Google Scholar]
- Pal, G. D. , Shaikh, M. , Forsyth, C. B. , Ouyang, B. , Keshavarzian, A. & Shannon, K. M. (2015). Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Frontiers in Neuroscience 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson, B. Ø. (2006). Systems Biology: Properties of Reconstructed Networks. Cambridge University Press, Cambridge. [Google Scholar]
- Park, K. Y. , Chung, P. W. , Kim, Y. B. , Moon, H. S. , Suh, B. C. , Won, Y. S. , Kim, J. M. , Youn, Y. C. & Kwon, O. S. (2015). Serum Vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovascular Diseases 40, 73–80. [DOI] [PubMed] [Google Scholar]
- Parmar, J. H. , Davis, G. , Shevchuk, H. & Mendes, P. (2017). Modeling the dynamics of mouse iron body distribution: hepcidin is necessary but not sufficient. BMC Systems Biology 11, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N. S. , Paris, D. , Mathura, V. , Quadros, A. N. , Crawford, F. C. & Mullan, M. J. (2005). Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. Journal of Neuroinflammation 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer, E. , Gomez‐Arango, L. F. , Barrett, H. L. & Nitert, M. D. (2016). Maternal health and the placental microbiome. Placenta 64, 30–37. [DOI] [PubMed] [Google Scholar]
- Perlstein, T. S. , Pande, R. , Berliner, N. & Vanasse, G. J. (2011). Prevalence of 25‐hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood 117, 2800–2806. [DOI] [PubMed] [Google Scholar]
- Perron, N. R. & Brumaghim, J. L. (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochemistry and Biophysics 53, 75–100. [DOI] [PubMed] [Google Scholar]
- Perron, N. R. , Wang, H. C. , Deguire, S. N. , Jenkins, M. , Lawson, M. & Brumaghim, J. L. (2010). Kinetics of iron oxidation upon polyphenol binding. Dalton Transactions 39, 9982–9987. [DOI] [PubMed] [Google Scholar]
- Peters, D. G. , Connor, J. R. & Meadowcroft, M. D. (2015). The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer's disease: two sides of the same coin. Neurobiology of Disease 81, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, D. R. & Doorn, J. A. (2004). Reactions of 4‐hydroxynonenal with proteins and cellular targets. Free Radical Biology and Medicine 37, 937–945. [DOI] [PubMed] [Google Scholar]
- Peterson, A. , Mattek, N. , Clemons, A. , Bowman, G. L. , Buracchio, T. , Kaye, J. & Quinn, J. (2012). Serum vitamin D concentrations are associated with falling and cognitive function in older adults. Journal of Nutritional Health & Aging 16, 898–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, A. L. , Murchison, C. , Zabetian, C. , Leverenz, J. B. , Watson, G. S. , Montine, T. , Carney, N. , Bowman, G. L. , Edwards, K. & Quinn, J. F. (2013). Memory, mood, and vitamin D in persons with Parkinson's disease. Journal of Parkinson's Disease 3, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova, J. , Manolov, V. , Vasilev, V. , Tzatchev, K. & Marinov, B. (2016). Ischemic stroke, inflammation, iron overload ‐ Connection to a hepcidin. International Journal of Stroke 11, NP16–NP17. [DOI] [PubMed] [Google Scholar]
- Pich, O. Q. & Merrell, D. S. (2013). The ferric uptake regulator of Helicobacter pylori: a critical player in the battle for iron and colonization of the stomach. Future Microbiology 8, 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay, K. & Govender, P. (2013). Amylin uncovered: a review on the polypeptide responsible for type II diabetes. BioMed Research International 2013, 826706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz, S. , Gaksch, M. , Kienreich, K. , Grübler, M. , Verheyen, N. , Fahrleitner‐Pammer, A. , Treiber, G. , Drechsler, C. , ó Hartaigh, B. , Obermayer‐Pietsch, B. , Schwetz, V. , Aberer, F. , Mader, J. , Scharnagl, H. , Meinitzer, A. , Lerchbaum, E. , Dekker, J. M. , Zittermann, A. , März, W. & Tomaschitz, A. (2015). Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension 65, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Pindjakova, J. , Sartini, C. , Lo Re, O. , Rappa, F. , Coupe, B. , Lelouvier, B. , Pazienza, V. & Vinciguerra, M. (2017). Gut dysbiosis and adaptive immune response in diet‐induced obesity vs. systemic inflammation. Frontiers in Microbiology 8, 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirillo, A. , Catapano, A. L. & Norata, G. D. (2015). HDL in infectious diseases and sepsis. Handbook of Experimental Pharmacology 224, 483–508. [DOI] [PubMed] [Google Scholar]
- Pirmohamed, M. , James, S. , Meakin, S. , Green, C. , Scott, A. K. , Walley, T. J. , Farrar, K. , Park, B. K. & Breckenridge, A. M. (2004). Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. British Medical Journal 329, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa, D. , Alonso, R. , Fernandez‐Fernandez, A. M. , Rabano, A. & Carrasco, L. (2017). Polymicrobial infections in brain tissue from Alzheimer's disease patients. Scientific Reports 7, 5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano, G. , Lombardi, R. & Fracanzani, A. L. (2016). Vascular damage in patients with nonalcoholic fatty liver disease: possible role of iron and ferritin. International Journal of Molecular Sciences 17, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitassi, L. H. U. , Magalhães, R. F. , Barjas‐Castro, M. L. , de Paula, E. V. , Ferreira, M. R. & Velho, P. E. (2007). Bartonella henselae infects human erythrocytes. Ultrastructural Pathology 31, 369–372. [DOI] [PubMed] [Google Scholar]
- Pludowski, P. , Jaworski, M. , Niemirska, A. , Litwin, M. , Szalecki, M. , Karczmarewicz, E. & Michalkiewicz, J. (2014). Vitamin D status, body composition and hypertensive target organ damage in primary hypertension. Journal of Steroid Biochemistry and Molecular Biology 144(Pt. A), 180–184. [DOI] [PubMed] [Google Scholar]
- Podmore, C. , Meidtner, K. , Schulze, M. B. , Scott, R. A. , Ramond, A. , Butterworth, A. S. , Di Angelantonio, E. , Danesh, J. , Arriola, L. , Barricarte, A. , Boeing, H. , Clavel‐Chapelon, F. , Cross, A. J. , Dahm, C. C. , Fagherazzi, G. , et al. (2016). Association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC‐InterAct Study. Diabetes Care 39, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak, A. , He, X. L. , Smirnova, I. , Liu, M. Y. , Van Huffel, C. , Du, X. , Birdwell, D. , Alejos, E. , Silva, M. , Galanos, C. , Freudenberg, M. , Ricciardi‐Castagnoli, P. , Layton, B. & Beutler, B. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- Poole, K. E. S. , Loveridge, N. , Barker, P. J. , Halsall, D. J. , Rose, C. , Reeve, J. & Warburton, E. A. (2006). Reduced vitamin D in acute stroke. Stroke 37, 243–245. [DOI] [PubMed] [Google Scholar]
- Poole, S. , Singhrao, S. K. , Kesavalu, L. , Curtis, M. A. & Crean, S. (2013). Determining the presence of periodontopathic virulence factors in short‐term postmortem Alzheimer's disease brain tissue. Journal of Alzheimers Disease 36, 665–677. [DOI] [PubMed] [Google Scholar]
- Posey, J. E. & Gherardini, F. C. (2000). Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653. [DOI] [PubMed] [Google Scholar]
- Postgate, J. R. (1967). Viability measurements and the survival of microbes under minimum stress. Advances in Microbial Physiology 1, 1–23. [Google Scholar]
- Postgate, J. R. (1969). Viable counts and viability. Methods in . Microbiology 1, 611–628. [Google Scholar]
- Postgate, J. R. (1976). Death in microbes and macrobes In In The Survival of Vegetative Microbes (eds Gray T. R. G. and Postgate J. R.), pp. 1–19. Cambridge University Press, Cambridge. [Google Scholar]
- Potempa, J. , Mydel, P. & Koziel, J. (2017). The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nature Reviews Rheumatology 13, 606–620. [DOI] [PubMed] [Google Scholar]
- Potgieter, M. , Bester, J. , Kell, D. B. & Pretorius, E. (2015). The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiology Reviews 39, 567–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound, M. W. & May, D. B. (2005). Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions. Journal of Clinical Pharmacy and Therapeutics 30, 291–295. [DOI] [PubMed] [Google Scholar]
- Prabhakar, P. , Majumdar, V. , Kulkarni, G. B. & Christopher, R. (2015). Genetic variants of vitamin D receptor and susceptibility to ischemic stroke. Biochemical and Biophysical Research Communications 456, 631–636. [DOI] [PubMed] [Google Scholar]
- Prendergast, M. M. & Moran, A. P. (2000). Lipopolysaccharides in the development of the Guillain‐Barré syndrome and Miller Fisher syndrome forms of acute inflammatory peripheral neuropathies. Journal of Endotoxin Research 6, 341–359. [PubMed] [Google Scholar]
