ABSTRACT
BACKGROUND AND PURPOSE
Brain death determination (BDD) is primarily a clinical diagnosis, where death is defined as the permanent loss of brainstem function. In scenarios where clinical examinations are inaccurate, ancillary imaging tests are required. The choice of ancillary imaging test is variable, but the common denominator for all of them is to establish a lack of cerebral blood flow. The purpose of this study was to compare the diagnostic accuracy and interrater reliability of different ancillary imaging tests used for BDD.
METHODS
Archival data were retrospectively analyzed for all patients who underwent any ancillary imaging test for BDD at our institution. The results of ancillary imaging tests were compared with, the reference standard, the clinical checklist for declaration of brain death. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of different ancillary imaging tests for BDD were performed. Interobserver agreement between two observers was measured using kappa statistics for each of the imaging modalities.
RESULTS
A total of 74 patients underwent 41 computer tomography perfusion (CTP), 54 CT angiogram, 15 radionuclide scans, 1 cerebral angiogram, 3 magnetic resonance imaging, and 71 nonenhanced CT (NECT) head for BDD. All ancillary tests (except NECT head) showed 100% specificity and PPV. CTP had the highest sensitivity and NPV. All ancillary imaging tests demonstrated very high interrater reliability.
CONCLUSIONS
The uses of ancillary imaging tests for BDD are increasing. Within this study's limitations, CTP followed by radionuclide scan were found to be the most accurate and reliable ancillary imaging test for BDD.
Keywords: Imaging, brain death, CT perfusion
Introduction
Brain death is primarily a clinical diagnosis. In certain clinical scenario, when the clinical diagnosis of brain death is not straightforward, an ancillary imaging test may be required.1, 2 Clinical determination of brain death requires establishment of irreversible coma etiology, absent brainstem reflexes, and apnea.3, 4, 5 In Canada, the minimum clinical criteria used for determination of brain death include: an established etiology capable of causing neurological death, in the absence of reversible conditions; deep unresponsive coma with bilateral absence of motor responses, excluding spinal reflexes; absent brainstem reflexes, including absent gag and cough reflexes, and bilateral absence of corneal responses, pupillary responses to light, and vestibulo‐ocular responses; absent respiratory effort based on apnea testing; and absence of confounding factors.6
Numerous confounding variables can affect whether the clinician can perform the complete clinical brain death exam, and more importantly whether the clinical results are valid without ambiguity. Confounding factors include: unresuscitated shock; hypothermia (defined as a core temperature <34 ⁰C); severe metabolic disorders or abnormalities with glucose, phosphate, calcium, and magnesium; liver and renal dysfunction that may be capable of causing a reversible coma; peripheral nerve or muscle dysfunction, or neuromuscular blockade; significant drug intoxications (eg, alcohol, barbiturates, sedatives, and hypnotics); complex spinal reflexes; false‐positive triggering of the ventilator; invalid apnea testing such as with high carbon dioxide retainers; and high cervical cord injury or trauma to the eyes, middle, or inner ears.2, 3, 6, 7, 8
Brain death confirmation with ancillary testing is used for timely resolution for all parties involved (eg, patients, families, and clinicians).7 Establishing an early and accurate brain death using a neuroimaging ancillary test decreases the risk of incorrect diagnosis, improves organ transplantation, and saves some of the precious health care resources. Confirmation of brain death with ancillary testing and the particular test used is highly variable depending on individual institution's capabilities, and region or country of practice.8, 9 The common denominator that must be established with the more commonly used ancillary tests for confirmation of brain death is a lack of whole brain cerebral blood flow (CBF). Accuracy and reliability of ancillary imaging test for brain death determination (BDD) need to be very high, if not absolute. The purpose of this study was to compare diagnostic accuracy and interrater reliability of different ancillary imaging tests for the determination of brain death.
Methods
Archival data were retrospectively analyzed at our institution for all patients who had ancillary imaging done for BDD studies. The study was approved by the institutional research ethics board (study number—1020480). All study procedures were performed in compliance with ethical standards of the institutional research committee and formal consent was waived for this type of study.
