Abstract
Nevirapine (NVP) therapy is associated with a high risk of serious liver injury and skin rash. Treatment of Brown Norway rats with NVP causes an immune-mediated skin rash. Even though NVP does not cause serious liver injury in wildtype animals, incubation of hepatocytes with NVP leads to the release of presumably danger-associated molecular pattern molecules (DAMPs), which activate macrophages. In this study, we examined the liver biopsies of Brown Norway rats treated with NVP to determine the histologic correlate to the release of DAMPs by hepatocytes. In vivo, debris from necrotic hepatocytes and endothelial cells were present in the liver sinusoids, a condition that can trigger an immune response. In addition to mitochondrial, hepatocytic, and endothelial damage, the drug induced large hepatocytic inclusions composed of lipid droplets surrounded by concentric whorls of smooth endoplasmic reticulum (SER) cisternae—lipid-SER (LSER) inclusions, which were deposited in the sinusoids. NVP is lipid soluble, and these LSER inclusions may be sinks of NVP or its metabolites. LSERs are deposited in the blood stream where they may be picked up by lymph nodes and contribute to initiation of an immune response leading to serious liver injury or skin rash. LSERs migration from liver to the blood stream may signify a novel mechanism of drug exocytosis.
Keywords: Antiretroviral therapies (ARTs), endothelium, hepatocytes, highly active antiretroviral therapy (HAART), lipid droplets, liver, mitochondria, skin rash, smooth endoplasmic reticulum (SER)
Introduction
The effectiveness of antiretroviral therapies (ARTs), such as highly active antiretroviral therapy (HAART), in treating HIV-infected patients has improved life expectancy of AIDS patients.1 Although ART has increased average life expectancy while decreased AIDS-related deaths, it has also increased susceptibility to nonalcoholic fatty liver disease (NAFLD) and mitochondrial dysfunction.1–4 Liver disease, which accounts for nearly 7% of all HIV-related deaths, is now the second leading cause of death among HIV-infected patients after AIDS.1
ART-induced hepatotoxicity may manifest as hypersensitivity, idiosyncratic hepatotoxicity, mitochondrial degeneration, immune reconstitution syndrome, and hepatic steatosis.5,6 Certain nucleoside reverse transcriptase inhibitors (NRTIs) used in ART can cause reduction in the mitochondrial DNA, disruption of fatty acid oxidation, and accumulation of microvesicular steatosis in the liver.1 ART therapies are also suspected of having detrimental effects on glucose control, and lipid metabolism while potentially contributing to insulin resistance.1,3 Liver ultrastructural aberrations in a patient cohort receiving ART have encompassed sinusoidal degeneration, hepatocytic aberrations, mitochondrial dysfunction, accumulation of bulky lipid droplets (steatosis), and occlusion of sinusoidal lumina.6
While both HIV and HAART have been implicated in hepatotoxicity, it is important to study the individual roles of these causative factors and elucidate their mechanism of action. One of these drugs is nevirapine (NVP), which is a non-nucleoside reverse transcriptase inhibitor (NNRTI).7,8 NVP is associated with a hypersensitivity reaction in 6–10% of patients, usually occurring within the first 4–6 weeks of drug administration, a late hepatotoxic sequela surfacing 6–12 months from starting the medication9, maculopapular exanthema, Stevens–Johnson syndrome (SJS), and toxic epidermal necrolysis.10 These hypersensitivity reactions are presumably immune-mediated and are associated with specific HLA genotypes.11,12
The metabolism of NVP is complex, but with an animal model we demonstrated that the skin rash is caused by a benzylic sulfate formed in the skin.13 In contrast, direct oxidation of NVP to a reactive quinone methide is responsible for most of the covalent binding in the liver.14 Although NVP causes an immune-mediated skin rash in Brown Norway rats, it does not cause significant hepatic necrosis as measured by serum alanine transaminase (ALT). This is presumably because the dominant immune response in the liver is immune tolerance. However, we did observe a significant increase in ALT in an animal model in which immune checkpoints were inhibited.15 Furthermore, incubation of NVP with hepatocytes led to the release of danger-associated molecular pattern molecules (DAMPs), which activate inflammasomes.16 This may be the mechanism by which NVP induces an immune response that can lead to hypersensitivity reactions such as toxic epidermal necrolysis and liver failure.
