Abstract
We report a novel phase 2 clinical trial in patients with poor prognosis refractory non-Hodgkin lymphoma (NHL) testing the efficacy of haploidentical donor natural killer (NK) cell therapy (NK dose 0.5–3.27 × 107 NK cells/kg) with rituximab and IL-2 (clinicaltrials.gov NCT01181258). Therapy was tolerated without graft-versus-host disease, cytokine release syndrome, or neurotoxicity. Of 14 evaluable patients, 4 had objective responses (29%; 95% CI 12–55%) at 2 months: 2 had complete response lasting 3 and 9 months. Circulating donor NK cells persisted for at least 7 days after infusion at the level 0.6–16 donor NK cells/µl or 0.35–90% of total CD56 cells. Responding patients had lower levels of circulating host-derived Tregs (17 ± 4 vs. 307 ± 152 cells/µL; p = 0.008) and myeloid-derived suppressor cells at baseline (6.6 ± 1.4% vs. 13.0 ± 2.7%; p = 0.06) than non-responding patients. Lower circulating Tregs correlated with low serum levels of IL-10 (R 2 = 0.64; p < 0.003; n = 11), suggestive of less immunosuppressive milieu. Low expression of PD-1 on recipient T cells before therapy was associated with response. Endogenous IL-15 levels were higher in responders than non-responding patients at the day of NK cell infusion (mean ± SEM: 30 ± 4; n = 4 vs. 19.0 ± 4.0 pg/ml; n = 8; p = 0.02) and correlated with day 14 NK cytotoxicity as measured by expression of CD107a (R 2 = 0.74; p = 0.0009; n = 12). In summary, our observations support development of donor NK cellular therapies for advanced NHL as a strategy to overcome chemoresistance. Therapeutic efficacy may be further improved through disruption of the immunosuppressive environment and infusion of exogenous IL-15.
Electronic supplementary material
The online version of this article (10.1007/s00262-017-2100-1) contains supplementary material, which is available to authorized users.
Keywords: Adoptive transfer NK cells, Cellular therapy, Chemotherapy-refractory non-Hodgkin lymphoma, IL-2, Immunosuppressive environment
Introduction
Patients with chemotherapy-refractory non-Hodgkin lymphoma (NHL) have poor prognosis [1]. Recent advances in cellular therapies and immunotherapies suggest that targeting tumor with an activated immune system overcomes chemoresistance, and early clinical experience with these approaches has yielded remarkable therapeutic success [2]. Evidence suggests that the immunosuppressive environment surrounding cancer cells contributes to the poor efficacy of therapeutic antibodies and chemotherapy [3]. We hypothesized that natural killer (NK) cells in patients with refractory NHL exhibit poor function with impaired capacity to mediate direct cytotoxic and antibody-mediated killing [4, 5]. NK cells are innate effectors, residing in lymphoid tissues, spleen and peripheral blood at low frequency (5–10% of lymphocytes). Immunophenotypically defined by expression of CD56 and the lack of CD3, NK cells express a variable combination of activating and inhibitory receptors that bind cognate HLA and HLA-independent ligands and exhibit killing of viral-infected or malignant transformed cells with downregulated self-class I MHC [6]. Several known triggers engage NK cells and transform them into powerful cellular killers including cytokines such as IL-2 or IL-15 and antibodies. The most powerful NK cell activating signal is triggered by antibody Fc binding CD16 receptor, which mediates robust target-cell killing with antigen specificity called antibody-dependent cell-mediated cytotoxicity (ADCC) [7]. NK cell killing is either direct (measured by CD107a expression after exposure to target) or mediated by secretion of cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNFα). We hypothesized that infusion of activated allogeneic donor-derived NK cells combined with CD20-targeting monoclonal antibody rituximab and IL-2 would result in anti-tumor responses against lymphoma expressing CD20 and overcome chemotherapy resistance. We report the results of a phase 2 clinical trial testing the efficacy of haploidentical donor NK cell infusions plus rituximab for refractory NHL.
Patients and methods
Eligibility criteria and treatment
We conducted a prospective clinical trial approved by the Institutional Review Board (IRB) of the University of Minnesota (clinicaltrials.gov NCT01181258). Eligible patients had multiple relapsed or refractory NHL or chronic lymphocytic leukemia expressing CD20, Karnofsky performance status (KPS) ≥ 80%, and adequate organ function. All patients were evaluated by positron emission tomography (PET) within 4 weeks of treatment and at 2 months post-NK cell infusion. We confirmed CD20 expression by immunohistochemistry on tumor biopsy at the time of last relapse or at the time of enrollment. Treatment consisted of lymphodepleting chemotherapy pentostatin 3.75 mg × 2 days, (n = 6), cyclophosphamide (60 mg/kg IV day − 5; n = 6) and IL-2 receptor-targeting agent to deplete regulatory T cells (Treg) denileukin diftitox (18 μg/kg/day IV day − 1; n = 5). We prospectively planned to increase the dose of denileukin diftitox to 18 μg/kg/day IV × 3 days (day − 4, − 3, − 2) for lack of Treg depletion (defined as no increase in Treg at day 14 compared to pre-therapy) in first 5 patients. After we enrolled 1 more patient, denileukin was withdrawn from the market (and our study) by the manufacturer (Eisai). We subsequently amended the protocol and replaced the lymphodepleting therapy with fludarabine 25 mg/m2 × 4 days (n = 10), cyclophosphamide (60 mg/kg IV day − 5; n = 10) and methylprednisolone (1 mg/kg day − 2 through day + 9; n = 10) with the aim to enhance NK cell function (Supplementary Figure 1). All patients received rituximab 375 mg/m2 IV weekly × 4 doses starting day − 8. The NK-enriched cellular product was infused intravenously on day 0 followed by subcutaneous infusion of IL-2 at 9 million IU every other day × 6 doses.
