Abstract
Background and purpose
Treatments to facilitate recovery after traumatic brain injury (TBI) are urgently needed. We conducted a 9‐month pilot, randomized placebo‐controlled clinical trial to examine the safety and potential effects of the herbal supplement MLC901 (NeuroAiD II™) on cognitive functioning following TBI.
Methods
Adults aged 18–65 years at 1–12 months after mild or moderate TBI were randomized to receive MLC901 (0.8 g capsules 3 times daily) or placebo for 6 months. The primary outcome was cognitive functioning as assessed by the CNS Vital Signs online neuropsychological test. Secondary outcomes included the Cognitive Failures Questionnaire, the Rivermead Post‐concussion Symptom Questionnaire (neurobehavioral sequelae), Quality of Life after Brain Injury, Hospital Anxiety and Depression Scale, Modified Fatigue Impact Scale and extended Glasgow Outcome Scale (physical disability). Assessments were completed at baseline and at 3‐, 6‐ and 9‐month follow‐up. Linear mixed‐effects models were conducted, with the primary outcome time‐point of 6 months.
Results
A total of 78 participants [mean age 37.5 ± 14.8 years, 39 (50%) female] were included in the analysis. Baseline variables were similar between groups (treatment group, n = 36; control group, n = 42). Linear mixed‐effects models controlling for time, group allocation, repeated measurements, adherence and baseline assessment scores revealed significant improvements in complex attention (P = 0.04, d = 0.6) and executive functioning (P = 0.04, d = 0.4) at 6 months in the MLC901 group compared with controls. There were no significant differences between the groups for neurobehavioral sequelae, mood, fatigue, physical disability or overall quality of life at 6 months. No serious adverse events were reported.
Conclusions
MLC901 was safe and well tolerated post‐TBI. This study provided Class I/II evidence that, for patients with mild to moderate TBI, 6 months of MLC901 improved cognitive functioning.
Keywords: cognitive deficits, concussion, MLC901, NeuroAiD II™, quality of life, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of disability and death in young adults 1, 2 and has a significant impact not only on the individual, but also on their family, friends and society 3, 4, 5. TBI is caused by external forces directly injuring the brain and/or as a result of rotational forces as the brain moves within the skull. Persistent cognitive deficits have been reported to affect 15–40% of adults post‐TBI 6, 7. Most commonly, longer‐term deficits are observed in the specific cognitive domains of complex attention (multi‐tasking), executive functioning (planning and decision making) and cognitive flexibility (switching quickly between tasks) 6, 8. People can also experience neurobehavioral sequelae (including headaches, dizziness and noise sensitivity), anxiety, depression and fatigue 7, 9. These deficits can profoundly impact a person's day‐to‐day functioning, often affecting their ability to return to work, social relationships and quality of life 10, 11.
Following TBI, the brain has the ability to restore and regenerate damaged cells and neuronal connections. However, following injury, disturbances in the balance between antioxidant defenses and the production of toxic reactive oxygen species (free radicals) known as oxidative stress can occur, causing secondary injury and hindering recovery. Supplementing the body's natural supply of antioxidants may help to reduce oxidative damage caused by reactive oxygen species and facilitate recovery 12. Indeed, emerging evidence suggests that antioxidant therapies facilitated stabilization of edema, improved cognitive functioning, neurobehavioral sequelae and decreased mortality post‐TBI 13. MLC901 (NeuroAiD II™) is a traditional Chinese medicine that may help to facilitate the restoration of neuronal circuits through its antioxidant properties, promotion of cell proliferation and stimulation of axonal and dendritic neuronal circuits after TBI 14, 15. In rodent models, MLC901 has been shown to both prevent cell death and to stimulate the generation of new neural cells, connections and pathways 14, 15. Rodents given MLC901 after an ischemic injury showed improved survival, neurological recovery, decreased neurodegeneration and improved cognitive functioning 14, 15. This pilot study aimed to test the safety and effects of MLC901 on cognitive functioning in adult humans after mild to moderate TBI, in addition to secondary outcomes of neurobehavioral sequelae, mood, fatigue, physical disability and quality of life. We hypothesized that MLC901 is safe and well tolerated by people with mild to moderate TBI and has a positive effect on cognitive functioning.
Methods
Study design
This was a pilot, double‐blind, placebo‐controlled, randomized clinical trial known as BRAin Injury and Neuroaid Supplementation (BRAINS) performed in the Auckland and Hamilton regions of New Zealand.