- Pretorius, E. (2011). The use of a desktop scanning electron microscope as a diagnostic tool in studying fibrin networks of thrombo‐embolic ischemic stroke. Ultrastructural Pathology 35, 245–250. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Akeredolu, O.‐O. , Soma, P. & Kell, D. B. (2017a). Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Experimental Biology and Medicine 242, 355–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Mbotwe, S. & Kell, D. B. (2017b). Lipopolysaccharide‐binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular comorbidities. Scientific Reports 7, 9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Bester, J. & Kell, D. B. (2016a). A bacterial component to Alzheimer‐type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease. Journal of Alzheimers Disease 53, 1237–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , du Plooy, J. N. & Bester, J. (2016b). A comprehensive review on eryptosis. Cellular Physiology & Biochemistry 39, 1977–2000. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Bester, J. , Vermeulen, N. , Alummoottil, S. , Soma, P. , Buys, A. V. & Kell, D. B. (2015). Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin‐generated fibrin: implications for diagnostics. Cardiovascular Diabetology 13, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Bester, J. , Vermeulen, N. , Lipinski, B. , Gericke, G. S. & Kell, D. B. (2014a). Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents. PLoS One 9, e85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Swanepoel, A. C. , Buys, A. V. , Vermeulen, N. , Duim, W. & Kell, D. B. (2014b). Eryptosis as a marker of Parkinson's disease. Aging 6, 788–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. & Kell, D. B. (2014). Diagnostic morphology: biophysical indicators for iron‐driven inflammatory diseases. Integrative Biology 6, 486–510. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Mbotwe, S. , Bester, J. , Robinson, C. J. & Kell, D. B. (2016c). Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. Journal of the Royal Society Interface 123, 20160539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. & Oberholzer, H. M. (2009). Ultrastructural changes of platelets and fibrin networks in human asthma: a qualitative case study. Blood Coagulation & Fibrinolysis 20, 146–149. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Page, M. J. , Engelbrecht, L. , Ellis, G. C. & Kell, D. B. (2017c). Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid‐selective fluorescent stains. Cardiovascular Diabetology 16, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Page, M. J. , Hendricks, L. , Nkosi, N. B. , Benson, S.R. , Kell, D. B. (2018). Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface 15, 20170941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Page, M. J. , Hendricks, L. , Nkosi, N. B. , Benson, S. R. & Kell, D. B. (2018a). Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface 20170941. 10.1098/rsif.2017.0941. [DOI] [PMC free article] [PubMed]
- Pretorius, E. , Page, MJ. , Mbotwe, S. , Kell, D. B. (2018b): Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson's disease. PlosOne 13, e0192121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, E. , Steyn, H. , Engelbrecht, M. , Swanepoel, A. C. & Oberholzer, H. M. (2011). Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagulation & Fibrinolysis 22, 696–700. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Swanepoel, A. C. , DeVilliers, S. & Bester, J. (2017d). Blood clot parameters: thromboelastography and scanning electron microscopy in research and clinical practice. Thrombosis Research 154, 59–63. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Vermeulen, N. , Bester, J. & Lipinski, B. (2013a). Novel use of scanning electron microscopy for detection of iron‐induced morphological changes in human blood. Microscopy Research and Technique 76, 268–271. [DOI] [PubMed] [Google Scholar]
- Pretorius, E. , Vermeulen, N. , Bester, J. , Lipinski, B. & Kell, D. B. (2013b). A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicology Mechanisms and Methods 23, 352–359. [DOI] [PubMed] [Google Scholar]
- Primas, H. (1981). Chemistry, Quantum Mechanics and Reductionism. Springer, Berlin. [Google Scholar]
- Prince, A. L. , Ma, J. , Kannan, P. S. , Alvarez, M. , Gisslen, T. , Harris, R. A. , Sweeney, E. L. , Knox, C. L. , Lambers, D. S. , Jobe, A. H. , Chougnet, C. A. , Kallapur, S. G. & Aagaard, K. M. (2016). The placental microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. American Journal of Obstetrics & Gynecology 214, 627.e1–627.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal, A. D. , Albert, P. J. & Marshall, T. G. (2013). The human microbiome and autoimmunity. Current Opinion in Rheumatology 25, 234–240. [DOI] [PubMed] [Google Scholar]
- Proal, A. D. , Albert, P. J. & Marshall, T. G. (2014). Inflammatory disease and the human microbiome. Discovery Medicine 17, 257–265. [PubMed] [Google Scholar]
- Proal, A. D. , Albert, P. J. & Marshall, T. G. (2015). Infection, autoimmunity, and vitamin D In Infection and Autoimmunity (eds Shoenfeld Y. and Rose N. R.), pp. 163–182. Academic Press, New York. [Google Scholar]
- Proal, A. D. , Lindseth, I. A. & Marshall, T. G. (2017). Microbe‐microbe and host‐microbe interactions drive microbiome dysbiosis and inflammatory processes. Discovery Medicine 23, 51–60. [PubMed] [Google Scholar]
- Prusiner, S. B. (1998). Prions. Proceedings of the National Academy of Sciences of the United States of America 95, 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S. B. (2012). A unifying role for prions in neurodegenerative diseases. Science 336, 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S. B. , Woerman, A. L. , Mordes, D. A. , Watts, J. C. , Rampersaud, R. , Berry, D. B. , Patel, S. , Oehler, A. , Lowe, J. K. , Kravitz, S. N. , Geschwind, D. H. , Glidden, D. V. , Halliday, G. M. , Middleton, L. T. , Gentleman, S. M. , et al. (2015). Evidence for alpha‐synuclein prions causing multiple system atrophy in humans with parkinsonism. Proceedings of the National Academy of Sciences of the United States of America 112, E5308–E5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pussinen, P. J. , Havulinna, A. S. , Lehto, M. , Sundvall, J. & Salomaa, V. (2011). Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34, 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri, S. M. , Bauer, J. , Zelenak, C. , Mahmud, H. , Kucherenko, Y. , Lee, S. H. , Ferlinz, K. & Lang, F. (2011). Sphingosine but not sphingosine‐1‐phosphate stimulates suicidal erythrocyte death. Cellular Physiology & Biochemistry 28, 339–346. [DOI] [PubMed] [Google Scholar]
- Qadri, S. M. , Donkor, D. A. , Bhakta, V. , Eltringham‐Smith, L. J. , Dwivedi, D. J. , Moore, J. C. , Pepler, L. , Ivetic, N. , Nazi, I. , Fox‐Robichaud, A. E. , Liaw, P. C. & Sheffield, W. P. (2016). Phosphatidylserine externalization and procoagulant activation of erythrocytes induced by Pseudomonas aeruginosa virulence factor pyocyanin. Journal of Cellular and Molecular Medicine 20, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri, S. M. , Mahmud, H. , Lang, E. , Gu, S. , Bobbala, D. , Zelenak, C. , Jilani, K. , Siegfried, A. , Föller, M. & Lang, F. (2012). Enhanced suicidal erythrocyte death in mice carrying a loss‐of‐function mutation of the adenomatous polyposis coli gene. Journal of Cellular and Molecular Medicine 16, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley, E. M. M. (2016). Leaky gut ‐ concept or clinical entity? Current Opinion in Gastroenterology 32, 74–79. [DOI] [PubMed] [Google Scholar]
- Rademacher, T. W. , Gumaa, K. & Scioscia, M. (2007). Preeclampsia, insulin signalling and immunological dysfunction: a fetal, maternal or placental disorder? Journal of Reproductive Immunology 76, 78–84. [DOI] [PubMed] [Google Scholar]
- Rambaran, R. N. & Serpell, L. C. (2008). Amyloid fibrils: abnormal protein assembly. Prion 2, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangé, H. , Labreuche, J. , Louedec, L. , Rondeau, P. , Planesse, C. , Sebbag, U. , Bourdon, E. , Michel, J. B. , Bouchard, P. & Meilhac, O. (2014). Periodontal bacteria in human carotid atherothrombosis as a potential trigger for neutrophil activation. Atherosclerosis 236, 448–455. [DOI] [PubMed] [Google Scholar]
- Rayman, M. P. , Barlis, J. , Evans, R. W. , Redman, C. W. & King, L. J. (2002). Abnormal iron parameters in the pregnancy syndrome preeclampsia. American Journal of Obstetrics & Gynecology 187, 412–418. [DOI] [PubMed] [Google Scholar]
- Reichert, C. O. , da Cunha, J. , Levy, D. , Maselli, L. M. F. , Bydlowski, S. P. & Spada, C. (2017). Hepcidin: homeostasis and diseases related to iron metabolism. Acta Haematologica 137, 220–236. [DOI] [PubMed] [Google Scholar]
- Reid, D. W. , Anderson, G. J. & Lamont, I. L. (2009). Role of lung iron in determining the bacterial and host struggle in cystic fibrosis. American Journal of Physiology ‐ Lung Cellular and Molecular Physiology 297, L795–L802. [DOI] [PubMed] [Google Scholar]
- Relman, D. A. , Schmidt, T. M. , Macdermott, R. P. & Falkow, S. (1992). Identification of the uncultured bacillus of Whipple's disease. New England Journal of Medicine 327, 293–301. [DOI] [PubMed] [Google Scholar]
- Remick, D. G. & Ward, P. A. (2005). Evaluation of endotoxin models for the study of sepsis. Shock 24(Suppl. 1), 7–11. [DOI] [PubMed] [Google Scholar]
- Renesto, P. , Crapoulet, N. , Ogata, H. , La Scola, B. , Vestris, G. , Claverie, J. M. & Raoult, D. (2003). Genome‐based design of a cell‐free culture medium for Tropheryma whipplei . Lancet 362, 447–449. [DOI] [PubMed] [Google Scholar]
