Abstract
We evaluated treatment outcomes in a prospective registry of human immunodeficiency virus/hepatitis C virus (HCV)–coinfected patients treated with interferon‐free direct‐acting antiviral agent–based therapy in hospitals from the region of Madrid between November 2014 and August 2016. We assessed sustained viral response at 12 weeks after completion of treatment and used multivariable logistic regression to identify predictors of treatment failure. We evaluated 2,369 patients, of whom 59.5% did not have cirrhosis, 33.9% had compensated cirrhosis, and 6.6% had decompensated cirrhosis. The predominant HCV genotypes were 1a (40.9%), 4 (22.4%), 1b (15.1%), and 3 (15.0%). Treatment regimens included sofosbuvir (SOF)/ledipasvir (61.9%), SOF plus daclatasvir (14.6%), dasabuvir plus ombitasvir/paritaprevir/ritonavir (13.2%), and other regimens (10.3%). Ribavirin was used in 30.6% of patients. Less than 1% of patients discontinued therapy owing to adverse events. The frequency of sustained viral response by intention‐to‐treat analysis was 92.0% (95% confidence interval, 90.9%‐93.1%) overall, 93.8% (92.4%‐95.0%) for no cirrhosis, 91.0% (88.8%‐92.9%) for compensated cirrhosis, and 80.8% (73.7%‐86.6%) for decompensated cirrhosis. The factors associated with treatment failure were male sex (adjusted odds ratio, 1.75; 95% confidence interval, 1.14‐2.69), Centers for Diseases Control and Prevention category C (adjusted odds ratio, 1.65; 95% confidence interval, 1.12‐2.41), a baseline cluster of differentiation 4–positive (CD4+) T‐cell count <200/mm3 (adjusted odds ratio, 2.30; 95% confidence interval, 1.35‐3.92), an HCV RNA load ≥800,000 IU/mL (adjusted odds ratio, 1.63; 95% confidence interval, 1.14‐2.36), compensated cirrhosis (adjusted odds ratio, 1.35; 95% confidence interval, 0.96‐1.89), decompensated cirrhosis (adjusted odds ratio, 2.92; 95% confidence interval, 1.76‐4.87), and the use of SOF plus simeprevir, SOF plus ribavirin, and simeprevir plus daclatasvir. Conclusion: In this large real‐world study, direct‐acting antiviral agent–based therapy was safe and highly effective in coinfected patients; predictors of failure included gender, human immunodeficiency virus–related immunosuppression, HCV RNA load, severity of liver disease, and the use of suboptimal direct‐acting antiviral agent–based regimens. (Hepatology 2018;68:32‐47).
Abbreviations
- ART
antiretroviral therapy
- CD4
cluster of differentiation 4
- CDC
Centers for Disease Control and Prevention
- DAA
direct‐acting antiviral agent
- DCV
daclatasvir
- DSV
dasabuvir
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ITT
intent‐to‐treat
- LDV
ledipasvir
- Madrid‐CoRe
Madrid Coinfection Registry
- m‐ITT
modified ITT
- OBV/PTV/r
ombitasvir/paritaprevir/ritonavir
- RBV
ribavirin
- SERMAS
Madrid Regional Health Service
- SMV
simeprevir
- SOF
sofosbuvir
- SVR
sustained viral response.
The introduction of direct‐acting antiviral agents (DAAs) represents a breakthrough in the treatment of infection by hepatitis C virus (HCV).1 This therapeutic advance provided new opportunities for the treatment of persons coinfected by HCV and the human immunodeficiency virus (HIV), one of the most difficult‐to‐treat populations in the interferon plus ribavirin (RBV) era, with sustained viral response (SVR) rates in real‐world settings of slightly more than 30%.2
Current guidelines recommend that HIV/HCV‐coinfected persons be treated using the approach followed for non–HIV‐infected individuals because the efficacy of currently licensed DAA regimens does not appear to differ between HCV‐monoinfected and coinfected individuals.3, 4 In the field of anti‐HCV therapy, concern has been raised about the generalizability of inclusion criteria from clinical trials of different DAAs to the highly heterogeneous population of HIV/HCV‐coinfected patients.5 Several studies have reported on the real‐world safety and effectiveness of DAAs in coinfected individuals. However, except for a report from the Veterans Affairs Health Care System on 996 patients,6 series are limited by small sample size or by the inclusion of specific patient groups such as those with cirrhosis or patients taking specific DAA regimens.7, 8, 9, 10 This contrasts with real‐world cohorts comprising thousands of HCV‐monoinfected individuals treated with a wide spectrum of DAA‐based regimens.11
We evaluated the response to treatment in a large prospective registry of HIV/HCV‐coinfected persons receiving DAA‐based HCV therapy in the region of Madrid (Spain) and analyzed factors associated with treatment failure.
Materials and Methods
DESIGN AND PATIENT SELECTION
In the region of Madrid, as in other parts of Spain, anti‐HCV therapy is provided by hospital pharmacies and is covered by the National Health System, to which the Madrid Regional Health Service (SERMAS) belongs. In November 2014, SERMAS created a compulsory prospective registry of individuals receiving DAAs for HCV infection in SERMAS hospitals. Providing baseline data for this online registry is mandatory for the retrieval of DAAs in SERMAS hospital pharmacies. Likewise, providing exhaustive follow‐up data is a condition for reimbursement.
The Madrid Coinfection Registry (Madrid‐CoRe) was created to determine the effectiveness and safety of all‐oral DAAs in HIV/HCV‐coinfected patients in the region of Madrid. The study protocol was approved by the ethics committee of Hospital Universitario La Paz for the analysis of anonymous routine clinical data without written informed consent for purposes of scientific publication.
In this analysis, patients included in the SERMAS online registry were eligible if they were 18 years or older, were infected with HIV, were receiving treatment with all‐oral DAAs for HCV infection, and were scheduled to finish treatment on or before August 31, 2016. Retreatments were excluded from this analysis.
TREATMENT
During the study period, the criteria for access to DAA therapy within SERMAS were confirmed HCV infection and presence of significant fibrosis (METAVIR F2 or F3 in liver biopsy or liver stiffness >7 kPa by transient elastography) or cirrhosis. In addition, DAA therapy could be administered irrespective of fibrosis stage to patients with significant extrahepatic manifestations of HCV, such as symptomatic cryoglobulinemia, and to patients at risk of transmitting HCV, such as injection drug users, men who have sex with men with high‐risk sexual practices for sexually acquired HCV, and women of childbearing age who wish to become pregnant. Available individual or fixed combinations of DAAs during the study period included sofosbuvir (SOF), simeprevir (SMV), daclatasvir (DCV), ledipasvir (LDV)/SOF, ombitasvir/paritaprevir/ritonavir (OBV/PTV/r), and dasabuvir (DSV). The decision to treat and the selection of the regimen, including duration and use or not of concomitant RBV, were taken by the treating physician according to current guidelines.
MEASUREMENTS
Baseline and follow‐up data were entered into the SERMAS registry by health care personnel at each institution.
Baseline data not related to HIV included demographics, HCV genotype and subtype, HCV RNA load, prior history of anti‐HCV therapy, liver fibrosis stage, and presence or absence of cirrhosis. In patients with cirrhosis, additional data collected included the method of diagnosis, history and type of decompensation, history of hepatocellular carcinoma, whether the patient was on the liver transplantation list or had undergone liver transplantation, Child‐Pugh‐Turcotte score, and Model for End‐Stage Liver Disease score. The date of initiation and type of DAA regimen, use of RBV, and planned treatment duration were also recorded.
Baseline HIV‐related data collected prospectively since November 2014 included whether the patient was HIV‐infected and was receiving antiretroviral therapy (ART). In September 2016, a case report form was used to collect the following HIV‐related variables offline: HIV transmission category, Centers for Disease Control and Prevention (CDC) clinical category, baseline and nadir cluster of differentiation 4–positive (CD4+) T‐cell counts, and baseline HIV viral load. In March 2017, the online registry was modified to include all of the variables related to HIV infection mentioned above. Since then, this information has been registered prospectively.
Fibrosis stage and cirrhosis were determined by liver biopsy or transient elastography (FibroScan; Echo‐Sens, Paris, France), in which liver stiffness was defined as a value >12.5 kPa. Cirrhosis was also defined by clinical evidence of liver decompensation. For descriptive purposes and analysis, patients with hepatocellular carcinoma were considered to have decompensated cirrhosis. The remaining liver stiffness cutoffs were as follows: ≤7 kPa, the cutoff to rule out null or mild fibrosis; <9.5 kPa, the cutoff to rule out advanced fibrosis‐cirrhosis; and ≤19.5 kPa, the cutoff to rule out high risk of esophageal varices among patients with cirrhosis.12
HCV RNA measurements were performed at baseline, weeks 12 and 24 of therapy (if applicable), and 12 weeks after completion of treatment. Real‐time PCR assays for the quantification of HCV RNA included Roche COBAS AmpliPrep/COBAS TaqMan HCV (Roche Molecular Systems, Pleasanton, CA; lower limit of detection, 15 IU/mL), Abbott RealTime HCV assay (Abbott Laboratories, Abbott Park, IL; lower limit of detection, 12 IU/mL), or Siemens Versant HCV RNA version 1.0 (Siemens Healthcare GmbH, Erlangen, Germany; lower limit of detection, 15 IU/mL).
