Abstract
Salivary duct carcinoma (SDC) is a subtype of salivary gland cancer with a dismal prognosis and a need for better prognostication and novel treatments. The aim of this national cohort study was to investigate clinical outcome, prognostic factors, androgen receptor (AR) and human epidermal growth factor receptor 2 (HER2) expression. SDC patients diagnosed between 1990 and 2014 were identified by the Nationwide Network and Registry of Histo‐ and Cytopathology in the Netherlands (PALGA). Subsequently, medical records were evaluated and pathological diagnoses reviewed. Data were analyzed for overall survival (OS), disease‐free survival (DFS), distant metastasis‐free survival (DMFS) and prognostic factors. AR was evaluated by immunohistochemistry (IHC), HER2 by IHC and fluorescent in‐situ hybridization. A total of 177 patients were included. The median age was 65 years, 75% were male. At diagnosis, 68% presented with lymph node metastases and 6% with distant metastases. Median OS, DFS and DMFS were 51, 23 and 26 months, respectively. In patients presenting without distant metastases, the absolute number of positive lymph nodes was associated with poor OS and DMFS in a multivariable analysis. AR and HER2 were positive in 161/168 (96%) and 44/153 (29%) tumors, respectively, and were not prognostic factors. SDC has a dismal prognosis with primary lymph node involvement in the majority of patients. The absolute number of lymph node metastases was found to be the only prognostic factor for DMFS and OS. AR expression and—to a lesser extent—HER2 expression hold promise for systemic treatment in the metastatic and eventually adjuvant setting.
Keywords: salivary duct carcinoma, salivary gland neoplasms, androgen receptors, receptor, ErbB‐2, prognosis, survival, in situ hybridization, fluorescence, immunohistochemistry
Short abstract
What's new?
Salivary duct carcinoma (SDC) is a rare and often fatal malignancy. Little is known about associations between its pathological features and clinical outcome. In this study, clinicopathological factors were analyzed for 177 patients diagnosed with SDC in The Netherlands between 1990 and 2014. The data show that median overall survival (OS) and distant metastasis‐free survival (DMFS) were 51 and 26 months, respectively. At diagnosis, 68% of patients presented with lymph node metastases. Lymph node positivity was associated with poor OS and poor DMFS. The absolute number of metastatic lymph nodes was the only significant prognostic factor for survival in a multivariate analysis. Androgen receptor and human epidermal growth factor 2 (HER2) were positive in 96% and 29%, respectively and were not a prognostic factor.
Salivary duct carcinoma (SDC) is a rare subtype of salivary gland cancer (SGC). It was first described in 1968,1 and defined as a distinctive entity in 1990.2 SDC usually affects middle‐aged men and the tumor is often located in the parotid gland. Patients frequently present with locally advanced disease. Primary treatment consists of resection of the primary tumor and neck dissection, usually followed by radiotherapy. SDC is characterized by a high rate of distant metastases resulting in a limited overall survival (OS).3
Immunohistochemically, SDC resembles prostate cancer, because of common expression of the androgen receptor (AR).4 Androgen deprivation therapy (ADT) in a small series showed a 50% clinical benefit rate with a median duration of 12 months.5 Morphologically, SDC shows similarities with invasive ductal carcinoma of the breast. However, SDC only rarely shows estrogen and progesterone receptor expression.
Expression of the Human Epidermal Growth Factor Receptor 2 (HER2) was reported in 44% of 32 patients with SDCs.6
Due to the rarity of disease, only relatively small cohorts have been described and prognostic factors remain to be elucidated. The largest studies with 495 SDC patients based on the National Cancer Database (NCDB) and with 228 SDC patients based on the Surveillance, Epidemiology, and End Results Program (SEER) database, lack vital information on the occurrence of local and regional recurrences, distant metastases, the use of systemic therapy and AR or HER2 expression.3 Furthermore, no pathological review was performed in both studies.
Thanks to the unique collaboration between the Dutch Pathology Network PALGA and the national network of head and neck centers, we collected data of patients diagnosed with SDC in the Netherlands and aimed to evaluate clinicopathological characteristics (such as AR and HER2 expression and primary treatment) in relation to clinical outcome and prognostic factors.
Patients and Methods
Patient selection
Patients diagnosed with SDC between 1990 and 2014 were identified by means of a retrospective search by the Nationwide Network and Registry of Histo‐ and Cytopathology in the Netherlands (PALGA).7 As all Dutch pathology laboratories participate in this network, all patients with a registered diagnosis of SDC in the Netherlands were enrolled. All patients were coded by PALGA and clinical data could be correlated with the pathological features in a coded procedure.
Clinical data
Clinical data were collected from the medical records and were obtained with permission of treating physicians according to Dutch national laws and Good Clinical Practice. Review by a medical ethical committee was not obligatory by Dutch law due to the retrospective nature of the observations.