- Reyes, L. , Herrera, D. , Kozarov, E. , Roldán, S. & Progulske‐Fox, A. (2013). Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. Journal of Periodontology 84, S30–S50. [DOI] [PubMed] [Google Scholar]
- Ribet, D. & Cossart, P. (2015). How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes & Infection 17, 173–183. [DOI] [PubMed] [Google Scholar]
- Richardson, D. R. , Huang, M. L. , Whitnall, M. , Becker, E. M. , Ponka, P. & Rahmanto, Y. S. (2010). The ins and outs of mitochondrial iron‐loading: the metabolic defect in Friedreich's ataxia. Journal of Molecular Medicine 88, 323–329. [DOI] [PubMed] [Google Scholar]
- Ridker, P. M. & Silvertown, J. D. (2008). Inflammation, C‐reactive protein, and atherothrombosis. Journal of Periodontology 79, 1544–1551. [DOI] [PubMed] [Google Scholar]
- Ritter, C. , da Cunha, A. A. , Echer, I. C. , Andrades, M. , Reinke, A. , Lucchiari, N. , Rocha, J. , Streck, E. L. , Menna‐Barreto, S. , Moreira, J. C. & Dal‐Pizzol, F. (2006). Effects of N‐acetylcysteine plus deferoxamine in lipopolysaccharide‐induced acute lung injury in the rat. Critical Care Medicine 34, 471–477. [DOI] [PubMed] [Google Scholar]
- Rittershaus, E. S. C. , Baek, S. H. & Sassetti, C. M. (2013). The normalcy of dormancy: common themes in microbial quiescence. Cell Host & Microbe 13, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival, T. , Page, R. M. , Chandraratna, D. S. , Sendall, T. J. , Ryder, E. , Liu, B. , Lewis, H. , Rosahl, T. , Hider, R. , Camargo, L. M. , Shearman, M. S. , Crowther, D. C. & Lomas, D. A. (2009). Fenton chemistry and oxidative stress mediate the toxicity of the beta‐amyloid peptide in a Drosophila model of Alzheimer's disease. European Journal of Neuroscience 29, 1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera, M. F. , Lee, J. Y. , Aneja, M. , Goswami, V. , Liu, L. , Velsko, I. M. , Chukkapalli, S. S. , Bhattacharyya, I. , Chen, H. , Lucas, A. R. & Kesavalu, L. N. (2013). Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoEnull mice. PLoS One 8, e57178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard, P. Y. , Dekker, G. , Iacobelli, S. & Chaouat, G. (2016). An essay of reflection: why does preeclampsia exist in humans, and why are there such huge geographical differences in epidemiology? Journal of Reproductive Immunology 114, 44–47. [DOI] [PubMed] [Google Scholar]
- Rodriguez, G. M. & Smith, I. (2003). Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Molecular Microbiology 47, 1485–1494. [DOI] [PubMed] [Google Scholar]
- Rosen, D. A. , Hooton, T. M. , Stamm, W. E. , Humphrey, P. A. & Hultgren, S. J. (2007). Detection of intracellular bacterial communities in human urinary tract infection. PLoS Medicine 4, e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault, T. A. (2016). Mitochondrial iron overload: causes and consequences. Current Opinion in Genetics & Development 38, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryynänen, J. , Neme, A. , Tuomainen, T. P. , Virtanen, J. K. , Voutilainen, S. , Nurmi, T. , de Mello, V. D. , Uusitupa, M. & Carlberg, C. (2014). Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Molecular Nutrition & Food Research 58, 2036–2045. [DOI] [PubMed] [Google Scholar]
- Saá, P. & Cervenakova, L. (2015). Protein misfolding cyclic amplification (PMCA): current status and future directions. Virus Research 207, 47–61. [DOI] [PubMed] [Google Scholar]
- Sachidanandham, R. & Yew‐Hoong Gin, K. (2009). A dormancy state in nonspore‐forming bacteria. Applied Microbiology and Biotechnology 81, 927–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakawi, Y. , Tarpey, M. , Chen, Y. F. , Calhoun, D. A. , Connor, M. G. , Chestnut, D. H. & Parks, D. A. (2000). Evaluation of low‐dose endotoxin administration during pregnancy as a model of preeclampsia. Anesthesiology 93, 1446–1455. [DOI] [PubMed] [Google Scholar]
- Sakka, S. G. , Kochem, A. J. , Disqué, C. & Wellinghausen, N. (2009). Blood infection diagnosis by 16S rDNA broad‐spectrum polymerase chain reaction: the relationship between antibiotic treatment and bacterial DNA load. Anesthesia & Analgesia 109, 1707–1708. [DOI] [PubMed] [Google Scholar]
- Saksa, N. , Neme, A. , Ryynänen, J. , Uusitupa, M. , de Mello, V. D. F. , Voutilainen, S. , Nurmi, T. , Virtanen, J. K. , Tuomainen, T. P. & Carlberg, C. (2015). Dissecting high from low responders in a vitamin D3 intervention study. Journal of Steroid Biochemistry and Molecular Biology 148, 275–282. [DOI] [PubMed] [Google Scholar]
- Sakura, T. , Morioka, T. , Shioi, A. , Kakutani, Y. , Miki, Y. , Yamazaki, Y. , Motoyama, K. , Mori, K. , Fukumoto, S. , Shoji, T. , Emoto, M. & Inaba, M. (2017). Lipopolysaccharide‐binding protein is associated with arterial stiffness in patients with type 2 diabetes: a cross‐sectional study. Cardiovascular Diabetology 16, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem, F. , Bjorndahl, T. C. , Ladner, C. L. , Perez‐Pineiro, R. , Ametaj, B. N. & Wishart, D. S. (2014). Lipopolysaccharide induced conversion of recombinant prion protein. Prion 8, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam, T. , Ito, A. , Rong, X. , Kim, J. , van Stijn, C. , Chamberlain, B. T. , Jung, M. E. , Chao, L. C. , Jones, M. , Gilliland, T. , Wu, X. , Su, G. L. , Tangirala, R. K. , Tontonoz, P. & Hong, C. (2014). The macrophage LBP gene is an LXR target that promotes macrophage survival and atherosclerosis. Journal of Lipid Research 55, 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson, D. R. , Welsh, D. A. & Shellito, J. E. (2015). Regulation of lung immunity and host defense by the intestinal microbiota. Frontiers in Microbiology 6, 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu, K. V. , Sherwin, E. , Schellekens, H. , Stanton, C. , Dinan, T. G. & Cryan, J. F. (2017). Feeding the microbiota‐gut‐brain axis: diet, microbiome, and neuropsychiatry. Translational Research 179, 223–244. [DOI] [PubMed] [Google Scholar]
- SanMiguel, A. & Grice, E. A. (2015). Interactions between host factors and the skin microbiome. Cellular and Molecular Life Sciences 72, 1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, R. M. , Barbieiro, J. , Lima, M. M. , Dombrowski, P. A. , Andreatini, R. & Vital, M. A. B. F. (2010). Depressive‐like behaviors alterations induced by intranigral MPTP, 6‐OHDA, LPS and rotenone models of Parkinson's disease are predominantly associated with serotonin and dopamine. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 34, 1104–1114. [DOI] [PubMed] [Google Scholar]
- Sato, J. , Kanazawa, A. , Ikeda, F. , Yoshihara, T. , Goto, H. , Abe, H. , Komiya, K. , Kawaguchi, M. , Shimizu, T. , Ogihara, T. , Tamura, Y. , Sakurai, Y. , Yamamoto, R. , Mita, T. , Fujitani, Y. , et al. (2014). Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes. Diabetes Care 37, 2343–2350. [DOI] [PubMed] [Google Scholar]
- Sayinalp, N. , Haznedaroğlu, I. C. , Büyükaşik, Y. , Göker, H. , Aksu, S. , Koçoğlu, H. , Özcebe, O. I. , Koşar, A. , Kirazli, Ş. & Dündar, S. V. (2004). Protein C inhibitor and serum amyloid A in immune thrombocytopaenic purpura. Journal of International Medical Research 32, 62–65. [DOI] [PubMed] [Google Scholar]
- Schaffer, J. E. (2003). Lipotoxicity: when tissues overeat. Current Opinion in Lipidology 14, 281–287. [DOI] [PubMed] [Google Scholar]
- Schaffer, J. E. (2016). Lipotoxicity: many roads to cell dysfunction and cell death: introduction to a thematic review series. Journal of Lipid Research 57, 1327–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber, J. , Dorschner, R. A. , Coda, A. B. , Buchau, A. S. , Liu, P. T. , Kiken, D. , Helfrich, Y. R. , Kang, S. , Elalieh, H. Z. , Steinmeyer, A. , Zugel, U. , Bikle, D. D. , Modlin, R. L. & Gallo, R. L. (2007). Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D‐dependent mechanism. Journal of Clinical Investigation 117, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer, M. , Smeekens, S. P. , Vlamakis, H. , Jaeger, M. , Oosting, M. , Franzosa, E. A. , Jansen, T. , Jacobs, L. , Bonder, M. J. , Kurilshikov, A. , Fu, J. , Joosten, L. A. , Zhernakova, A. , Huttenhower, C. , Wijmenga, C. , et al. (2016). Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1125–1136 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, K. , Wienken, M. , Keller, C. W. , Balcarek, P. , Münz, C. & Schmidt, J. (2017). IL‐1beta‐induced accumulation of amyloid: macroautophagy in skeletal muscle depends on ERK. Mediators of Inflammation 2017, 5470831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, D. S. & Ayres, J. S. (2008). Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Reviews Immunology 8, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, S. A. (2016). Neurodegenerations with Brain Iron Accumulation. Parkinsonism and Related Disorders 22(Suppl. 1), S21–S25. [DOI] [PubMed] [Google Scholar]
- Schroeder, B. O. & Bäckhed, F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nature Medicine 22, 1079–1089. [DOI] [PubMed] [Google Scholar]