OUTCOMES
Follow‐up data in the online registry included the following: (1) SVR, defined as an undetectable plasma HCV RNA at 12 weeks after completion of treatment; (2) relapse, defined as detectable posttreatment HCV RNA after undetectable HCV RNA at the end of therapy; and (3) viral breakthrough, defined as detectable HCV RNA at the end of treatment and follow‐up week 12. Discontinuations due to adverse events or for reasons other than adverse events, losses to follow‐up, and deaths were also registered.
STATISTICAL ANALYSIS
Efficacy results were analyzed using the intent‐to‐treat (ITT) approach and a modified ITT (m‐ITT) approach, in which nonvirological failures for reasons other than discontinuation of treatment secondary to adverse events or death were not considered in the analysis. Multivariable logistic regression models were used to identify independent baseline factors associated with treatment failure by ITT analysis (the primary analysis) and by m‐ITT analysis. Analyses were performed for the entire data set and for subgroups of liver disease severity (absence of cirrhosis, compensated cirrhosis, and decompensated cirrhosis). Baseline factors were included in multivariable models if P ≤ 0.1. Wald tests were used to derive P values. The analyses were performed using Stata version 14 (StataCorp, College Station, TX).
Results
PATIENT CHARACTERISTICS
During the study period, 2,435 HIV/HCV‐coinfected patients initiating all‐oral DAAs for HCV in 25 hospitals from the region of Madrid were eligible for this study (Fig. 1). After exclusion of 39 patients for whom information about treatment completion was unavailable and 27 with pending SVR results after completion of therapy, the final study population comprised 2,369 patients. A total of 482 patients from Madrid‐CoRe were previously included in a study analyzing differences in treatment outcomes of DAA therapy between HCV‐monoinfected and HIV/HCV‐coinfected patients.13 Also, fewer than 100 patients from Madrid‐CoRe were included in another study assessing the frequency and predictors of treatment failure to all‐oral DAA therapy in patients who completed a course of all‐oral DAA therapy before December 2015 in three Spanish hospitals, two of which contributed to Madrid‐CoRe.14
Figure 1.
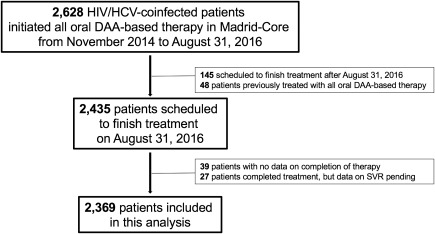
Flowchart.
According to the severity of liver disease, there were 1,410 patients without cirrhosis (59.5%), 803 patients with compensated cirrhosis (33.9%), and 156 patients with decompensated cirrhosis (6.6%). The baseline characteristics are shown in Table 1. In brief, 78.2% were men, the median age was 51 years, and 63.9% were naive for anti‐HCV therapy. The predominant HCV genotypes were 1a (40.9%), 4 (22.4%), 1b (15.1%), and 3 (15.0%). The median HCV RNA was 6.3 log IU/mL. At baseline, 98.0% patients were on ART. Full data on HIV‐related characteristics (collected offline) were available for analysis from only two thirds of the patients in Madrid‐CoRe as data from patients from eight of the 25 hospitals were not available at the time of analysis (Table 1). In comparison to patients with complete HIV data, those with incomplete HIV data were on average 1 year older and had a lower frequency of both compensated and decompensated cirrhosis (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). Information about modification of ART prior to the initiation of DAA therapy was available for 1,613 patients, 476 of whom (29.5%) had their ART regimen modified to prevent potentially clinically significant drug interactions between antiretroviral drugs and DAAs.
Table 1.
Baseline Characteristics of Study Population
|
No Cirrhosis |
Compensated Cirrhosis |
Decompensated Cirrhosis |
Total | |
|---|---|---|---|---|
| Variables | n = 1,410 (59.5) | n = 803 (33.9) | n = 156 (6.6) | N = 2,369 |
| Age, median (IQR) | 50 (47‐53) | 51 (48‐54) | 51 (48‐54) | 51 (47‐54) |
| Male sex, n (%) | 1,085 (76.9) | 656 (81.7) | 111 (71.1) | 1,852 (78.2) |
| Prior anti‐HCV therapy, n (%) | ||||
| No | 953 (67.6) | 464 (57.8) | 96 (61.5) | 1,513 (63.9) |
| Yes | 457 (32.4) | 338 (42.1) | 60 (38.5) | 855 (36.1) |
| Unknown | 0 | 1 (0.1) | 0 | 1 (0.04) |
| Genotype, n (%) | ||||
| 1 | ||||
| 1a | 596 (42.3) | 318 (39.6) | 54 (34.6) | 968 (40.9) |
| 1b | 196 (13.9) | 130 (16.2) | 32 (20.5) | 358 (15.1) |
| 1 nonsubtyped | 58 (4.1) | 38 (4.7) | 7 (4.5) | 103 (4.3) |
| 2 | 18 (1.3) | 6 (0.7) | 3 (1.9) | 27 (1.1) |
| 3 | 179 (12.7) | 152 (18.9) | 24 (15.4) | 355 (15.0) |
| 4 | 349 (24.7) | 149 (18.6) | 32 (20.5) | 530 (22.4) |
| Mixed | 12 (0.8) | 9 (1.1) | 3 (1.9) | 24 (1.0) |
| Indeterminate | 2 (0.1) | 1 (0.1) | 1 (0.6) | 4 (0.2) |
| HCV RNA, n (%) | ||||
| Unknown | 0 | 0 | 0 | 0 |
| Known | 1,410 (100) | 803 (100) | 156 (100) | 2,369 (100) |
| Log IU/mL, median (IQR) | 6.3 (5.8‐6.7) | 6.2 (5.8‐6.6) | 6.0 (5.5‐6.4) | 6.3 (5.8‐6.7) |
| Transient elastography, n (%) | ||||
| No | 12 (0.8) | 12 (1.5) | 14 (9.0) | 38 (1.6) |
| Yes | 1,398 (99.2) | 791 (98.5) | 142 (91.0) | 2,331 (98.4) |
| Stiffness, kPa‐Median (IQR) | 8.2 (7.3‐9.9) | 21.3 (15.3‐32.8) | 31.8 (20.3‐48.0) | 10.4 (7.9‐18.0) |
| HIV risk factor, n (%) | ||||
| Injection drug use | 744 (52.8) | 529 (65.9) | 97 (62.2) | 1,370 (57.8) |
| Men who have sex with men | 82 (5.8) | 21 (2.6) | 5 (3.2) | 108 (4.6) |
| Heterosexual relations | 68 (4.8) | 27 (3.4) | 7 (4.5) | 102 (4.3) |
| Transfusions | 10 (0.7) | 5 (0.6) | 1 (0.6) | 16 (0.7) |
| Mother to child | 2 (0.1) | 1 (0.1) | 0 | 3 (0.1) |
| Other/unknown | 504 (35.7) | 220 (27.4) | 46 (29.5) | 770 (32.5) |
| CDC clinical category, n (%) | ||||
| A | 356 (25.2) | 222 (27.6) | 39 (25.0) | 617 (26.0) |
| B | 235 (16.7) | 133 (16.6) | 36 (23.1) | 404 (17.1) |
| C | 319 (22.6) | 226 (28.1) | 38 (24.4) | 583 (24.6) |
| Unknown | 500 (35.5) | 222 (27.6) | 43 (27.6) | 765 (32.3) |
| Nadir CD4+/mm3, n (%) | ||||
| >500 | 84 (6.0) | 40 (5.0) | 4 (2.6) | 128 (5.4) |
| 200‐499 | 347 (24.6) | 183 (22.8) | 27 (17.3) | 557 (23.5) |
| <200 | 477 (33.8) | 358 (44.6) | 82 (52.6) | 917 (38.7) |
| Unknown | 502 (35.6) | 222 (27.6) | 43 (27.6) | 767 (32.4) |
| Baseline CD4+/mm3, n (%) | ||||
| Unknown | 535 (37.9) | 248 (30.9) | 48 (30.8) | 831 (35.1) |
| Known | 875 (62.1) | 555 (69.1) | 108 (69.2) | 1,538 (64.9) |
| Median (IQR) | 628 (429‐820) | 523 (324‐777) | 374 (233‐591) | 575 (369‐814) |
| HIV RNA, n (%) | ||||
| Unknown | 483 (34.3) | 208 (25.9) | 43 (27.6) | 734 (31.0) |
| Known | 927 (65.7) | 595 (74.1) | 113 (72.4) | 1,635 (69.0) |
| Detectable | 44 (4.7) | 36 (6.0) | 3 (2.6) | 83 (5.1) |
| Undetectable | 883 (95.3) | 559 (94.0) | 110 (97.4) | 1,552 (94.9) |
| ART, n (%) | ||||
| No | 23 (1.6) | 17 (2.1) | 7 (4.5) | 47 (2.0) |
| Yes | 1,387 (98.4) | 786 (97.9) | 149 (95.5) | 2,322 (98.0) |
| ART regimen before DAA Rx, n (%) | ||||
| 2nRTI+1PI | 77 (30.3) | 58 (32.2) | 21 (50.0) | 156 (32.8) |
| 2nRTI+1 INSTI | 13 (5.1) | 19 (10.6) | 2 (4.8) | 34 (7.1) |
| 2nRTI+1nnRTI | 115 (45.3) | 74 (41.1) | 12 (28.6) | 201 (42.2) |
| PI‐based dual therapy | 10 (3.9) | 2 (1.1) | 2 (4.8) | 14 (2.9) |
| PI monotherapy | 9 (3.5) | 7 (3.9) | 0 | 16 (3.4) |
| Other | 29 (11.4) | 18 (10.0) | 5 (11.9) | 52 (10.9) |
| Unknown | 1 (0.4) | 2 (1.1) | 0 | 3 (0.6) |
| ART change prior to DAA Rx, n (%) | ||||
| No | 658 (47.4) | 409 (52.0) | 70 (47.0) | 1,137 (49.0) |
| Yes | 254 (18.3) | 180 (22.9) | 42 (28.2) | 476 (20.5) |
| Unknown | 475 (34.2) | 197 (25.1) | 37 (24.8) | 709 (30.5) |
Abbreviations: INSTI, integrase strand transfer inhibitor; IQR, interquartile range; nnRTI, non‐nucleoside reverse transcriptase inhibitor; nRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; Rx, therapy.