Pathology
For all patients, hematoxylin and eosin (H&E) stained slides, formalin‐fixed paraffin‐embedded (FFPE) tumor blocks and corresponding anonymous pathological reports were requested. All patient materials used in this study were obtained during routine patient care, and used for scientific research with permission by Dutch Law (“Code for Secondary Use of Human Tissue,” Dutch Federation of Medical Scientific Societies). H&E slides were used for re‐evaluation of the diagnosis and to mark areas of primary tumor by an experienced pathologist (UF). From each “donor” block, one to three cores of primary tumor were transferred into the “recipient” tissue micro array (TMA) block, using the TMA Grandmaster by Sysmex. From the new TMA “recipient blocks,” slides were produced for further analysis. Each TMA slide was analyzed for AR and HER2 and scored by the pathologist (UF), who was blinded for the clinical outcome. AR (immunohistochemistry (IHC)) and HER2 (either IHC or fluorescent in‐situ hybridization (FISH)) acquired during routine clinical care procedures was permitted in case it was not possible to determine AR or HER2 with a TMA. In case of heterogeneity between cores or between TMA and clinically obtained results, the highest score was used.
For AR expression, the AR polyclonal antibody of Santa Cruz was used, dilution 1:200, pretreatment with citrate (pH 6.0) for 10 min in a pretreatment module (Labvision/thermo scientific by Klinipath/VWR). Then, immunostaining was carried out with the detection system (Brightvision) of Immunologic, Duiven, the Netherlands. AR immunhistochemistry was executed in the Radboudumc. AR was scored positive or negative based on diffuse nuclear staining, as described in the WHO classification of SDC.8
HER2 was determined upfront by both IHC and FISH. The Hercepkit of DAKO was used according to protocol for HER2 immunostaining. For HER2 FISH, the probe of Kreatech (location of hybridization on 17q12cep17) was used. The probe was incubated according to standard ISH protocol. Scoring of HER2 was performed according to guidelines from the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) analogous to breast cancer.9 HER2 IHC and FISH were scored independently. After scoring IHC and FISH, the results were compared. As the guideline does not mention how to report on discrepancies between IHC and FISH, we considered the result of HER2 FISH directive in discordant cases, that is, HER2 IHC3+ and HER2 FISH negative was scored as HER2 negative.
Definitions and statistical analysis
Lymph node ratio (LNR) was defined as the number of tumor‐positive lymph nodes divided by the total number of lymph nodes resected. Date of diagnosis was defined as date of obtaining the first histological proof of SDC. In case the diagnosis was confirmed in a later stadium, the original date of obtaining the histopathological material was used. Overall survival (OS) was measured from date of diagnosis until death of any cause. Patients alive at last known follow‐up date were censored. Disease‐free survival (DFS) was measured from date of surgery until date of local or regional recurrence, distant metastases or death of any cause, whichever came first. Patients alive without disease at last known follow‐up were censored. Distant metastasis free survival (DMFS) was defined as date of diagnosis until date of distant metastases or death of any cause, whatever came first. Patients alive without distant metastasis were censored. Survival curves were estimated with the Kaplan–Meier method. To investigate association between patient and tumor characteristics and survival, univariate Cox proportional hazards regression models were fitted first. Next, a multivariable Cox regression model was estimated with a forward selection procedure based on the Wald test at significance level of 0.05. The variables used in the multivariable analysis for OS and DMFS included gender, age (categorical), T‐ and N‐stage, number of positive lymph nodes (categorical), AR, HER2, carcinoma ex pleomorphic adenoma, primary tumor site and resection margins. Patients with metastatic disease at diagnosis and patients with missing values in one or more of the variables were excluded from the multivariable analysis. Data were analyzed using SPSS version 22.0.
Results
Patient and tumor characteristics
Pathological review led to the inclusion of 177 eligible SDC patients out of 294 patients in the PALGA database (Fig. 1). Patient characteristics are shown in Table 1. The median age was 65 years [range 38–92], and the majority was male (75%). The parotid gland was the most affected (82%) salivary gland. Thirty‐six percent of patients had an SDC arising from a pleomorphic adenoma (carcinoma ex pleomorphic adenoma). One‐hundred and twenty patients (68%) had lymph node metastases. Eleven patients (6%) presented with distant metastases. Ninety‐six percent of 168 evaluable tumors were AR positive. Twenty‐nine percent of the 153 evaluable tumors were HER2 positive. One‐hundred and forty patients were evaluated for AR and HER2 using the TMA, the scores of the remaining patients were based on routine clinical evaluations. Table 2 shows the number of patients evaluated by FISH and IHC. Four patients had HER2 IHC3+ but had a negative HER2 FISH, and were scored as HER‐2 negative.