Cellular product and NK cell evaluation
Donor selection criteria included HLA-haploidentical-related donor aged 12–75 years who is able and willing to undergo leukapheresis. We preferred younger donors if multiple were available. HLA-haploidentical-related donors underwent 5-h lymphapheresis with COBE Spectra Apheresis System (TerumoBCT, Lakewood, CO, USA). Apheresis product was depleted of T cells (CD3) and B cells (CD19) using the Miltenyi Biotec CliniMACS® Cell Selection System and CD3 and CD19 MicroBeads and reagent (Miltenyi Biotec, Auburn, CA, USA) and cultured in serum-free media (X-VIVO 15, Cambrex BioScience, Walkersville, MD, USA) supplemented with 10% human AB serum and 1000 U/mL IL-2 (Chiron Corporation, Emeryville, CA, USA) [8]. After 8–16 h of incubation, cells were washed twice with a 5% human serum albumin. An aliquot of the cell product was analyzed by flow cytometry to determine the number of T, B, and NK cells, and NK cell phenotype (fluorochrome-conjugated antibodies detailed in Supplementary Table 1). The planned target NK cell dose to be infused was between 1.5 and 8 × 107/kg. Lot release criteria for allogeneic IL-2-activated NK cell product (BB-IND 8847) included viability > 70%, NK cell (CD56 + CD3−) >20%, T cell content ≤ 3.0 × 105/kg, B cell content < 3%, endotoxin < 5EU/kg and no organism by Gram stain. If product contained less then 1.5 × 107 kg or < 20% NK cells, than all cells were given but NK expansion outcome was reported separately. In vivo circulating peripheral blood (PB) donor-derived NK cells were examined in fresh samples in all evaluable patients and enumerated as absolute lymphocyte count/μL × % CD56+/CD3− NK cells (in lymphocyte gate) × % whole blood donor chimerism using standard very short tandem repeat (VNTR) testing from the whole blood. In recipient–donor pairs differentially expressing HLA-A2 or HLA-A24 antigens, we performed peripheral blood (PB) flow cytometry analyzing donor-specific HLA alleles to quantify the donor NK cells. For each recipient, we determined panel reactive antibody (PRA) by Flow/Luminex assay: flow bead immunoassays utilized purified antigen coated on latex beads as targets for binding of specific antibody in human serum. PRA was correlated with the level of donor NK cell expansion and clinical response.
Sample collection for correlative studies
We collected PB at baseline, after chemotherapy, and on days 3, 7, 14, and 28. We processed mononuclear cells (PBMC) by Ficoll method and stored cells in liquid nitrogen and serum at − 80 °C. For comparison, we also obtained PB from healthy volunteers and newly diagnosed patients with NHL prior to receiving any therapy. All volunteer subjects and patients signed an IRB-approved consent for study of their PB samples.
Flow cytometry analysis and ELISA
Cryopreserved PBMCs were rested overnight in RPMI medium to recover from freezing and then cultured for 6 h in the presence of target cells (K562) and IL-12 (5 ng/ml) and IL-18 (50 ng/ml) prior to staining. Cells were stained with fluorochrome-conjugated antibodies detailed in Supplementary Table 1. Detection of CD107a (degranulation), Ki67 (proliferation), and IFN-γ production was performed after fixation and permeabilization (eBioscience) according to the manufacturer’s instructions. Tregs were defined by the expression of Foxp3 and elevated CD25. Myeloid-derived suppressor cells were defined as CD33+CD14+HLA-DR−/Low. All cells were acquired by LSRII and analyzed by FlowJo 10.0. Plasma levels of IL-2, IL-15, IL-10, and IL-2R were performed on Luminex-platform (EMD Millipore, Billerica, MA, USA), and IL-6 was determined by commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA). Correlative studies were performed on cryopreserved samples for 12 patients with stored full-paired sample set (two patients had missing samples).
Study definitions and statistical methods
The primary objective of the prospective trial was overall response rate at 2 months. Secondary objectives were safety and tolerability of this therapy and in vivo expansion of allogeneic NK cells. All patients were evaluated by positron emission tomography/computed tomography (PET/CT) and bone marrow biopsy (if prior marrow involvement) per recent criteria for NHL [9]. Trial size calculation was based on Simon method using a minimax two-stage design with early stopping rule if less than 20% of patients achieve an objective response and a 50% response was a rate worthy of further study. The maximum sample size of 17 patients was sufficient to maintain an overall type I error of 5% while providing 80% statistical power. The first enrollment included 6 patients with an early stop if 2 or fewer patients respond. With January 2012, revision of the preparative regimen removed denileukin diftitox (taken off the market by Eisai) and added low-dose methylprednisolone (1 mg/kg days − 2 to + 9); stage 1 enrollment was restarted. Response in < 6 patients or <25% of patients achieve NK cell expansion was considered not worthy of further study. Cell frequencies were summarized using descriptive statistics. Statistical analysis was performed using a paired or unpaired Student t test and Pearson correlation (Graphpad Prism, version 6; ElCamino, CA). p values from secondary endpoints were subject to multiple comparison adjustment using the false-discovery rate (FDR) [10]. A p value < 0.05 (raw p value and FDR) was considered statistically significant. Representative histograms or images were chosen based on average values ± SEM.