TREATMENT REGIMENS
Table 2 shows the all‐oral DAA regimens used and the duration of the treatment in weeks. In brief, SOF/LDV was the most frequently used regimen in approximately two thirds of the patients, followed by SOF+DCV, DSV+OBV/PTV/r, and OBV/PTV/r. Less commonly used regimens were SOF+SMV, SOF+RBV, SMV+DCV, SOF+SMV+DCV, and SOF+OBV/PTV/r. The distribution of all‐oral DAA regimens categorized by severity of liver disease is shown in Table 3. The distribution of all‐oral DAA regimens categorized by genotype is shown in http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo.
Table 2.
Treatment Regimens (Total N = 2,369)
| Regimen, no. (%)a |
SOF/LDV 1,467 (61.9) |
SOF+DCV 346 (14.6) |
DSV+OBV/PTV/r 314 (13.2) |
OBV/PTV/r 132 (5.6) |
SOF+SMV 71 (3.0) |
SOF+RBV 32 (1.3) |
|---|---|---|---|---|---|---|
| 8 weeks | 129 | — | 1 | — | — | |
| 12 weeks | 832 | 140 | 105 | 6 | 27 | — |
| 12 weeks + RBV | 104 | 58 | 151 | 114 | 18 | 13 |
| 16 weeks | 1 | 3 | — | — | — | — |
| 16 weeks + RBV | — | 3 | — | — | — | 1 |
| 24 weeks | 303 | 69 | 3 | — | 21 | — |
| 24 weeks + RBV | 98 | 73 | 54 | 12 | 5 | 18 |
Other regimens not shown in the table: SMV+DCV, n = 4; SOF+SMV+DCV, n = 2; SOF+OBV/PTV/r, n = 1.
Table 3.
Treatment Regimens According to Severity of Liver Disease
| Regimen, n (%) |
No Cirrhosis (n = 1,410) |
Compensated Cirrhosis (n = 803) |
Decompensated Cirrhosis (n = 156) |
Total (N = 2,369) |
|---|---|---|---|---|
| SOF/LDV | 864 (61.3) | 523 (65.1) | 80 (51.3) | 1,467 (61.9) |
| SOF+DCV | 177 (12.5) | 132 (16.4) | 37 (23.7) | 346 (14.6) |
| DSV+OBV/PTV/r | 223 (15.8) | 87 (10.8) | 4 (2.6) | 314 (13.2) |
| OBV/PTV/r | 120 (8.5) | 12 (1.5) | — | 132 (5.6) |
| SOF+SMV | 9 (0.6) | 36 (4.5) | 26 (16.7) | 71 (3.0) |
| SOF+RBV | 16 (1.1) | 8 (1.0) | 8 (5.1) | 32 (1.3) |
| SMV+DCV | 1 (0.1) | 2 (0.2) | 1 (0.6) | 4 (0.2) |
| SOF+SMV+DCV | — | 2 (0.2) | — | 2 (0.1) |
| SOF+OBV/PTV/r | — | 1 (0.1) | — | 1 (0.04) |
| Use of RBV | 328 (23.3) | 323 (40.2) | 73 (46.8) | 724 (30.6) |
TREATMENT RESPONSE
Treatment Response According to Liver Disease Severity
Treatment response according to liver disease categories by ITT and by m‐ITT analyses is shown in Fig. 2. The frequency of SVR by ITT and by m‐ITT analysis was 92.0% and 94.1%, respectively. The respective values were 93.8% and 95.9% for patients without cirrhosis, 91.0% and 93.1% for patients with compensated cirrhosis, and 80.8% and 82.4% for patients with decompensated cirrhosis.
Figure 2.
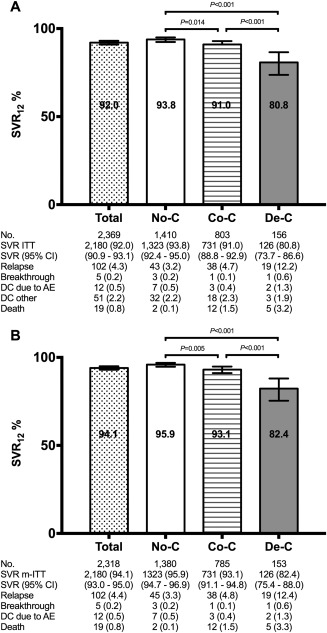
Treatment outcomes by severity of liver disease by ITT (A) and by m‐ITT analyses (B). Abbreviations: AE, adverse events; CI, confidence interval; Co‐C, compensated cirrhosis; DC, treatment discontinuations (number [%]); De‐C, decompensated cirrhosis; No‐C, no cirrhosis.
Treatment Response According to DAA Regimen, Genotype, and Liver Disease Severity
The response (by ITT and m‐ITT analyses) to the DAA regimens categorized by genotype and the severity of liver disease is shown in Table 4 and the http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo.
Table 4.