Patient Selection
Consecutive patients who had any ancillary imaging test request for determination of brain death (including nonenhanced computer tomography [NECT], CT perfusion [CTP], CT angiogram [CTA], nuclear medicine perfusion scan, digital subtraction angiogram [DSA], and magnetic resonance imaging [MRI]) were included. These patients were identified via a search in our picture archiving and communication system in the Department of Diagnostic Radiology at our institute using the keywords: “brain death,” “neurological determination of death,” and “NDD.”
Image Acquisition
For CTA, standard CTA protocol included both single‐phase and two‐phase CTA where two acquisitions were done 1 minute apart, after injection of contrast injection. CTP has been obtained based on our previously published protocol.10, 11 The MRI done for this indication included T1, T2, fluid‐attenuated inversion recovery (FLAIR), and diffusion‐weighted imaging (DWI) of the brain in axial plane. For radionuclide scan, Tc99m HMPAO isotope was injected intravenously, 30 minutes after reconstitution. Images were acquired 15–20 minutes after injection.
Clinical Confirmation
Findings from clinical examination were considered gold standard for determination of brain death. Imaging findings were compared with the results of clinical examination for determination of brain death that were recorded in a “checklist for declaration of brain death,” used in our institution. Clinical examination information was collected through chart review and findings from the checklist for declaration of brain death. When this checklist was not recorded or not available, the clinical examination that was conducted closest in time to the imaging test was used for comparison. The time interval between the clinical examination and the imaging tests was recorded. Information on clinical status of each patient was also collected in patients at 7 days from the time of the imaging test.
Image Analysis
NECT—All NECTs of head were evaluated for the presence of diffuse loss of gray‐white matter differentiation using a binary outcome (yes/no).
CTA—Contrast opacification was assessed as a binary outcome of yes/no for extracranial vessels, intracranial internal carotid artery (ICA) (supraclinoid), middle cerebral artery (MCA) branches (M1, M2), anterior cerebral artery (ACA) branches (A1, A2), intracranial vertebral artery, intracranial basilar artery, internal cerebral vein, and vein of Galen. CTA was assessed on 4‐point, 7‐point, and 10‐point scales.12, 13, 14
Radionuclide scan—For radionuclide scan, tracer activity in the brain, in the brainstem, and lack of tracer activity in the brainstem but preserved tracer activity in the rest of the brain were evaluated. A no tracer activity was recorded when a “hollow skull/empty light bulb” sign was seen.15
CTP—CTP source images were analyzed for the appearance and subsequent disappearance of contrast media in superficial scalp vessels (eg, superficial temporal artery branches) to establish successful intravascular injection of the contrast, as well as complete coverage of a cardiac cycle. Marked‐matched decrease of CBF and cerebral blood volume (CBV) in the whole brain, in the brainstem, and isolated brain stem defect (matched defect on CBF and CBV in the brainstem but preservation in the rest of the brain) were recorded.10, 11
The images were analyzed by a third‐year radiology resident (DM) and a fellowship trained neuroradiologist with more than 7 years of experience (JS). Interobserver agreement was measured between the two observers for each of the imaging modalities. In the event of disagreement between the two observers, a consensus was reached for final analysis.
Statistics‐contingency table analysis with sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs) of the different ancillary imaging tests for diagnosis of brain death were performed and receiver‐operating characteristic (ROC) curves were generated. Interobserver agreement between the two observers for each of the imaging modalities was measured using kappa statistics.
