Abstract
Chromosome fragile sites are a fascinating cytogenetic phenomenon now widely implicated in a slew of human diseases ranging from neurological disorders to cancer. Yet, the paths leading to these revelations were far from direct, and the number of fragile sites that have been molecularly cloned with known disease-associated genes remains modest. Moreover, as more fragile sites were being discovered, research interests in some of the earliest discovered fragile sites ebbed away, leaving a number of unsolved mysteries in chromosome biology. In this review we attempt to recount some of the early discoveries of fragile sites and highlight those phenomena that have eluded intense scrutiny but remain extremely relevant in our understanding of the mechanisms of chromosome fragility. We then survey the literature for disease association for a comprehensive list of fragile sites. We also review recent studies addressing the underlying cause of chromosome fragility while highlighting some ongoing debates. We report an observed enrichment for R-loop forming sequences in fragile site-associated genes than genomic average. Finally, we will leave the reader with some lingering questions to provoke discussion and inspire further scientific inquiries.
Keywords: Chromosome fragility, Common and rare fragile sites, DNA double-strand breaks, DNA replication stress, R-loops, Aphidicolin, Folate stress, Neurological disorders, Cancer
21.1 Introduction
Chromosome fragile sites are defined as gaps, constrictions, or breaks on metaphase chromosomes that are induced by various DNA replication inhibitors. The Human Genome Database currently documents 120 chromosome fragile sites (30 of the rare type and 90 of the common type, Tables 21.1 and 21.2). According to the HUGO Gene Nomenclature Committee, each fragile site is reported as “FRA” followed by the chromosome number and a letter denoting the order of nomenclature, such as FRA1A. In the last decade, fragile sites have been the subject of many comprehensive reviews with respect to the molecular basis, mechanisms of expression, and relevance to human diseases (Debacker and Kooy 2007; Durkin and Glover 2007; Fungtammasan et al. 2012; Lukusa and Fryns 2008; Mirkin 2006; Thys et al. 2015). Here we approach this subject from an alternative angle. By retracing some of the early developments in fragile site discoveries, we hope to identify the gaps in our knowledge and bring awareness to some “cold cases,” so to speak, in chromosome fragile sites (we apologize to countless researchers whose work may have been omitted from this review due to space limitation). We review the classification of common vs. rare fragile sites and provide some insights into the DNA structural determinants of fragility. We also provide updated information regarding disease associations of known fragile sites in the database as well as summarize recent genome-wide studies of fragile sites. Finally, we highlight three “unsolved mysteries” in chromosome fragile sites and provide speculations.
Table 21.1.
Updated summary of RFSs
