Abstract
Background
The aim of the study was to analyze the characteristics and survival of a group of patients with COPD according to their clinical phenotype.
Patients and methods
The study population was selected from patients undergoing scheduled spirometry between January 1, 2011 and June 30, 2011 at the respiratory function laboratory of a teaching hospital and comprised those with a previous and confirmed diagnosis of COPD and forced expiratory volume in the first second (FEV1) of <70%. The patients selected were classified into 4 groups: positive bronchodilator response, non-exacerbator, exacerbator with emphysema, and exacerbator with chronic bronchitis. Patients were followed up until April 2017.
Results
We recruited 273 patients, of whom 89% were men. The distribution by phenotype was as follows: non-exacerbator, 47.2%; positive bronchodilator response, 25.8%; exacerbator with chronic bronchitis, 13.8%; and exacerbator with emphysema, 13.0%. A total of 90 patients died during follow-up (32.9%). Taking patients with a positive bronchodilator response as the reference category, the risk factors that were independently associated with death were older age (HR, 1.06; 95% CI, 1.03–1.09), lower FEV1 (HR, 0.98; 95% CI, 0.96–0.99), and exacerbator with chronic bronchitis phenotype (HR, 3.28; 95% CI, 1.53–7.03).
Conclusion
Classification of COPD patients by phenotype makes it possible to identify subgroups with different prognoses. Thus, mortality was greater in exacerbators with chronic bronchitis and lower in those with a positive bronchodilator response.
Keywords: COPD, phenotypes, positive bronchodilator response, chronic bronchitis, emphysema, exacerbator, mortality
Introduction
COPD is a common, preventable, and treatable condition that is characterized by persistent limitation of airflow as a consequence of exposure to harmful gases or particles.1
In clinical practice, we can see that the clinical presentation, response to therapy, and progression of COPD differ between patients. Consequently, it is increasingly clear that to evaluate the complexity of the disease, we cannot base our approach exclusively on the degree of airflow obstruction. In this sense, symptoms and exacerbations are particularly important and can help to identify different clinical forms of the disease. A phenotype of COPD is considered a set of attributes that, either alone or in combination, enable us to describe the differences between COPD patients based on clinically significant parameters (symptoms, exacerbations, response to treatment, rate of disease progression, and mortality).2
Spanish COPD guidelines (GesEPOC)3 classify patients according to their predominant pattern, emphysema, or chronic bronchitis, together with the frequency of exacerbations. Thus, GesEPOC also describes the mixed phenotype asthma-COPD overlap (ACO).
Several studies have analyzed the prognosis for each of the phenotypes. The chronic bronchitis phenotype is clearly associated with exacerbations, admissions to hospital due to exacerbation, and mortality.4–8 The case of ACO is more controversial. While some authors report a higher incidence of exacerbation9,10 and mortality in this phenotype,11,12 others have not detected a higher incidence of exacerbations.13 Compared with the other phenotypes of COPD, ACO has even been reported to have a “protective effect” against mortality.3–15
The objective of the present study was to analyze the characteristics and survival of a group of patients with COPD according to their clinical phenotype.
Patients and methods
This study was approved by the Gregorio Marañon Hospital Ethics Committee. Patients provided written informed consent to participate in the study. The study population was selected from all patients who underwent scheduled spirometry between January 1, 2001 and June 30, 2011 at the respiratory function laboratory of a teaching hospital and included those with a previous and subsequently confirmed diagnosis of COPD by spirometry (by confirming the existence of a forced expiratory volume in the first second [FEV1]/forced vital capacity ratio <0.70 after the bronchodilator test) and FEV1 of <70%.
The patients selected were classified into 4 groups: positive bronchodilator response, non-exacerbator, exacerbator with emphysema, and exacerbator with chronic bronchitis. First, all patients with an increase in FEV1>12% and >200 mL in the bronchodilator test result were included in the positive bronchodilator response group. Next, all patients who had visited the emergency department less than twice for a respiratory complaint but did not require admission for the complaint during the year before the initial spirometry test were included in the non-exacerbator group. Patients who had been admitted for a respiratory complaint during the year before undergoing spirometry were included in the exacerbator with emphysema group or exacerbator with chronic bronchitis group according to the information in the clinical history and the following criteria. Patients were identified as belonging to the exacerbator with emphysema phenotype when, in addition to the criteria for exacerbation, they had radiological and/or functional abnormalities (diffusion capacity of carbon monoxide) compatible with pulmonary emphysema. Patients in the exacerbator with chronic bronchitis group had to present the criteria for exacerbation and have had productive cough for 3 months per year for at least 2 consecutive years.