In this study, we have used a rat model for NVP-induced skin rash to examine the ultrastructural changes noted in the livers of NVP-treated rats. These changes provide clues to the mechanism by which NVP induces an immune response that can lead to serious liver injury or skin rash. The effects of NVP are described here at the ultra-structural level, revealing the transformations that take place within the rats’ hepatocytes, sinusoid endothelial cells (ECs), and lumina, as well as possible pathological consequences. Additionally, we are presenting a working hypothesis about the sequestration of NVP and its derivatives within hepatocytes and their subsequent release into the sinusoids.
Materials and methods
Our investigation is focused on NVP-induced liver aberration in an animal model that consistently develops NVP-related skin rash.7,17 Therefore, we have used Brown Norway female rats for this study since they have shown 100% occurrence of skin rash when treated with NVP. Details of the materials and methods are in Shenton et al.7 Briefly, Brown Norway Female rats (n = 32, 150–175 g) (Charles River, Montreal QC, Canada) were given a daily NVP (Boehringer-Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA) dose of 150 mg/kg/day for 8 weeks.7 Livers were harvested and fixed in 2.5% buffered glutaraldehyde then processed for transmission electron microscopy (TEM) according to Ogilvy et al.18 Briefly, liver tissue was doubly-fixed in a PBS-buffered glutaraldehyde (2.5% at pH 7.4) and osmium tetroxide (0.5%), then embedded in epoxy resin. Thin sections (90 nm) were collected on 200-mesh copper grids, air-dried, and doubly-stained with uranyl acetate and lead citrate. Sections were viewed with a JEOL JEM-1010 electron microscope, and photographed.
Results
Nevirapine-induced heterogeneous damage in hepatocytes
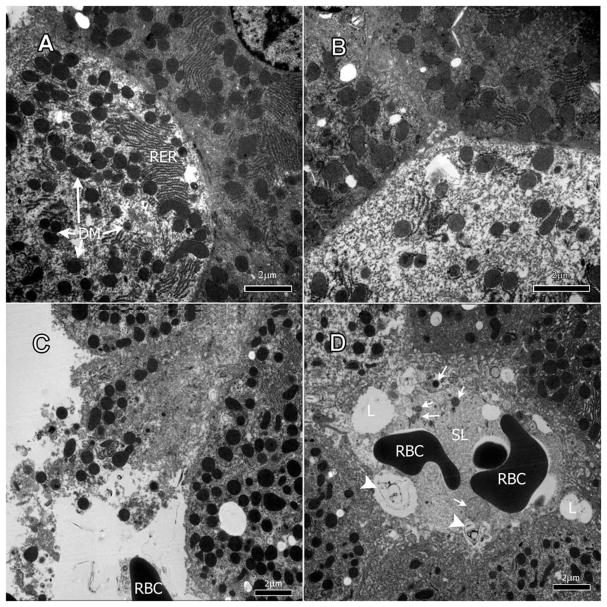
Liver tissue from the treated rats showed a heterogeneous pattern of damage where some hepatocytes appeared to have been affected by the drug and developed necrotic cytoplasm as well as degenerate mitochondria while others appeared unaffected (Figure 1A & B). The damage in the affected hepatocytes varied in degrees of cellular degeneration to necrosis (Figure 1B & C). The cytoplasm of some cells was granular and depleted (Figure 1B). Debris from necrotic cells could be found around the hepatocytes (Figure 1C). In some areas, the membrane of hepatocytes had severely deteriorated leading to cell contents entering sinusoids (Figure 1C & D, arrows).