| Fragile site | Type | Repeat | Cyto-band | Population freq. (%)a | Size (kb) | No. of RLFSs | No. of RLFSs/kb | No. of genes with > = 1 RLFS/kb | Freq. (%) in folate stressa | Freq. (%) in APHb | Assoc. genes | Disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FRA1M | Folate | 1p21.3 | 4,900 | 48 | 0.010 | 0 | 3.333 | ID (D’Angelo et al. 2015) | ||||
| FRA2A | Folate | CGG | 2q11.2 | 0.0014 | 6,400 | 266 | 0.042 | 1 | 27.00 | 0.089 | AFF3 | Schizophrenia (Chen et al. 1998); ID (Metsu et al. 2014a) |
| FRA2B | Folate | 2q13 | 5,200 | 177 | 0.034 | 0 | 0.067 | |||||
| FRA2K | Folate | 2q22.3 | 3,700 | 20 | 0.005 | 0 | 0.626 | ID (Mulatinho et al. 2012) | ||||
| FRA5G | Folate | 5q35 | 13,458 | 898 | 0.067 | 6 | 0.120 | Found in a FX patient at 50% expression and a normal brother at 36% (Howell et al. 1990) | ||||
| FRA6A | Folate | 6p23 | 2,000 | 99 | 0.050 | 1 | 0.009c | Schizophrenia (Olavesen et al. 1995) | ||||
| FRA7A | Folate | CGG | 7p11.2 | 3,500 | 89 | 0.025 | 1 | 0.058 | ZNF713 | ASD (Metsu et al. 2014b) | ||
| FRA8A | Folate | 8q22.3 | 4,500 | 164 | 0.036 | 2 | 0.018 | |||||
| FRA9A | Folate | 9p21 | 0.0041 | 12,900 | 50 | 0.004 | 0 | 21.00 | 0.102 | |||
| FRA9B | Folate | 9q32 | 2,800 | 136 | 0.049 | 0 | 1.513 | |||||
| FRA10A | Folate | CGG | 10q23.3 | 0.0028 | 16,707 | 168 | 0.010 | 0 | 35.67 | 0.124 | FRA10AC1 | Single case of turner syndrome (Li et al. 2015; Maltby and Higgins 1987); Alzheimer’s (Li et al. 2015) |
| FRA11A | Folate | CGG | 11q13.3 | 1,500 | 165 | 0.110 | 2 | 0.075 | C11orf8c/MPPED2 | |||
| FRA11B | Folate | CGG | 11q23.3 | 5,300 | 459 | 0.087 | 1 | 0.098 | CBL2 | Jacobsen syndrome (Jones et al. 1994, 1995; Michaelis et al. 1998) | ||
| FRA12A | Folate | CGG | 12q13.1 | 0.0007 | 4,678 | 382 | 0.082 | 8 | 17.67 | 0.075 | DIP2B | ASD and MR (Winnepenninckx et al. 2007) |
| FRA12D | Folate | 12q24.13 | 2,000 | 93 | 0.047 | 0 | Not induced | Segregating in FX families (Amarose et al. 1987; Barletta et al. 1991; Sutherland and Baker 1993) | ||||
| FRA16A | Folate | CGG | 16p13.11 | 0.0007 | 2,000 | 116 | 0.058 | 1 | 12.00 | 0.053 | N.D. | |
| FRA19B | Folate | 19p13 | 19,800 | 4,650 | 0.235 | 88 | 0.036 | |||||
| FRA20A | Folate | 20p11.23 | 3,400 | 70 | 0.021 | 0 | 0.027c | |||||
| FRA22A | Folate | 22q13 | 0.0014 | 13,791 | 2,445 | 0.177 | 17 | 5.67 | 0.328 | MR (Webb and Thake 1984) | ||
| FRAXA | Folate | CGG | Xq27.3 | 5,000 | 17 | 0.003 | 0 | Not induced | FMR1, ASFMR1/FMR4 | FXS, MR, ID, FXTAS, FXPOI (Galloway and Nelson 2009; Santoro et al. 2012; Usdin et al. 2014) | ||
| FRAXE | Folate | CGG | Xq28 | 8,014 | 615 | 0.077 | 13 | 0.018c | AFF2/FMR2 | MR (Bensaid et al. 2009) | ||
| FRAXF | Folate | CGG | Xq28 | 8,014 | 615 | 0.077 | 13 | 0.018c | FAM11A | MR; ASD; psychosis, pyramidal signs, macroordism (Holden et al. 1996; Lindsay et al. 1996; Vianna-Morgante et al. 1996) | ||
| FRA8E | DA | 8q24.1 | 28,526 | 1,394 | 0.049 | 24 | 0.799 | Azoospermia (Seki et al. 1992); hereditary multiple exostoses 1 gene and two overlapping Langer-Giedion syndrome deletion endpoints (Hill et al. 1997) | ||||