The variables recorded at inclusion were age, sex, body mass index (BMI), smoking history, comorbidities (arterial hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, atrial fibrillation, cerebrovascular accident), blood eosinophil count, lung function parameters, and treatment of respiratory disease.
Follow-up was until April 1, 2017. Patients’ death was recorded on this date; if the patient had not died, the last date the patient was known to be alive was recorded. During follow-up, dates of and reasons for visits to the emergency department and admissions to hospital, specifically for exacerbations of COPD and pneumonia, were recorded. Dates and causes of death were obtained from the clinical history.
Statistical analysis
The results for continuous variables are presented as mean and CI; the results for categorical variables are presented as frequency and percentage.
Categorical data and proportions were analyzed using the χ2 or Fisher exact test, as applicable. Continuous variables were compared using an analysis of variance or the Kruskal–Wallis test.
Survival was analyzed taking the date of inclusion in the study as the reference point. The study ran from the day the initial spirometry test was performed until death or the last date the patient was known to be alive. A Kaplan–Meier analysis was performed to estimate the cumulative probability of death according to phenotype. The log-rank test was used for univariate comparisons between groups. Lastly, multivariate Cox regression analysis was used to test the association between phenotype and death, with an adjustment for age, sex, and FEV1.
The statistical analysis was performed using R, version 3.4.3. Statistical significance was set at p<0.05.
Results
We recruited 273 patients, of whom 89% were men (Table 1). Mean age was 67.99 years (95% CI, 66.72–69.25). Mean BMI was 28.05 kg/m2 (95% CI, 27.40–28.71). Most patients were smokers (33.7%) and ex-smokers (64.5%); 1.1% of the patients were nonsmokers. The most common comorbidities were arterial hypertension (53.5%), dyslipidemia (39.6%), diabetes mellitus (26%), ischemic heart disease (13.9%), atrial fibrillation (12.8%), and cerebrovascular accidents (8.1%). The mean eosinophil percentage was 2.42% (95% CI, 2.22%–2.63%), and the mean FEV1 was 48.64% (95% CI, 47.14%–50.14%). As for treatment, 93% of patients were receiving anticholinergic drugs, 88.6% long-acting β2-adrenergic agonists, and 77.7% inhaled corticosteroids.
Table 1.
General characteristics of the study patients
| Parameter | Value |
|---|---|
| Patients, n | 273 |
| Age, years | 67.99 (66.72–69.25) |
| Men, n (%) | 243 (89) |
| BMI, kg/m2 | 28.05 (27.40–28.71) |
| Smoking status, n (%) | |
| Nonsmoker | 3 (1.1) |
| Smoker | 92 (33.7) |
| Ex-smoker | 176 (64.5) |
| Unknown | 2 (0.7) |
| Diabetes mellitus, n (%) | 71 (26) |
| Arterial hypertension, n (%) | 146 (53.5) |
| Dyslipidemia, n (%) | 108 (39.6) |
| Ischemic heart disease, n (%) | 38 (13.9) |
| Atrial fibrillation, n (%) | 35 (12.8) |
| Cerebrovascular accident, n (%) | 22 (8.1) |
| Eosinophils, absolute value | 202.93 (185.18–220.68) |
| Eosinophils, % | 2.42 (2.22–2.63) |
| FEV1, mL | 1,221.39 (1,171.76–1,271.02) |
| FEV1, % | 48.64 (47.14–50.14) |
| Treatment, n (%) | |
| LAMA | 254 (93) |
| LABA | 242 (88.6) |
| Inhaled corticosteroid | 212 (77.7) |
| Roflumilast | 7 (2.6) |
| Theophylline | 15 (5.5) |
| COH | 91 (33.5) |
| CPAP | 31 (11.4) |
| BiPAP | 14 (5.1) |
| Mortality, n (%) | 90 (32.9) |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in the first second; LABA, long-acting β2-adrenergic agonists; CPAP, continuous positive airway pressure; BiPAP, bilevel positive airway pressure; COH, continuous home oxygen therapy.