- Schwandner, R. , Dziarski, R. , Wesche, H. , Rothe, M. & Kirschning, C. J. (1999). Peptidoglycan‐ and lipoteichoic acid‐induced cell activation is mediated by toll‐like receptor 2. Journal of Biological Chemistry 274, 17406–17409. [DOI] [PubMed] [Google Scholar]
- Schwartz, D. J. , Chen, S. L. , Hultgren, S. J. & Seed, P. C. (2011). Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infection & Immunity 79, 4250–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedhelm, E. & Boger, R. H. (2003). Application of gas chromatography‐mass spectrometry for analysis of isoprostanes: their role in cardiovascular disease. Clinical Chemistry and Laboratory Medicine 41, 1552–1561. [DOI] [PubMed] [Google Scholar]
- Scioscia, M. , Robillard, P. Y. , Hall, D. R. , Rademacher, L. H. , Williams, P. J. & Rademacher, T. W. (2012). Inositol phosphoglycan P‐type in infants of preeclamptic mothers. Journal of Maternal‐Fetal & Neonatal Medicine 25, 193–195. [DOI] [PubMed] [Google Scholar]
- Scioscia, M. , Williams, P. J. , Gumaa, K. , Fratelli, N. , Zorzi, C. & Rademacher, T. W. (2011). Inositol phosphoglycans and preeclampsia: from bench to bedside. Journal of Reproductive Immunology 89, 173–177. [DOI] [PubMed] [Google Scholar]
- Seal, J. B. , Morowitz, M. , Zaborina, O. , An, G. & Alverdy, J. C. (2010). The molecular Koch's postulates and surgical infection: a view forward. Surgery 147, 757–765. [DOI] [PubMed] [Google Scholar]
- See, S. , Scott, E. K. & Levin, M. W. (2005). Penicillin‐induced Jarisch‐Herxheimer reaction. Annals of Pharmacotherapy 39, 2128–2130. [DOI] [PubMed] [Google Scholar]
- Segre, J. A. (2013). What does it take to satisfy Koch's postulates two centuries later? Microbial genomics and Propionibacteria acnes . Journal of Investigative Dermatology 133, 2141–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim, M. H. & Ratan, R. R. (2004). The role of iron neurotoxicity in ischemic stroke. Ageing Research Reviews 3, 345–353. [DOI] [PubMed] [Google Scholar]
- Sengupta, U. , Nilson, A. N. & Kayed, R. (2016). The role of amyloid‐beta oligomers in toxicity, propagation, and immunotherapy. eBioMedicine 6, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar, Z. , Gür, E. & Develioğlu, O. (2006). Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochemistry and Function 24, 209–215. [DOI] [PubMed] [Google Scholar]
- Serpell, L. C. (2000). Alzheimer's amyloid fibrils: structure and assembly. Biochimica et Biophysica Acta 1502, 16–30. [DOI] [PubMed] [Google Scholar]
- Serpell, L. C. , Benson, M. , Liepnieks, J. J. & Fraser, P. E. (2007). Structural analyses of fibrinogen amyloid fibrils. Amyloid 14, 199–203. [DOI] [PubMed] [Google Scholar]
- Serrano, M. , Moreno‐Navarrete, J. M. , Puig, J. , Moreno, M. , Guerra, E. , Ortega, F. , Xifra, G. , Ricart, W. & Fernández‐Real, J. M. (2013). Serum lipopolysaccharide‐binding protein as a marker of atherosclerosis. Atherosclerosis 230, 223–227. [DOI] [PubMed] [Google Scholar]
- Seubert, A. , Schulein, R. & Dehio, C. (2002). Bacterial persistence within erythrocytes: a unique pathogenic strategy of Bartonella spp. International Journal of Medical Microbiology 291, 555–560. [DOI] [PubMed] [Google Scholar]
- Shah, D. , Zhang, Z. , Khodursky, A. , Kaldalu, N. , Kurg, K. & Lewis, K. (2006). Persisters: a distinct physiological state of E. coli . BMC Microbiology 6, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey‐Toppen, T. P. , Tewari, A. K. & Raman, S. V. (2014). Iron and atherosclerosis: nailing down a novel target with magnetic resonance. Journal of Cardiovascular Translational Research 7, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, J. , Chakraborty, A. , Nag, A. , Chattopadyay, A. , Dasgupta, A. K. & Bhattacharyya, M. (2017). Intracellular iron overload leading to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients. European Journal of Haematology 99, 399–408. [DOI] [PubMed] [Google Scholar]
- Sheelakumari, R. , Madhusoodanan, M. , Radhakrishnan, A. , Ranjith, G. & Thomas, B. (2016). A potential biomarker in amyotrophic lateral sclerosis: can assessment of brain iron deposition with SWI and corticospinal tract degeneration with DTI help? American Journal of Neuroradiology 37, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C. H. , Chou, C. H. , Liu, F. C. , Lin, T. Y. , Huang, W. Y. , Wang, Y. C. & Kao, C. H. (2016). Association between tuberculosis and Parkinson disease: a nationwide, population‐based cohort study. Medicine (Baltimore) 95, e2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, L. & Ji, H. F. (2015). Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta‐analysis. Nutrition Journal 14, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin, E. , Dinan, T. G. & Cryan, J. F. (2017). Recent developments in understanding the role of the gut microbiota in brain health and disease. Annals of the New York Academy of Sciences 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- Shim, R. & Wong, C. H. Y. (2016). Ischemia, immunosuppression and infection‐‐tackling the predicaments of post‐stroke complications. International Journal of Molecular Sciences 17, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, C. S. , Moon, B. S. , Park, K. S. , Kim, S. Y. , Park, S. J. , Chung, M. H. & Lee, H. K. (2001). Serum 8‐hydroxy‐guanine levels are increased in diabetic patients. Diabetes Care 24, 733–737. [DOI] [PubMed] [Google Scholar]
- Shovlin, C. L. , Awan, I. , Abdulla, F. N. , Govani, F. S. , Mollet, I. & Patel, D. (2015). One in twenty patients with hereditary hemorrhagic telangiectasia report iron treatments precipitate nosebleeds. Angiogenesis 18, 566–567. [Google Scholar]
- Shovlin, C. L. , Gilson, C. , Busbridge, M. , Patel, D. , Shi, C. , Dina, R. , Abdulla, F. N. & Awan, I. (2016). Can iron treatments aggravate epistaxis in some patients with hereditary hemorrhagic telangiectasia? Laryngoscope 126, 2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, S. K. , Cook, D. , Meyer, J. , Vernon, S. D. , Le, T. , Clevidence, D. , Robertson, C. E. , Schrodi, S. J. , Yale, S. & Frank, D. N. (2015). Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS One 10, e0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siakallis, L. , Tziakouri‐Shiakalli, C. & Georgiades, C. S. (2014). Amyloidosis: review and imaging findings. Seminars in Ultrasound, CT and MRI 35, 225–239. [DOI] [PubMed] [Google Scholar]
- Silva, C. J. , Vazquez‐Fernández, E. , Onisko, B. & Requena, J. R. (2015). Proteinase K and the structure of PrPSc: the good, the bad and the ugly. Virus Research 207, 120–126. [DOI] [PubMed] [Google Scholar]
- Simcox, J. A. & McClain, D. A. (2013). Iron and diabetes risk. Cell Metabolism 17, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, Z. & Chadha, P. (2016). Assessment of DNA damage as an index of genetic toxicity in welding microenvironments among iron‐based industries. Toxicology and Industrial Health 32, 1817–1824. [DOI] [PubMed] [Google Scholar]
- Sipe, J. D. , Benson, M. D. , Buxbaum, J. N. , Ikeda, S. , Merlini, G. , Saraiva, M. J. & Westermark, P. (2014). Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21, 221–224. [DOI] [PubMed] [Google Scholar]
- Sivick, K. E. & Mobley, H. L. T. (2010). Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infection & Immunity 78, 568–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaaby, T. , Husemoen, L. L. , Thuesen, B. H. & Linneberg, A. (2015). Prospective population‐based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 50, 231–238. [DOI] [PubMed] [Google Scholar]
- Skaaby, T. , Husemoen, L. L. , Thuesen, B. H. , Pisinger, C. , Jørgensen, T. , Fenger, R. V. & Linneberg, A. (2014). Vitamin D status and chronic obstructive pulmonary disease: a prospective general population study. PLoS One 9, e90654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar, E. P. (2010). The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathogens 6, e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, B. G. , McColl, B. W. , Allmendinger, R. , Pahle, R. , Lopez‐Castejon, G. , Rothwell, N. J. , Knowles, J. , Mendes, P. , Brough, D. & Kell, D. B. (2011). Efficient discovery of anti‐inflammatory small molecule combinations using evolutionary computing. Nature Chemical Biology 7, 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C. J. , Nakagomi, A. , Chandar, S. , Cai, H. , Lim, I. G. S. , McNeil, H. P. , Freedman, S. B. & Geczy, C. L. (2006). C‐reactive protein contributes to the hypercoagulable state in coronary artery disease. Journal of Thrombosis and Haemostasis 4, 98–106. [DOI] [PubMed] [Google Scholar]
- Spaulding, C. N. , Dodson, K. W. , Chapman, M. R. & Hultgren, S. J. (2015). Fueling the fire with fibers: bacterial amyloids promote inflammatory disorders. Cell Host & Microbe 18, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritharan, M. (2006). Iron and bacterial virulence. Indian Journal of Medical Microbiology 24, 163–164. [PubMed] [Google Scholar]
- Stadler, N. , Lindner, R. A. & Davies, M. J. (2004). Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arteriosclerosis, Thrombosis & Vascular Biology 24, 949–954. [DOI] [PubMed] [Google Scholar]