Figure 1.
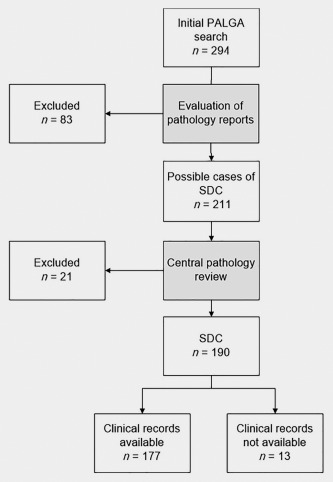
Consort diagram of inclusion of SDC patients. PALGA is the Nationwide Network and Registry of Histo‐ and Cytopathology in the Netherlands.
Table 1.
Patient's and tumor characteristics
| Characteristics | Number of patients (n = 177) |
|---|---|
| Median age, in years | 65 |
| Range, in years | 38–92 |
| Age, in years, n (%) | |
| ≤50 | 19 (11) |
| 51–60 | 43 (24) |
| 61–70 | 54 (31) |
| 71–80 | 41 (23) |
| >80 | 20 (11) |
| Gender, n (%) | |
| Male | 133 (75) |
| Female | 44 (25) |
| Primary tumor, n (%) | |
| Parotid gland | 145 (82) |
| Submandibular gland | 19 (11) |
| Sublingual gland | 2 (1) |
| Other | |
| Minor salivary glands | 7 (4) |
| Lacrimal glanda | 1 (1) |
| Unknown | 3 (2) |
| Presenting symptoms, n (%) | |
| Painless mass | 84 (48) |
| Painful mass | 27 (15) |
| Facial nerve paralysis | 51 (29) |
| Unknown | 15 (9) |
| Carcinoma ex pleomorphic adenoma, n (%) | |
| Yes | 63 (36) |
| No (“de novo”) | 114 (64) |
| TNM stadium | |
| T1/T2/T3/T4/Tx | 29/49/20/68/11 |
| (%) | (16/28/11/38/6) |
| N0/N1/N2/N3 | 57/15/104/1 |
| (%) | (32/8/59/1) |
| M0/M1 | 166/11 |
| (%) | (94/6) |
| Overall stage | |
| I/II/III/IV/unknown | 16/18/10/130/3 |
| (%) | (9/10/5/73/2) |
|
Primary treatment with curative intent
b
(n = 162)
Surgery, n (%) |
|
| Resection primary tumor with neck dissection | 123 (76) |
| Resection primary tumor without neck dissection | 36 (22) |
| Neck dissection only | 3 (2) |
| Postoperative radiotherapy, n (%) | |
| Yes | 149 (91) |
| No | 13 (9) |
| Neck dissection c | |
| Median number of resected lymph nodes, range | 27 (1–122) |
| Median number of positive lymph nodes, range | 4 (0–97) |
| Median lymph node ratio, range | 0.20 [0–1.00] |
| Androgen receptor (AR), n (%) d | |
| Positive | 162 (96) |
| Negative | 6 (4) |
| HER2, n (%) e | |
| Positive | 45 (29) |
| Negative | 108 (71) |
| HER2 determination methods, n (%) | |
| FISH and IHC | 140 (92) |
| FISH | 5 (3) |
| IHC | 8 (5) |
Histopathological appearance of SDC despite its localization in the lacrimal gland.
One patient underwent primary surgery despite distant metastases on baseline imaging in retrospect.
n = 126 patients.
Nine patients had no AR result. n = 168 patients.
Twenty‐four patients had no HER2 result. n = 153 patients.
Table 2.