In relation to the distribution of phenotypes, non-exacerbators accounted for 47.2% of patients, 25.8% of patients had a positive bronchodilator response, 13.8% were exacerbators with chronic bronchitis, 13.0% were exacerbators with emphysema, with mean values of diffusion capacity of carbon monoxide (DLCO) and KCO (DLCO/alveolar volume) of 57.54 (95% CI 45.41–69.67) and 61.20 (95% CI 48.72–73.68), respectively, in this last group. Table 2 shows the characteristics of patients according to phenotype. We found significant differences in age (lower in patients with a positive bronchodilator response), smoking (more active smokers in patients with a positive bronchodilator response), some comorbid conditions (lower percentage of patients with hypertension and ischemic heart disease among patients with a positive bronchodilator response), the eosinophil count and percentage (higher in patients with a positive bronchodilator response), and FEV1 (higher in patients with a positive bronchodilator response). Significant differences were also found for mortality, which was lower in patients with a positive bronchodilator response.
Table 2.
Characteristics of the study population by phenotype
| Variable | Positive bronchodilator response | Non-exacerbator | Exacerbator with emphysema | Exacerbator with chronic bronchitis | p-value |
|---|---|---|---|---|---|
| Patients, n | 71 | 130 | 36 | 38 | |
| Age, years | 63.44 (60.83–66.04) | 69.67 (68.07–71.27) | 68.44 (64.88–72.01) | 71.16 (68.15–74.16) | 0.001 |
| Men, n (%) | 63 (88.7) | 119 (91.5) | 31 (86.1) | 32 (84.2) | 0.558 |
| BMI, kg/m2 | 28.15 (26.94–29.36) | 28.61 (27.61–29.61) | 26.69 (25.06–28.32) | 27.01 (25.94–28.08) | 0.121 |
| Smoking status, n (%) | |||||
| Nonsmoker | 0 (0) | 3 (2.3) | 0 (0) | 0 (0) | 0.336 |
| Smoker | 35 (49.3) | 35 (27.1) | 11 (30.6) | 13 (35.1) | 0.017 |
| Ex-smoker | 36 (50.7) | 91 (70.5) | 25 (69.4) | 24 (64.9) | 0.039 |
| Unknown | 0 (0) | 1 (0.8) | 0 (0) | 1 (2.6) | 0.441 |
| Diabetes mellitus, n (%) | 16 (22.5) | 32 (24.6) | 13 (36.1) | 11 (28.9) | 0.45 |
| Arterial hypertension, n (%) | 28 (39.4) | 80 (61.5) | 18 (50) | 22 (57.9) | 0.023 |
| Dyslipidemia, n (%) | 24 (33.8) | 56 (43.1) | 10 (27.8) | 12 (31.6) | 0.245 |
| Ischemic heart disease, n (%) | 4 (5.6) | 18 (13.8) | 4 (11.1) | 14 (36.8) | <0.001 |
| Atrial fibrillation, n (%) | 4 (5.6) | 18 (13.8) | 5 (13.9) | 9 (23.7) | 0.063 |
| Cerebrovascular accident, n (%) | 3 (4.2) | 14 (10.8) | 3 (8.3) | 2 (5.3) | 0.373 |
| Eosinophils, absolute value | 235.21 (200.11–270.32) | 215.38 (190.99–239.78) | 150 (111.16–188.84) | 118.42 (82.48–154.36) | <0.001 |