Treatment Response Categorized by Genotype, Severity of Liver Disease, and Treatment Regimen in Madrid‐CoRe
| Genotype | ||||||
|---|---|---|---|---|---|---|
| 1a | 1b | 3 | 4 | Other | All | |
| Regimen—treated (% SVR‐12)—patients without cirrhosis (ITT analysis) | ||||||
| n = 596 | n = 196 | n = 179 | n = 349 | n = 90 | N = 1,410 | |
| SOF/LDV | 470 (92.5) | 101 (97.0) | 8 (100) | 230 (92.2) | 55 (96.4) | 864 (93.3) |
| SOF+DCV | — | — | 170 (95.3) | 1 (100) | 6 (100) | 177 (95.5) |
| DSV+OBV/PTV/r | 118 (95.8) | 93 (96.8) | — | 1 (100) | 11 (90.9) | 223 (96.0) |
| OBV/PTV/r | 2 (100) | 1 (100) | — | 116 (92.2) | 1 (100) | 120 (92.5) |
| SOF+SMV | 5 (100) | 1 (100) | — | 1 (100) | 2 (100) | 9 (100) |
| SOF+RBV | — | — | 1 (100) | — | 15 (86.7) | 16 (87.5) |
| SMV+DCV | 1 (0) | — | — | — | — | 1 (0) |
| Regimen—treated (% SVR‐12)—patients without cirrhosis (m‐ITT analysis) | ||||||
| n = 585 | n = 194 | n = 174 | n = 338 | n = 89 | N = 1,380 | |
| SOF/LDV | 459 (94.8) | 100 (98.0) | 8 (100) | 222 (95.5) | 54 (98.1) | 843 (95.6) |
| SOF+DCV | — | — | 165 (98.2) | 1 (100) | 6 (100) | 172 (98.3) |
| DSV+OBV/PTV/r | 118 (95.8) | 92 (97.8) | — | 1 (100) | 11 (90.9) | 222 (96.4) |
| OBV/PTV/r | 2 (100) | 1 (100) | — | 113 (94.7) | 1 (100) | 117 (94.9) |
| SOF+SMV | 5 (100) | 1 (100) | — | 1 (100) | 2 (100) | 9 (100) |
| SOF+RBV | — | — | 1 (100) | — | 15 (86.7) | 16 (87.5) |
| SMV+DCV | 1 (0) | — | — | — | — | 1 (0) |
| Regimen—treated (% SVR‐12)—patients with compensated cirrhosis (ITT analysis) | ||||||
| n = 318 | n = 130 | n = 152 | n = 149 | n = 54 | N = 803 | |
| SOF/LDV | 220 (94.5) | 84 (94.0) | 65 (90.8) | 116 (92.2) | 38 (89.5) | 523 (93.1) |
| SOF+DCV | 29 (86.2) | 6 (83.3) | 83 (91.6) | 10 (70.0) | 4 (75.0) | 132 (87.9) |
| DSV+OBV/PTV/r | 52 (94.2) | 31 (87.1) | — | — | 4 (100) | 87 (91.9) |
| OBV/PTV/r | — | — | — | 12 (91.7) | — | 12 (91.7) |
| SOF+SMV | 15 (73.3) | 8 (87.5) | — | 9 (44.4) | 4 (100) | 36 (72.2) |
| SOF+RBV | — | — | 4 (50.0) | — | 4 (100) | 8 (75.0) |
| SMV+DCV | — | 1 (100) | — | 1 (100) | — | 2 (100) |
| SOF+SMV+DCV | 2 (100) | — | — | — | — | 2 (100) |
| SOF+OBV/PTV/r | — | — | — | 1 (100) | — | 1 (100) |
| Regimen—treated (% SVR‐12)—patients with compensated cirrhosis (m‐ITT analysis) | ||||||
| n = 310 | n = 129 | n = 148 | n = 146 | n = 52 | N = 785 | |
| SOF/LDV | 213 (97.6) | 83 (95.2) | 64 (92.2) | 114 (93.9) | 36 (94.4) | 510 (95.5) |
| SOF+DCV | 29 (86.2) | 6 (83.3) | 80 (95.0) | 10 (70.0) | 4 (75.0) | 129 (89.9) |
| DSV+OBV/PTV/r | 51 (96.1) | 31 (87.1) | — | — | 4 (100) | 86 (93.0) |
| OBV/PTV/r | — | — | — | 11 (100) | — | 11 (100) |
| SOF+SMV | 15 (73.3) | 8 (87.5) | — | 9 (44.4) | 4 (100) | 36 (72.2) |
| SOF+RBV | — | — | 4 (50.0) | — | 4 (100) | 8 (75.0) |
| SMV+DCV | — | 1 (100) | — | 1 (100) | — | 2 (100) |
| SOF+SMV+DCV | 2 (100) | — | — | — | — | 2 (100) |
| SOF+OBV/PTV/r | — | — | — | 1 (100) | — | 1 (100) |
| Regimen—treated (% SVR‐12)—patients with decompensated cirrhosis (ITT analysis) | ||||||
| n = 54 | n = 32 | n = 24 | n = 32 | n = 14 | N = 156 | |
| SOF/LDV | 29 (79.3) | 16 (93.7) | 10 (80.0) | 20 (80.0) | 5 (100) | 80 (83.7) |
| SOF+DCV | 9 (100) | 6 (66.7) | 11 (72.7) | 7 (100) | 4 (75.0) | 37 (83.8) |
| DSV+OBV/PTV/r | 4 (100) | — | — | — | — | 4 (100) |
| SOF+SMV | 10 (60.0) | 9 (88.9) | — | 5 (60.0) | 2 (100) | 26 (73.1) |
| SOF+RBV | 1 (100) | 1 (100) | 3 (66.7) | — | 3 (33.3) | 8 (62.5) |
| SMV+DCV | 1 (0) | — | — | — | — | 1 (0) |
| Regimen—treated (% SVR‐12)—patients with decompensated cirrhosis (m‐ITT analysis) | ||||||
| n = 52 | n = 32 | n = 23 | n = 32 | n = 14 | N = 153 | |
| SOF/LDV | 27 (85.2) | 16 (93.7) | 10 (80.0) | 20 (80.0) | 5 (100) | 78 (85.9) |
| SOF+DCV | 9 (100) | 6 (66.7) | 11 (72.7) | 7 (100) | 4 (75.0) | 37 (83.8) |
| DSV+OBV/PTV/r | 4 (100) | — | — | — | — | 4 (100) |
| SOF+SMV | 10 (60.0) | 9 (88.9) | — | 5 (60.0) | 2 (100) | 26 (73.1) |
| SOF+RBV | 1 (100) | 1 (100) | 2 (100) | — | 3 (33.3) | 7 (71.4) |
| SMV+DCV | 1 (0) | — | — | — | — | 1 (0) |
GENOTYPE 1a
For genotype 1a, the SVR rates of SOF/LDV by ITT and m‐ITT analyses were 92.5% and 94.8%, respectively, for patients without cirrhosis, 94.5% and 97.6% for patients with compensated cirrhosis, and 79.3% and 85.2% for patients with decompensated cirrhosis. The SVR rates of DSV+OBV/PTV/r by ITT and m‐ITT analyses were both 95.8% for patients without cirrhosis and 94.2% and 96.1%, respectively, for patients with compensated cirrhosis.
GENOTYPE 1b
For genotype 1b, the SVR rates of SOF/LDV by ITT and m‐ITT analyses were 97.0% and 98.0%, respectively, for patients without cirrhosis, 94.0% and 95.2% for patients with compensated cirrhosis, and 93.7% by both analyses for patients with decompensated cirrhosis. The SVR rates of DSV+OBV/PTV/r by ITT and m‐ITT analyses were 96.8% and 97.8%, respectively, for patients without cirrhosis and 87.1% by both analyses for patients with compensated cirrhosis.
GENOTYPE 3
In patients without cirrhosis who had HCV genotype 3, the SVR rates of SOF+DCV by ITT and m‐ITT analyses were 95.3% and 98.2%, respectively. A total of 119 patients without cirrhosis received SOF+DCV without RBV for 12 weeks, with SVR rates by ITT and m‐ITT analyses of 95.0% and 97.4%, respectively (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). In patients with HCV genotype 3 with compensated cirrhosis, the SVR rates of SOF+ DCV by ITT and m‐ITT analyses were 91.6% and 95.0%, respectively. A total of 48 patients with compensated cirrhosis received SOF+DCV plus RBV for 24 weeks, with SVR rates by ITT and m‐ITT analyses of 93.7% and 97.8%, respectively (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). In patients with HCV genotype 3 with decompensated cirrhosis, the SVR rates of SOF+DCV by ITT and m‐ITT analyses were both 72.7%, although only 11 patients were included in this last category. For genotype 3, the SVR rates of SOF/LDV by ITT and m‐ITT analyses were 100% in patients without cirrhosis, although only 8 patients were included. For patients with compensated cirrhosis, the SVR rates for this regimen were 90.8% and 92.2%, respectively. A total of 52 patients with compensated cirrhosis received SOF/LDV plus RBV for 24 weeks, with SVR rates by ITT and m‐ITT analyses of 88.5% and 90.2%, respectively (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). In patients with HCV genotype 3 and decompensated cirrhosis, the SVR rates of SOF/LDV by ITT and m‐ITT analyses were both 80.0%, although only 10 patients were included in this last category.
GENOTYPE 4
For genotype 4, the SVR rates of SOF/LDV by ITT and m‐ITT analyses were 92.2% and 95.5%, respectively, for patients without cirrhosis and 92.2% and 93.9% for patients with compensated cirrhosis. Among patients without cirrhosis, 218 received SOF/LDV for 12 weeks and 111 received OBV/PTV/r for 12 weeks, with SVR rates by ITT and m‐ITT analyses of 91.7%‐95.2% and 91.9%‐94.4%, respectively (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). A total of 74 patients with compensated cirrhosis received SOF/LDV without RBV for 24 weeks, with SVR rates by ITT and m‐ITT analyses of 93.2% and 94.5%, respectively (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). In patients with HCV genotype 4 and decompensated cirrhosis, the SVR rates of SOF/LDV by ITT and m‐ITT analyses were both 80.0%, although only 20 patients were included in this last category. For this same genotype, the SVR rates of OBV/PTV/r by ITT and m‐ITT analyses were 92.2% and 94.7%, respectively, for patients without cirrhosis. The figures were 91.7% and 100%, respectively, for patients with compensated cirrhosis, although only 11 patients were included in this category.