Figure 1.
Nevirapine effects on hepatocytes. A & B: nevirapine-induced a heterogeneous pattern of damage in the liver where some hepatocytes appeared to have necrotic cytoplasm and degenerate mitochondria (DM) (see hepatocyte on the left) while others appeared mostly unaffected (hepatocyte on the right). C & D: cellular debris of necrotic cells (arrows) scattered around hepatocytes and in sinusoidal lumen (SL). L: lipid droplets; RBC: red blood cell.
Nevirapine-induced lipid-SER inclusions in hepatocytes
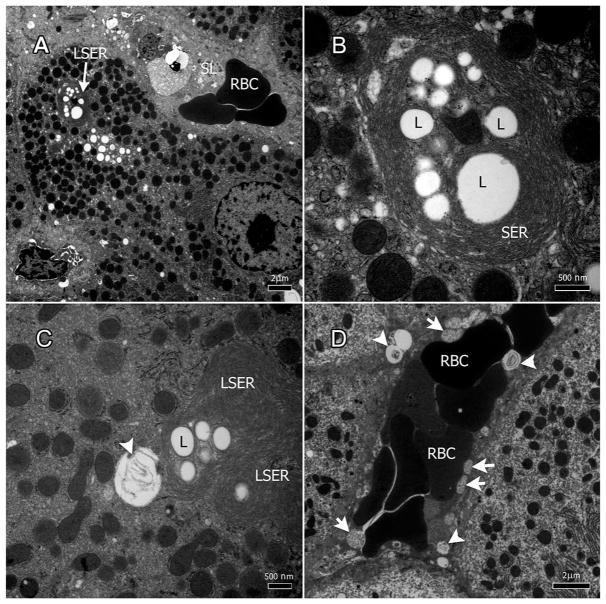
Large lipid droplets were present in hepatocytes throughout the specimens (Figure 2A–D). Concentric whirls of smooth endoplasmic reticulum (SER) cisternae surrounded a number of fat droplets forming a structure that we termed lipid-SER (LSER) inclusions (Figure 2A & B). The size of the lipid droplets within the LSER inclusions averaged 0.85 μm (±0.54). Some of the large lipid droplets may have resulted from the fusion of several smaller droplets; an LSER inclusion may have more than 10 lipid droplets (Figure 2C & D). Some LSER inclusions appeared with degenerate SER membranes inside them (Figures 1D and 2C & D, arrow heads). SER whorls were reduced or absent around LSER inclusions close to or within sinusoids (Figures 1D and 2D, arrow heads).
Figure 2.
Nevirapine induces lipid-SER (LSER) inclusions in hepatocytes. A–C: Concentric whirls of smooth endoplasmic reticulum (SER) surround lipid droplets to produce LSER inclusions. C: Some LSERs had degenerate SER membranes inside them. D. LSERs carrying internal degenerate SER (arrow heads) and degenerate mitochondria (arrows) within the sinusoidal lumen.
Nevirapine affected the endothelium and sinusoids
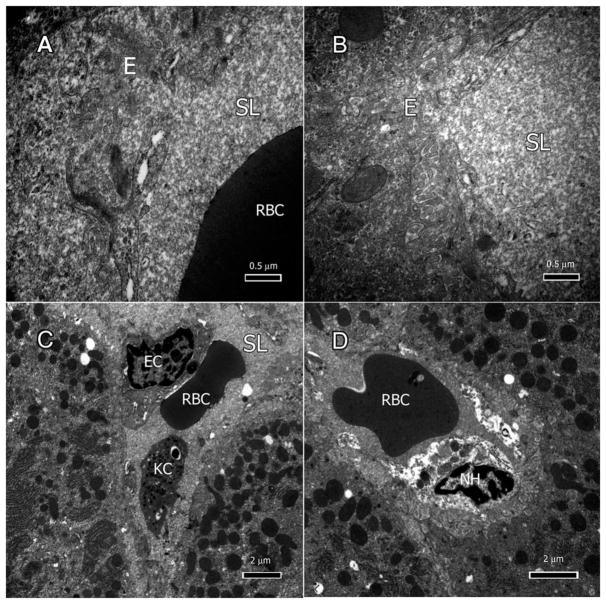
All the liver biopsies from treated rats displayed varying degrees of endothelial abnormalities (Figure 3A–D). The sinusoidal endothelium exhibited signs of degeneration, and most lacked a well-defined cellular membrane (Figure 3A & B). Some Kupffer cells (KCs) and loose ECs with hypertrophic nuclei were seen in the sinusoid (Figure 3C). Necrotic hepatocytes and their contents were present in the sinusoidal lumen (Figure 3D).