Results
Patient, disease and product characteristics
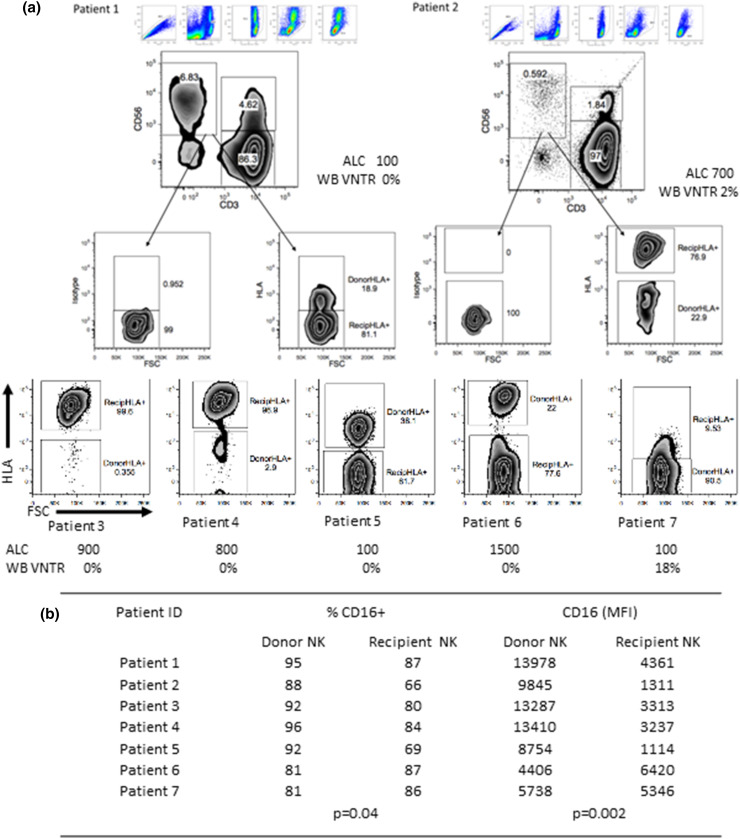
Sixteen patients were enrolled in the study (Table 1) between 10/19/2010 and 7/9/2015. Patients had a median age of 52 years (range 48–67) and 13 were males. Diffuse large B cell lymphoma was the most common diagnosis (n = 11; 5 transformed and 6 de novo). All patients were rituximab and chemotherapy refractory and failed at least 3 prior therapies. Most patients had B-symptoms and extranodal disease at the time of treatment. The cellular product was infused with a median total nuclear cell (TNC) dose of 4.3 × 107/kg (range 1.9–7.7) and contained 40.2% NK cells (median; range 12–88%) and 48% monocytes (median, range 12–63%). The final product characteristics are detailed in Table 1. Clinical product NK cell characteristics included high expression of CD16 (median 86%) and high cytotoxicity against K562 targets. Final product NK cells produced higher IFNγ, and had higher proliferation compared to before activation (Supplementary Figure 2a). Expression of NKp44, NKp46, CD69, TIGIT and PD1 on the activated NK product and NK cell inhibitory receptor expression are detailed in Supplementary Figure 2 and Supplementary Table 2.
Table 1.
Patient, disease, and treatment characteristics (included are all patients by intention to treat)
| N = 16 | |
|---|---|
| Age median (range) | 52 (48–67 years) |
| Gender: male/female | 13/3 |
| Diagnosis | |
| Diffuse large B cell lymphoma de novo | 6 |
| Diffuse large B cell lymphoma transformed | 5 |
| Mantle cell lymphoma | 2 |
| Follicular lymphoma | 1 |
| Lymphoplasmacytic lymphoma | 1 |
| Chronic lymphocytic leukemia with 17p del | 1 |
| Disease status | |
| Refractory with prior remission | 5 |
| Primary refractory | 11 |
| Disease stage | |
| I–II | 3 |
| III–IV | 13 |
| B-symptoms present | 10 |
| Extranodal disease present | 12 |
| Bone marrow involved | 8 |
| IPI at the time of treatment | |
| 1–2 | 4 |
| 3 | 7 |
| 4–5 | 5 |
| Prior therapy | |
| > 3 prior lines of therapy | 12 |
| Prior radiation therapy | 4 |
| Prior autologous HCT | 2 |
| Donor | |
| Haploidentical child | 10 |
| Haploidentical sibling | 5 |
| Lymphodepleting chemotherapy | |
| Rituximab/Cy/pentostatin/denileukin difitox | 6 |
| Rituximab/Cy/fludarabine/methylPND | 10 |
| TNC/kg apheresis (median; range) | 3.2 × 108 (1.15–4.45) |
| Infused IL-2-activated product | 4.3 × 107 (1.9–7.7) |
| TNC dose/kg (median; range) | |
| Infused NK cells/kg (median; range) | 1.9 × 107 (0.5–3.27) |
| Infused NK % (median; range) | 40.2% (12–88%) |
| Infused monocytes % (median; range) | 48.4% (12–63%) |
| Infused CD3 cell dose median (range) | 4.8 × 104 (0.19–18) |
| Infused CD3% (median; range) | 0.13% (0.02–0.33%) |
Cy cyclophosphamide, TNC total nucleated cell, NK natural killer, HCT hematopoietic cell transplantation, f/u follow-up
Clinical response assessment
Clinical outcomes are summarized in Table 2. Fifteen patients were evaluable and 14 had clinical response assessment on day 28. Four responded (28.5%) including two complete responses and two partial responses at 2 months. Three responding patients with extensive bulky disease at the time of enrollment had robust tumor regressions (≥ 80%). One patient developed tumor lysis syndrome and required rasburicase. Three responding patients proceeded to allogeneic donor transplantation in sustained remission at 2, 3, and 9 months after NK cell therapy. There was no correlation between the clinical response and infused cell dose between responders (NK cell dose 1.6 × 107/kg; T cell dose 4.5 × 104) and non-responders (NK cell dose 2.1 × 107/kg; T cell dose 4.8 × 104). Notably one of the responding patients received low NK cell dose (1.15 × 107/kg, less than the planned target cell dose).
Table 2.
Clinical response evaluation of 14 evaluable subjects
| Overall response at 2 months | 4 (28%) |
| Complete response* | 2 |
| Partial response* | 2 |
| Progressive disease | 10 |
| Subsequent allogeneic HCT | 3 (2, 3, 8 months post-NK therapy) |
| NK cell detected in PB day 7 post-infusion | |
| Flow cytometry assay for donor HLA | 7/7 (positive/tested) |
| VNTR assay for donor DNA chimerism | 3/14 (positive/tested) |
| Donor PB NK cell present at day 7 in responders | 75% (3 out of 4) |
HLA human leukocyte antigen, HCT hematopoietic cell transplantation, NK natural killer cells, PB peripheral blood
* Complete responses occurred in lymphoplasmacytic lymphoma and transformed lymphoma. Partial remissions occur in follicular lymphoma and diffuse large B cell lymphoma
We observed expected hematologic toxicity (NCI) common terminology criteria for adverse events (CTCAE) grade 1–3 thrombocytopenia, anemia and neutropenia in all subjects. Therapy was associated with NCI CTCAE non-hematologic grade 3 adverse effects (tumor lysis syndrome, infection due pneumocystis; Clostridium difficile colitis, neutropenic fever, thrombotic microangiopathy related to pentostatin). Two patients (out of 16) died before disease response assessment: one developed sepsis prior to NK cell infusion, and the second died of overwhelming culture-negative sepsis 10 days after NK cell infusion, both deaths are possibly attributed to study therapy. IL-6 levels at day 7 in all 15 patients varied from 3.99 to 21.5 pg/ml; these levels did not suggest cytokine release syndrome. We observed no symptoms or signs of graft-versus-host disease, neurotoxicity, or persistent marrow aplasia.