| Eosinophils, % | 2.73 (2.34–3.12) | 2.6 (2.31–2.9) | 1.76 (1.33–2.18) | 1.58 (1.15–2.02) | <0.001 |
| FEV1, % | 53.71 (51.12–56.31) | 48.69 (46.57–50.8) | 42.31 (38.92–45.7) | 42.87 (39.32–46.42) | <0.001 |
| FEV1, mL | 1,450.7 (1,358.4–1,543.01) | 1,197.62 (1,131.72–1,263.51) | 995.56 (912.42–1,078.69) | 985.79 (889.16–1,082.42) | <0.001 |
| Mortality, n (%) | 12 (16.9) | 44 (33.8) | 15 (41.7) | 19 (50) | 0.002 |
| Treatment, n (%) | |||||
| Anticholinergic drugs | 60 (84.5) | 124 (95.4) | 35 (97.2) | 38 (100) | 0.004 |
| LABA | 66 (93) | 107 (82.3) | 35 (97.2) | 37 (97.4) | 0.006 |
| Inhaled corticosteroids | 61 (85.9) | 87 (66.9) | 33 (91.7) | 35 (92.1) | <0.001 |
| Roflumilast | 2 (2.8) | 2 (1.5) | 2 (5.6) | 1 (2.6) | 0.6 |
| Theophylline | 1 (1.4) | 5 (3.8) | 5 (13.9) | 6 (15.8) | 0.003 |
| COH | 14 (19.7) | 38 (29.5) | 21 (58.3) | 24 (63.2) | <0.001 |
| CPAP, BiPAP | 10 (14.1) | 16 (12.3) | 3 (8.3) | 2 (5.3) | 0.497 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in the first second; LABA, long-acting β2-adrenergic agonists; CPAP, continuous positive airway pressure; BiPAP, bilevel positive airway pressure; COH, continuous home oxygen therapy.
Ninety patients (32.9%) died during follow-up. Table 3 shows the causes of death according to phenotype. The most frequent cause was respiratory disease (acute exacerbation of COPD/pneumonia/sepsis), irrespective of the phenotype analyzed, followed by cancer.
Table 3.
Cause of death according to phenotype
| Cause | Total
|
Positive bronchodilator response
|
Non-exacerbator
|
Exacerbator with emphysema
|
Exacerbator with chronic bronchitis
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Acute exacerbation of COPD/pneumonia/respiratory sepsis | 42 | 46.67 | 5 | 41.67 | 18 | 40.91 | 9 | 60 | 10 | 52.63 |
| Cancer | 23 | 25.56 | 4 | 33.33 | 11 | 25 | 3 | 20 | 5 | 26.32 |
| Heart disease | 2 | 2.22 | 0 | 0 | 2 | 4.55 | 0 | 0 | 0 | 0 |
| Other | 3 | 3.33 | 0 | 0 | 1 | 2.27 | 1 | 6.67 | 1 | 5.26 |
| Not available | 20 | 22.22 | 3 | 25 | 12 | 27.27 | 2 | 13.33 | 3 | 15.79 |
Survival is shown according to phenotype in Figure 1. The factors that were independently associated with survival – age, FEV1, and the exacerbator with chronic bronchitis phenotype – are shown in Table 4. The risk of mortality increased with age (HR, 1.06; 95% CI, 1.03–1.09), and increased with the decrease in FEV1 (HR, 0.98; 95% CI, 0.96–0.99). The risk of mortality was significantly greater in exacerbators with chronic bronchitis than in patients with a positive bronchodilator response (HR, 3.28; 95% CI, 1.53–7.03).
Figure 1.