Figure 3.
Nevirapine effects on endothelium and sinusoids. A: Degenerate sinusoidal endothelium (E) lacking cytoplasmic membrane. B: Sinusoids filled with dense amorphous proteinaceous material. C: Kupffer cell (KC) and endothelial cell (EC) in the sinusoid. D: Necrotic hepatocytes (NH) and their contents in the sinusoidal lumen.
Nevirapine affected mitochondria of the hepatocytes and endothelium
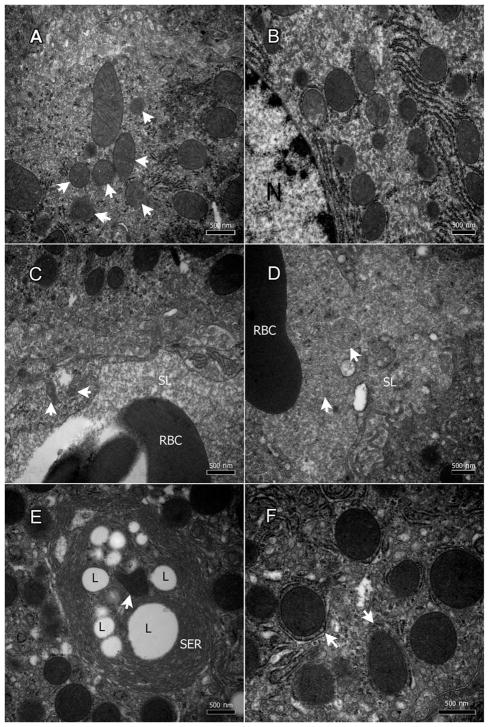
Liver specimens displayed varying degrees of mitochondrial degeneration in both the hepatocytes (Figure 4A & B, arrows) and ECs (Figure 4C, arrows). In the hepatocytes, few mitochondria appeared normal with distinct intact inner and outer membranes as well as defined cristae formations (Figure 4A). The majority had damaged inner (and sometimes outer) membranes at different stages of degeneration as well as advanced deterioration (Figure 4B). In some hepatocytes, the mitochondria were surrounded by extra membranes indicating the formation of what could be autophagosomes (Figure 4F, arrows).
Figure 4.
Nevirapine damages the mitochondria of the hepatocytes and the endothelium. A & B: degenerate mitochondrial (mostly the inner membrane) in the hepatocytes (arrows), and C: endothelial cells (arrows). D: degenerate mitochondria within the sinusoidal lumina (arrows). E: a mitochondrion within LSER inclusions (arrow). F: Mitochondria surrounded by extra membranes indicating the formation of what could be autophagosomes.
In the ECs, most mitochondria were completely degenerate, and others were in varying states of deterioration (Figure 4C); however, few remaining endothelial mitochondria were healthy with well-defined membranes and cristae (Figure 4C). Some degenerate mitochondria were seen in the sinusoidal lumina (Figure 4D), and others within the LSER inclusions (Figure 4E, arrow).