Univariate and multivariable analyses for overall survival and distant metastasis free survival
| Univariate | |||||
|---|---|---|---|---|---|
| OS | DMFS | ||||
| Factor | No of patients | HR + 95% CI | p | HR + 95% CI | p |
| Increasing age in years | 177 | 1.02 [1.00–1.03] | 0.09 | 1.00 [0.99–1.02] | 0.58 |
| Age, categories a, b | 0.31 | 0.67 | |||
| ≤50 years | 19 | 1.00 | 1.00 | ||
| 51–60 years | 43 | 2.46 [1.01–6.00] | 0.048 | 1.66 [0.83–3.30] | 0.15 |
| 61–70 years | 54 | 2.02 [0.83–4.95] | 0.12 | 1.48 [0.74–2.93] | 0.27 |
| 71–80 years | 41 | 1.98 [0.79–4.98] | 0.15 | 1.40 [0.69–2.83] | 0.35 |
| >80 years | 20 | 2.74 [1.01–7.42] | 0.048 | 1.24 [0.54–2.86] | 0.62 |
| Gender a, b | |||||
| Female | 44 | 1.00 | 1.00 | ||
| Male | 133 | 2.24 [1.24–4.06] | 0.008 | 2.10 [1.27–3.49] | 0.004 |
| Carcinoma ex pleomorphic adenoma a, b | |||||
| No (“de novo”) | 114 | 1.00 | 1.00 | ||
| Yes | 63 | 0.80 [0.50–1.26] | 0.33 | 0.88 [0.59–1.29] | 0.50 |
| T‐stadium a, b | 0.18 | 0.008 | |||
| T1/T2 | 77 | 1.00 | 1.00 | ||
| T3/T4 | 89 | 1.50 [0.95–2.36] | 0.08 | 1.88 [1.26–2.79] | 0.002 |
| Tx | 11 | 1.56 [0.68–3.54] | 0.29 | 1.65 [0.77–3.55] | 0.20 |
| N‐stadium a, b | |||||
| N0 | 57 | 1.00 | 1.00 | ||
| N1/N2/N3 | 120 | 2.28 [1.36–3.81] | 0.002 | 2.24 [1.44–3.49] | 0.000 |
| Number of positive lymph nodes a, b | 159 | 0.000 | 0.000 | ||
| 0 | 56 | 1.00 | 1.00 | ||
| 1–2 | 27 | 1.13 [0.54–2.40] | 0.74 | 1.15 [0.60–2.23] | 0.67 |
| 3–15 | 45 | 2.03 [1.11–3.72] | 0.022 | 2.03 [1.20–3.45] | 0.009 |
| >15 | 31 | 3.83 [1.98–7.43] | 0.000 | 4.38 [2.47–7.78] | 0.000 |
| Lymph node ratio (LNR) | |||||
| <0.20 | 64 | 1.00 | 1.00 | ||
| >0.20 | 60 | 2.43 [1.42–4.16] | 0.001 | 2.36 [1.48–3.78] | 0.000 |
| M‐stadium c | |||||
| M0 | 166 | 1.00 | |||
| M1 | 11 | 4.26 [2.08–8.71] | 0.000 | ||
| Resection margins a, b | 0.31 | 0.57 | |||
| Free | 16 | 1.00 | 1.00 | ||
| Close | 15 | 0.58 [0.17–1.92] | 0.37 | 1.08 [0.44–2.66] | 0.87 |
| Tumor‐positive margins | 127 | 1.23 [0.59–2.59] | 0.58 | 1.36 [0.70–2.64] | 0.37 |
| Primary tumor site a, b | 0.90 | 0.96 | |||
| Parotid gland | 145 | 1.00 | 1.00 | ||
| Submandibular gland | 19 | 0.85 [0.43–1.72] | 0.66 | 0.93 [0.51–1.71] | 0.82 |
| Other | 10 | 1.03 [0.44–2.39] | 0.95 | 0.93 [0.43–2.02] | 0.86 |
| Androgenreceptor a, b | |||||
| Negative | 6 | 1.00 | 1.00 | ||
| Positive | 162 | 1.69 [0.53–5.39] | 0.38 | 1.41 [0.52–3.86] | 0.50 |
| HER2 a, b | |||||
| Negative | 108 | 1.00 | 1.00 | ||
| Positive | 45 | 1.08 [0.65–1.81] | 0.76 | 1.23 [0.80–1.89] | 0.35 |
| Multivariable c | |||||
|---|---|---|---|---|---|
| OS | DMFS | ||||
| Factor | No of patients | HR + 95% CI | p | HR + 95% CI | p |
| Number of positive lymph nodes | 136 | 0.003 | 0.000 | ||
| 0 | 49 | 1.00 | 1.00 | ||
| 1–2 | 22 | 1.20 [0.50–2.86] | 0.69 | 1.26 [0.60–2.66] | 0.54 |
| 3–15 | 41 | 2.17 [1.09–4.32] | 0.028 | 2.25 [1.25–4.06] | 0.007 |
| >15 | 25 | 3.96 [1.84–8.55] | 0.000 | 4.73 [2.48–9.00] | 0.000 |
Abbreviations: 95%CI, 95% confidence interval; DMFS, distant metastasis free survival; HR, hazard ratio; OS = overall survival.
Variables included in multivariable analyses for OS.
Variables included in multivariable analyses for DMFS.
Patients who presented with primarily metastatic disease were not included in the multivariable analysis.
Primary treatment with curative intent
Of the 177 patients, 162 patients underwent primary surgery with curative intent.
Fourteen patients did not have surgery because of an irresectable tumor in 3 patients or distant metastases at diagnosis in 11 patients. One patient underwent primary surgery of the primary tumor and neck dissection, but in retrospect had distant metastases on baseline imaging, and was not considered as having been treated with curative intent.