Results
Between December 2006 and February 2016, 74 patients (male/female: 40/34; mean age: 45.2 years; median age: 50 years; range: 16–74 years) underwent ancillary imaging tests for determination of brain death for various clinical presentation (Table 1). The number of imaging tests done for determination of brain death have increased over the years (Fig 1). Most patients underwent more than one ancillary imaging test and only 9 patients had just one ancillary imaging test. The ancillary imaging tests included 41 CTP scans, 54 computed CTA scans (16 single‐phase CTAs, 6 dual‐phase CTAs, 32 CTAs analyzed from CTP source images), 15 radionuclide scans, 1 DSA, 3 MRI, and 71 NECT head scans. Three patients underwent repeated ancillary tests (one radionuclide scan and 2 CTP and CTA) due to changes in their clinical status. For the analysis, the two sets of tests were considered independent tests and were compared with separate clinical examination done closest to the imaging tests. Since there were only one DSA and three MRI, these were excluded from further analysis.
Table 1.
Clinical Presentation for Patients Included for Ancillary Imaging Tests for Confirmation of Brain Death
| Clinical Presentation for Patients Included | Number of Patients (N = 74) |
|---|---|
| Anoxic brain injuries | 24 |
| Cardiac arrests | 10 |
| Hangings | 7 |
| Drug overdoses | 4 |
| Carbon monoxide poisoning | 1 |
| Drowning | 1 |
| Traumatic brain injuries | 19 |
| Subarachnoid hemorrhages | 16 |
| Intracranial hemorrhages | 11 |
| Stroke | 3 |
| Rapidly progressive meningitis. | 1 |
N = number of patients
Figure 1.
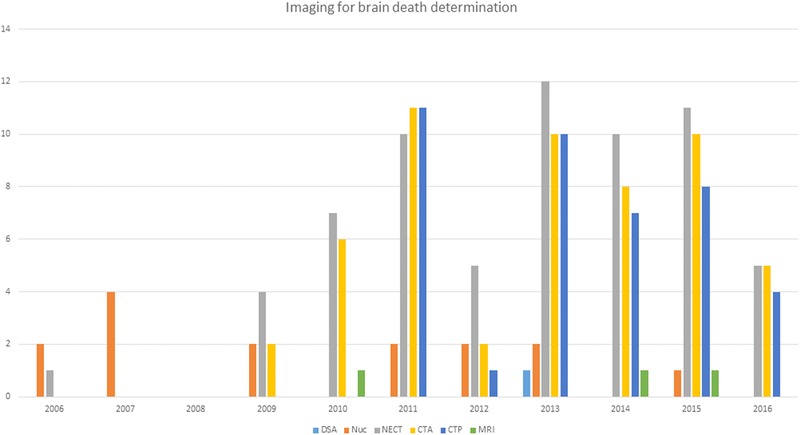
Number of imaging tests used for brain death determination between December 2006 and February 2016. DSA = digital subtraction angiography; Nuc = nuclear scintigraphy; NECT = nonenhanced computed tomography; CTA = computed tomography angiography; CTP = computed tomography perfusion; MRI = magnetic resonance imaging.
The number of patients undergoing ancillary imaging tests increased over the years in our institution from 3 in 2006 and 2007 to 12 in 2013 and 11 in 2015. The choice of imaging test changed from DSA and radionuclide angiograms in earlier years to CTA and CTP in more recent years. The imaging tests were done within a median of 2.5 hours (mean = 4.7 hours) of clinical examination for confirmation of brain death.
All ancillary imaging tests (except NECT head) were 100% specificity and had 100% PPV. In other words, none of the tests demonstrated signs of brain death when the patient was not clinically dead (Table 2 and Fig 2). The ancillary tests differed in their sensitivity and NPV. NECT head showed the least sensitivity and specificity; whereas CTP showed the highest sensitivity and specificity. In 1 patient, CTP findings of preserved brainstem perfusion were not consistent with clinical findings of brain death. It is important to note that the checklist for declaration of brain death was completed 32 hours after CTP was done. As such, this discrepancy between CTP and the clinical exam may reflect change in the clinical status of the patient in this long time interval. If we exclude this outlier patient, the sensitivity, specificity, PPV, and NPV of CTP would become 100% in predicting the clinical determination of brain death.
Table 2.