Discussion
Although NNRTIs, such as NVP, are known to cause hepatotoxicity6,19, the details of their ultra-structural effects have not been studied; therefore, the entire spectrum of their physiological interactions has not been completely revealed. NVP induces a hypersensitivity reaction that is characterized by fever, abnormal liver enzymes, and skin rash.9 Various mechanisms of injury have been postulated regarding NVP-induced liver damage and its induction of skin rash.14,17,20 Furthermore, certain genetic variations in CCHCR1 and HLA genes are strongly associated with NVP-induced rash.11,12 In this study, we focused on the detailed ultrastructural changes in rat livers that developed severe skin lesions due to an idiosyncratic reaction to NVP, thus, our report adds to the existing hypotheses about the possible mechanisms of NNRTI-induced liver injury and skin rash.
It has been postulated that NVP’s adverse reactions appear to be immune-mediated and independent of HIV infections because, if anything, the risk is higher when the drug is used prophylactically in non-infected patients.20 The chemical species responsible for the rash and liver injury appear to be different. Specifically, the skin rash is caused by a reactive benzylic sulfate formed in the skin as evidenced by the fact that a topical sulfotransferase inhibitor prevented covalent binding in the skin and rash where it was applied.13 Mice do not develop a skin rash, and they appear to lack the required sulfotransferase. In contrast, most of the covalent binding of NVP in the liver and involves direct oxidation of NVP to a reactive quinone methide. The covalent binding in the liver is quite extensive and leads to inhibition of cytochromes P450 followed by a compensatory induction of P450 synthesis.
Of the several effects of NVP on the liver that we describe in this survey, the most striking is the formation of large lipid droplet inclusions surrounded by SER cisternae, referred to here as lipid-SER (LSER) inclusions. Hepatocytes are rich in SER whose enzymes detoxify many lipid-soluble compounds.21 LSER inclusions have been described in benign hepatomas and well differentiated hepatocellular carcinomas.21 Liver hepatocytes do not seem to carry out any further processing of LSERs, but rather expels them into the blood stream where they will end up in the intestines or excreted through the skin.22 Furthermore, LSERs may be picked up by lymph nodes and contribute to initiation of an immune response leading to serious liver injury or skin rash. Aside from the obvious lipid content of LSER, their exact chemical content is not yet known; however, it is assumed that they contain reactive derivative(s) of NVP. Therefore, the formation of LSER inclusions appears to be a mechanism of sequestration and evacuation of NVP or its metabolites from hepatocytes.
NVP has been identified as a lipid soluble and hydrophobic compound.23 In hepatocytes, NVP is converted through the induction of CYP enzymes (such as 3A4 and 2B6) into 2-, 3-, 8-, and 12-hydroxynevirapine followed by the glucuronidation of these hydroxyl metabolites; the glucuronides are secreted in the blood stream and exit the body through urinary excretion.24–26 The production of LSER inclusions may be due to the failure of the hepatocyte to glucuronidate one of hydroxynevirapines.
Mechanisms of drug sequestration may vary depending on the organ, and are often sequestered within autophagosomes, endoplasmic reticulum, lysosomes, and mitochondria.27–29 Transporters within the liver are known to sequester substrates into subcellular compartments within hepatocytes.27 We have previously suggested that ART medications induced large lipid droplets within human hepatocytes and that certain ART medications may be sequestered in the lipid droplets. However, these lipid droplets were not surrounded by SER membranes.6
The results of our ultrastructural analysis of the liver specimens revealed aberrations within the hepatocytes and sinusoidal endothelium that were induced by NVP. This deterioration was similar to other HAART-induced hepatotoxicity.6 Hepatocyte injury was demonstrated by the presence of granular cytoplasm, abnormal lipid inclusions, and the prevalent destruction of mitochondrial inner membrane. The latter is characteristic of mitochondrial toxicity due to both the NRTIs and non-nucleoside drugs such as NVP in HAART.5,6
The damage to the liver sinusoidal ECs found in the specimens is possibly a characteristic of anti-retroviral-induced liver injury. Degeneration was severe in parts of the specimens, while endothelial damage was prevalent throughout the specimens.