Surgery
One hundred and sixty‐two patients had primary surgery, of which 123 patients had a resection of the primary tumor and neck dissection, 36 patients only had a resection of the primary tumor. Three patients only underwent a neck dissection, because no primary tumor could be detected. In patients who underwent a neck dissection (n = 126), the median number of tumor positive lymph nodes was 4 [range 0–97] (Table 1). Figure 2 shows the number of tumor positive lymph nodes plotted against the total number of lymph nodes examined in the resected specimens (number of patients = 126). The median LNR was 0.20.
Figure 2.
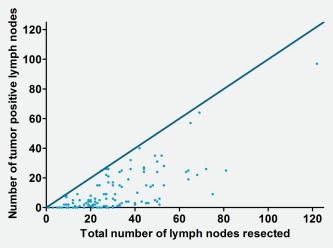
Tumor‐positive lymph nodes are plotted against the total number of lymph nodes during neck dissection (number of patients = 126). The dark blue line represents the line at which all resected lymph nodes would have been tumor positive. [Color figure can be viewed at http://wileyonlinelibrary.com]
Radiotherapy
One hundred and forty‐nine of 162 patients (91%) received postoperative radiotherapy. The median dose was 66 Gy (range 14–70 Gy). Only one of these patients underwent adjuvant concurrent chemoradiotherapy (CRT) (radiotherapy combined with weekly Cisplatin).
Patterns of recurrences and distant metastases
Eighty‐seven out of 162 patients (54%) developed locoregional recurrence and/or distant metastases after primary treatment with curative intent. Figure 3 a shows the Venn diagram of local, regional and distant recurrences in 162 patients treated with curative intent. Eighty‐four out of 177 patients (47%) had distant metastases during the course of disease; 11 (6%) patients had distant metastatic disease at diagnosis and 73/162 (45%) patients developed distant metastases after primary treatment with curative intent. Figure 3 b shows the sites of distant metastases for 84 patients who had distant metastases at diagnosis (n = 11) or developed distant metastases after treatment with curative intent (n = 73). Pulmonary (54%), bone (46%) and lymph nodes (42%) metastases were most frequently encountered. Brain metastases occurred in 15 (18%) patients. Of these 15 patients with brain metastases, the HER2 status was available in 13. Five out of 13 patients (38%) were HER2 positive, and the other 8 patients (62%) were HER2 negative. The median time until the occurrence of distant metastases was 16 months (range 1–69 months).
Figure 3.
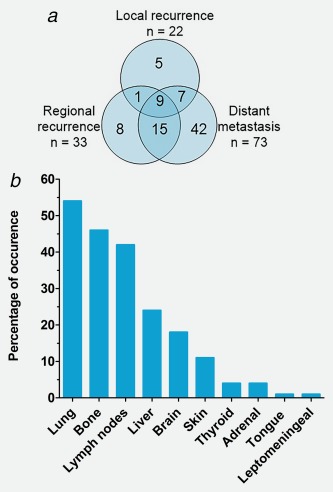
Patterns of disease recurrence. (a) Breakdown of local and regional recurrences and distant metastases in 87 patients with a recurrence. The numbers in the circles represent the absolute number of patients with local and regional recurrences and the presence of distant metastases. Patients with primarily metastatic disease are not included in this figure. (b) Localization of distant metastases sorted by percentage of presence in 84 patients with distant metastases. Patients with primarily metastatic disease were included in this figure. [Color figure can be viewed at http://wileyonlinelibrary.com]
Treatment with palliative intent
In total, 84 patients had distant metastases (11 at time of diagnosis and 73 after treatment with curative intent) and three patients had unresectable disease. One of these three patients received primary radiotherapy. The other two patients were treated with palliative ADT. Thirty‐six patients with distant metastatic SDC received ADT as first‐ or second‐line palliative treatment. Fifteen (18%) patients underwent chemotherapy and four (5%) patients targeted therapy. Most regimens included either taxanes (docetaxel or paclitaxel) or platinum (cisplatin or carboplatin) based chemotherapy. Some patients received multiple lines of systemic therapy. Forty‐four (54%) of 84 patients with distant metastases received only best supportive care. A total of 39 (46%) patients with distant metastases underwent radiotherapy with palliative intent.
Survival
After a median follow‐up of 26 months, 84 of 177 patients had died. The 5‐ and 10‐years survival were estimated as 43% [95% CI 33–52%] and 26% [95%CI 15–37%], respectively. The 5‐ and 10‐year DFS were estimated as 28% [95% CI 20–36%] and 17% [95% CI 8–25%], respectively. The 5‐ and 10‐year DMFS were 32% [95% CI 24–40%] and 20% [95% CI 2–29%], respectively.