Comparison of Imaging Tests to Clinical Examination in the Determination of Brain Death
| CTA (54) | |||||||
|---|---|---|---|---|---|---|---|
| Imaging Tests (Number) | NECT (71) | Radionuclide Scan (15) | 4‐Point | 7‐ Point | 10‐ Point | CTP‐ Whole Brain (41) | CTP‐ Whole Brain + Brainstem (41) |
| Sensitivity | 32.7 | 92.9 | 59.1 | 59.1 | 52.3 | 80 | 96.7 |
| Specificity | 73.3 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPV | 81.8 | 100 | 100 | 100 | 100 | 100 | 100 |
| NPV | 22.9 | 50 | 35.7 | 35.7 | 32.3 | 62.5 | 90.9 |
| AUC | 0.51 | 0.95 | 0.79 | 0.79 | 0.76 | 0.90 | 0.92 |
| Kappa | 0.90 | 1 | 1 | 0.92 | 0.92 | 1 | 0.86 |
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) along with area under the curve (AUC) of different ancillary imaging tests for determination of brain death in comparison to the clinical examination done closest to the ancillary test. Interrater reliability (measured as kappa value) between the two raters for each of the ancillary imaging tests is also shown. NECT = nonenhanced computed tomography; CTA = computed tomography angiography; CTP = computed tomography perfusion.
Figure 2.
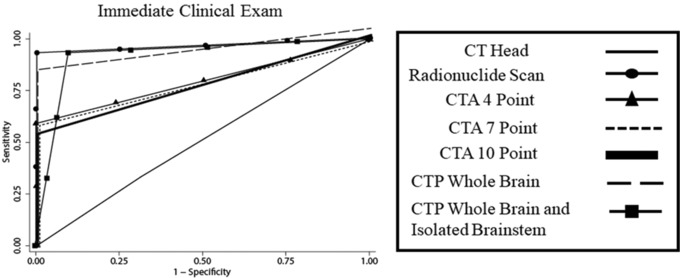
Receiver‐operating characteristic curve shows the diagnostic accuracy of all ancillary imaging tests for determination of brain death. CT = computed tomography; CTA = computed tomography angiography; CTP = computed tomography perfusion; MRI = magnetic resonance imaging.
In 5 patients (12.2%), the CTP demonstrated isolated brainstem defects, with preservation of perfusion in rest of the brain (Fig 3). These patients were clinically declared brain‐dead. Grouping these patients with isolated brain death10, 11 together with those who showed features of whole brain death increased the diagnostic accuracy of the CTP for predicting clinical determination of brain death. Six additional patients showed focal matched defect in the region of brainstem (Figs 3A and 3B) that was not consistent with the definition of isolated brainstem death.10, 11 These patients demonstrated preservation of brainstem function on clinical examination. However, all of these patients were declared dead within a week of the CTP.
Figure 3.
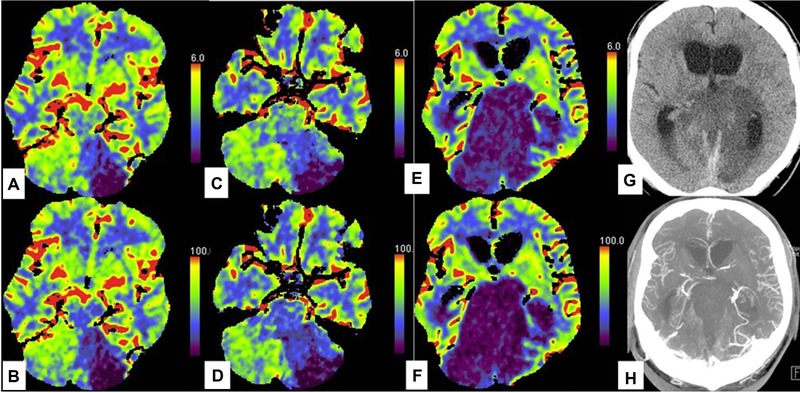
Example of isolated brainstem defects with preservation of perfusion in rest of the brain. A man in his early 60s presented with vertebro‐basilar artery thrombosis. CT perfusion (CTP) done at presentation showed large area of decreased cerebral blood volume (CBV; A, C) and blood flow (CBF; B, D) in the left cerebellar hemisphere and also focal‐matched defect in the midbrain (A, B). The patient had some preserved brainstem function on clinical examination. Patient changed clinically 47 hours later and had no brainstem function on clinical examination. A repeat CTP showed matched CBF and CBV defect in the brainstem but some preserved perfusion in the cerebellum and complete preservation of perfusion in the supratentorial compartment. Nonenhanced CT head (G) done at the same time showed preserved gray‐white matter differentiation in the supratentorial compartment. CT angiogram (H) at this time had preserved opacification of intracranial arteries and veins including the intracranial vertebral artery and posterior cerebral arteries.