Standard protocol approvals, registrations and patient consents
All study procedures were conducted in accordance with good clinical practice guidelines and were approved by the Central Regional Ethics Committee (13/CEN/175) and the Auckland University of Technology Ethics Committee (14/28). Approval was obtained from the New Zealand Medicines and Medical Devices Safety Authority (MEDSAFE, 13/SCOTT/100). Written informed consent was obtained from all participants. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12613000711718).
Participants and procedure
Clinicians based in local hospitals or concussion clinics were asked to identify potential participants and to provide them with brief information about the study. Additionally, advertisements were placed in medical facilities and the local press to facilitate self‐referrals. Study processes are outlined in Fig. S1.
Inclusion criteria were: (i) aged 18–65 years; (ii) 1–12 months after mild or moderate TBI and (iii) experiencing cognitive functioning difficulties as indicated by a score of >30 on the Cognitive Failures Questionnaire 16. Exclusion criteria were: (i) coexisting injury or medical condition, which is severe or unstable, that could adversely impact on the outcome measures, such as spinal cord injury or other severe conditions with life expectancy of <5 years; (ii) severe TBI defined by Glasgow Coma Scale score of ≤8; (iii) current participation in another clinical trial; (iv) dependent on others for everyday activities before the onset of the brain injury; (v) pregnant or breast feeding; (vi) not fluent in English or aphasia/dysphasia that precludes ability to complete the assessments; (vii) known allergy to any of the components of MLC901 or (viii) unknown date of injury. A member of the research team contacted each potential participant via telephone to screen for eligibility. TBI was defined according to World Health Organization criteria as an injury to the brain resulting from external physical forces 17. TBI was classified as mild based on a Glasgow Coma Scale score of 13–15 and as moderate based on a score of 9–12. Any cases where a Glasgow Coma Scale score was not recorded were classified as mild and unclear cases of TBI were reviewed by a neurologist 17. Recruitment stopped at 79 participants following identification of significant treatment effects in the interim analysis.
Randomization
Eligible participants were randomized to receive either the MLC901 supplement or placebo using 1:1 minimization randomization 18. Participants were stratified by study locality (Hamilton/Auckland), time since injury (1–2 months/3–12 months) and sex. The manufacturer of the supplement and placebo capsules held the group allocation codes until data analysis was completed so that the research team remained blind to group allocation.
Experimental intervention and placebo
MLC901 contains nine herbal components: Radix astragali, Radix salvia miltiorrhizae, Radix paeoniae rubra, Rhizoma chuanxiong, Radix angelicae sinesis, Carthamus tinctorius, Prunus persica, Radix polygalae and Rhizoma acori tatarinowii. The dose was two capsules (0.4 g/capsule) taken orally three times per day for 6 months. Each placebo capsule contained dextrin and magnesium stearate and they were visually indistinguishable from the active pills in terms of the shape/smell and color.
At baseline, 1 and 3 months, participants were given their supply of capsules in blister cards by the researcher. Packaging was visually identical for both groups except for being marked A or B. Participants were advised to take two capsules three times per day. All participants continued to receive standard medical care, with any changes in medical treatment being recorded. If a participant missed one dose, they were advised to take that dose as soon as they remembered. If more than one dose was missed, the participant was advised to take one dose and to continue treatment as usual. Side effects were monitored via telephone calls to participants each week for the first 2 weeks, as well as at each follow‐up visit. After the 9‐month assessment, participants in the control group were offered a 1‐month supply of the MLC901 supplement by a researcher independent of the study team.
Outcome assessment
Following eligibility assessment, an in‐person meeting was arranged at the participant's home or other suitable location. Using a repeated‐measures design, all measures were administered at baseline, 1, 3, 6 and 9 months to explore the duration and extent of any observed effects.
Primary outcome
Cognitive functioning was assessed by an online neuropsychological test (CNS Vital Signs) 19. Speed and accuracy on six tests were used to calculate the level of functioning across eight cognitive domains, including verbal and visual memory, complex attention, psychomotor speed, cognitive flexibility, processing speed, executive function and reaction time. Further details of tests and scoring are outlined in Table S2. Raw scores were transformed to standard scores, with a mean of 100 and SD of 10, based on normative data to account for age and gender effects using an integrated algorithm. Scores <90 indicate below average levels of functioning, with higher scores indicative of better cognitive functioning. CNS Vital Signs has demonstrated good discriminant and concurrent validity with conventional neuropsychological tests 19 and is sensitive to impairments across TBI severity, with evidence of good test–retest reliability 20, 21.