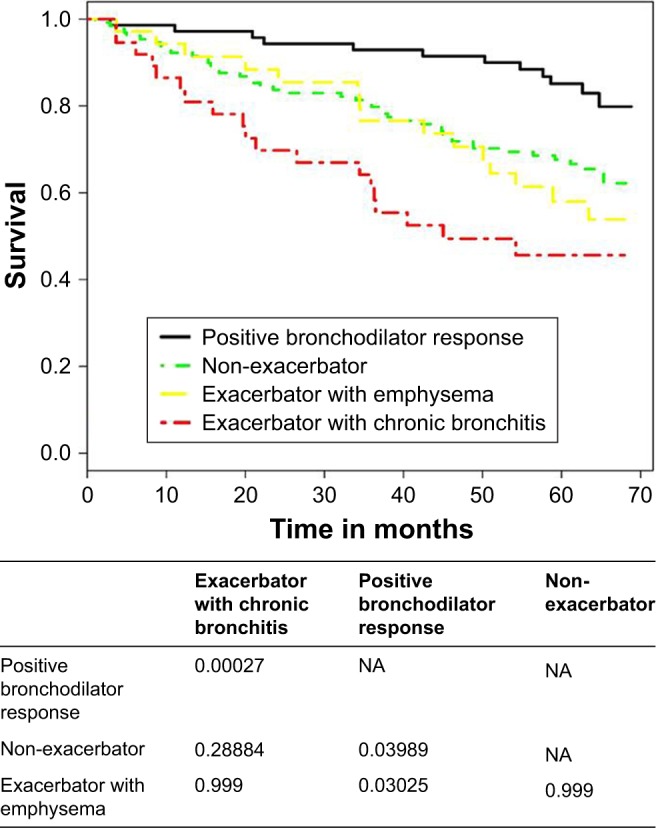
Kaplan–Meier survival curves according to the phenotype of COPD.
Note: p-value for the comparison of survival curves using the log-rank test.
Abbreviation: NA, not applicable.
Table 4.
Factors independently associated with mortality (Cox regression)
| Variable | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Age | 1.06 | 1.03–1.09 | <0.001 |
| Sex | |||
| Female | 1 | ||
| Male | 2.10 | 0.74–5.91 | 0.158 |
| FEV1 | 0.98 | 0.96–0.99 | 0.044 |
| Phenotype | |||
| Positive bronchodilator response | 1 | ||
| Non-exacerbator | 1.67 | 0.87–3.20 | 0.122 |
| Exacerbator with emphysema | 2.04 | 0.91–4.56 | 0.079 |
| Exacerbator with chronic bronchitis | 3.28 | 1.53–7.03 | 0.002 |
Abbreviation: FEV1, forced expiratory volume in 1 second.
Discussion
The main conclusion of our study is that the classification by phenotype among patients with COPD makes it possible to identify subgroups with different prognoses. Thus, survival was greater in patients with a positive bronchodilator response and lower in exacerbators with chronic bronchitis.
Previous studies have shown that 6%–25% of patients with COPD have ACO.14–21 In our study, 25.8% of patients had a positive bronchodilator response. This figure fell within the range of reversibility detected by other authors in a similar percentage of patients (21%–27.6%).13,22 Furthermore, the FEV1 of patients with a positive bronchodilator response in our study was higher than that of the other phenotypes. Similar results were reported by Calle Rubio et al,18 although this finding was not reported elsewhere.10,14
Non-exacerbators accounted for 47.2% of patients, exacerbators with chronic bronchitis accounted for 13.8%, and exacerbators with emphysema accounted for 13.0%. This distribution is similar to that reported in other studies, which show non-exacerbator to be the most frequent phenotype.18–21 However, a recent study performed in primary care in Russia showed that the most common phenotype was exacerbator with chronic bronchitis, with only 36% of patients classified as non-exacerbators. The authors also indicated that these results were associated with the difficulty in diagnosing COPD in Russia.23 Consistent with previous studies, patients with chronic bronchitis were frequent among exacerbators.20,21
Severe exacerbations seem to be an independent risk factor for death,24–26 with the result that the frequency of exacerbations increases the risk of death. The risk of death increases 2-fold in patients with 1 or 2 severe exacerbations (requiring a visit to the emergency department or admission to hospital) compared with non-exacerbators; the risk is 4-fold greater in patients with 3 or more severe exacerbations per year.24 These data have been confirmed in other studies, which show that the risk of death is even independent of the BODE index.25 Other authors have also shown that the risk of death increases with the number of hospitalizations, as does the risk of a subsequent admission.26 We found that exacerbators with chronic bronchitis have a 3-fold greater risk of death than patients with a positive bronchodilator response. In this way, it is important to optimize the treatment of these patients. The first step of treatment in patients with exacerbator phenotype with chronic bronchitis is the double bronchodilator therapy, and the next step is to identify the best option for each patient according to their characteristics. These options include drugs such as inhaled corticosteroids and mucolytics, as well as long-term phosphodiesterase 4 inhibitors and antibiotics.27
Disease progression is the main cause of death in patients with COPD.28 In our setting, 50%–80% of COPD patients died of exacerbation (30%–50%), lung cancer (8.5%–27%), or other respiratory diseases.29–33 In the early stages of COPD, the causes of death are mainly non-respiratory, although most are associated with smoking (cancer, ischemic heart disease, and cerebrovascular accident).28 In the case of patients with COPD and chronic respiratory insufficiency, Zielinski et al34 observed that the main causes of death were exacerbation of chronic respiratory insufficiency (38%), heart failure (13%), respiratory infection (11%), pulmonary thromboembolism (10%), cardiac arrhythmias (8%), and lung cancer (7%). In our study, the trend was similar, with respiratory disease (exacerbation of COPD, pneumonia, and respiratory sepsis) being the main cause, followed by cancer, mainly lung cancer.