Estimates for the median OS, DFS and DMFS were 51 months (95% CI 40–61 months), 23 months (95% CI 18–27 months) and 26 months (95% CI 20–34 months), respectively. The Kaplan–Meier curves for OS, DFS and DMFS are shown in Figures 4 a–4 c.
Figure 4.
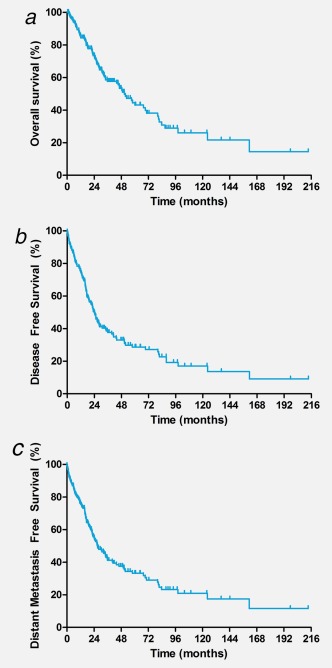
Kaplan–Meier curves for overall survival (OS), disease‐free survival (DFS) and distant metastasis free survival (DMFS) based on data of 177 SDC patients. (a) Kaplan–Meier curve for OS based on data of 177 SDC patients. Estimated median OS was 51 months (95% CI 40–61 months). (b) Kaplan–Meier curves for DFS based on data of 177 SDC patients. Estimated median DFS was 23 months (95% CI 18–27 months). (c) Kaplan–Meier curve of DMFS based on data of 177 SDC patients. Estimated median DMFS was 26 months (95% CI 20–34 months). [Color figure can be viewed at http://wileyonlinelibrary.com]
Prognostic factors
Patient selection
All 177 patients were included in the univariate analysis. Patients with distant metastases at diagnosis were not included in multivariable analyses for OS and DMFS. Owing to missing values, only 136 patients were included in the multivariable predictive model for OS and DMFS.
Overall survival
Univariate analysis showed that male gender, high N‐stadium, increasing number of tumor positive lymph nodes, LNR and primarily metastatic disease at diagnosis were associated with poor OS. The multivariable prediction model only contained the number of positive lymph nodes as independent variable: an increasing number of positive lymph nodes negatively affects survival (overall p = 0.003) (3–15 lymph nodes vs. 0 lymph nodes HR 2.17, 95% CI 1.09–4.32, p = 0.028; >15 lymph nodes vs. 0 lymph nodes HR 3.96, 95% CI 1.84–8.55, p = 0.000). The number of lymph nodes was categorized due to a nonlinear correlation with OS.
No significant association was found between AR and HER2 and OS in the univariate as well as the multivariable analysis. The results of the univariate and multivariable analyses for OS are shown in Table 2.
Distant metastasis free survival
Male gender, high T‐stadium, high N‐stadium, increasing numbers of positive lymph nodes and LNR were associated with poor DMFS using univariate analysis. The final multivariable prediction model only contained the number of positive lymph nodes as independent variable; an increasing number of positive lymph nodes is a prognostic factor for poor DMFS (overall p = 0.000) (3–15 lymph nodes vs. 0 lymph nodes HR 2.25, 95% CI 1.25–4.06, p = 0.007; >15 lymph nodes vs. 0 lymph nodes HR 4.73, 95% CI 2.48–9.00, p = 0.000). The number of lymph nodes was categorized due to nonlinear correlation with DMFS. From the univariate and multivariable analysis, no significant association was found between DMFS and AR and HER2. The results of the univariate and multivariable analysis for DMFS are displayed in Table 2.
Discussion
In this article, we present 177 patients with SDC, which represents the largest series of SDC patients with pathological review worldwide. These data provide extensive insight in treatment, clinical outcome, AR and HER2 expression/amplification and prognostic factors in SDC patients. This study underscores the aggressive clinical course characterized by a high rate of distant metastases (47%), and a median OS of 51 months. AR and HER2 were positive in 96 and 29% respectively; both were of no prognostic value as they were not significantly associated with OS and DMFS. The number of positive lymph nodes was the only factor independently associated with poor OS and DMFS.
SDC has a high propensity for lymph node and distant metastases; 68% of our patients presented with lymph node metastases, which is higher than the 46.6% and 49% reported by Osborn and Jayaprakesh et al.3, 10 An abundancy of tumor positive lymph nodes was observed in neck dissections. Furthermore, distant metastases were observed relatively short after primary diagnosis with a median time until distant metastases of only 16 months. Although distant metastases occurred mostly in the lungs, bones and lymph nodes, a wide variety of metastatic sites were seen, of which the 18% rate of brain metastasis was most notable. Local and regional recurrences were often accompanied by distant metastases. In this study, 67% of patients with a local or regional recurrence also had distant metastases, as shown in Figure 3 a, which corresponds to the 23–75% found in two other reports.11, 12 Therefore, in case of local or regional recurrences, we suggest thorough screening for distant metastases, as this may change treatment from curative to palliative intent. Moreover, despite locoregional control, distant metastases were encountered during follow‐up in 42 patients. Notably, 54% of patients with metastatic disease did not receive any form of systemic treatment. Possible explanations for this may be the extensiveness of disease, performance status, co‐morbidity and unfamiliarity of physician with the treatment of this rare tumor type, especially during the early years of the time period that we have studied. This may have influenced the overall survival of SDC patients in general.