Secondary outcomes
The Cognitive Failures Questionnaire 22, 23 was included as a self‐report measure of attentional lapses. Participants are asked to rate the frequency with which they experience 25 common perception, memory and motor lapses in everyday life, with higher scores indicative of greater attentional lapses. This measure has demonstrated good internal consistency and test–retest reliability 23.
The Rivermead Post‐Concussion Symptoms Questionnaire 24, 25 assesses neurobehavioral sequelae and consists of two subscales including the RPQ3, which includes symptoms of headaches, dizziness and nausea, and the RPQ13 comprising 13 other common symptoms such as restlessness, noise and light sensitivity, sleep disturbance, blurred vision and balance difficulties. Higher scores indicate greater frequency and severity of symptoms. The two subscales (RPQ3 and RPQ13) have demonstrated good external construct validity and test–retest reliability 24.
Mood was assessed by the Hospital Anxiety and Depression Scale 26. Participants were asked to rate the extent to which they have been feeling the way described by each item in the past week, yielding separate subscale scores for anxiety and depression. The measure has demonstrated good test–retest reliability 27 and good sensitivity and specificity 28.
Perceived levels of physical, cognitive and psychosocial aspects of fatigue were assessed using the Modified Fatigue Impact Scale 29. Although initially developed in multiple sclerosis, the measure has been validated in mild to moderate brain injury 30. Participants were asked to read each statement and to indicate how often fatigue has affected them in each way during the past 4 weeks. A total score is calculated, with higher scores indicative of higher fatigue. This measure has demonstrated excellent test–retest reliability, sensitivity to change and predictive validity 31, 32.
The Quality of Life after Brain Injury measure 33 contains 37 items assessing perceived satisfaction with cognitive, self, autonomy in daily life, social, emotional and physical domains of quality of life. Average total subscale scores are converted to a 0–100 scale by subtracting 1 from the mean and multiplying by 25. Higher scores indicate better quality of life. The measure has demonstrated good internal consistency for the subscale and good test–retest reliability 34.
Physical disability was assessed using the extended Glasgow Outcome Scale 35. This measure utilizes a structured interview exploring levels of independence. Responses ranged between 1 (dead) and 8 (upper levels of good recovery). The measure has demonstrated good reliability and validity 35.
Statistical analysis
Tests of difference determined if there were any group differences on sociodemographic and clinical variables at baseline. Results expressed as means and SDs across measures at each time‐point reveal change over time. Safety and tolerability were assessed by the frequency and nature of any potential side effects recorded. Levels of adherence to the treatment regime were determined based on the number of capsules not taken. All further analyses were conducted by intention to treat.
Repeated‐measures analyses using mixed linear regression models adjusting for age, sex, severity of injury, baseline scores, adherence, group and natural recovery over time determined group differences due to group allocation at the primary outcome time‐point of 6 months. The participant was used to represent the random effect. Model selection was undertaken with each outcome using standard selection heuristics. Covariates such as education, marital status and occupation were selected based on improving the overall efficiency of the model by pooling the SD observed for the outcome in each arm (MLC901 and placebo) creating an F‐value. If the mean difference for an outcome between each arm exceeded this F‐value then it was included as a covariate in the model. Trajectories over time were displayed using smoothed spline from a linear model. Effect sizes were calculated using Cohen's d, with small effects classified as d = 0.2–0.5, moderate as 0.6–1.19 and large as 1.2–1.99) 36.
Results
A total of 79 participants were enrolled and randomized into the study. One participant was advised by a clinician to withdraw from the study due to high stress levels. As stress is likely to influence cognitive functioning 37, data collected for this participant were excluded from the analysis. There was no statistical difference in the number of withdrawals between groups (χ 2=0.19, P = 0.66). Post hoc power calculations revealed that, with 78 participants, the pilot trial was powered at 80% to observe an effect size of 0.6.
There were no significant differences between the two groups with regard to demographic and other baseline characteristics as shown in Table 1. The majority of TBIs were classified as being mild, with n = 2 (3%) injuries classified as moderate in severity. Mean time since injury to enrolment in the study was 148 days (equivalent to 5 months).
Table 1.