Our results were obtained under real-world conditions and show that a simple positive bronchodilator response, which is therefore less demanding than the current criteria for ACO, makes it possible to identify a subgroup of patients with COPD and a more favorable course than the other phenotypes. They also show that mortality is higher in exacerbators with chronic bronchitis. The worst prognosis found in patients with exacerbator phenotype with chronic bronchitis is in line with other studies that have been previously published. For example, De Abajo Larriba et al35 have recently described that these patients are more severe, have more exacerbations, and require more income than the other phenotypes.
Our study is subject to a series of limitations. First, the study was performed in a single center, thus limiting the generalizability of the results. Second, due to the design characteristics of our study, it was not possible to collect the exacerbations diagnosed and treated ambulatory by primary care physicians; only exacerbations management in the emergency department were considered. Third, the lack of active follow-up implies that for patients who died outside the hospital, we could not register the cause of death in some cases. Lastly, some of the variables that might have influenced the results – cumulative smoking, duration of COPD, and treatment of comorbidities – were not available.
In conclusion, classification of COPD patients by phenotype makes it possible to identify subgroups with different prognoses. Mortality was higher in patients with the exacerbator with chronic bronchitis phenotype than in the positive bronchodilator response phenotype. It is clear that the treatment and the clinical progress of patients with COPD must be improved in exacerbators with chronic bronchitis.
Acknowledgments
The authors would like to thank Menarini for grant support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. [Accessed July 7, 2018]. Available from: http://goldcopd.org.
- 2.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Guía española de la EPOC (GesEPOC). Actualización 2014. [Spanish guideline for COPD (GesEPOC). Update 2014] Arch Bronconeumol. 2014;50(Suppl 1):1–16. doi: 10.1016/S0300-2896(14)70070-5. Spanish. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Prescott E, Lange P, Copenhagen City Heart Study Group Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 5.Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130(4):1129–1137. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 6.Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8(8):1333–1338. doi: 10.1183/09031936.95.08081333. [DOI] [PubMed] [Google Scholar]
- 7.Kim V, Criner GJ. The chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implications. Curr Opin Pulm Med. 2015;21(2):133–141. doi: 10.1097/MCP.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahousse L, Seys LJM, Joos GF, Franco OH, Stricker BH, Brusselle GG. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J. 2017;50(2):1602470. doi: 10.1183/13993003.02470-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardin M, Silverman EK, Barr RG, et al. COPDGene Investigators The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai JW, Mao B, Yang WL, Liang S, Lu HW, Xu JF. Asthma–COPD overlap syndrome showed more exacerbations however lower mortality than COPD. QJM. 2017;110(7):431–436. doi: 10.1093/qjmed/hcx005. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Guzman E, Khosravi M, Mannino DM. Asthma, chronic obstructive pulmonary disease, and mortality in the U.S. population. COPD. 2011;8(6):400–407. doi: 10.3109/15412555.2011.611200. [DOI] [PubMed] [Google Scholar]
- 12.Hospers JJ, Postma DS, Rijcken B, Weiss ST, Schouten JP. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet. 2000;356(9238):1313–1317. doi: 10.1016/S0140-6736(00)02815-4. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Makita H, Konno S, et al. Hokkaido COPD Cohort Study Investigators Asthma-like features and clinical course of chronic obstructive pulmonary disease: an analysis from the Hokkaido COPD cohort study. Am J Respir Crit Care Med. 2016;194(11):1358–1365. doi: 10.1164/rccm.201602-0353OC. [DOI] [PubMed] [Google Scholar]