The origin of the LSER inclusions and the role they play in the NVP metabolism in the liver seemed to differ from those of the fatty droplets that appear under hypoxic conditions.6 The latter formed when a cell fails to metabolize acetyl-coA in the tricarboxylic acid (TCA) cycle, and instead used it to synthesize fatty acids in the cytoplasm which are then stored in lipid droplets. LSER inclusions observed here were larger in size and visible with light microscopy7, while traditionally fatty droplets are smaller and can hardly be seen using light microscopy.6 LSER inclusions’ size, content, confinement by SER, and their migration to the blood stream indicated a novel mechanism of drug exocytosis.
Although we first reported on the NVP-induced LSER inclusion, we did not elaborate on their damaging effects to the hepatocytes and endothelium as well as their possible role in causing skin rash.7 Due to their relatively large size as well as their entry into the blood stream, we think that LSER inclusions may be part of a NNRTI sequestration and evacuation mechanism. This provides an insight into the mechanisms of the idiosyncratic reaction to NVP. Furthermore, early formation of LSER inclusions in the liver could be a predictor of the idiosyncratic reaction in the patients. This study offers valuable progress in the research of NNRTI damage and ultrastructural elucidation of the reaction mechanisms that the liver carries out during drug metabolism.
Acknowledgments
Funding
The research was funded by the intramural program of the National Eye Institute, NIH, Bethesda, MD, USA.
Footnotes
Financial Disclosures
The authors declare they have no conflicting financial interests.
References
- 1.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377(9772):1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 2.Sherman KE, Rockstroh J, Thomas D. Human immunodeficiency virus and liver disease: an update. Hepatology. 2015;62(6):1871–1882. doi: 10.1002/hep.28150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurlimann D, Weber R, Enseleit F, Luscher TF. HIV infection, antiretroviral therapy, and endothelium. Herz. 2005;30(6):472–480. doi: 10.1007/s00059-005-2740-3. [DOI] [PubMed] [Google Scholar]
- 4.Ryom L, Lundgren JD, De Wit S, et al. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. Aids. 2016;30(11):1731–1743. doi: 10.1097/QAD.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 5.Paemanee A, Sornjai W, Kittisenachai S, et al. Nevirapine induced mitochondrial dysfunction in HepG2 cells. Sci Rep. 2017;7(1):9194. doi: 10.1038/s41598-017-09321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chwiki S, Campos MM, McLaughlin ME, et al. Adverse effects of antiretroviral therapy on liver hepatocytes and endothelium in HIV patients: an ultrastructural perspective. Ultrastruct Pathol. 2017;41(2):186–195. doi: 10.1080/01913123.2017.1282066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenton JM, Teranishi M, Abu-Asab MS, Yager JA, Uetrecht JP. Characterization of a potential animal model of an idiosyncratic drug reaction: nevirapine-induced skin rash in the rat. Chem Res Toxicol. 2003;16(9):1078–1089. doi: 10.1021/tx034064+. [DOI] [PubMed] [Google Scholar]
- 8.Pollard RB, Robinson P, Dransfield K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin Ther. 1998;20(6):1071–1092. doi: 10.1016/s0149-2918(98)80105-7. [DOI] [PubMed] [Google Scholar]