- Stankiewicz, J. M. , Neema, M. & Ceccarelli, A. (2014). Iron and multiple sclerosis. Neurobiology of Aging 35(Suppl. 2), S51–S58. [DOI] [PubMed] [Google Scholar]
- Stanley, N. , Stadler, N. , Woods, A. A. , Bannon, P. G. & Davies, M. J. (2006). Concentrations of iron correlate with the extent of protein, but not lipid, oxidation in advanced human atherosclerotic lesions. Free Radical Biology and Medicine 40, 1636–1643. [DOI] [PubMed] [Google Scholar]
- Stefani, M. (2012). Structural features and cytotoxicity of amyloid oligomers: implications in Alzheimer's disease and other diseases with amyloid deposits. Progress in Neurobiology 99, 226–245. [DOI] [PubMed] [Google Scholar]
- Stefanova, K. I. , Delcheva, G. T. , Maneva, A. I. , Batalov, A. Z. , Geneva‐Popova, M. G. , Karalilova, R. V. & Simitchiev, K. K. (2016). Pathobiochemical mechanisms relating iron homeostasis to parameters of inflammatory activity and autoimmune disorders in rheumatoid arthritis. Folia Medica (Plovdiv) 58, 257–263. [DOI] [PubMed] [Google Scholar]
- Stephenson, E. , Nathoo, N. , Mahjoub, Y. , Dunn, J. F. & Yong, V. W. (2014). Iron in multiple sclerosis: roles in neurodegeneration and repair. Nature Reviews Neurology 10, 459–468. [DOI] [PubMed] [Google Scholar]
- Sternberg, Z. , Hu, Z. , Sternberg, D. , Waseh, S. , Quinn, J. F. , Wild, K. , Jeffrey, K. , Zhao, L. & Garrick, M. (2017). Serum hepcidin levels, iron dyshomeostasis and cognitive loss in Alzheimer's disease. Aging & Disease 8, 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stijlemans, B. , Beschin, A. , Magez, S. , Van Ginderachter, J. A. & De Baetselier, P. (2015). Iron homeostasis and trypanosoma brucei associated immunopathogenicity development: a battle/quest for iron. BioMed Research International 2015, 819389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll, L. L. , Denning, G. M. & Weintraub, N. L. (2004). Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arteriosclerosis, Thrombosis & Vascular Biology 24, 2227–2236. [DOI] [PubMed] [Google Scholar]
- Stromer, T. & Serpell, L. C. (2005). Structure and morphology of the Alzheimer's amyloid fibril. Microscopy Research and Technique 67, 210–217. [DOI] [PubMed] [Google Scholar]
- Sturm, A. & Dworkin, J. (2015). Phenotypic diversity as a mechanism to exit cellular dormancy. Current Biology 25, 2272–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, J. H. , Chung, Y. C. , Lee, H. C. , Tseng, I. C. & Chang, M. C. (2009). Ferrous iron‐binding protein Omb of Salmonella enterica serovar Choleraesuis promotes resistance to hydrophobic antibiotics and contributes to its virulence. Microbiology 155, 2365–2374. [DOI] [PubMed] [Google Scholar]
- Subashchandrabose, S. & Mobley, H. L. T. (2015). Back to the metal age: battle for metals at the host‐pathogen interface during urinary tract infection. Metallomics 7, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S. , Blanton, L. V. , Frese, S. A. , Charbonneau, M. , Mills, D. A. & Gordon, J. I. (2015). Cultivating healthy growth and nutrition through the gut microbiota. Cell 161, 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, J. L. (2009). Iron in arterial plaque: a modifiable risk factor for atherosclerosis. Biochimica et Biophysica Acta 1790, 718–723. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Yu, Z. , Ye, X. , Zou, S. , Li, H. , Yu, D. , Wu, H. , Chen, Y. , Dore, J. , Clement, K. , Hu, F. B. & Lin, X. (2010). A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33, 1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Pan, A. , Hu, F. B. , Manson, J. E. & Rexrode, K. M. (2012). 25‐Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta‐analysis. Stroke 43, 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutak, R. , Lesuisse, E. , Tachezy, J. & Richardson, D. R. (2008). Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends in Microbiology 16, 261–268. [DOI] [PubMed] [Google Scholar]
- Suwalsky, M. , Bolognin, S. & Zatta, P. (2009). Interaction between Alzheimer's amyloid‐beta and amyloid‐beta‐metal complexes with cell membranes. Journal of Alzheimers Disease 17, 81–90. [DOI] [PubMed] [Google Scholar]
- Szeto, F. L. , Sun, J. , Kong, J. , Duan, Y. , Liao, A. , Madara, J. L. & Li, Y. C. (2007). Involvement of the vitamin D receptor in the regulation of NF‐kappaB activity in fibroblasts. Journal of Steroid Biochemistry and Molecular Biology 103, 563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta, H. P. , Berthelot, K. , Coulary‐Salin, B. , Castano, S. , Desbat, B. , Bonnafous, P. , Lambert, O. , Alves, I. , Cullin, C. & Lecomte, S. (2012). A yeast toxic mutant of HET‐s amyloid disrupts membrane integrity. Biochimica et Biophysica Acta 1818, 2325–2334. [DOI] [PubMed] [Google Scholar]
- Tang, F. & Saier, M. H. Jr. (2014). Transport proteins promoting Escherichia coli pathogenesis. Microbial Pathogenesis 71‐72, 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi, C. , Agostoni, C. & Kim, K. S. (2014). The placental microbiome and pediatric research. Pediatric Research 76, 218–219. [DOI] [PubMed] [Google Scholar]
- Teeuw, W. J. , Slot, D. E. , Susanto, H. , Gerdes, V. E. , Abbas, F. , D'Aiuto, F. , Kastelein, J. J. & Loos, B. G. (2014). Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta‐analysis. Journal of Clinical Periodontology 41, 70–79. [DOI] [PubMed] [Google Scholar]
- Telling, N. D. , Everett, J. , Collingwood, J. F. , Dobson, J. , van der Laan, G. , Gallagher, J. J. , Wang, J. & Hitchcock, A. P. (2017). Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer's disease. Cell Chemical Biology 24, 1205–1215 e3. [DOI] [PubMed] [Google Scholar]
- The Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl, I. , Theurl, M. , Seifert, M. , Mair, S. , Nairz, M. , Rumpold, H. , Zoller, H. , Bellmann‐Weiler, R. , Niederegger, H. , Talasz, H. & Weiss, G. (2008). Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 111, 2392–2399. [DOI] [PubMed] [Google Scholar]
- Thevaranjan, N. , Puchta, A. , Schulz, C. , Naidoo, A. , Szamosi, J. C. , Verschoor, C. P. , Loukov, D. , Schenck, L. P. , Jury, J. , Foley, K. P. , Schertzer, J. D. , Larche, M. J. , Davidson, D. J. , Verdu, E. F. , Surette, M. G. & Bowdish, D. M. (2017). Age‐associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe 21, 455–466 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn, A. N. , Macia, L. & Mackay, C. R. (2014). Diet, metabolites, and "western‐lifestyle" inflammatory diseases. Immunity 40, 833–842. [DOI] [PubMed] [Google Scholar]
- Thuret, I. (2013). Post‐transfusional iron overload in the haemoglobinopathies. Comptes Rendus Biologies 336, 164–172. [DOI] [PubMed] [Google Scholar]
- Thwaites, G. E. & Gant, V. (2011). Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nature Reviews Microbiology 9, 215–222. [DOI] [PubMed] [Google Scholar]
- Toldi, G. , Stenczer, B. , Molvarec, A. , Takáts, Z. , Bekö, G. , Rigó, J. Jr. & Vásárhelyi, B. (2010). Hepcidin concentrations and iron homeostasis in preeclampsia. Clinical Chemistry and Laboratory Medicine 48, 1423–1426. [DOI] [PubMed] [Google Scholar]
- Tomás, I. , Diz, P. , Tobías, A. , Scully, C. & Donos, N. (2012). Periodontal health status and bacteraemia from daily oral activities: systematic review/meta‐analysis. Journal of Clinical Periodontology 39, 213–228. [DOI] [PubMed] [Google Scholar]
- Toyofuku, T. , Inoue, Y. , Kurihara, N. , Kudo, T. , Jibiki, M. , Sugano, N. , Umeda, M. & Izumi, Y. (2011). Differential detection rate of periodontopathic bacteria in atherosclerosis. Surgery Today 41, 1395–1400. [DOI] [PubMed] [Google Scholar]
- Traoré, H. N. & Meyer, D. (2007). Necrosis of host cells and survival of pathogens following iron overload in an in vitro model of co‐infection with human immunodeficiency virus (HIV) and Mycobacterium tuberculosis . International Journal of Antimicrobial Agents 29, 465–470. [DOI] [PubMed] [Google Scholar]
- Trikha, S. & Jeremic, A. M. (2013). Distinct internalization pathways of human amylin monomers and its cytotoxic oligomers in pancreatic cells. PLoS One 8, e73080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsemekhman, K. , Goldschmidt, L. , Eisenberg, D. & Baker, D. (2007). Cooperative hydrogen bonding in amyloid formation. Protein Science 16, 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikas, D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Analytical Biochemistry 524, 13–30. [DOI] [PubMed] [Google Scholar]
- Tufekci, K. U. , Genc, S. & Genc, K. (2011). The endotoxin‐induced neuroinflammation model of Parkinson's disease. Parkinson's Disease 2011, 487450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo, Q. Z. , Lei, P. , Jackman, K. A. , Li, X. L. , Xiong, H. , Li, X. L. , Liuyang, Z. Y. , Roisman, L. , Zhang, S. T. , Ayton, S. , Wang, Q. , Crouch, P. J. , Ganio, K. , Wang, X. C. , Pei, L. , et al. (2017). Tau‐mediated iron export prevents ferroptotic damage after ischemic stroke. Molecular Psychiatry 22, 1520–1530. [DOI] [PubMed] [Google Scholar]