Baseline demographic and medical characteristics of the study participants
| MLC901 group (n = 36) | Control group (n = 42) | Test of difference significance | |
|---|---|---|---|
| Males [n (%)] | 17 (47.22) | 22 (52.38) | P = 0.82 |
| Age (years) [mean (SD)] | 38.58 (14.12) | 38.40 (15.74) | P = 0.96 |
| Time since injury (days) [median (IQR)] | 98.00 (196.75) | 94.5 (160.0) | P = 0.59 |
| Ethnicity | |||
| New Zealand European [n (%)] | 25 (69.44) | 24 (57.14) | P = 0.20 |
| Highest level of education | |||
| Tertiary education or above [n (%)] | 12 (33.33) | 16 (38.10) | P = 0.81 |
| Employment status | |||
| Employed [n (%)] | 19 (52.78) | 29 (69.05) | P = 0.17 |
| Marital status | |||
| Married or living with partner [n (%)] | 17 (47.22) | 15 (35.71) | P = 0.36 |
| TBI severity assessed | |||
| Confirmed severity by GCS score | 14 (38.89) | 12 (28.57) | P = 0.34 |
| Unclassified severity | 22 (61.11) | 30 (71.43) | |
| Mechanism of injury [n (%)] | |||
| Motor vehicle accident | 6 (16.67) | 7 (16.67) | P = 0.94 |
| Assault | 12 (33.33) | 13 (30.95) | |
| Fall | 14 (38.89) | 15 (35.71) | |
| Hit by object/other | 4 (11.11) | 7 (16.67) | |
| Other injuries sustained | |||
| Yes [n (%)] | 19 (52.78) | 18 (42.86) | P = 0.50 |
| Prior TBI | |||
| Yes [n (%)] | 17 (47.22) | 27 (64.29) | P = 0.19 |
| Baseline cognitive functioning level | |||
| Below average | 21 (58.33) | 22 (52.38%) | P = 0.60 |
GCS, Glasgow Coma Scale; IQR, interquartile range; TBI, traumatic brain injury.
Fifty participants (64%) completed the full 9‐month protocol (Fig. 1). Eight (10%) participants across the two groups reported side effects during the course of the study. In the intervention group, one participant reported headache, one reported a sore tongue and one reported experiencing itchiness. In the control group, adverse events included difficulty sleeping, headache, itchiness and upset stomach. One further participant in the control group reported experiencing blood in their urine, which, following medical attention, was found to be due to a secondary medical condition. Overall adherence in taking the capsules varied between 84% at 1 month, 89% at 3 months and 85% at 6 months.
Figure 1.
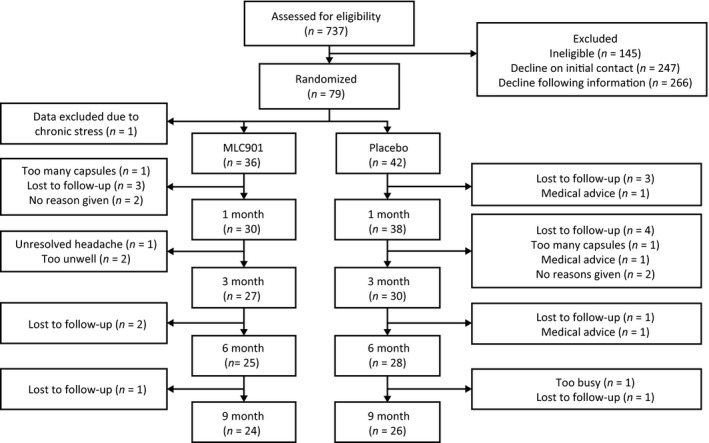
Consort diagram of participants.
Primary outcome
Means and SDs across time‐points for all cognitive functioning domains are shown in Table S1. The results of significant regression models are shown in Table 2. Participants randomized to receive MLC901 had significant improvements in complex attention and executive functioning on CNS Vital Signs at 6 months in comparison to controls, with group allocation being independently predictive of complex attention. There was a mean difference of 11.88 for complex attention (>1 SD) and 7.16 for executive functioning, suggesting clinically meaningful improvements at 6 months, with moderate to small effect sizes (d = 0.6 and d = 0.4 respectively). There were no significant differences between the groups on any other domains of cognitive functioning at 6 months. Using the mean differences observed here (α = 0.05) with 80% power, a total sample of 174 participants (87 per group) would be required to determine efficacy of MLC901 on complex attention as part of a full trial. Sensitivity analysis excluding the two cases of moderate TBI from the regression models did not significantly change the results. Group allocation remained a significant independent predictor of complex attention at 6 months (t = −2.14, P = 0.0371).