- 9.Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis. 2007;11(3):615–39. vii–viii. doi: 10.1016/j.cld.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Carr DF, Bourgeois S, Chaponda M, et al. Genome-wide association study of nevirapine hypersensitivity in a sub-Saharan African HIV-infected population. J Antimicrob Chemother. 2017;72(4):1152–1162. doi: 10.1093/jac/dkw545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19(2):139–146. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 12.Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. Genome-wide association study identifies variations in 6p21.3 associated with nevirapine-induced rash. Clin Infect Dis. 2011;53(4):341–348. doi: 10.1093/cid/cir403. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AM, Novalen M, Tanino T, Uetrecht JP. 12-OH-nevirapine sulfate, formed in the skin, is responsible for nevirapine-induced skin rash. Chem Res Toxicol. 2013;26(5):817–827. doi: 10.1021/tx400098z. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Mannargudi BM, Xu L, Uetrecht J. Demonstration of the metabolic pathway responsible for nevirapine-induced skin rash. Chem Res Toxicol. 2008;21(9):1862–1870. doi: 10.1021/tx800177k. [DOI] [PubMed] [Google Scholar]
- 15.Mak A, Uetrecht J. The combination of anti-CTLA-4 and PD1-/- mice unmasks the potential of isoniazid and nevirapine to cause liver injury. Chem Res Toxicol. 2015;28(12):2287–2291. doi: 10.1021/acs.chemrestox.5b00305. [DOI] [PubMed] [Google Scholar]
- 16.Kato R, Uetrecht J. Supernatant from hepatocyte cultures with drugs that cause idiosyncratic liver injury activates macrophage inflammasomes. Chem Res Toxicol. 2017;30(6):1327–1332. doi: 10.1021/acs.chemrestox.7b00065. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Sharma AM, Uetrecht J. Identification of danger signals in nevirapine-induced skin rash. Chem Res Toxicol. 2013;26(9):1378–1383. doi: 10.1021/tx400232s. [DOI] [PubMed] [Google Scholar]
- 18.Ogilvy AJ, Shen D, Wang Y, Chan CC, Abu-Asab MS. Implications of DNA leakage in eyes of mutant mice. Ultrastruct Pathol. 2014;38(5):335–343. doi: 10.3109/01913123.2014.927406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morse CG, McLaughlin M, Matthews L, et al. Nonalcoholic steatohepatitis and hepatic fibrosis in HIV-1-monoinfected adults with elevated aminotransferase levels on antiretroviral therapy. Clin Infect Dis. 2015;60(10):1569–1578. doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho T, Uetrecht J. How reactive metabolites induce an immune response that sometimes leads to an idiosyncratic drug reaction. Chem Res Toxicol. 2017;30(1):295–314. doi: 10.1021/acs.chemrestox.6b00357. [DOI] [PubMed] [Google Scholar]
- 21.Erlandson RA. Diagnostic Transmission Electron Microcsopy of Tumors: With Clinicopathological, Immunohistochemical, and Cytogenetic Correlations. New York, USA: Raven Press; 1994. p. xvii.p. 857. [Google Scholar]
- 22.Zhou SS, Li D, Zhou YM, Cao JM. The skin function: a factor of anti-metabolic syndrome. Diabetol Metab Syndr. 2012;4(1):15. doi: 10.1186/1758-5996-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chudasama AS, Patel VV, Nivsarkar M, Vasu KK, Shishoo CJ. In vivo evaluation of self emulsifying drug delivery system for oral delivery of nevirapine. Indian J Pharm Sci. 2014;76(3):218–224. [PMC free article] [PubMed] [Google Scholar]
- 24.Popovic M, Shenton JM, Chen J, et al. Handb Exp Pharmacol. Vol. 196. Springer; Berlin, Heidelberg: 2010. Nevirapine hypersensitivity; pp. 437–451. [DOI] [PubMed] [Google Scholar]
- 25.Riska P, Lamson M, MacGregor T, et al. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos. 1999;27(8):895–901. [PubMed] [Google Scholar]
- 26.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27(12):1488–1495. [PubMed] [Google Scholar]
- 27.Chu X, Korzekwa K, Elsby R, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94(1):126–141. doi: 10.1038/clpt.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo B, Tam A, Santi SA, Parissenti AM. Role of autophagy and lysosomal drug sequestration in acquired resistance to doxorubicin in MCF-7 cells. BMC Cancer. 2016;16(1):762. doi: 10.1186/s12885-016-2790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhitomirsky B, Assaraf YG. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat. 2016;24:23–33. doi: 10.1016/j.drup.2015.11.004. [DOI] [PubMed] [Google Scholar]