NK cells in patients with refractory NHL are hypofunctional
As compared to healthy controls, NK cells in patients with newly diagnosed NHL (not enrolled to this trial) and chemorefractory NHL (all enrolled to this trial) exhibited poor degranulation capacity (CD107a) and impaired production of pro-apoptotic cytokines IFNγ (Fig. 1a). Moreover, the expression of CD16, which mediates ADCC, was lowest in patients with refractory NHL (Fig. 1a). NK cells from patients with NHL expressed higher levels of the suppressive receptor “T cell immunoreceptor with Ig and ITIM domains” (TIGIT) and lower levels of the T cell immunoglobulin mucin-3 (TIM-3) receptor with activating function on NK cells compared to healthy controls (Fig. 1b) [11–13]. NK cell expression of programmed cell death 1 receptor (PD1) was below 2% in patients and controls.
Fig. 1.
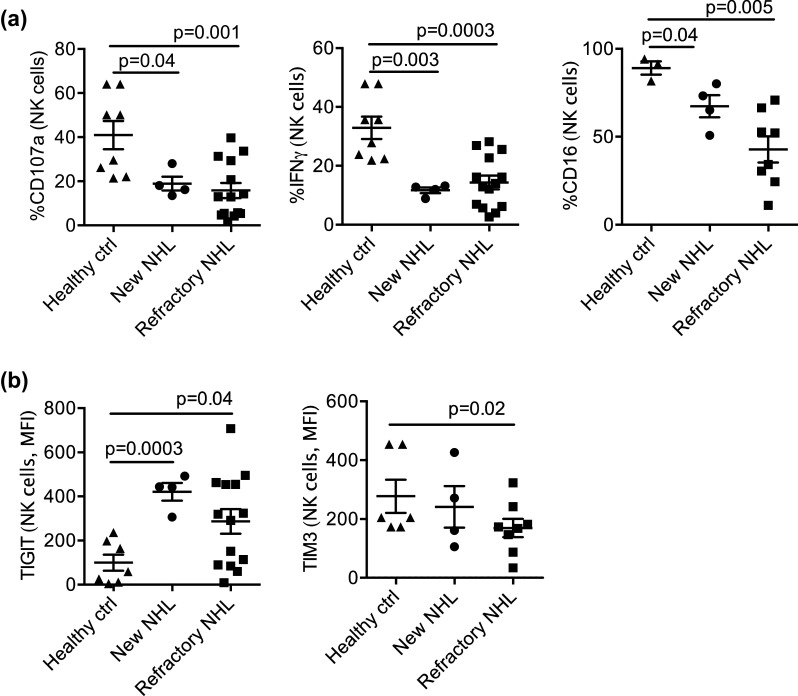
NK cell phenotype and function in patients with relapsed/refractory lymphoma as compared to newly diagnosed NHL patients and healthy controls. a PBMC from NHL patients (n = 14) and healthy controls (n = 8) were rested overnight in medium and stimulated with target cells (K562, 20:1) and cytokines 6 h prior to staining. NK cell degranulation (CD107a), IFNγ production, and frequency of CD16-expressing NK cells were evaluated by flow cytometry. b Overnight-rested PBMCs from newly diagnosed (n = 4) or refractory NHL (8–14) and healthy controls (n = 6–7) were stained for TIGIT and TIM3 and evaluated by flow cytometry. Data are shown as mean ± SEM, and statistical analyses were done on pooled data using the Student’s unpaired t test. Each symbol represents an individual donor. New NHL newly diagnosed NHL
Transient donor NK cell persistence and function correlated with endogenous IL-15
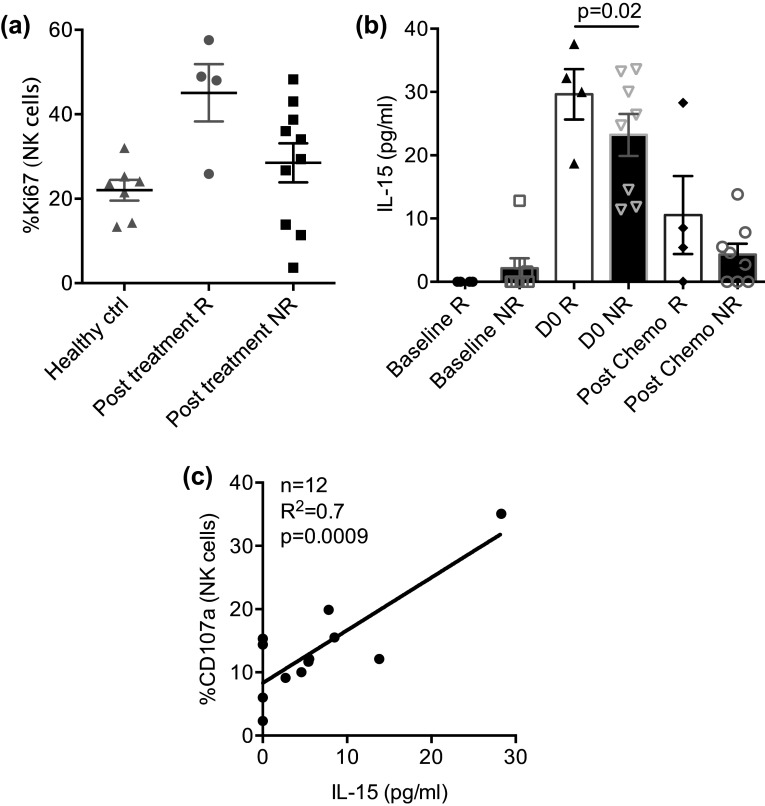
We determined the frequency of circulating donor NK cells at defined time points by two methods designed to differentiate recipient vs. donor by interrogating DNA (VNTR) or HLA expression (flow cytometry). Donor NK cells persisted in the PB for at least a week after infusion (in 7 of 7 tested patients with available antibodies against donor-specific HLA alleles) ranging between 0.6 and 16 cells/µl (median 3 cells/µl), and 0.35–90% of PB NK cells exhibited a donor-specific HLA phenotype (Fig. 2a). We separately analyzed 3 patients infused with < 1.5 × 107 TNC/kg (outside of lot release criteria goal) and determined that all 3 had evidence of donor cells by HLA-specific immunophenotype (Patients 3, 5, 7). Donor-derived NK cells exhibited higher CD16 expression compared with patients’ NK cell CD16 (mean MFI ± SEM: 9900 ± 1460 vs. 3590 ± 740, p = 0.002, FDR = 0.007 Fig. 2b). Whole blood VNTR detected donor DNA in 3 patients out of 14 tested samples at day 7 at levels 2, 8, 18% donor chimerism, suggesting lower sensitivity as compared to HLA allele-directed flow cytometry. In one responding patient, donor NK cells were sustained beyond day 28 (3% on DNA chimerism by VNTR). Although not significant, NK cells proliferated more vigorously in responders as compared to non-responders (day 14: Ki67 mean ± SEM: 45.1 ± 7.0, n = 4 vs. 28.5 ± 4.6, n = 10; p = 0.07, FDR = 0.01; Fig. 3a); yet, PB NK cell function as measured by CD107, IFNγ, and TNFα was similar in responders and non-responders at baseline and after treatment (Supplementary Figure 3). Endogenous IL-15 serum levels peaked at the time of NK cell infusion (range 11–33 pg/dl). Responders had higher IL-15 levels as compared to non-responders at day 0 (mean ± SEM: 30.0 ± 4.0 (n = 4) vs 19.0 ± 4.0 pg/ml (n = 8); p = 0.02, FDR = 0.04) but not day 14 post-chemotherapy (10.5 ± 6.0 (n = 4) vs 4 ± 2 pg/ml (n = 8; p = NS) (Fig. 3b). Serum IL-15 levels correlated with functional NK cell killing at day 14 as measured by expression of CD107a (R 2 = 0.74; p = 0.0009, FDR = 0.006 (adjusted slope); n = 12, Fig. 3c), suggesting that higher endogenous IL-15 improved NK cell cytotoxic capacity. We next examined the effect of pre-existing recipient anti-HLA antibodies on donor NK cells’ persistence. Fifteen recipients were tested at baseline and 2 patients had high-level donor HLA allele-specific antibodies. Both patients with high titer PRA exhibited donor NK cell expansion at day 7 (10 and 6 NK cells/µL) and one was a responder, suggesting lack of an adverse effect of PRA on NK cell persistence and clinical outcomes.
Fig. 2.
Donor NK cells’ persistence. a Donor NK cell expansion in peripheral blood at day 7 depicted in histograms showing donor vs. recipient HLA gated on CD56+CD3− cells. All 7 subjects with differentiating donor/recipient HLA antigens (A2 or A24) are shown. Patients 1, 2 and 3 were responders; patients 4, 5 and 6 were non-responders and patient 7 died from neutropenic sepsis at day 10 after NK cell infusion. Absolute lymphocyte cell (ALC) in cells/µL and whole blood standard very short tandem repeat (WB VNTR) at day 7 after NK cell infusion is shown for each patient. b Donors’ and recipients’ CD16 expression 7 days following infusion are shown as mean ± SEM, and statistical analysis were done on pooled data using the Student’s unpaired t test
Fig. 3.
Correlation of response with endogenous IL-15. a PBMC from NHL patients (n = 14) and healthy controls (n = 7) were rested overnight in medium and evaluated for proliferation (Ki67) by flow cytometry. b Endogenous IL-15 serum levels were evaluated at different time points in NHL patients (n = 12) comparing responders and non-responders. Data are shown as mean ± SEM, and statistical analyses were done on pooled data using the Student’s unpaired t test. Each symbol represents an individual donor. c Correlation analyses (n = 12) evaluating the relationship between NK cell degranulation and endogenous IL-15 level after therapy (day 14). p value was calculated on adjusted slope
High expression of PD-1 and TIGIT correlates with lack of response
We next examined T cells from the NHL patients and random healthy donors for expression of key inhibitory checkpoints: programmed cell death receptor 1 (PD-1) and T cell immunoreceptor with Ig and ITIM domains (TIGIT) at baseline and at day 14 post-therapy. Responding patients had similar levels of PD-1 expression to healthy controls at baseline and day 14; however, non-responders exhibited significantly higher levels of PD-1 on T cells before and particularly after therapy. Similarly, T cell TIGIT expression was higher in non-responders at both time points (Fig. 4a, b). Separating PB T cells into CD3+CD4+ and CD3+CD8+, we observed a higher pattern of PD-1 and TIGIT expression on CD3+CD4+ cells and CD8+ T effectors in non-responders as compared to responders (Fig. 4b).
Fig. 4.