Table 2.
Significant linear regression models of cognitive outcomes at 6 months
| Estimate | Standard error | P | |
|---|---|---|---|
| Complex attention | |||
| Intercept | 91.14 | 8.36 | 0.0000 |
| Education | 13.38 | 5.92 | 0.0238 |
| Occupation (manager) | |||
| Community worker | −22.04 | 9.59 | 0.0214 |
| Laborer | −9.48 | 9.73 | 0.3320 |
| Technician | −4.88 | 6.79 | 0.4715 |
| Machine worker | −15.58 | 10.02 | 0.1188 |
| Sales | −7.97 | 11.63 | 0.4902 |
| Marital status (in a relationship) | 10.59 | 5.29 | 0.0455 |
| Time since intervention | |||
| 1 month | 7.64 | 1.81 | 0.0000 |
| 3 months | 7.58 | 2.09 | 0.0003 |
| 6 months | 8.58 | 2.47 | 0.0005 |
| Group allocation | −10.13 | 4.90 | 0.0385 |
| Executive functioning | |||
| Intercept | 102.40 | 8.55 | 0.0000 |
| Sex (male) | −8.14 | 4.24 | 0.0549 |
| Education (tertiary) | 11.27 | 4.07 | 0.0056 |
| Current employment (employed) | −6.67 | 4.21 | 0.1141 |
| Mechanism of injury (assault) | |||
| Fall | −12.33 | 4.79 | 0.0102 |
| Hit by object | −7.99 | 6.28 | 0.2041 |
| Traffic incident | −12.78 | 6.26 | 0.0414 |
| Time since intervention | |||
| 1 month | 9.73 | 1.66 | 0.0000 |
| 3 months | 8.90 | 1.79 | 0.0000 |
| 6 months | 12.74 | 1.99 | 0.0000 |
| Group allocation | −10.13 | 4.90 | 0.1527 |
| Quality of life (cognition domain) | |||
| Intercept | 44.22 | 9.33 | 0.0000 |
| Ethnicity (European) | |||
| Maori | −10.59 | 6.24 | 0.0891 |
| Asian/other | −7.77 | 9.14 | 0.3953 |
| Occupation (manager) | −22.04 | 9.59 | 0.3320 |
| Community worker | −9.48 | 9.73 | 0.3320 |
| Laborer | −4.88 | 6.79 | 0.4715 |
| Technician | −15.58 | 10.02 | 0.1188 |
| Machine worker | −7.97 | 11.63 | 0.4902 |
| Sales | 10.59 | 5.29 | 0.0455 |
| Marital status (in a relationship) | −10.53 | 6.15 | 0.0873 |
| Time since intervention | |||
| 1 month | 10.48 | 1.99 | 0.0000 |
| 3 months | 7.69 | 2.23 | 0.0006 |
| 6 months | 11.08 | 2.57 | 0.0000 |
| Group allocation | 14.31 | 5.61 | 0.0108 |
Trajectories over time for the cognitive domains of complex attention and executive functioning are shown in Figs 2 and 3. For complex attention, improvements were observed in the MLC901 group that were cumulative over time, whereas the control group remained relatively stable. For executive functioning, improvements were observed for both groups with improvement accelerated in the MLC901 group. Improvements in cognitive functioning slowed between 6 and 9 months following cessation of treatment.
Figure 2.
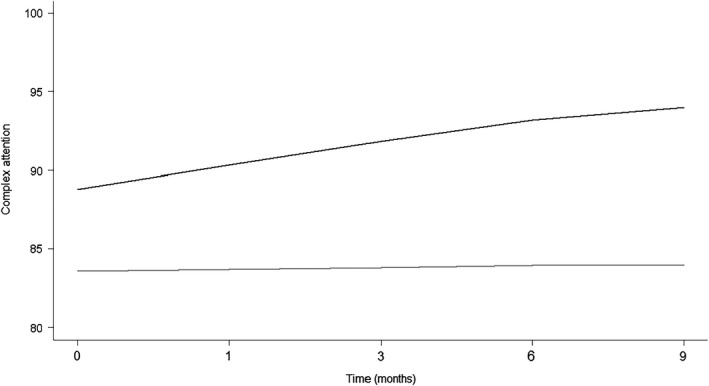
Trajectories of complex attention over time between groups. Grey line, control; black line, MLC901.
Figure 3.