- Tuomi, K. & Logomarsino, J. V. (2016). Bacterial lipopolysaccharide, lipopolysaccharide‐binding protein, and other inflammatory markers in obesity and after bariatric surgery. Metabolic Syndrome and Related Disorders 14, 279–288. [DOI] [PubMed] [Google Scholar]
- Turetsky, A. , Goddeau, R. P. Jr. & Henninger, N. (2015). Low serum vitamin D Is independently associated with larger lesion volumes after ischemic stroke. Journal of Stroke and Cerebrovascular Diseases 24, 1555–1563. [DOI] [PubMed] [Google Scholar]
- Turnbaugh, P. J. , Ley, R. E. , Hamady, M. , Fraser‐Liggett, C. M. , Knight, R. & Gordon, J. I. (2007). The human microbiome project. Nature 449, 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko, R. (2016). Structure of aggregates revealed. Nature 537, 492–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko, R. & Wickner, R. B. (2013). Molecular structures of amyloid and prion fibrils: consensus versus controversy. Accounts of Chemical Research 46, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill, D. M. , Ozinsky, A. , Hajjar, A. M. , Stevens, A. , Wilson, C. B. , Bassetti, M. & Aderem, A. (1999). The Toll‐like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815. [DOI] [PubMed] [Google Scholar]
- Urieli‐Shoval, S. , Shubinsky, G. , Linke, R. P. , Fridkin, M. , Tabi, I. & Matzner, Y. (2002). Adhesion of human platelets to serum amyloid A. Blood 99, 1224–1229. [DOI] [PubMed] [Google Scholar]
- Uversky, V. N. (2010). Mysterious oligomerization of the amyloidogenic proteins. FEBS Journal 277, 2940–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia‐Shelton, F. & Loeffelholz, M. (2014). Nonculture techniques for the detection of bacteremia and fungemia. Future Microbiology 9, 543–559. [DOI] [PubMed] [Google Scholar]
- Valincius, G. , Heinrich, F. , Budvytyte, R. , Vanderah, D. J. , McGillivray, D. J. , Sokolov, Y. , Hall, J. E. & Losche, M. (2008). Soluble amyloid beta‐oligomers affect dielectric membrane properties by bilayer insertion and domain formation: implications for cell toxicity. Biophysical Journal 95, 4845–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek, J. H. G. M. , Kirkwood, T. B. L. & Bassingthwaighte, J. B. (2016). Understanding the physiology of the ageing individual: computational modelling of changes in metabolism and endurance. Interface Focus 6, 20150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen, T. A. , Harmsen, H. J. M. , Bootsma, H. , Spijkervet, F. K. L. , Kroese, F. G. M. & Vissink, A. (2016). The microbiome systemic diseases connection. Oral Diseases 22, 719–724. [DOI] [PubMed] [Google Scholar]
- van der Schaft, J. , Koek, H. L. , Dijkstra, E. , Verhaar, H. J. , van der Schouw, Y. T. & Emmelot‐Vonk, M. H. (2013). The association between vitamin D and cognition: a systematic review. Ageing Research Reviews 12, 1013–1023. [DOI] [PubMed] [Google Scholar]
- van Duijn, S. , Bulk, M. , van Duinen, S. G. , Nabuurs, R. J. A. , van Buchem, M. A. , van der Weerd, L. & Natté, R. (2017). Cortical iron reflects severity of Alzheimer's disease. Journal of Alzheimers Disease 60, 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg, J. J. , Lin, H. , Gao, X. , Toh, E. , Fortney, K. R. , Ellinger, S. , Zwickl, B. , Janowicz, D. M. , Katz, B. P. , Nelson, D. E. , Dong, Q. & Spinola, S. M. (2015). The human skin microbiome associates with the outcome of and is influenced by bacterial infection. MBio 6, e01315‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn, B. B. , Bruinse, H. W. , Veerbeek, J. H. , Post Uiterweer, E. D. , Koenen, S. V. , van der Bom, J. G. , Rijkers, G. T. , Roest, M. & Franx, A. (2016). Postpartum Circulating markers of inflammation and the systemic acute‐phase response after early‐onset preeclampsia. Hypertension 67, 404–414. [DOI] [PubMed] [Google Scholar]
- Vasil, M. L. & Ochsner, U. A. (1999). The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Molecular Microbiology 34, 399–413. [DOI] [PubMed] [Google Scholar]
- Vaubel, R. A. & Isaya, G. (2013). Iron‐sulfur cluster synthesis, iron homeostasis and oxidative stress in Friedreich ataxia. Molecular and Cellular Neuroscience 55, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velsko, I. M. , Chukkapalli, S. S. , Rivera, M. F. , Lee, J. Y. , Chen, H. , Zheng, D. , Bhattacharyya, I. , Gangula, P. R. , Lucas, A. R. & Kesavalu, L. (2014). Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One 9, e97811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergès, B. , Duvillard, L. , Lagrost, L. , Vachoux, C. , Garret, C. , Bouyer, K. , Courtney, M. , Pomié, C. & Burcelin, R. (2014). Changes in lipoprotein kinetics associated with type 2 diabetes affect the distribution of lipopolysaccharides among lipoproteins. Journal of Clinical Endocrinology & Metabolism 99, E1245–E1253. [DOI] [PubMed] [Google Scholar]
- Versalovic, J. , Carroll, K. C. , Funke, G. , Jorgensen, J. H. , Landry, M. L. & Warnock, D. W. (2011). Manual of Clinical Microbiology, Tenth Edition (). American Society of Microbiology, Washington, DC. [Google Scholar]
- Vientós‐Plotts, A. I. , Ericsson, A. C. , Rindt, H. , Grobman, M. E. , Graham, A. , Bishop, K. , Cohn, L. A. & Reinero, C. R. (2017). Dynamic changes of the respiratory microbiota and its relationship to fecal and blood microbiota in healthy young cats. PLoS One 12, e0173818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vientós‐Plotts, A. I. , Ericsson, A. C. , Rindt, H. & Reinero, C. R. (2017). Oral probiotics alter healthy feline respiratory microbiota. Frontiers in Microbiology 8, 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimaleswaran, K. S. , Cavadino, A. , Berry, D. J. , LifeLines Cohort Study investigators , Jorde, R. , Dieffenbach, A. K. , Lu, C. , Alves, A. C. , Heerspink, H. J. , Tikkanen, E. , Eriksson, J. , Wong, A. , Mangino, M. , Jablonski, K. A. , Nolte, I. M. , et al. (2014). Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes & Endocrinology 2, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinchi, F. , Muckenthaler, M. U. , Da Silva, M. C. , Balla, G. , Balla, J. & Jeney, V. (2014). Atherogenesis and iron: from epidemiology to cellular level. Frontiers in Pharmacology 5, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A. , Rigante, D. , Lopalco, G. , Brizi, M. G. , Caso, F. , Franceschini, R. , Denaro, R. , Galeazzi, M. , Punzi, L. , Iannone, F. , Lapadula, G. , Simpatico, A. , Marrani, E. , Costa, L. , Cimaz, R. & Cantarini, L. (2014). Serum amyloid‐A in Behcet's disease. Clinical Rheumatology 33, 1165–1167. [DOI] [PubMed] [Google Scholar]
- Votyakova, T. V. , Kaprelyants, A. S. & Kell, D. B. (1994). Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in extended stationary phase. The population effect. Applied and Environmental Microbiology 60, 3284–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyoral, D. & Jiri, P. (2017). Therapeutic potential of hepcidin ‐ the master regulator of iron metabolism. Pharmacological Research 115, 242–254. [DOI] [PubMed] [Google Scholar]
- Waldron, J. L. , Ashby, H. L. , Cornes, M. P. , Bechervaise, J. , Razavi, C. , Thomas, O. L. , Chugh, S. , Deshpande, S. , Ford, C. & Gama, R. (2013). Vitamin D: a negative acute phase reactant. Journal of Clinical Pathology 66, 620–622. [DOI] [PubMed] [Google Scholar]
- Wallace, M. B. , Vazquez‐Roque, M. , Bojarski, C. & Schulzke, J. D. (2014). Imaging the leaky gut. Gastroenterology 147, 952–954. [DOI] [PubMed] [Google Scholar]
- Walter, J. & Ley, R. (2011). The human gut microbiome: ecology and recent evolutionary changes. Annual Review of Microbiology 65, 411–429. [DOI] [PubMed] [Google Scholar]
- Wälti, M. A. , Ravotti, F. , Arai, H. , Glabe, C. G. , Wall, J. S. , Böckmann, A. , Güntert, P. , Meier, B. H. & Riek, R. (2016). Atomic‐resolution structure of a disease‐relevant Abeta(1‐42) amyloid fibril. Proceedings of the National Academy of Sciences of the United States of America 113, E4976–E4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. S. , Jagadamma, S. , Mayes, M. A. , Schadt, C. W. , Steinweg, J. M. , Gu, L. H. & Post, W. M. (2015a). Microbial dormancy improves development and experimental validation of ecosystem model. ISME Journal 9, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Fang, X. & Wang, F. (2015b). Pleiotropic actions of iron balance in diabetes mellitus. Reviews in Endocrine and Metabolic Disorders 16, 15–23. [DOI] [PubMed] [Google Scholar]
- Wang, G. S. , Mayes, M. A. , Gu, L. H. & Schadt, C. W. (2014). Representation of dormant and active microbial dynamics for ecosystem modeling. PLoS One 9, e89252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Altemus, J. , Niazi, F. , Green, H. , Calhoun, B. C. , Sturgis, C. , Grobmyer, S. R. & Eng, C. (2017). Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget Gerontology 9, 88122–88138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Funchain, P. , Bebek, G. , Altemus, J. , Zhang, H. , Niazi, F. , Peterson, C. , Lee, W. T. , Burkey, B. B. & Eng, C. (2017). Microbiomic differences in tumor and paired‐normal tissue in head and neck squamous cell carcinomas. Genome Medicine 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Harrington, L. , Trebicka, E. , Shi, H. N. , Kagan, J. C. , Hong, C. C. , Lin, H. Y. , Babitt, J. L. & Cherayil, B. J. (2009). Selective modulation of TLR4‐activated inflammatory responses by altered iron homeostasis in mice. Journal of Clinical Investigation 119, 3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Zhang, X. , Chen, S. , Zhang, X. , Zhang, S. , Youdium, M. & Le, W. (2011). Prevention of motor neuron degeneration by novel iron chelators in SOD1G93A transgenic mice of amyotrophic lateral sclerosis. Neurodegenerative Diseases 8, 310–321. [DOI] [PubMed] [Google Scholar]