Recently, a few cohort studies on patients with SDC were published. The cohort based on the National Cancer Database (NCDB) is the largest cohort of SDC patients with 495 patients (no median OS described for all patients) followed by the SEER database with 228 patients (median OS 79 months).3, 10 However, lack of pathology review has a risk of including patients with other diagnosis as the histological diagnosis of SDC is notoriously difficult. The median OS in this study (51 months) was comparable to a cohort study of 56 patients in a single institution in Korea (OS of 48 months).13 In a Japanese study with 141 SDC patients, where all tumors were pathologically reviewed, three‐year OS was 73% versus 57% in our series.11
In our series, 96% of tested tumors had a positive AR. This is comparable with other series of SDC patients reporting AR positivity up to 89%.4 AR positivity, in the presence of typical morphological features, is strongly suggestive for SDC, although other subtypes of SGC may express AR.14 ADT is an interesting therapeutic option for AR‐positive SDC.5 The results of first‐line ADT in our patients will be published in a separate article.15
We confirmed the presence of HER2 in 29% of tested cases, which is in line with the previously described HER2 amplification/expression in 27% (of 41), 32% (of 31) and 44% (of 32) SDC cases.6, 16, 17 We reported on four patients with HER2 IHC3+ with HER2 FISH‐negative tumor samples. Although this is unusual, it is known from comparative studies that this may occur.18 Recently, preliminary data for 45 patients with HER2‐positive advanced unresectable SGC (of the ductal subtype) treated with docetaxel and trastuzumab in a phase 2 trial showed promising results, that is, overall response rate of 69%, median PFS of 11.3 months and median OS of 38.0 months.19 These results seem to support treating HER2‐positive SDC patients with trastuzumab plus docetaxel.
Unfortunately, it was not possible to correlate clinical outcomes to other genetic alterations such as TP53 and PI3KCA. Future research may include characterization of genetic alterations and clinical outcomes of SDC patients.
Studies demonstrated a correlation between lymph node metastases and OS. There seems to be no consensus on the best way of categorizing lymph node metastases, whereby classifications comparing N0–1 versus N2–3, N2b‐c versus N0‐N2a or N0 versus any N+ disease are used.3, 12, 13, 20 We demonstrated in the univariate analysis that lymph node metastases is indeed a significant prognostic factor. However, in the multivariable analysis, the absolute number of tumor positive lymph nodes is a stronger prognostic factor than the N‐stage and LNR.21 We therefore suggest a categorizing system according to the absolute number of tumor positive lymph nodes (categorized as 0, 1–2, 3–15 and >15 lymph nodes), although this needs to be validated in other SDC cohorts.
One may argue if the patient with SDC of the lacrimal gland should be included in the analysis; however, the histopathological features in this particular patient included comedo‐type necrosis and AR positivity. Recently, another case of AR‐positive SDC of the lacrimal gland was described in literature.22
The main strengths of this study are the large number of patients data collected using a nationwide search strategy by PALGA, which covers 95–100% of all cancer patients, and especially the pathological review of SDC cases. A major advantage of our data, as compared to the larger, national NCDB and SEER databases, is the availability of individual patient data in our cohort. Extensive data on diagnosis, treatment, recurrence patterns and survival could be collected and has given valuable insights not only in the presentation but also in the course of the disease. We were therefore able to analyze meaningful prognostic factors in univariate and multivariable analyses. Limitations of this study are mainly due to its’ retrospective nature, the absence of FFPE blocks for AR and HER2 testing in some cases, and a not 100% coverage of all detailed information. Only tumors classified as SDC were included; therefore, patients that may have been wrongfully classified, that is, as another subcategory of the SGCs, may have been missed.
In conclusion, we presented 177 SDC patients with pathological review of the diagnosis. The median OS was just over 4 years, and the disease was characterized by a high initial lymph node involvement and development of a high rate of distant metastases. In the multivariable analysis, the absolute number of positive lymph nodes was the only significant prognostic factor for both poor OS and DMFS. We advocate the determination of AR and HER2 as this may have therapeutic consequences, although these are not prognostic factors. Given the high recurrence rate, future clinical research could encompass adjuvant treatment in high‐risk lymph node‐positive patients.