LDV/SOF for 8 Weeks
A total of 129 patients, 128 without cirrhosis and 1 with compensated cirrhosis, received SOF/LDV without RBV for 8 weeks. The HCV genotype was 1 in 127 (98.4%) patients and 4 in 2 (1.6%) patients. Eleven (8.5%) patients had previously been exposed to pegylated interferon plus RBV. The median (interquartile range) liver stiffness was 7.6 kPa (7.0‐8.6 kPa), and the median (interquartile range) HCV RNA was 1,100,000 IU/mL (333,907‐3,140,000 IU/mL). The overall SVR (95% confidence interval) rate of LDV/SOF for 8 weeks was 93.0% (87.2%‐96.8%) by ITT analysis and 93.8% (88.1%‐97.3%) by m‐ITT analysis. In Madrid‐CoRe, a total of 308 anti‐HCV treatment‐naive patients without cirrhosis with HCV genotype 1 and with an HCV‐RNA <6 million (6.8 Log) IU/mL were treated with LDV/SOF without RBV. Of these, 104 (33.8%) were treated for 8 weeks and 204 for 12 weeks. The SVR by ITT and m‐ITT analyses for patients treated for 8 and 12 weeks were 92.3%‐93.2% and 93.1%‐95.0%, respectively. The relapse rates for patients treated for 8 and 12 weeks were 5.8% and 3.9%, respectively (P = 0.46) (Fig. 3).
Figure 3.
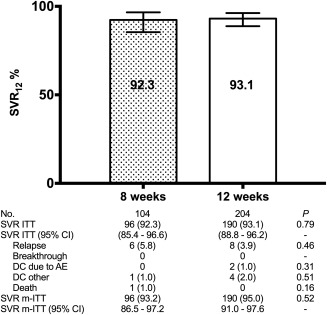
Treatment outcomes for SOF/LDV without RBV for HCV genotype 1 (1a, 1b, or nonsubtyped 1) in treatment‐naive, patients without cirrhosis who had an HCV RNA <6 million (6.8 log) IU/mL. Abbreviations: AE, adverse events; CI, confidence interval; DC, treatment discontinuations (number [%]); No., number of patients.
VARIABLES ASSOCIATED WITH TREATMENT FAILURE
Table 5 shows the results of univariable and multivariable logistic regression models to identify baseline variables associated with treatment failure by ITT analysis for the full data set. In brief, factors independently associated with an increased odds of treatment failure by ITT analysis were male sex, CDC clinical category C, a baseline CD4+ T‐cell count <200/mm3, an HCV RNA load ≥800,000 IU/mL, liver cirrhosis, decompensated liver disease, and the use of specific DAA regimens (SOF+SMV, SOF+RBV, and SMV+DCV). Similar findings were observed by m‐ITT analysis (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo).
Table 5.
Results From Univariable and Multivariable Logistic Regression Models to Identify Independent Baseline Factors Predictive of Treatment Failure by ITT Analysis Considering All Categories of Liver‐Disease Severity (N = 2,369)
| Treatment Failures (n = 189) | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Variable | n (%) | OR (95% CI) | P | OR (95% CI) | P |
| Age (years) | 0.03 | 0.089 | |||
| <45 | 16 (5.6) | 1.00 | 1.00 | ||
| 45‐54 | 123 (7.3) | 1.40 (0.82‐2.40) | 1.27 (0.73‐2.19) | ||
| ≥55 | 50 (10.7) | 2.03 (1.13‐3.64) | 1.77 (0.98‐3.22) | ||
| Sex | 0.01 | 0.01 | |||
| Female | 27 (5.2) | 1.00 | 1.00 | ||
| Male | 162 (8.7) | 1.74 (1.14‐2.65) | 1.75 (1.14‐2.69) | ||
| HIV transmission category risk | 0.39 | ||||
| Non‐IDU | 13 (5.7) | 1.00 | |||
| IDU | 111 (8.1) | 1.46 (0.81‐2.65) | |||
| Other/unknown | 65 (8.4) | 1.53 (0.83‐2.83) | |||
| CDC clinical category | 0.01 | 0.04 | |||
| A/B | 64 (6.3) | 1.00 | 1.00 | ||
| C | 60 (10.3) | 1.72 (1.19‐2.48) | 1.65 (1.12‐2.41) | ||
| Unknown | 65 (8.5) | 1.39 (0.97‐1.99) | 1.30 (0.67‐2.54) | ||
| Nadir CD4+ T‐cell count, cells/mm3 | 0.02 | ||||
| ≥ 200 | 38 (5.5) | 1.00 | |||
| < 200 | 85 (9.3) | 1.74 (1.17‐2.58) | |||
| Unknown | 66 (8.6) | 1.60 (1.06‐2.42) | |||
| Baseline CD4+ T‐cell count, cells/mm3 | <0.001 | 0.01 | |||
| ≥200 | 95 (6.7) | 1.00 | 1.00 | ||
| <200 | 21 (16.0) | 2.64 (1.58‐4.40) | 2.30 (1.35‐3.92) | ||
| Unknown | 73 (8.8) | 1.33 (0.97‐1.83) | 1.22 (0.65‐2.30) | ||
| Baseline HIV RNA copies/mL | 0.90 | ||||
| <50 | 122 (7.9) | 1.00 | |||
| ≥50 | 6 (7.2) | 0.91 (0.39‐2.14) | |||
| Unknown | 61 (8.3) | 1.06 (0.77‐1.46) | |||
| Combination ART | 0.23 | ||||
| Yes | 183 (7.9) | 1.00 | |||
| No | 6 (12.8) | 1.71 (0.72‐4.08) | |||
| Liver stiffness, kPaa | <0.001 | ||||
| <9.5 | 66 (6.6) | 1.00 | |||
| 9.5‐12.5 | 21 (5.2) | 0.77 (0.46‐1.27) | |||
| 12.6‐19.4 | 18 (4.7) | 0.70 (0.41‐1.19) | |||
| ≥19.5 | 79 (14.4) | 2.38 (1.68‐3.36) | |||
| Unknown | 5 (13.2) | 2.13 (0.81‐5.65) | |||
| HCV genotype | 0.26 | ||||
| 1 | 102 (7.1) | 1.00 | |||
| 2 | 4 (14.8) | 2.26 (0.77‐6.67) | |||
| 3 | 29 (8.2) | 1.16 (0.75‐1.78) | |||
| 4 | 51 (9.6) | 1.39 (0.97‐1.97) | |||
| Other | 3 (10.7) | 1.56 (0.46‐5.26) | |||
| HCV RNA IU/mL | 0.047 | 0.01 | |||
| <800,000 | 46 (6.3) | 1.00 | 1.00 | ||
| ≥800,000 | 143 (8.7) | 1.42 (1.01‐2.00) | 1.63 (1.14‐2.36) | ||
| Naive for anti‐HCV therapy | 0.84 | ||||
| Yes | 122 (8.1) | 1.00 | |||
| No |
67 (7.8) 0 |
0.97 (0.71‐1.32) | |||
| Liver disease category | <0.001 | <0.001 | |||
| No cirrhosis | 87 (6.2) | 1.00 | 1.00b, c | ||
| Compensated cirrhosis | 72 (9.0) | 1.50 (1.08‐2.07) | 1.35 (0.96‐1.89)b, d | ||
| Decompensated cirrhosis | 30 (19.2) | 3.62 (2.30‐5.70) | 2.92 (1.76‐4.87)c, d | ||
| Anti‐HCV regimen | <0.001 | <0.001 | |||
| SOF/LDV | 107 (7.3) | 1.00 | 1.00 | ||
| SOF+DCV | 30 (8.7) | 1.21 (0.79‐1.84) | 1.10 (0.71‐1.70) | ||
| DSV+OBV/PTV/r | 16 (5.1) | 0.68 (0.40‐1.17) | 0.73 (0.42‐1.27) | ||
| OBV/PTV/r | 10 (7.6) | 1.04 (0.53‐2.04) | 1.40 (0.70‐2.79) | ||
| SOF+SMV | 17 (23.9) | 4.00 (2.24‐7.14) | 2.84 (1.53‐5.29) | ||
| SOF+RBV | 7 (21.9) | 3.56 (1.50‐8.42) | 3.41 (1.39‐8.36) | ||
| SMV+DCV | 2 (50.0) | 12.71 (1.77‐91.1) | 11.77 (1.59‐ 87.27) | ||
| SOF+SMV+DCV | 0 | — | — | ||
| SOF+OBV/PTV/r | 0 | — | — | ||
| Anti‐HCV treatment duration | 0.12 | ||||
| 8 weeks | 9 (6.9) | 0.94 (0.47‐1.90) | |||
| 12 weeks | 115 (7.3) | 1.00 | |||
| 16 weeks | 0 | — | |||
| 24 weeks | 65 (9.8) | 1.38 (1.01‐1.90) | |||
| Ribavirin use | 0.48 | ||||
| No | 127 (7.7) | 1.00 | |||
| Yes | 62 (8.6) | 1.12 (0.82‐1.54) |
Liver stiffness cutoffs: <9.5 kPa, cutoff accurate to rule out advanced fibrosis‐cirrhosis (METAVIR F3‐F4); ≤12.5 kPa, cutoff accurate to rule out liver cirrhosis; ≤19.5 kPa, cutoff accurate to rule out high‐risk of esophageal varices.