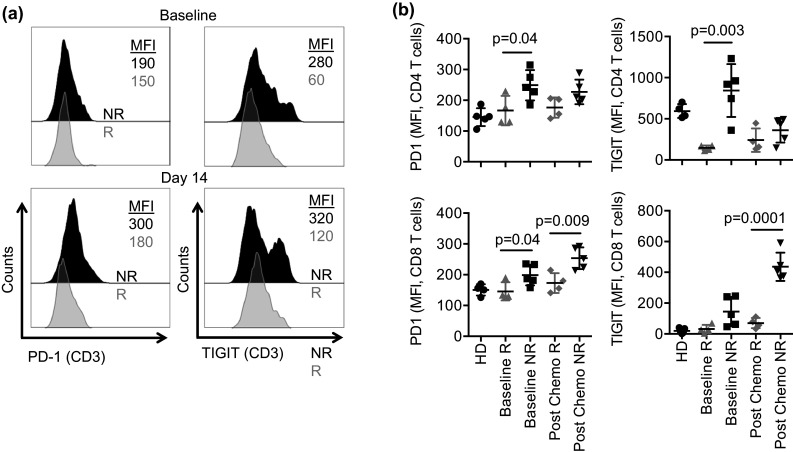
Expression of suppressive receptors on circulating T cells. a PBMC from NHL patients were rested overnight in medium and then staining for PD-1 and TIGIT and evaluated by flow cytometry. Representative histograms showing PD-1 and TIGIT expression in bulk T cells (CD3) in responders vs. non-responders before (upper panel) and 14 days after treatment (lower panel). b Different T cell subsets (CD4, and CD8) were evaluated for PD-1 and TIGIT expression in responders (R) and non-responders (NR). Data are shown as mean ± SEM, and statistical analyses were done on pooled data using the Student’s unpaired t test. Each symbol represents an individual donor
Low Treg and myeloid suppressor cell levels correlate with response
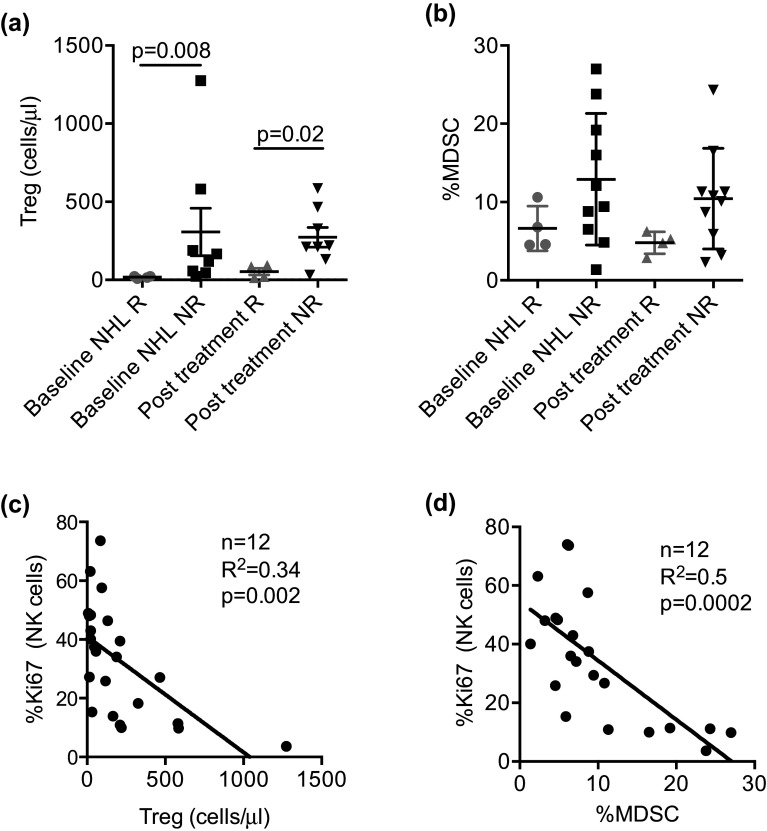
We next examined the impact of immune suppressor cells on NK cell expansion and clinical response. Responding patients (n = 4) had lower levels of absolute number of Treg at baseline (mean ± SEM: 17 ± 4 vs. 307 ± 152 cells/µL; p = 0.008, FDR = 0.02) and 14 days after therapy (53 ± 21 vs. 273 ± 63 cells/µL; p = 0.02, FDR = 0.04) as compared to non-responders (n = 8) (Fig. 5a). Treatment with denileukin diftitox did not result in change in PB Treg frequency (day 14 Treg with denileukin mean ± SEM: 240 ± 56 cells/µL vs. no denileukin 160 ± 90) and had no effect on in vivo NK cell expansion (data not shown). High PB Treg levels correlated with serum IL-10 (R 2 = 0.7; p < 0.0001, FDR = 0.001; n = 12) and IL-2 receptor-α (IL-2Rα R 2 = 0.4; p = 0.006, FDR = 0.02; n = 12), suggestive of an accentuated immunosuppressive milieu. Although not statistically significant, frequencies of PB myeloid-derived suppressor cells (MDSC) were low in responders and higher in non-responders at baseline (mean ± SEM: 6.6 ± 1.4% vs. 13 ± 2.7%) and after therapy (day 14 mean ± SEM: 4.8 ± 0.7%; vs. 10.0 ± 2.0%; Fig. 5b). Notably, low levels of circulating Tregs and MDSCs correlated with NK cell proliferation (n = 12, R 2 = 0.25; p = 0.035, FDR = 0.054 and R 2 = 0.5; p = 0.002, FDR = 0.007; Fig. 5c, d).
Fig. 5.
Circulating MDSC and regulatory T cell correlate with clinical response and NK cell proliferation. Circulating regulatory T cells and MDSC in NHL patients before and after therapy comparing responders (n = 4) and non-responders (n = 8–10). a, b PBMCs from NHL patients were rested overnight and stained, and then the frequencies of MDSCs and Tregs were determined by flow cytometry. Each symbol represents an individual donor. c, d Correlation analyses (n = 12) evaluating the relationship between NK cell proliferation and the numbers and frequency of Tregs and MDSCs in patients with NHL before and 14 days after treatment. Statistical analyses were done using Pearson correlation
Discussion
Our clinical experience using haploidentical NK cells with IL-2 and rituximab suggests that this therapy is well tolerated and produces remission in over 1/4th of highly refractory NHL patients. We showed a transient persistence of donor NK cells in most subjects and improved sensitivity of donor NK detection by flow cytometry for donor-specific DNA as compared to PCR techniques. Our data also show that autologous NK cells in refractory NHL patients exhibited poor function, express lower CD16, higher levels of the immunosuppressive receptor TIGIT and lower expression of activating receptor TIM3 as compared to NK cells from healthy controls. These findings suggest several potential mechanisms of immunotherapy resistance in patients with advanced disease. Monoclonal antibodies are often used to focus autologous NK cells to have tumor specificity; however, CD16 downregulation can render antibodies less effective. We showed that transient homeostatic expansion of highly functional CD16 expressing donor NK cells may be clinically effective in some refractory NHL patients.