- Wang, W. (2005). Protein aggregation and its inhibition in biopharmaceutics. International Journal of Pharmacology 289, 1–30. [DOI] [PubMed] [Google Scholar]
- Wardman, P. & Candeias, L. P. (1996). Fenton chemistry: an introduction. Radiation Research 145, 523–531. [PubMed] [Google Scholar]
- Watanabe, K. , Yamashita, Y. , Ohgawara, K. , Sekiguchi, M. , Satake, N. , Orino, K. & Yamamoto, S. (2001). Iron content of rat serum ferritin. Journal of Veterinary Medical Science 63, 587–589. [DOI] [PubMed] [Google Scholar]
- Waterhouse, J. C. , Perez, T. H. & Albert, P. J. (2009). Reversing bacteria‐induced vitamin D receptor dysfunction is key to autoimmune disease. Annals of the New York Academy of Sciences 1173, 757–765. [DOI] [PubMed] [Google Scholar]
- Waters, K. M. , Cummings, B. S. , Shankaran, H. , Scholpa, N. E. & Weber, T. J. (2014). ERK oscillation‐dependent gene expression patterns and deregulation by stress response. Chemical Research in Toxicology 27, 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, S. W. , Novitsky, T. J. , Quinby, H. L. & Valois, F. W. (1977). Determination of bacterial number and biomass in the marine environment. Applied and Environmental Microbiology 33, 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, J. C. , Condello, C. , Stohr, J. , Oehler, A. , Lee, J. , DeArmond, S. J. , Lannfelt, L. , Ingelsson, M. , Giles, K. & Prusiner, S. B. (2014). Serial propagation of distinct strains of Abeta prions from Alzheimer's disease patients. Proceedings of the National Academy of Sciences of the United States of America 111, 10323–10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, E. D. (1978). Iron and infection. Microbiological Reviews 42, 45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, E. D. (2009). Iron availability and infection. Biochimica et Biophysica Acta 1790, 600–605. [DOI] [PubMed] [Google Scholar]
- Weinberg, E. D. & Miklossy, J. (2008). Iron withholding: a defense against disease. Journal of Alzheimers Disease 13, 451–463. [DOI] [PubMed] [Google Scholar]
- Weinreb, O. , Mandel, S. , Youdim, M. B. H. & Amit, T. (2013). Targeting dysregulation of brain iron homeostasis in Parkinson disease by iron chelators. Free Radical Biology and Medicine 62, 52–64. [DOI] [PubMed] [Google Scholar]
- Wen, W. , Li, Y. , Cheng, Y. , He, J. , Jia, R. , Li, C. , Guo, J. , Sun, X. & Li, Z. (2018). Lipopolysaccharide‐binding protein is a sensitive disease activity biomarker for rheumatoid arthritis. Clinical and Experimental Rheumatology. [PubMed] [Google Scholar]
- Weng, S. L. , Chiu, C. M. , Lin, F. M. , Huang, W. C. , Liang, C. , Yang, T. , Yang, T. L. , Liu, C. Y. , Wu, W. Y. , Chang, Y. A. , Chang, T. H. & Huang, H. D. (2014). Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS One 9, e110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling‐Resnick, M. (2010). Iron homeostasis and the inflammatory response. Annual Review of Nutrition 30, 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester, A. L. , Melby, K. K. , Wuyller, T. B. & Dahle, U. R. (2014). E. coli bacteremia strains ‐ high diversity and associations with age‐related clinical phenomena. Clinical Microbiology 3, 1000140. [Google Scholar]
- Westermark, G. T. & Westermark, P. (2009). Serum amyloid A and protein AA: molecular mechanisms of a transmissible amyloidosis. FEBS Letters 583, 2685–2690. [DOI] [PubMed] [Google Scholar]
- Westermark, P. , Lundmark, K. & Westermark, G. T. (2009). Fibrils from designed non‐amyloid‐related synthetic peptides induce AA‐amyloidosis during inflammation in an animal model. PLoS One 4, e6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwell‐Roper, C. , Dai, D. L. , Soukhatcheva, G. , Potter, K. J. , van Rooijen, N. , Ehses, J. A. & Verchere, C. B. (2011). IL‐1 blockade attenuates islet amyloid polypeptide‐induced proinflammatory cytokine release and pancreatic islet graft dysfunction. Journal of Immunology 187, 2755–2765. [DOI] [PubMed] [Google Scholar]
- Westwell‐Roper, C. Y. , Chehroudi, C. A. , Denroche, H. C. , Courtade, J. A. , Ehses, J. A. & Verchere, C. B. (2015). IL‐1 mediates amyloid‐associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia 58, 575–585. [DOI] [PubMed] [Google Scholar]
- Westwell‐Roper, C. Y. , Ehses, J. A. & Verchere, C. B. (2014). Resident macrophages mediate islet amyloid polypeptide‐induced islet IL‐1beta production and beta‐cell dysfunction. Diabetes 63, 1698–1711. [DOI] [PubMed] [Google Scholar]
- Williams, P. J. , Gumaa, K. , Scioscia, M. , Redman, C. W. & Rademacher, T. W. (2007). Inositol phosphoglycan P‐type in preeclampsia: a novel marker? Hypertension 49, 84–89. [DOI] [PubMed] [Google Scholar]
- Williamson, R. D. , McCarthy, C. , Kenny, L. C. & O'Keeffe, G. W. (2016). Magnesium sulphate prevents lipopolysaccharide‐induced cell death in an in vitro model of the human placenta. Pregnancy Hypertension 6, 356–360. [DOI] [PubMed] [Google Scholar]
- Wilson, I. D. & Nicholson, J. K. (2017). Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Translational Research 179, 204–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. B. (2006). Iron dysregulation in Friedreich ataxia. Seminars in Pediatric Neurology 13, 166–175. [DOI] [PubMed] [Google Scholar]
- Winner, M. W. III , Sharkey‐Toppen, T. , Zhang, X. , Pennell, M. L. , Simonetti, O. P. , Zweier, J. L. , Vaccaro, P. S. & Raman, S. V. (2015). Iron and noncontrast magnetic resonance T2* as a marker of intraplaque iron in human atherosclerosis. Journal of Vascular Surgery 61, 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham, M. , Kennedy, G. , Belch, J. , Hill, A. & Khan, F. (2014). Association between vitamin D status and markers of vascular health in patients with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). International Journal of Cardiology 174, 139–140. [DOI] [PubMed] [Google Scholar]
- Witham, M. D. , Adams, F. , McSwiggan, S. , Kennedy, G. , Kabir, G. , Belch, J. J. F. & Khan, F. (2015). Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome‐‐a randomised controlled trial. Nutrition, Metabolism & Cardiovascular Diseases 25, 287–294. [DOI] [PubMed] [Google Scholar]
- Woerman, A. L. , Kazmi, S. A. , Patel, S. , Freyman, Y. , Oehler, A. , Aoyagi, A. , Mordes, D. A. , Halliday, G. M. , Middleton, L. T. , Gentleman, S. M. , Olson, S. H. & Prusiner, S. B. (2018). MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathologica 35, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerman, A. L. , Stöhr, J. , Aoyagi, A. , Rampersaud, R. , Krejciova, Z. , Watts, J. C. , Ohyama, T. , Patel, S. , Widjaja, K. , Oehler, A. , Sanders, D. W. , Diamond, M. I. , Seeley, W. W. , Middleton, L. T. , Gentleman, S. M. , et al. (2015). Propagation of prions causing synucleinopathies in cultured cells. Proceedings of the National Academy of Sciences of the United States of America 112, E4949–E4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, H. (2015). Iron ‐ the missing link between ApoE and Alzheimer disease? Nature Reviews Neurology 11, 369. [DOI] [PubMed] [Google Scholar]
- Wood, T. K. , Knabel, S. J. & Kwan, B. W. (2013). Bacterial persister cell formation and dormancy. Applied and Environmental Microbiology 79, 7116–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyke, T. , Doud, D. F. R. & Schulz, F. (2017). The trajectory of microbial single‐cell sequencing. Nature Methods 14, 1045–1054. [DOI] [PubMed] [Google Scholar]
- Wu, S. , Liao, A. P. , Xia, Y. , Li, Y. C. , Li, J. D. , Sartor, R. B. & Sun, J. (2010). Vitamin D receptor negatively regulates bacterial‐stimulated NF‐kappaB activity in intestine. American Journal of Pathology 177, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Xia, Y. , Liu, X. & Sun, J. (2010). Vitamin D receptor deletion leads to reduced level of IkappaBalpha protein through protein translation, protein‐protein interaction, and post‐translational modification. International Journal of Biochemistry and Cell Biology 42, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, P. P. , Zheng, M. M. , Gong, P. , Lin, C. M. , Zhou, J. J. , Li, Y. J. , Shen, L. , Diao, Z. Y. , Yan, G. J. , Sun, H. X. & Hu, Y. L. (2015). Single administration of ultra‐low‐dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PLoS One 10, e0124001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, W. F. , Hellewell, A. L. , Gosal, W. S. , Homans, S. W. , Hewitt, E. W. & Radford, S. E. (2009). Fibril fragmentation enhances amyloid cytotoxicity. Journal of Biological Chemistry 284, 34272–34282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, W. F. , Hellewell, A. L. , Hewitt, E. W. & Radford, S. E. (2010). Fibril fragmentation in amyloid assembly and cytotoxicity: when size matters. Prion 4, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, M. , Terao, Y. , Mori‐Yamaguchi, Y. , Domon, H. , Sakaue, Y. , Yagi, T. , Nishino, K. , Yamaguchi, A. , Nizet, V. & Kawabata, S. (2013). Streptococcus pneumoniae invades erythrocytes and utilizes them to evade human innate immunity. PLoS One 8, e77282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi, H. , Iyama, S. , Yamaguchi, Y. , Kanakura, Y. & Iwatani, Y. (2002). Relation between iron content of serum ferritin and clinical status factors extracted by factor analysis in patients with hyperferritinemia. Clinical Biochemistry 35, 523–529. [DOI] [PubMed] [Google Scholar]