P = 0.015.
P < 0.001.
P < 0.001.
Abbreviations: CI, confidence interval; OR, odds ratio; IDU, injection drug user.
We also performed separate logistic regression models to identify baseline variables associated with treatment failure for the different categories of liver disease severity. The results are shown in http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo. In brief, ITT analysis showed that the only variables independently associated with treatment in patients without cirrhosis were male sex, CDC clinical category C, and a baseline CD4+ T‐cell count <200/mm3 (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). In patients with compensated cirrhosis, the factors independently associated with treatment failure by ITT analysis were increasing age, liver stiffness ≥19.5 kPa, and the use of SOF+SMV as anti‐HCV regimens (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo). Finally, in patients with decompensated cirrhosis, the factors independently associated with treatment failure by ITT analysis were Child‐Pugh‐Turcotte category C and a history of hepatocellular carcinoma (http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo).
Discussion
We prospectively assessed the effectiveness and safety of all‐oral DAA therapy in approximately 2,400 HIV/HCV‐coinfected patients, of whom 40% had liver cirrhosis. The frequency of SVR by ITT and m‐ITT analyses was 92% and 94%, respectively. Less than 1% of patients discontinued therapy because of adverse events. In the ITT analysis, the factors independently associated with an increased odds of treatment failure were male sex, CDC clinical category C, baseline CD4+ T‐cell count <200/mm3, HCV RNA load ≥800,000 IU/mL, cirrhosis, decompensated liver disease, and the use of specific DAA regimens (SOF+ SMV, SOF+RBV, and SMV+DCV).
Slightly more than 1,400 patients in Madrid‐CoRe were infected with HCV genotype 1a or 1b, which were the predominant infecting genotypes. The rates of SVR were high with LDV/SOF and DSV+OBV/PTV/r in patients with compensated liver disease and did not differ from the rates reported for this genotype in clinical trials in HCV‐monoinfected and HIV/HCV‐coinfected individuals for LDV/SOF15, 16, 17 and DSV+OBV/PTV/r.18, 19, 20 Our findings also agree with the real‐world results of the Veterans Affairs Health Care System, in which no differences in SVR rates were found between DSV+OBV/PTV/r and LDV/SOF regimens in a group of more than 5,000 patients with HCV genotype 1, the clear majority of whom were HCV‐monoinfected.21
Genotype 4 was the second most frequent infecting genotype in Madrid‐CoRe. Among patients without cirrhosis who had this genotype, SOF/LDV and OBV/PTV/r yielded very high SVR rates that were similar to those reported in clinical trials for patients without cirrhosis who had HCV genotype 4 and were treated with the same regimens.22, 23, 24 In Madrid‐CoRe, 116 genotype 4–infected patients with cirrhosis were treated with SOF/LDV and 11 with OBV/PTV/r. The SVR rates for SOF/LDV were the same as those recorded in patients without cirrhosis and were 100% for OBV/PTV/r.
In patients without cirrhosis who had genotype 3, the SVR rates of SOF+DCV without RBV for 12 weeks were the same as those found with this same regimen in genotype 3–infected patients without cirrhosis in the ALLY‐3 clinical trial.25 A total of 83 patients with genotype 3 and compensated cirrhosis were treated with SOF+DCV with or without RBV for different durations. Of these, 48 patients were treated with SOF+DCV with RBV for 24 weeks, with SVR rates of 94% and 98% by ITT and m‐ITT analyses. The SVR rates were below 90% for SOF+ DCV with RBV for 12 weeks or SOF+DCV without RBV for 24 weeks, although the numbers of patients treated with these two regimens were small. In the ALLY‐3 trial, a 12‐week regimen of SOF+DCV without RBV in 32 patients with cirrhosis yielded an SVR rate of 63%.25 In the ALLY‐3+ trial, a regimen of SOF+DCV with RBV for 12 or 16 weeks in patients with cirrhosis (18 patients in each arm) yielded SVR rates of 83% and 89%, respectively.26 The results of Madrid‐CoRe support the current recommendations of the European Association for the Study of the Liver to use SOF+DCV with RBV for 24 weeks for genotype 3 infection in patients with compensated cirrhosis, even though no clinical trials support this recommendation.3
In Madrid‐CoRe, one quarter of genotype 3–infected patients, most of whom had cirrhosis, were treated with SOF/LDV, a regimen that was prioritized for this genotype during the first months of the study period owing to the lower costs of LDV/SOF compared with SOF+DCV in the SERMAS system. A total of 52 patients with compensated cirrhosis were treated with SOF/LDV plus RBV for 24 weeks, with SVR rates of 89% and 90% by ITT and m‐ITT analyses; these results were substantially lower than the SVR rates found with SOF+DCV with RBV for 24 weeks (see above). SOF/LDV is not recommended for patients infected with HCV genotype 3 because LDV is less potent against genotype 3 than DCV or velpatasvir.3, 4, 27, 28 In a trial with LDV/SOF plus RBV for 12 weeks in genotype 3 patients, the SVR rate was 100% in 26 treatment‐naive patients, 6 of whom had cirrhosis; however, in the 50 treatment‐experienced patients, the rates of SVR were 73% and 89% in those with and without cirrhosis, respectively.29 In a second trial with 111 naive patients with HCV genotype 3 infections treated for 12 weeks with LDV/SOF plus RBV, the SVR rates were 94% in 72 patients without cirrhosis and 79% in 39 patients with compensated cirrhosis.30 Taken together, the results of these trials and the real‐world experience of Madrid‐CoRe suggest that SOF/LDV should be considered a suboptimal regimen for genotype 3 infections in patients with cirrhosis.
According to current guidelines, treatment of HCV genotype 1 with LDV/SOF can be shortened to 8 weeks in treatment‐naive patients without cirrhosis if their baseline HCV RNA level is <6 million (6.8 log) IU/mL.3, 4 This recommendation is based on the observation that among ION‐3 patients with baseline HCV RNA <6 million IU/mL, relapse rates were similar in those receiving 8 weeks of treatment and those receiving 12 weeks of treatment.31, 32 In Madrid‐CoRe, 308 patients met the required conditions for a short 8‐week regimen of SOF/LDV without RBV. Of these, 104 patients were treated for 8 weeks and 204 for 12 weeks with SVR rates by ITT and m‐ITT analyses of 92%‐93% and 93%‐95%, respectively. The relapse rates were 5.8% and 3.9% in the 8‐week and 12‐week arms, respectively. These results are similar to those reported in the ION‐3 trial, in which 215 patients received SOF/LDV for 8 weeks, with an SVR rate of 94% and a relapse rate of 5%.32 Real‐world cohort data have also shown the comparable effectiveness of SOF/LDV for 8 and 12 weeks in HCV genotype 1–infected, treatment‐naive patients without cirrhosis.21, 33, 34, 35, 36 Of note, the proportions of patients with METAVIR F3 fibrosis (or an equivalent by transient elastography) treated with SOF/LDV for 8 weeks were 13% in the ION‐3 trial and <25% in Madrid‐CoRe. Thus, the question remains as to whether the 8‐week LDV/SOF regimen is appropriate for patients with METAVIR F3 in liver biopsy or equivalent by noninvasive methods.
The high SVR rates of most currently licensed all oral DAA‐based regimens against HCV and the relatively small number of patients included in registration trials have made it difficult to identify predictors of treatment failure. Nevertheless, genotype 3, cirrhosis, liver decompensation, and preexisting resistance‐associated variants all seem to reduce the probability of SVR.11 The large size of the Madrid‐CoRe cohort permitted us to evaluate predictors of treatment failure. Multivariable logistic regression modeling showed that gender, infection‐related variables (HIV and HCV), severity of liver disease, and treatment‐related variables were independently associated with response to treatment.