Interrater agreement was found to be very high (kappa = .86 to 1) for all ancillary imaging tests (Table 2) with the highest (kappa = 1) for CTP whole brain, radionuclide scan, and CTA four‐point score.
Discussion
Brain death can be declared when all brain function has ceased. This is defined as irreversible loss of the capacity for consciousness combined with the irreversible loss of all brainstem functions. A complete and accurate clinical evaluation is considered the gold standard for the determination of brain death. In clinical practice, ancillary imaging tests are being increasingly used for determination of brain death. The choice of ancillary tests is variable4, 9 and diagnostic accuracy and reliability is unclear. The most commonly used imaging test in our study was NECT scan of the head. NECT of the head was found to be the least sensitive and specific for determination of brain death and had the lowest interrater reliability. All other ancillary imaging tests (radionuclide scan, CTA, and CTP) showed 100% specificity and PPV. In other words, none of these ancillary imaging tests demonstrated signs of brain death when the patients were not clinically dead. The sensitivity and NPV differed among the ancillary tests. CTP was found to have the highest sensitivity, specificity, PPV, and NPV. The high interrater reliability for all ancillary imaging test was very reassuring.
Previous studies on ancillary imaging tests have reported only on patients who were clinically declared dead.10, 12, 13, 16, 17, 18 So, these studies were good for reporting only the sensitivity of imaging in declaration of brain death. Our study reported diagnostic accuracy of these ancillary imaging tests in declaration of patients when they were clinically not dead. Thus, our study is the first one to report the specificity of the ancillary imaging tests. Since brain death is such a critical decision, an ideal ancillary imaging test should have the highest sensitivity, specificity, PPV, and NPV. Barring one outlier patient, our study suggested that CTP has 100% sensitivity, specificity, PPV, and NPV for predicting clinical declaration of brain death. Consistent use of this ancillary test will help boost the confidence of the family members and physicians who must make critical decisions about continuing the care of patients who may be brain dead. Our study confirms the previous reports of high sensitivity of CTP.10, 16, 17 Our study goes one step further by also reporting the specificity of CTP, which was not reported by previous studies.
The internationally developed definition of death now includes permanent loss of brainstem function.2 For ancillary tests, we look for the absence of CBF in whole brain (rather than brainstem only). This clinical‐imaging discordance was perhaps due to the absence of an imaging modality that could resolve the reduced CBF in brainstem only as compared to the whole brain. CTP was the first ancillary imaging test reported to show the phenomenon of isolated brainstem death.10, 11 Although this may be controversial, the current study confirmed the phenomenon of isolated brainstem death in 5 patients and these results concurred with the clinical exams demonstrating the absence of brainstem function. Six additional patients in our study showed focal matched defect in the brainstem that was not consistent with the current definition of isolated brainstem death.10, 11 All 6 patients had partial brainstem function present on clinical examination but all of them were declared dead within a week of CTP. These are novel imaging findings, not reported before in the literature. The focal matched defect on CTP in brainstem in a critical care setting may represent a poor prognostic factor that needs to be assessed further.