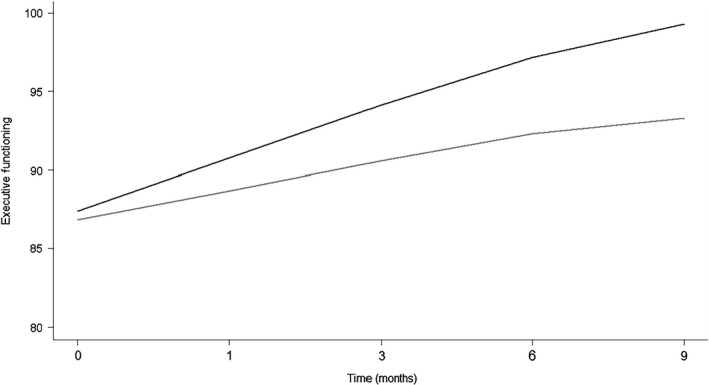
Trajectories of executive functioning over time between groups. Grey line, control; black line, MLC901.
Secondary outcomes
Both groups showed improvements over time across all secondary outcomes. There were no significant differences between the groups on the neurobehavioral sequelae of mood, fatigue and overall quality of life at the P < 0.05 level across time‐points. As shown in Table 2, participants receiving MLC901 showed significant improvements on the cognitive domain of quality of life with a mean difference of 1.62; however, the effect size was considered small (d = 0.1).
Discussion
The findings provide initial evidence that MLC901 may help to facilitate neural recovery on complex attention and executive functioning following TBI, with small to moderate effect sizes. Few side effects were reported by participants, supporting the safety and tolerability profile of MLC901 post‐TBI. There were no significant differences between the groups at follow‐up for self‐reported cognitive failures, neurobehavioral sequelae, fatigue, mood, physical disability or overall quality of life.
Complex attention and executive functioning are the most common areas of cognitive functioning to be affected post‐TBI 6, 8. Significant improvements on these two domains suggest potential clinical utility of MLC901 post‐TBI. Trends in improvement in complex attention and executive functioning in the MLC901 group were observed at 3 months, becoming significant at 6 months. This suggests a cumulative and consistent treatment effect, with rate of improvement declining following cessation of treatment. The findings of improved cognitive functioning following treatment with MLC901 support the results of experimental trials of MLC901 on cognitive functioning in rodents 14, 15. The impact of MLC901 on cognitive functioning was strengthened by significant differences observed in both the neuropsychological test and the self‐reported cognitive domain of quality of life. The lack of a significant difference on the self‐reported measure of attentional lapses (Cognitive Failures Questionnaire) may reflect the focus of this measure on memory and motor lapses rather than complex attention, executive function or impact of these cognitive difficulties on everyday life.
There was no significant difference in post‐concussion symptoms, mood, fatigue, physical disability or other domains of quality of life. The lack of significance for mood and disability is likely to reflect that at baseline, scores for both anxiety and depression were in the normal to mild range. Levels of physical functioning indicating that people were functioning independently made it unlikely that any treatment effect would be observed. Improvements in neurobehavioral sequelae have been observed using other antioxidant treatments although research remains limited 13. It may be the case that changes have been observed for specific symptoms but are obscured by use of the total symptom score. Analysis by symptom rather than by total symptom severity may be worthy of further exploration.
The brain has been demonstrated to be highly plastic following a TBI with continued improvements observed over the year following injury 6. Indeed, a key predictor of outcome across both groups was time, indicative of a process of natural recovery. Group allocation was an independent predictor of complex attention, suggesting that MLC901 further facilitates the natural recovery process. As effects were sustained at 9 months following cessation of treatment, this supports an ongoing treatment effect; However, it remains unclear how long improvements may be sustained. Participants were recruited within 12 months post‐injury as it is more likely that the anti‐inflammatory and antioxidative effects of MLC901 will affect recovery in the early phase post‐injury and evidence supports efficacy up to 12 months post‐injury 38. However, many participants did not enter the trial until 5–6 months post‐injury and it remains unclear if earlier administration of MLC901 may further increase the treatment effect or whether there are treatment effects for cognitive difficulties sustained many years following TBI.
Recruitment of participants proved challenging with many participants citing the need to take capsules three times per day over a 6‐month period as a barrier to participation, as well as a reason for withdrawal. Adherence was also reported to be challenging, particularly within the context of cognitive difficulties experienced. Condensing the dosages into once or twice daily administration may facilitate recruitment and adherence 39. Additionally, aids to support memory could be integrated into the protocol to support adherence. For example, adherence has been found to be increased when medications are taken at the same time and integrated into daily routines 39.