Our multivariable model showed that the probability of failure was highest for decompensated cirrhosis, intermediate for compensated cirrhosis, and lowest for absence of cirrhosis, with statistically significant pair‐wise comparisons between the three groups. The importance of the severity of liver disease in treatment outcomes is further emphasized by our observation that among patients with compensated cirrhosis the presence of a liver stiffness value ≥19.5 kPa was associated with a significantly higher probability of treatment failure. This cutoff defines a group of patients at risk of having clinically significant portal hypertension and esophageal varices12 and corresponds to the new category of compensated advanced chronic liver disease in the report of the Baveno VI Consensus Workshop.37
Male sex was a predictor of treatment failure in Madrid‐CoRe. Male sex, a recognized predictor of treatment failure in the interferon plus RBV era,38 has also been associated with treatment failure in a large real‐world report of patients infected with HCV genotype 1 treated with SOF/LDV or DSV+OBV/PTV/r within the US Veterans Affairs Health Care System.33
Of note, we found that CDC clinical category C and a baseline CD4+ T‐cell count <200/mm3 were independently associated with increased odds of treatment failure. These findings suggest that the immune response may play a role in clearance of HCV during DAA‐based therapies, possibly through the recognition and elimination by T cells of viral variants with resistance to DAAs.39 In a German real‐world cohort in the DAA era (395 HIV/HCV‐coinfected patients), the variables associated with reduced odds of SVR were liver cirrhosis, a CD4+ T‐cell count <350/mm3, and a CD4+ T‐cell percentage <20%; however, in the multivariable analysis, only liver cirrhosis remained statistically significantly associated with treatment failure.40 Sufficiently large cohorts of HIV/HCV‐coinfected patients and HCV‐monoinfected patients may reveal statistically significant differences (albeit not clinically relevant) in efficacy rates between both treatment groups after DAA therapy.
In Madrid‐CoRe, HCV RNA load ≥800,000 IU/mL was the only HCV‐related factor independently associated with treatment failure. To date, an association between an HCV RNA load ≥800,000 IU/mL and treatment failure has been reported in patients with HCV genotype 1a treated with the combination of elbasvir/grazoprevir.41
Finally, we found that the use of DAA regimens including SOF+SMV, SOF+RBV, and SMV+DCV was independently associated with treatment failure. SOF+RBV and SMV+DCV are no longer included in the recommended regimens for treating HCV infection.3, 4 However, SOF+SMV is currently one of the recommended regimens for genotype 1a or 1b in treatment‐naive and treatment‐experienced, DAA‐naive patients without cirrhosis in the American Association for the Study of Liver Diseases–Infectious Diseases Society of America guidelines4 and for genotype 4 in treatment‐naive and treatment‐experienced, DAA‐naive patients without cirrhosis and patients with compensated cirrhosis in the European Association for the Study of the Liver guidelines.3
The main limitation of our study was that some baseline HIV‐related variables were collected retrospectively. Moreover, this information was not available at the time of the data analysis in approximately one third of patients, who were attended in eight out of the 25 participating hospitals and who did not send the information on time. However, in our univariable and multivariable adjusted models, patients for whom data were unavailable were classified as “unknown.” Our study is also limited by the lack of data on concomitant medication, including proton pump inhibitors,42 and adherence. Furthermore, the absence of information on preexisting viral variants with resistance‐associated substitutions prevented us from analyzing their prevalence and their impact on treatment outcomes.43 Nevertheless, to our knowledge, Madrid‐CoRe is the largest real‐world study of interferon‐free regimens in HIV/HCV‐coinfected patients reported to date, with two and a half times more patients than the large series recently reported from the Veterans Administration Health Care System (996 patients)6 and almost 6 times more patients than all other reported series (<400 patients).7, 8, 10 This huge sample, which compares favorably with the large real‐world studies reported for HCV‐monoinfected patients,11 gave us the opportunity to assess treatment outcomes for various regimens against different genotypes in different liver disease categories and to evaluate predictors of treatment response, which is difficult to assess for a therapy with failure rates <10%.
In conclusion, in this large real‐world prospective study, interferon‐free DAA therapy was found to be safe and highly effective in HIV/HCV‐coinfected patients. Our findings support the use of an 8‐week regimen of LDV/SOF without RBV for treatment‐naive, coinfected patients without cirrhosis with an HCV RNA level <6 million IU/mL. They also support the use of a 24‐week regimen of SOF+DCV with RBV for genotype 3 infection in coinfected patients with compensated cirrhosis. The variables found to be independently associated with treatment failure included gender, CDC clinical category, baseline CD4+ T‐cell count, HCV RNA load, cirrhosis, decompensated liver disease, and the use of DAA regimens currently considered suboptimal in some guidelines.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo.
Supporting Information 1
Acknowledgment
The authors thank Thomas O'Boyle for writing assistance.
The Madrid‐CoRe Study Group
Hospital General Universitario Gregorio Marañón: Berenguer J, Aldámiz T, Miralles T, López JC, Parras F, Gijón P, Padilla B, Montilla P, Fernández‐Cruz A, Valerio M, Bermúdez E, Catalán P, Rodríguez C
Hospital La Paz‐Carlos III: González JJ, Montes ML, Martín L, Moreno V, Valencia E, Pérez I, Bernardino I, Jiménez I, Moreno F
Subdirección General de Farmacia y Productos Sanitarios/SERMAS: Gil A, Alcaraz M, Aranguren A, Calvo MJ, Cruz E
Hospital Universitario Ramon y Cajal: Moreno A, Quereda C, Casado J, Perez MJ, Vivancos MJ, Diaz A, Navas E, Fortún J, Moreno S, Serrano S, García M, Rodríguez MA
Hospital Universitario Doce de Octubre: Pulido F, Rubio R, Domínguez L, Matarranz M, de Lagarde M, Fernández I, Muñoz R, Martín A, Pinar O
Hospital Clínico Universitario San Carlos: Téllez MJ, Estrada V, Vergas J, Cabello N, Saénz M, Santiago A
Hospital Universitario de la Princesa: Santos I, Martínez C
Hospital Universitario Príncipe de Asturias: Sanz J, De Miguel J, Arranz A, Casas E, Víctor V, Herrero M
Hospital Universitario Infanta Leonor: Ryan P, Troya J, Cuevas G, Esteban C
Hospital Universitario Puerta de Hierro: Benítez L, Arias A, Díaz A, Baños I, Duca A, Menchen B, Santiago M
Hospital Universitario de Getafe: Gaspar G, Sánchez‐Rubio J
Fundación Hospital Jiménez Díaz: Górgolas A. Alvarez B, Polo B, Varela A, González A, Cabello A, Calvo R, Porres JC, Bonilla M
Hospital Universitario Severo Ochoa: Torres R, Cervero M, Jusadado JJ, Díaz E
Hospital Universitario de Móstoles: Merino F, Barros C, Corrales L
Hospital Fundación de Alcorcón: Losa JE, Hervas R, Velasco M, Moreno L, Henríquez C, Pérez M, Polanco M
Hospital de Fuenlabrada: San Martín J, Canalejo E, Hinojosa J, Ruiz‐Giardin JM, Aguilar C, Hernández B
Hospital de Torrejón: Arponen S, Gimeno A, Montero MC
Hospital del Henares: Serrano R, Sanz P, Egües E, Tovar M
Hospital del Tajo: Monsalvo R, Terrancle I, Pedraza LA
Hospital Infanta Elena: Vegas A, del Portillo A, Collado V
Hospital Infanta Cristina: De Guzman MT, Martínez JA, Pérez JL, Melero JA, Matilla E
Hospital del Sureste: García MT, Peñalver R, Capilla C, Fernández‐Amago MT
Hospital Rey Juan Carlos: Gotuzzo L, Marcos J, García A
Hospital Infanta Sofía: Malmierca E, Suárez I, Portillo L
Hospital El Escorial: Belda L, Sanchez S
Hospital Gómez Ulla: Menéndez MA
Instituto de Salud Carlos III: Jarrín I
Facultad de Farmacia, Universidad Complutense: Benedi J
Potential conflict of interest: Dr. Berenguer consults for and received grants from AbbVie, Gilead, MSD, and ViiV. He consults for Janssen. Dr. González‐García advises, is on the speakers' bureau of, and received grants from Gilead, Janssen, AbbVie, MSD, and ViiV. Dr. Monsalvo is on the speakers' bureau for Viiv, Gilead, and MSD.
Dr. Juan Berenguer is an investigator of the Programa de Intensificación de la Actividad Investigadora en el Sistema Nacional de Salud (I3SNS; ref. INT16/00100). Clinical research at Hospital General Universitario Gregorio Marañón, Hospital Universitario Ramón y Cajal, Hospital La Paz, Hospital Universitario 12 de Octubre, and Hospital Universitario de la Princesa is supported in part by the Spanish AIDS Research Network (RD16/0025/0017), which is included in the Spanish I+D+I Plan and cofinanced by ISCIII‐Subdirección General de Evaluacion and European Funding for Regional Development (FEDER).