One of the advantages of CTP over more commonly used ancillary imaging test of CTA was the ability of CTP to demonstrate isolated brainstem death. While CTA is ubiquitously used and studied,10, 11, 12, 13, 14, 16, 19, 20, 21, 22, 23 it does not permit functional assessment of brainstem function. Radionuclide scan could, in principle, demonstrate the isolated brainstem death; however, the number of patients in our study was small and the poor spatial resolution of radionuclide scan could be a limitation. Studies using digital phantoms to examine the lower limits of perfusion analysis with CTP found that the technology is capable of discriminating between severe hypoperfusion (2%, 1.2 mL/100 g/minute) from an absence (0%).24 A CBF below 35 mL/100 g/minute correlates with cessation of neuronal protein synthesis; below 20 mL/100 g/minute with modification of synaptic transmission (ie, ischemic activity symptomatically noticed by patients) and absence of EEG activity, and <10 mL/100 g/minute with irreversible damage and neuronal death.8, 17 Although, being able to detect <10 mL/100 g/minute, CTP has been shown to be capable of detecting more profound hypoperfusion as low as 1.2 mL/100 g/minute.
Despite being the largest study to report the accuracy of ancillary imaging tests for BDD, our study is small in terms of number of patients. Our study confirms the phenomenon of isolated brainstem defect/death; however, this must be demonstrated in larger studies. A large prospective ongoing study would help ascertain and establish the high diagnostic accuracy and reliability of CTP for determination of brain death.25 As is true for CT scans of head, CTP is limited due to presence of streak artifacts in the posterior fossa. Isolated brainstem death was declared only when the matched perfusion defect was seen in more than one axial slice and when streak artifacts were absent in these slices. Large coverage of the brain in z axis, preferably the whole brain, is desirable for CTP in BDD. In the current study, 1 patient was excluded due to inadequate coverage of brainstem on CTP. An additional limitation of our study was the wide time interval between the ancillary imaging tests and the clinical examination for BDD. Since clinical status of critically ill patients is dynamic, the diagnostic accuracy of an ancillary test should only be compared with the clinical examination that is conducted very close in time to the ancillary tests. Although the median time interval between the ancillary imaging test and clinical examination in our study was 2.5 hours, in 1 patient, clinical examination was done 32 hours after the ancillary imaging test. The long time interval makes the comparison unreliable. In any future prospective study, the time interval between the ancillary imaging test and clinical examination should be kept to minimum for optimal comparison.
One of the important limitations of our study is that different ancillary imaging tests were not done on the same group of patients. This makes a direct comparison between the tests difficult. However, the reference standard for comparison for all ancillary tests was clinical confirmation of brain death and our study compares the relative accuracy of different ancillary tests compared to the reference standard.
In conclusion, the uses of ancillary imaging tests for BDD are increasing over the years. Within the limitation of retrospective study, CTPs followed by radionuclide scan were found to be the most accurate and reliable ancillary imaging test for BDD.