- Yang, W. S. & Stockwell, B. R. (2016). Ferroptosis: death by lipid peroxidation. Trends in Cell Biology 26, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates, S. L. , Burgess, L. H. , Kocsis‐Angle, J. , Antal, J. M. , Dority, M. D. , Embury, P. B. , Piotrkowski, A. M. & Brunden, K. R. (2000). Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP‐1 cells and murine microglia. Journal of Neurochemistry 74, 1017–1025. [DOI] [PubMed] [Google Scholar]
- Ye, Z. , Sharp, S. J. , Burgess, S. , Scott, R. A. , Imamura, F. , InterAct, C. , Langenberg, C. , Wareham, N. J. & Forouhi, N. G. (2015). Association between circulating 25‐hydroxyvitamin D and incident type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes & Endocrinology 3, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim, I. , Hur, E. & Kokturk, F. (2013). Inflammatory markers: C‐reactive protein, erythrocyte sedimentation rate, and leukocyte count in vitamin d deficient patients with and without chronic kidney disease. International Journal of Endocrinology 2013, 802165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, L. , Unger, E. L. , Jellen, L. C. , Earley, C. J. , Allen, R. P. , Tomaszewicz, A. , Fleet, J. C. & Jones, B. C. (2012). Systems genetic analysis of multivariate response to iron deficiency in mice. American Journal of Physiology ‐ Regulative, Integrative and Comparative Physiology 302, R1282–R1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, L. M. , Tu, L. H. , Raleigh, D. P. , Ashcroft, A. E. & Radford, S. E. (2017). Understanding co‐polymerization in amyloid formation by direct observation of mixed oligomers. Chemical Science 8, 5030–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, D. A. , Miller, C. W. , El‐Abbassi, A. M. , Cutchins, D. C. , Cutchins, C. , Grant, W. B. & Peiris, A. N. (2011). Antimicrobial implications of vitamin D. Dermato‐Endocrinology 3, 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. , Guo, P. , Xie, X. , Wang, Y. & Chen, G. (2017). Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. Journal of Cellular and Molecular Medicine 21, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer, U. , Lindner, B. , Inamura, S. , Heine, H. & Alexander, C. (2008). TLR2 ‐ promiscuous or specific? A critical re‐evaluation of a receptor expressing apparent broad specificity. Immunobiology 213, 205–224. [DOI] [PubMed] [Google Scholar]
- Zaman, G. S. & Zaman, F. (2015). Relationship between postprandial endotoxemia in nonobese postmenopausal women and diabetic nonobese postmenopausal women. Journal of Natural Science, Biology and Medicine 6, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamolodchikov, D. , Berk‐Rauch, H. E. , Oren, D. A. , Stor, D. S. , Singh, P. K. , Kawasaki, M. , Aso, K. , Strickland, S. & Ahn, H. J. (2016). Biochemical and structural analysis of the interaction between beta‐amyloid and fibrinogen. Blood 128, 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamolodchikov, D. & Strickland, S. (2012). Abeta delays fibrin clot lysis by altering fibrin structure and attenuating plasminogen binding to fibrin. Blood 119, 3342–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky, J. , Young, B. , Wang, H. J. , Westerman, M. , Olbina, G. , Nemeth, E. , Ganz, T. , Rivera, S. , Nissenson, A. R. & Salusky, I. B. (2009). Hepcidin‐‐a potential novel biomarker for iron status in chronic kidney disease. Clinical Journal of the American Society of Nephrology 4, 1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein, S. , Rachidi, S. s. , Shami, N. , Sharara, I. , Cheikh‐Ali, K. , Gauchez, A. S. , Moulis, J. M. , Ayoubi, J. M. , Salameh, P. & Hininger‐Favier, I. (2017). Association between iron level, glucose impairment and increased DNA damage during pregnancy. Journal of Trace Elements in Medicine & Biology 43, 52–57. [DOI] [PubMed] [Google Scholar]
- Zewinger, S. , Drechsler, C. , Kleber, M. E. , Dressel, A. , Riffel, J. , Triem, S. , Lehmann, M. , Kopecky, C. , Saemann, M. D. , Lepper, P. M. , Silbernagel, G. , Scharnagl, H. , Ritsch, A. , Thorand, B. , de las Heras Gala, T. , et al. (2015). Serum amyloid A: high‐density lipoproteins interaction and cardiovascular risk. European Heart Journal 36, 3007–3016. [DOI] [PubMed] [Google Scholar]
- Zhan, X. , Cox, C. , Ander, B. P. , Liu, D. , Stamova, B. , Jin, L. W. , Jickling, G. C. & Sharp, F. R. (2015). Inflammation combined with ischemia produces myelin injury and plaque‐like aggregates of myelin, amyloid‐beta and AbetaPP in adult rat brain. Journal of Alzheimers Disease 46, 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, X. , Stamova, B. , Jin, L. W. , DeCarli, C. , Phinney, B. & Sharp, F. R. (2016). Gram‐negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87, 2324–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Jackson, A. P. , Zhang, Z. R. , Han, Y. , Yu, S. , He, R. Q. & Perrett, S. (2010). Amyloid‐like aggregates of the yeast prion protein ure2 enter vertebrate cells by specific endocytotic pathways and induce apoptosis. PLoS One 5, e12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Hu, R. , Li, M. , Li, F. , Meng, H. , Zhu, G. , Lin, J. & Feng, H. (2013). Deferoxamine attenuates iron‐induced long‐term neurotoxicity in rats with traumatic brain injury. Neurological Sciences 34, 639–645. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Miller, R. G. , Gascon, R. , Champion, S. , Katz, J. , Lancero, M. , Narvaez, A. , Honrada, R. , Ruvalcaba, D. & McGrath, M. S. (2009). Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). Journal of Neuroimmunology 206, 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Liu, H. , Chuang, C. L. , Li, X. , Au, M. , Zhang, L. , Phillips, A. R. , Scott, D. W. & Cooper, G. J. S. (2014). The pathogenic mechanism of diabetes varies with the degree of overexpression and oligomerization of human amylin in the pancreatic islet beta cells. FASEB Journal 28, 5083–5096. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Yew, W. W. & Barer, M. R. (2012). Targeting persisters for tuberculosis control. Antimicrobial Agents and Chemotherapy 56, 2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Zhang, K. , Du, X. & Li, Y. (2012). Neuroprotection of desferrioxamine in lipopolysaccharide‐induced nigrostriatal dopamine neuron degeneration. Molecular Medicine Reports 5, 562–566. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Sun, Y. , Ji, H. F. & Shen, L. (2013). Vitamin D levels in Alzheimer's and Parkinson's diseases: a meta‐analysis. Nutrition 29, 828–832. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Li, S. , Liu, G. , Yan, F. , Ma, X. , Huang, Z. & Tian, H. (2012). Body iron stores and heme‐iron intake in relation to risk of type 2 diabetes: a systematic review and meta‐analysis. PLoS One 7, e41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Xiao, X. , Zhang, Q. , Mao, L. , Yu, M. & Xu, J. (2015). The placental microbiome varies in association with low birth weight in full‐term neonates. Nutrients 7, 6924–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, T. , Han, H. & Yang, Z. (2014). Iron, Oxidative Stress and Gestational Diabetes. Nutrients 6, 3968–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimbro, M. J. , Power, D. A. , Miller, S. M. , Wilson, G. E. & Johnson, J. A. (2009). Difco & BBL Manual, Second Edition. BD Diagnostics, Sparks. [Google Scholar]
- Zoccali, C. , Benedetto, F. A. , Mallamaci, F. , Tripepi, G. , Cutrupi, S. , Parlongo, S. , Malatino, L. S. , Bonanno, G. , Rapisarda, F. , Fatuzzo, P. , Seminara, G. , Nicocia, G. & Buemi, M. (2003). Fibrinogen, inflammation and concentric left ventricular hypertrophy in chronic renal failure. European Journal of Clinical Investigation 33, 561–566. [DOI] [PubMed] [Google Scholar]
- Zughaier, S. M. , Alvarez, J. A. , Sloan, J. H. , Konrad, R. J. & Tangpricha, V. (2014). The role of vitamin D in regulating the iron‐hepcidin‐ferroportin axis in monocytes. Journal of Clinical and Translational Endocrinology 1, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zughaier, S. M. , Shafer, W. M. & Stephens, D. S. (2005). Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cellular Microbiology 7, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]