REFERENCES
- 1. Liang TJ, Ghany MG. Therapy of hepatitis C—back to the future. N Engl J Med 2014;370:2043‐2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berenguer J, Gonzalez‐Garcia J, Lopez‐Aldeguer J, Von‐Wichmann MA, Quereda C, Hernando A, et al. Pegylated interferon α2a plus ribavirin versus pegylated interferon α2b plus ribavirin for the treatment of chronic hepatitis C in HIV‐infected patients. J Antimicrob Chemother 2009;63:1256‐1263. [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017;66:153‐194. [DOI] [PubMed] [Google Scholar]
- 4. American Association for the Study of Liver Diseases, Infectious Diseases Society of America . HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org/. Accessed July 24, 2017. [DOI] [PMC free article] [PubMed]
- 5. Saeed S, Strumpf EC, Walmsley SL, Rollet‐Kurhajec K, Pick N, Martel‐Laferriere V, et al. How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016;62:919‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhattacharya D, Belperio PS, Shahoumian TA, Loomis TP, Goetz MB, Mole LA, et al. Effectiveness of all‐oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus genotype 1‐coinfected patients treated in routine practice. Clin Infect Dis 2017;64:1711‐1720. [DOI] [PubMed] [Google Scholar]
- 7. Rockstroh JK, Ingiliz P, Petersen J, Peck‐Radosavljevic M, Welzel TM, Van der Valk M, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, in real‐world patients with HIV‐HCV coinfection and advanced liver disease. Antivir Ther 2017;22:225‐236. [DOI] [PubMed] [Google Scholar]
- 8. Hawkins C, Grant J, Ammerman LR, Palella F, McLaughlin M, Green R, et al. High rates of hepatitis C virus (HCV) cure using direct‐acting antivirals in HIV/HCV‐coinfected patients: a real‐world perspective. J Antimicrob Chemother 2016;71:2642‐2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sogni P, Gilbert C, Lacombe K, Piroth L, Rosenthal E, Miailhes P, et al. All‐oral direct‐acting antiviral regimens in HIV/hepatitis C virus‐coinfected patients with cirrhosis are efficient and safe: real‐life results from the prospective ANRS CO13‐HEPAVIH cohort. Clin Infect Dis 2016;63:763‐770. [DOI] [PubMed] [Google Scholar]
- 10. Piroth L, Wittkop L, Lacombe K, Rosenthal E, Gilbert C, Miailhes P, et al. Efficacy and safety of direct‐acting antiviral regimens in HIV/HCV‐co‐infected patients—French ANRS CO13 HEPAVIH cohort. J Hepatol 2017;67:23‐31. [DOI] [PubMed] [Google Scholar]
- 11. Afdhal NH, Serfaty L. Effect of registries and cohort studies on HCV treatment. Gastroenterology 2016;151:387‐390. [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Muir AJ, Dieterich DT, Falck‐Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology 2017;152:1544‐1577. [DOI] [PubMed] [Google Scholar]
- 13. Montes ML, Olveira A, Ahumada A, Aldamiz T, Garcia‐Samaniego J, Clemente A, et al. Similar effectiveness of direct‐acting antiviral against hepatitis C virus in patients with and without HIV infection. AIDS 2017;31:1253‐1260. [DOI] [PubMed] [Google Scholar]
- 14. Arias A, Aguilera A, Soriano V, Benitez‐Gutierrez L, Lledo G, Navarro D, et al. Rate and predictors of treatment failure to all‐oral HCV regimens outside clinical trials. Antivir Ther 2017;22:307‐312. [DOI] [PubMed] [Google Scholar]
- 15. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483‐1493. [DOI] [PubMed] [Google Scholar]
- 16. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889‐1898. [DOI] [PubMed] [Google Scholar]
- 17. Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV‐1. N Engl J Med 2015;373:705‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1594‐1603. [DOI] [PubMed] [Google Scholar]
- 19. Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973‐1982. [DOI] [PubMed] [Google Scholar]
- 20. Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co‐dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co‐infected with HIV‐1: a randomized trial. JAMA 2015;313:1223‐1231. [DOI] [PubMed] [Google Scholar]
- 21. Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology 2016;151:457‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hezode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer‐Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment‐naive and treatment‐experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL‐I): a randomised, open‐label trial. Lancet 2015;385:2502‐2509. [DOI] [PubMed] [Google Scholar]
- 23. Smith MA, Mohammad RA. Ledipasvir‐sofosbuvir for hepatitis C genotype 4 infection. Lancet Infect Dis 2015;15:993‐995. [DOI] [PubMed] [Google Scholar]
- 24. Abergel A, Metivier S, Samuel D, Jiang D, Kersey K, Pang PS, et al. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology 2016;64:1049‐1056. [DOI] [PubMed] [Google Scholar]
- 25. Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All‐oral 12‐week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY‐3 phase 3 study. Hepatology 2015;61:1127‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY‐3+). Hepatology 2016;63:1430‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010;465:96‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol 2013;59:375‐382. [DOI] [PubMed] [Google Scholar]
- 29. Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015;149:1454‐1461. [DOI] [PubMed] [Google Scholar]
- 30. Feld JJ, Ramji A, Shafran SD, Willems B, Marotta P, Huchet E, et al. Ledipasvir‐sofosbuvir plus ribavirin in treatment‐naive patients with hepatitis C virus genotype 3 infection: an open‐label study. Clin Infect Dis 2017;65:13‐19. [DOI] [PubMed] [Google Scholar]
- 31. Kowdley KV, An D, Pang PS, Wyles D. Analysis of subgroup differences in the ION‐3 trial of ledipasvir‐sofosbuvir in chronic hepatitis C infection. Open Forum Infect Dis 2015;2:ofv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879‐1888. [DOI] [PubMed] [Google Scholar]
- 33. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real‐world effectiveness and predictors of sustained virological response with all‐oral therapy in 21,242 hepatitis C genotype‐1 patients. Antivir Ther 2017;22:481‐493. [DOI] [PubMed] [Google Scholar]
- 34. Ingiliz P, Christensen S, Kimhofer T, Hueppe D, Lutz T, Schewe K, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV‐monoinfected and HIV‐HCV‐coinfected individuals: results from the German hepatitis C cohort (GECCO‐01). Clin Infect Dis 2016;63:1320‐1324. [DOI] [PubMed] [Google Scholar]
- 35. Kowdley KV, Sundaram V, Jeon CY, Qureshi K, Latt NL, Sahota A, et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology 2017;65:1094‐1103. [DOI] [PubMed] [Google Scholar]
- 36. Terrault NA, Zeuzem S, Di Bisceglie AM, Lim JK, Pockros PJ, Frazier LM, et al. Effectiveness of ledipasvir‐sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology 2016;151:1131‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Franchis R, Baveno VI Faculty . Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743‐752. [DOI] [PubMed] [Google Scholar]
- 38. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 2011;55:245‐264. [DOI] [PubMed] [Google Scholar]
- 39. Ahlen G, Frelin L, Brenndorfer ED, Brass A, Weiland O, Chen M, et al. Containing “the great Houdini” of viruses: combining direct acting antivirals with the host immune response for the treatment of chronic hepatitis C. Drug Resist Updat 2013;16:60‐67. [DOI] [PubMed] [Google Scholar]
- 40. Boesecke C, Ingiliz P, Berger F, Lutz T, Schewe K, Schulze Zur Wiesch J, et al. Liver cirrhosis as a risk factor for direct‐acting antiviral therapy failure in real‐life hepatitis C virus/human immunodeficiency virus coinfection. Open Forum Infect Dis 2017;4:ofx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeuzem S, Rockstroh J, Kwo PY, Roth D, Lawitz E, Sulkowski M, et al. Predictors of response to grazoprevir/elbasvir among HCV genotype 1 (GT1)–infected patients: integrated analysis of phase 2‐3 trials. Hepatology 2015;62(Suppl. 1):554A‐555A. [Google Scholar]
- 42. Tapper EB, Bacon BR, Curry MP, Dieterich DT, Flamm SL, Guest LE, et al. Evaluation of proton pump inhibitor use on treatment outcomes with ledipasvir and sofosbuvir in a real‐world cohort study. Hepatology 2016;64:1893‐1899. [DOI] [PubMed] [Google Scholar]
- 43. Zeuzem S, Mizokami M, Pianko S, Mangia A, Han KH, Martin R, et al. NS5A resistance‐associated substitutions in patients with genotype 1 hepatitis C virus: prevalence and effect on treatment outcome. J Hepatol 2017;66:910‐918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29814/suppinfo.
Supporting Information 1