Acknowledgment and disclosure: This study was not funded. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1. Shemie SD, Lee D, Sharpe M, et al. Brain blood flow in the neurological determination of death: Canadian expert report. Can J Neurol Sci 2008;35:140‐5. [DOI] [PubMed] [Google Scholar]
- 2. Shemie SD, Hornby L, Baker A, et al. International guideline development for the determination of death. Intensive Care Med 2014;40:788‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wijdicks EFM. The diagnosis of brain death. N Engl J Med 2001;344:1215‐21. [DOI] [PubMed] [Google Scholar]
- 4. Wijdicks EFM. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology 2002;58:20‐5. [DOI] [PubMed] [Google Scholar]
- 5. Machado C. Diagnosis of brain death. Neurol Int 2010;2:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shemie SD, Doig C, Dickens B, et al. Severe brain injury to neurological determination of death: Canadian forum recommendations. CMAJ 2006;174:S1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wijdicks EFM, Varelas PN, Gronseth GS, et al. Evidence‐based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:1911‐8. [DOI] [PubMed] [Google Scholar]
- 8. Heran MKS, Heran NS, Shemie SD. A review of ancillary tests in evaluating brain death. Can J Neurol Sci 2008;35:409‐19. [DOI] [PubMed] [Google Scholar]
- 9. Citerio G, Crippa IA, Bronco A, et al. Variability in brain death determination in Europe: looking for a solution. Neurocrit Care 2014;21:376‐82. [DOI] [PubMed] [Google Scholar]
- 10. Shankar JJS, Vandorpe R. CT perfusion for confirmation of brain death. AJNR Am J Neuroradiol 2013;34:1175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shankar JJS, Stewart‐Perrin B, Quraishi A, et al. Computed tomography perfusion aides in the prognostication of comatose post‐cardiac arrest patients. Am J Cardiol 2018;121:874‐8. [DOI] [PubMed] [Google Scholar]
- 12. Frampas E, Videcoq M, de Kerviler E, et al. CT angiography for brain death diagnosis. AJNR Am J Neuroradiol 2009;30:1566‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dupas B, Gayet‐Delacroix M, Villers D, et al. Diagnosis of brain death using two‐phase spiral CT. AJNR Am J Neuroradiol 1998;19:641‐7. [PMC free article] [PubMed] [Google Scholar]
- 14. Sawicki M, Bohatyrewicz R, Safranow K, et al. Dynamic evaluation of stasis filling phenomenon with computed tomography in diagnosis of brain death. Neuroradiology 2013;55:1061‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdel‐Dayem HM, Bahar RH, Sigurdsson GH, et al. The hollow skull: a sign of brain death in Tc‐99m HM‐PAO brain scintigraphy. Clin Nucl Med 1989;14:912‐6. [DOI] [PubMed] [Google Scholar]
- 16. Bohatyrewicz R, Sawicki M, Walecka A, et al. Computed tomographic angiography and perfusion in the diagnosis of brain death. Transplant Proc 2010;42:3941‐6. [DOI] [PubMed] [Google Scholar]
- 17. Escudero D, Otero J, Marqués L, et al. Diagnosing brain death by CT perfusion and multislice CT angiography. Neurocrit Care 2009;11:261‐71. [DOI] [PubMed] [Google Scholar]
- 18. Sawicki M, Sołek‐Pastuszka J, Chamier‐Ciemińska K, et al. Computed tomography perfusion is a useful adjunct to computed tomography angiography in the diagnosis of brain death. Clin Neuroradiol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Şahin H, Pekçevik Y. CT angiography as a confirmatory test in diagnosis of brain death: comparison between three scoring systems. Diagn Interv Radiol 2015;21:177‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor T, Dineen RA, Gardiner DC, et al. Computed tomography (CT) angiography for confirmation of the clinical diagnosis of brain death. Cochrane Database Syst Rev 2014:CD009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kramer AH, Roberts DJ. Computed tomography angiography in the diagnosis of brain death: a systematic review and meta‐analysis. Neurocrit Care 2014;21:539‐50. [DOI] [PubMed] [Google Scholar]
- 22. Welschehold S, Boor S, Reuland K, et al. Technical aids in the diagnosis of brain death: a comparison of SEP, AEP, EEG, TCD and CT angiography. Dtsch Arztebl Int 2012;109:624‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berenguer CM, Davis FE, Howington JU. Brain death confirmation: comparison of computed tomographic angiography with nuclear medicine perfusion scan. J Trauma 2010;68:553‐9. [DOI] [PubMed] [Google Scholar]
- 24. Uwano I, Kudo K, Sasaki M, et al. CT and MR perfusion can discriminate severe cerebral hypoperfusion from perfusion absence: evaluation of different commercial software packages by using digital phantoms. Neuroradiology 2012;54:467‐74. [DOI] [PubMed] [Google Scholar]
- 25. CT‐perfusion for neurological diagnostic evaluation (INDex‐CTP) . In: ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03098511. Accessed February 1, 2018.