Acknowledgements
The authors thank M. Tomassen for his extensive help during pathological revision and construction of the TMAs. They thank P. J. Slootweg for his consultation on ambiguous cases of SDC. They also thank all participating pathologists and clinicians for their contribution to patient selection and inclusion.
| Pathologist | Affiliation |
|---|---|
| J. Meijer | Rijnstate Hospital, Arnhem, The Netherlands |
| J.E. van der Wal | Martini Hospital, Groningen, The Netherlands |
| L. Arensman | Meander MC, Amersfoort, The Netherlands |
| Tissue Bank, University Medical Center, Groningen, The Netherlands | |
| Stichting laboratorium Pathologie Oost‐Nederland |
This article was presented at the Annual Meeting of the American Society of Clinical Oncology (ASCO) Chicago, 2016.
Conflict of Interest: S.F. Oosting
Potential Financial Conflict: Research grant Pfizer, Research grant Novartis
References
- 1. Kleinsasser O, Klein HJ, Hubner G. Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 1968;192:100–5. [PubMed] [Google Scholar]
- 2. Seifert G, Brocheriou C, Cardesa A, et al. WHO International Histological Classification of Tumours. Tentative histological classification of salivary gland tumours. Pathol Res Pract 1990;186:555–81. [DOI] [PubMed] [Google Scholar]
- 3. Jayaprakash V, Merzianu M, Warren GW, et al. Survival rates and prognostic factors for infiltrating salivary duct carcinoma: analysis of 228 cases from the Surveillance, Epidemiology, and End Results database. Head Neck 2014;36:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nardi V, Sadow PM, Juric D, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment. Clin Cancer Res 2013;19:480–90. [DOI] [PubMed] [Google Scholar]
- 5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor‐positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol 2011;29:e473–6. [DOI] [PubMed] [Google Scholar]
- 6. Masubuchi T, Tada Y, Maruya S, et al. Clinicopathological significance of androgen receptor, HER2, Ki‐67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol 2015;20:35–44. [DOI] [PubMed] [Google Scholar]
- 7. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagoa T, Licitra L, Loening T, et al. Salivary duct carcinoma In: El‐Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO classification of head and neck tumours, 4th edn., vol. 9 World Health Organization (IARC), 2017. 173–174. [Google Scholar]
- 9. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 10. Osborn V, Givi B, Lee A, et al. Characterization, treatment and outcomes of salivary ductal carcinoma using the National Cancer Database. Oral Oncol 2017;71:41–6. [DOI] [PubMed] [Google Scholar]
- 11. Otsuka K, Imanishi Y, Tada Y, et al. Clinical outcomes and prognostic factors for salivary duct carcinoma: a multi‐institutional analysis of 141 patients. Ann Surg Oncol 2016;23:2038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston ML, Huang SH, Waldron JN, et al. Salivary duct carcinoma: treatment, outcomes, and patterns of failure. Head Neck 2016;38:E820–6. [DOI] [PubMed] [Google Scholar]
- 13. Roh JL, Lee JI, Choi SH, et al. Prognostic factors and oncologic outcomes of 56 salivary duct carcinoma patients in a single institution: high rate of systemic failure warrants targeted therapy. Oral Oncol 2014;50:e64–6. [DOI] [PubMed] [Google Scholar]
- 14. Locati LD, Perrone F, Losa M, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs). Oral Oncol 2009;45:986–90. [DOI] [PubMed] [Google Scholar]
- 15. Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor‐positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in The Netherlands. Head Neck. 2018;40:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang K, Russell JS, McDermott JD, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res 2016;22:6061–8. [DOI] [PubMed] [Google Scholar]
- 18. Eswarachary V, Mohammed IG, Jayanna PK, et al. HER2/neu testing in 432 consecutive breast cancer cases using FISH and IHC ‐ a comparative study. J Clin Diagn Res 2017;11:EC01–EC5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi H, Masubuchi T, Fushimi C, et al. Trastuzumab and docetaxel for HER2‐positive unresectable salivary gland carcinoma: Updated results of a phase II trial. 2016 International Conference on Head and Neck Cancer Abstract S207 Presented July 18, 2016.
- 20. Gilbert MR, Sharma A, Schmitt NC, et al. A 20‐year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg 2016;142:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong HR, Roh JL, Cho KJ, et al. Prognostic value of lymph node density in high‐grade salivary gland cancers. J Surg Oncol 2015;111:784–9. [DOI] [PubMed] [Google Scholar]
- 22. Rahimi S, Lambiase A, Brennan PA, et al. An androgen receptor‐positive carcinoma of the lacrimal drainage system resembling salivary duct carcinoma: case report and review of the literature. Appl Immunohistochem Mol Morphol 2016;24:e69–71. [DOI] [PubMed] [Google Scholar]