While prior data demonstrated that the tumor microenvironment plays an important role in disease severity and clinical outcomes in B cell NHL, most studies examined the composition of intratumoral T cells, whereas here, we probed the blood compartment [14–16]. T cell exhaustion is a status of T cell immune response induced by viral infection or tumor which results in reduced function and proliferation [15]. Our findings suggest that refractory NHL patients have a highly suppressive immune environment characterized by increased expression of PD-1 and TIGIT on circulating T cells. In contrast, low baseline expression of PD-1 and TIGIT on CD8 cells and lower Tregs in the blood compartment was associated with improved clinical responses to adoptive NK cell transfer. Together, these findings highlight the role of the immunosuppressive milieu in modulating an anti-tumor response. TIGIT is a novel key inhibitory checkpoint protein expressed on both NK and T cells with wide variation in its expression among individuals [17, 18]. Non-responding patients had elevated expression of TIGIT on T cells. Interestingly, we found increased levels of TIGIT in CD8 cells and marked decreased levels in CD4 T cells following treatment in non-responding patients. In line with our data, earlier studies have shown that tumor-engaged CD8 T cells have increased TIGIT, which is associated with exhaustion and poor clinical responses in acute myeloid leukemia (AML) patients, while CD4 T cell TIGIT expression did not correlate with clinical responses [19]. Our findings suggest that blockade of checkpoint proteins could enhance efficacy of NK cell adoptive therapies and potentially elicit higher clinical response rates in refractory NHL [4]. Recent in vitro report suggested that the blockade of the TIGIT pathway can increase NK cell IFNγ secretion and abrogate MDSC-mediated inhibition [20].
In this clinical trial, we showed no benefit of denileukin on Treg depletion, NK cell persistence or clinical response. Notably, three responders received methylprednisone as part of their therapysuggesting that the effect of steroids in adoptive NK cell therapy may not be detrimental. Our prior experience showed lack of consistent Treg depletion by denileukin [21]; yet, we observed significantly improved response rates in refractory AML, which did not occur in our NHL cohort. Given the relatively small number of treated patients with denileukin in this trial (due to withdrawal of denileukin from the market), our study was not powered to compare the two therapeutic approaches.
In terms of the product characteristics, NK cell-enriched product contained variable fraction of other cells, mostly monocytes that likely contributed to in vivo cytokine production such as IL-15, IL-18, IL-6, TNF, and IL-10 [22, 23]. Our prior data suggested that using CD56 positively selected NK cell products produced lower remission rate in AML compared to CD19/CD3-depleted products [21]. Many pre-clinical and clinical investigations continue to study the impact of NK cell dose, purity and expansion on in vivo NK cell persistence and clinical outcomes [24–29]. The size of this trial had limited statistical power to examine the effect of killer cell immunoglobulin-like receptor (KIR) genotype or KIR ligand mismatch on clinical outcomes.
Taken together, our observations allowed identification of host factors that may have interfered with response to adoptive NK cell infusion. We showed that high frequencies of MDSCs were associated with lack of clinical response. These data are concordant with pre-clinical results that showed that MDSCs mediate potent inhibition of conventional NK cells [30, 31]. In addition, increased numbers of MDSC have been detected in patients with diffuse large B cell lymphoma at diagnosis and correlated with worse outcomes [32, 33]. Furthermore, tumor infiltrating MDSC have been recognized to recruit Treg cells and be permissive of further tumor growth [34]. In our study, circulating Treg appears to limit NK cell proliferation. We suggest that MDSCs and Treg should be studied in future trials as cellular biomarkers of resistance to adoptive NK cell therapy. These findings also highlight the possibility of targeting MSDCs to enhance the efficacy of cellular therapies [35].
In this study we also confirmed the importance of endogenous cytokines released after lymphodepleting chemotherapy. Levels of IL-15 in PB after chemotherapy and prior NK cell infusion were almost twofold higher in patients who experienced clinical response. This finding supports the development of therapeutic cytokines, such as recombinant human IL-15 (NCI) or IL-15 superagonist ALT803 (Altor), in combination with NK cells to further improve clinical activity. Pilot clinical studies with NK cell therapies are underway (NCT03050216).
Our observation supports development of donor NK cellular therapies for advanced NHL as a treatment strategy. NK cell expansion and therapeutic efficacy might be further improved by checkpoint inhibitors, MDSC-depleting strategies, and IL-15 administration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank patients and families participating in this clinical study and clinical trial office personnel, particularly research nurse Dixie Lewis, RN, for her devotion to the patients. We also thank English editor Michael Franklin.
Abbreviations
- ACL
Absolute lymphocyte cell
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- AML
Acute myeloid leukemia
- ASH
American Society of Hematology
- CD
Cluster of differentiation
- CT
Computed tomography
- CTCAE
Common terminology criteria for adverse events
- FDR
False-discovery rate
- IFN-γ
Interferonγ
- IRB
Institutional Review Board
- ITIM
Immunoreceptor tyrosine-based inhibition motif
- KIR
Killer cell immunoglobulin-like receptor
- KPS
Karnofsky performance status
- MDSC
Myeloid-derived suppressor cell
- NCI
National Cancer Institute
- NHL
Non-Hodgkin lymphoma
- NK
Natural killer
- PB
Peripheral blood
- PBMC
Peripheral blood mononuclear cells
- PCR
Polymerase chain reaction
- PET
Positron emission tomography
- PRA
Panel reactive antibody
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- TIM-3
T cell immunoglobulin mucin-3
- TNC
Total nuclear cell
- TNFα
Tumor necrosis factorα
- Treg
Regulator T cells
- VNTR
Very short tandem repeat
- WB
Whole blood
Funding
Research reported in this publication was supported by NIH Grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core and the Translational Therapy Laboratory shared resources of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. This work was also supported by American Society of Hematology Scholar Award (Veronika Bachanova), P01 CA65493, P01 CA111412, R01 CA72669.