Only 3% of participants had sustained a moderate TBI and findings need to be interpreted with caution for this population. A limitation of the study is that safety and effects of MLC901 remain unknown for those who have experienced a severe TBI. Although few adverse events were reported, one person in the intervention did withdraw from the study due to persistent headaches. It is unknown whether or not this was directly attributable to the MLC901 and headaches should be specifically monitored as part of a full trial. The findings suggest that MLC901 is safe and well tolerated and may help to improve complex attention and executive functioning following mild to moderate TBI. A phase III randomized controlled trial is required to determine effectiveness.
Disclosure of conflicts of interest
The study was funded by Moleac Pte Ltd, Singapore, who manufacture the MLC901 supplement. The study was designed and conducted independently by the research team. The authors declare no financial or other conflicts of interest.
Supporting information
Figure S1. Outline of study processes.
Table S1. Means and SDs for the outcome measures across time‐points.
Table S2. Test descriptions and computation of domain scores.
Acknowledgements
We would like to acknowledge the financial support of Moleac Pte Ltd, Singapore. We thank the participants for their time and interest in taking part in this study and the service providers for their support with patient recruitment and discussion of the implications of the study findings.
References
- 1. Langlois JA, Rutland‐Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006; 21: 375–378. [DOI] [PubMed] [Google Scholar]
- 2. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neurorehabilitation 2007; 22: 341–353. [PubMed] [Google Scholar]
- 3. Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry 2005; 18: 301–317. [DOI] [PubMed] [Google Scholar]
- 4. Zumstein MA, Moser M, Mottini M, et al Long‐term outcome in patients with mild traumatic brain injury: a prospective observational study. J Trauma 2011; 71: 120–127. [DOI] [PubMed] [Google Scholar]
- 5. Anderson V, Brown S, Newitt H, Hoile H. Educational, vocational, psychosocial, and quality‐of‐life outcomes for adult survivors of childhood traumatic brain injury. J Head Trauma Rehabil 2009; 24: 303–312. [DOI] [PubMed] [Google Scholar]
- 6. Barker‐Collo S, Jones K, Theadom A, et al Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: A population‐based New Zealand study. Brain Inj 2015; 29: 1604–1616. [DOI] [PubMed] [Google Scholar]
- 7. McMahon P, Hricik A, Yue JK, et al Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK‐TBI study. J Neurotrauma 2014; 31: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hume P, Theadom A, Lewis GN, et al A comparison of cognitive function in former rugby union players compared with former non‐contact‐sport players and the impact of concussion history. Sports Med 2017; 47(6): 1209–1220. [DOI] [PubMed] [Google Scholar]
- 9. Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2003; 84: 1449–1457. [DOI] [PubMed] [Google Scholar]
- 10. Gilworth G, Eyres S, Carey A, Bhakta BB, Tennant A. Working with a brain injury: personal experiences of returning to work following a mild or moderate brain injury. J Rehabil Med 2008; 40: 334–339. [DOI] [PubMed] [Google Scholar]
- 11. Petchprapai N, Winkelman C. Mild traumatic brain injury: determinants and subsequent quality of life. A review of the literature. J Neurosci Nurs 2007; 39: 260–272. [PubMed] [Google Scholar]
- 12. Venegoni W, Shen Q, Thimmesch AR, et al The use of antioxidants in the treatment of traumatic brain injury. J Adv Nurs 2017; 73: 1331–1338. [DOI] [PubMed] [Google Scholar]
- 13. Shen Q, Hiebert JB, Hartwell J, Thimmesch AR, Pierce JD. Systematic review of traumatic brain injury and the impact of antioxidant therapy on clinical outcomes. Worldviews Evid Based Nurs 2016; 13: 380–389. [DOI] [PubMed] [Google Scholar]
- 14. Heurteaux C, Gandin C, Borsotto M, et al Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo . Neuropharmacology 2010; 58: 987–1001. [DOI] [PubMed] [Google Scholar]
- 15. Quintard H, Borsotto M, Veyssiere J, et al MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology 2011; 61: 622–631. [DOI] [PubMed] [Google Scholar]