- 14.Cosio BG, Soriano JB, López-Campos JL, et al. CHAIN Study Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149(1):45–52. doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi Y, Yasunaga H, Matsui H, et al. Comparison of in-hospital mortality in patients with COPD, asthma and asthma-COPD overlap exacerbations. Respirology. 2015;20(6):940–946. doi: 10.1111/resp.12556. [DOI] [PubMed] [Google Scholar]
- 16.Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197–219. doi: 10.1586/ecp.13.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474–481. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- 18.Calle Rubio M, Casamor R, Miravitlles M. Identification and distribution of COPD phenotypes in clinical practice according to Spanish COPD Guidelines: the FENEPOC study. Int J Chron Obstruct Pulmon Dis. 2017;12:2373–2383. doi: 10.2147/COPD.S137872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koblizek V, Milenkovic B, Barczyk A, et al. Phenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE Study. Eur Respir J. 2017;49(5):1601446. doi: 10.1183/13993003.01446-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miravitlles M, Barrecheguren M, Roman-Rodríguez M. Frequency and characteristics of different clinical phenotypes of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2015;19(8):992–998. doi: 10.5588/ijtld.15.0021. [DOI] [PubMed] [Google Scholar]
- 21.Cosio BG, Soriano JB, López-Campos JL, et al. Distribution and outcomes of a phenotype-based approach to guide COPD management: results from the CHAIN cohort. PLoS One. 2016;11(9):e0160770. doi: 10.1371/journal.pone.0160770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marín JM, Ciudad M, Moya V, et al. Airflow reversibility and long-term outcomes in patients with COPD without comorbidities. Respir Med. 2014;108(8):1180–1188. doi: 10.1016/j.rmed.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Arkhipov V, Arkhipova D, Miravitlles M, Lazarev A, Stukalina E. Characteristics of COPD patients according to GOLD classification and clinical phenotypes in the Russian Federation: the SUPPORT trial. Int J Chron Obstruct Pulmon Dis. 2017;12:3255–3262. doi: 10.2147/COPD.S142997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, Tordera MP, Sánchez PR. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2009;103(5):692–699. doi: 10.1016/j.rmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical practice guideline for the diagnosis and treatment of patients with chronic obstructive pulmonary disease (COPD) – the Spanish COPD Guideline (GesEPOC) Arch Bronconeumol. 2017;53(Suppl 1):2–64. 2017 version. [Google Scholar]
- 28.Garcia IS, Clarà PC. Causes of death and prediction of mortality in COPD. Arch Bronconeumol. 2010;46:343–346. doi: 10.1016/j.arbres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):680–685. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 30.Solanes I, Casan P, Sangenis M, Calaf N, Giraldo B, Guell R. Factores de riesgo de mortalidad en la EPOC. [Risk factors for mortality in COPD] Arch Bronconeumol. 2007;43(8):445–449. doi: 10.1016/s1579-2129(07)60101-3. Spanish. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FJ, Foster G, Curtis JL, et al. NETT Research Group Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122(11):823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 33.Moreno A, Montón C, Belmonte Y, Gallego M, Pomares X, Real J. Causas de muerte en pacientes con EPOC grave. Factores pronósticos. [Causes of death and risk factors for mortality in patients with severe COPD] Arch Bronconeumol. 2009;45(4):181–186. doi: 10.1016/j.arbres.2008.09.004. Spanish. [DOI] [PubMed] [Google Scholar]
- 34.Zielinski J, MacNee W, Wedzicha J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis. 1997;52(1):43–47. [PubMed] [Google Scholar]
- 35.De Abajo Larriba AB, Méndez Rodríguez E, González-Gallego J, et al. Estimating the prevalence of phenotypes in patients with pulmonary obstructive disease. ADEPOCLE study. Nutr Hosp. 2017;34(1):180–185. doi: 10.20960/nh.995. [DOI] [PubMed] [Google Scholar]