Compliance with ethical standards
Conflict of interest
Eisai Inc has provided denileukin diftitox for this trial and supported the correlative assays. Veronika Bachanova receives funding from GT Biopharma Inc, Novartis and serves on advisory board for Seattle-Genetics. Other authors have no relevant conflicts of interest to report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Note on previous publication: published as a poster at American Society of Hematology (ASH), Dec 8–12, 2016, San Diego, CA, USA. Blood 2016 128:3030 and at the 14th International Conference on Malignant Lymphoma, Palazzo dei Congressi, Lugano, Switzerland, 14–17 June, 2017. Hematological Oncology Volume 35, Issue Supplement S2; 261–62.
Veronika Bachanova and Dhifaf Sarhan contributed equally to this manuscript.
References
- 1.Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, Bouadballah R, Radford J, Bargetzi M, Ribrag V, Duhrsen U, Ma D, Briere J, Thieblemont C, Bachy E, Moskowitz CH, Glass B, Gisselbrecht C. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2017;52(2):216–221. doi: 10.1038/bmt.2016.213. [DOI] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA. Chemotherapy-refractory diffuse large B-Cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler NH, Cheah CY, Gascoyne RD, Gribben J, Neelapu SS, Ghia P, Bollard C, Ansell S, Curran M, Wilson WH, O’Brien S, Grant C, Little R, Zenz T, Nastoupil LJ, Dunleavy K. Role of the tumor microenvironment in mature B-cell lymphoid malignancies. Haematologica. 2016;101(5):531–540. doi: 10.3324/haematol.2015.139493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59(11):1739–1744. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy WJ, Parham P, Miller JS. NK cells–from bench to clinic. Biol Blood Marrow Transplant. 2012;18(1Suppl):2–7. doi: 10.1016/j.bbmt.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26(2):161–172. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna DH, Kadidlo DM, Miller JS, Orchard PJ, Wagner JE, McCullough J. The Minnesota molecular and cellular therapeutics facility: a state-of-the-art biotherapeutics engineering laboratory. Transfus Med Rev. 2005;19(3):217–228. doi: 10.1016/j.tmrv.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Lister TA, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 11.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang ZZ, Grote DM, Ziesmer SC, Xiu B, Novak AJ, Ansell SM. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5:e281. doi: 10.1038/bcj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang ZZ, Liang AB, Ansell SM. T-cell-mediated antitumor immunity in B-cell non-Hodgkin lymphoma: activation, suppression and exhaustion. Leuk Lymphoma. 2015;56(9):2498–2504. doi: 10.3109/10428194.2015.1011640. [DOI] [PubMed] [Google Scholar]
- 16.Kiaii S, Clear AJ, Ramsay AG, Davies D, Sangaralingam A, Lee A, Calaminici M, Neuberg DS, Gribben JG. Follicular lymphoma cells induce changes in T-cell gene expression and function: potential impact on survival and risk of transformation. J Clin Oncol. 2013;31(21):2654–2661. doi: 10.1200/JCO.2012.44.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186(3):1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015;45(10):2886–2897. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 19.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+T-cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016;22(12):3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 20.Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, Verneris MR, Blazar BR, Miller JS. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5696–5706. doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3866. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liew FY, McInnes IB. Role of interleukin 15 and interleukin 18 in inflammatory response. Ann Rheum Dis. 2002;61(Suppl2):ii100–i102. doi: 10.1136/ard.61.suppl_2.ii100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80(9):2221–2229. [PubMed] [Google Scholar]
- 24.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, Dandekar V, Mei Z, Jackson K, Vera J, Ando J, Ngo MC, Coustan-Smith E, Campana D, Szmania S, Garg T, Moreno-Bost A, Vanrhee F, Gee AP, Rooney CM. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14(9):1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah N, Li L, McCarty J, Kaur I, Yvon E, Shaim H, Muftuoglu M, Liu E, Orlowski RZ, Cooper L, Lee D, Parmar S, Cao K, Sobieiski C, Saliba R, Hosing C, Ahmed S, Nieto Y, Bashir Q, Patel K, Bollard C, Qazilbash M, Champlin R, Rezvani K, Shpall EJ. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457–466. doi: 10.1111/bjh.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JS, Rooney CM, Curtsinger J, McElmurry R, McCullar V, Verneris MR, Lapteva N, McKenna D, Wagner JE, Blazar BR, Tolar J. Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: implications for clinical therapy. Biol Blood Marrow Transplant. 2014;20(8):1252–1257. doi: 10.1016/j.bbmt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayello J, van de Ven C, Fortino W, Wade-Harris C, Satwani P, Baxi L, Simpson LL, Sanger W, Pickering D, Kurtzberg J, Cairo MS. Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering. Biol Blood Marrow Transplant. 2006;12(6):608–622. doi: 10.1016/j.bbmt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Vasu S, Berg M, Davidson-Moncada J, Tian X, Cullis H, Childs RW. A novel method to expand large numbers of CD56(+) natural killer cells from a minute fraction of selectively accessed cryopreserved cord blood for immunotherapy after transplantation. Cytotherapy. 2015;17(11):1582–1593. doi: 10.1016/j.jcyt.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182(1):240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 31.Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20(15):4096–4106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]
- 32.Tadmor T, Fell R, Polliack A, Attias D. Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma-possible link with monocytic myeloid-derived suppressor cells. Hematol Oncol. 2013;31(3):65–71. doi: 10.1002/hon.2019. [DOI] [PubMed] [Google Scholar]
- 33.Azzaoui I, Uhel F, Rossille D, Pangault C, Dulong J, Le Priol J, Lamy T, Houot R, Le Gouill S, Cartron G, Godmer P, Bouabdallah K, Milpied N, Damaj G, Tarte K, Fest T, Roussel M. T-cell defect in diffuse large B-cell lymphomas involves expansion of myeloid-derived suppressor cells. Blood. 2016;128(8):1081–1092. doi: 10.1182/blood-2015-08-662783. [DOI] [PubMed] [Google Scholar]
- 34.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189(12):5601–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 35.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, Epling-Burnette PK, Blazar BR, Weiner LM, Weisdorf DJ, Vallera DA, Miller JS. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+targets. Blood. 2014;123(19):3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.