- 16. Bejot Y, Caillier M, Ben Salem D, et al Ischemic stroke subtypes and associated risk factors: a French population‐based study. J Neurol Neurosurg Psychiatry 2008; 79: 1344–1348. [DOI] [PubMed] [Google Scholar]
- 17. Carroll LJ, Cassidy JD, Holm L, et al Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; 43(Suppl.): 113–125. [DOI] [PubMed] [Google Scholar]
- 18. Pocock SJ. Clinical Trials. A Practical Approach. Chichester: John Wiley, 1983. [Google Scholar]
- 19. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clinical Neuropsychol 2006; 21: 623–643. [DOI] [PubMed] [Google Scholar]
- 20. Gualtieri CT, Johnson LG, Benedict KB. Psychometric and clinical properties of a new, computerized neurocognitive asessment battery. Bal Harbor, FL: American Neuropsychiatric Association Annual Meeting, 2004. [Google Scholar]
- 21. Gualtieri CT, Johnson LG. A computerized test battery sensitive to mild and severe brain injury. Medscape J Med 2008; 10: 90. [PMC free article] [PubMed] [Google Scholar]
- 22. Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates.Br J Clin Psychol 1982; 21: 1–16. [DOI] [PubMed] [Google Scholar]
- 23. Wallace JC, Kass SJ, Stanny CJ. The Cognitive Failures Questionnaire revisited: dimensions and correlates. J Gen Psychol 2002; 129: 238–256. [DOI] [PubMed] [Google Scholar]
- 24. Eyres S, Carey A, Gilworth G, Neumann V, Tennant A. Construct validity and reliability of the Rivermead post‐concussion symptoms questionnaire. Clin Rehabil 2005; 19: 878–887. [DOI] [PubMed] [Google Scholar]
- 25. King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead post‐concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995; 242: 587–592. [DOI] [PubMed] [Google Scholar]
- 26. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 27. Herrmann C. International experiences with the Hospital Anxiety and Depression Scale – a review of validation data and clinical results. J Psychosom Res 1997; 42: 17–41. [DOI] [PubMed] [Google Scholar]
- 28. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 29. Fisk JD, Ritvo PG, Ross L, et al Measuring the functional impact of fatigue: initial validation of the Fatigue Impact Scale. Clin Infect Dis 1994; 18: S79–S83. [DOI] [PubMed] [Google Scholar]
- 30. Schiehser DM, Delano‐Wood L, Jak AJ, et al Validation of the Modified Fatigue Impact Scale in mild to moderate traumatic brain injury. J Head Trauma Rehabil 2015; 30: 116–121. [DOI] [PubMed] [Google Scholar]
- 31. Kos D, Kerckhofs E, Carrea I, et al Evaluation of the Modified Fatigue Impact Scale in four different European countries. Mult Scler 2005; 11: 76–80. [DOI] [PubMed] [Google Scholar]
- 32. Sendroy‐Terrill M, Whiteneck GG, Brooks CA. Aging with traumatic brain injury: cross‐sectional follow‐up of people receiving inpatient rehabilitation over more than 3 decades. Arch Phys Med Rehabil 2010; 91: 489–497. [DOI] [PubMed] [Google Scholar]
- 33. von Steinbüchel N, Wilson L, Gibbons H, et al Quality of Life after Brain Injury (QOLIBRI): scale validity and correlates of quality of life. J Neurotrauma 2010; 27: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 34. Hawthorne G, Kaye AH, Gruen R, Houseman D, Bauer I. Traumatic brain injury and quality of life: initial Australian validation of the QOLIBRI. J Clin Neurosci 2011; 18: 197–202. [DOI] [PubMed] [Google Scholar]
- 35. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998; 15: 573–585. [DOI] [PubMed] [Google Scholar]
- 36. Hopkins WGA. A scale of magnitudes for effect statistics In: A New View of Statistics. Auckland: W.G. Hopkins, 2002. [Google Scholar]
- 37. Österberg K, Karison B, Hansen AM. Cognitive performance in patients with burnout, in relation to diurnal salivary cortisol. Stress 2009; 2: 70–81. [DOI] [PubMed] [Google Scholar]
- 38. Rigg JL, Elovic EP, Greenwald BD. A review of the effectiveness of antioxidant therapy to reduce neuronal damage in acute traumatic brain injury. J Head Trauma Rehabil 2005; 20: 389–391. [DOI] [PubMed] [Google Scholar]
- 39. Atreja A, Bellam N, Levy SR. Strategies to enhance patient adherence: making it simple. Medscape Gen Med 2005; 7: 4. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Outline of study processes.
Table S1. Means and SDs for the outcome measures across time‐points.
Table S2. Test descriptions and computation of domain scores.