| FRA11I | DA | 11p15.1 | 5,609 | 175 | 0.031 | 1 | 0.510 | Azoospermia (Seki et al. 1992) | ||||
| FRA16B | DA | AT-rich MS | 16q22.1 | 4,200 | 491 | 0.117 | 9 | 0.488 | N.D. | Difficulty achieving pregnancy (Martorell et al. 2014); dominant inheritance of cleft palate, microstomia, and micrognathia (McKenzie et al. 2002) | ||
| FRA16E | DA | 16p12.1 | 5,900 | 178 | 0.030 | 1 | 0.027 | Batten disease (common form of neuronal ceroid lipofuscinoses [NCLs]) (Dooley et al. 1994) | ||||
| FRA17A | DA | 17p12 | 0.0049 | 4,700 | 91 | 0.019 | 2 | 20.00 | 0.049 | Azoospermia (Shabtai et al. 1982) | ||
| FRA10B | BrdU | AT-rich MS | 10q25.2 | 3,100 | 45 | 0.015 | 0 | 0.191 | N.D. | |||
| FRA12C | BrdU | 12q24 | 24,850 | 1,907 | 0.077 | 6 | 0.288 | Segregating in FX families (Amarose et al. 1987; Barletta et al. 1991; Sutherland and Baker 1993) | ||||
| FRA8F | UC | 8q13 | 7,900 | 104 | 0.013 | 0 | 0.004c |
Folate folate stress, DA distamycin A, BrdU bromodeoxyuridine, UC unclassified, MS minisatellite, RLFS R-loop forming sequence, ID intellectual disability, ASD autism spectrum disorder, MR mental retardation, FXTAS fragile X-associated tremor/ataxia syndrome, FXPOI fragile X-associated premature ovarian insufficiency, Freq. frequency of fragile site expression
Data derived from Kähkönen et al. (1989)
Data derived from Mrasek et al. (2010)
Fragile site detected in fewer than three individuals when induced by aphidicolin
Table 21.2.
Summary of CFS-associated genes and their implications in human diseases
| Fragile site | Induction method | Cyto-band | Freq. in APH (%)a | Assoc. genes | Disease |
|---|---|---|---|---|---|
| FRA1A | APH | 1p36 | 0.488 | ||
| FRA1B* | APH | 1p32 | 1.491 | DAB1 | Brain and endometrial cancer (McAvoy et al. 2008) |
| FRA1C | APH | 1p31.2 | IL23R-C1orf141 | Vogt-Koyanagi-Harada syndrome; LOH/MSI associated with advanced tumor stage in prostate cancer (Brys et al. 2013; Hou et al. 2014) | |
| FRA1D | APH | 1p22 | 0.009 | ||
| FRA1E* | APH | 1p21.2 | 0.067 | DPYD | Colorectal, breast, and ovarian cancer (Hormozian et al. 2007) |
| FRA1F | APH | 1q21 | 0.098 | ||
| FRA1G | APH | 1q25 | 0.799 | ||
| FRA1I | APH | 1q44 | 2.299 | Nasopharyngeal carcinoma (Xia et al. 1988) | |
| FRA1K | APH | 1q31 | 0.138 | ||
| FRA1L | APH | 1p31 | 0.479 | ||
| FRA2C* | APH | 2p24.2 2q24.3 |
1.513 | MYCN | Two FSs, FRA2Ccen and FRA2Ctel, flank the MYCN amplicon; locus-specific and genomic rearrangements in neuroblastoma and multiple cancers (Blumrich et al. 2011; Lipska et al. 2013) |
| FRA2D | APH | 2p16.2 | 2.325 | ||
| FRA2E | APH | 2p13 | 0.235 | Posttransplant diffuse large B-cell lymphoma (Rinaldi et al. 2010) | |
| FRA2F | APH | 2q21.3 | 0.994 | LRP1B | Alzheimer’s; Kawasaki disease (Lin et al. 2014; Shang et al. 2015) |
| FRA2G* | APH | 2q31 | 0.306 | GAD1 | Schizophrenia (Bharadwaj et al. 2013) |
| FRA2H* | APH | 2q32.1 | 3.905 | DIRC1, PMS1, MIRN589, MIRN1245 | Breakpoints characterized in K562 cells (Pelliccia et al. 2010) |
| FRA2I | APH | 2q33 | 1.722 | ||
| FRA2J | APH | 2q37.3 | 0.657 | ||
| FRA3A | APH | 3p24.2 | 1.327 | ||
| FRA3B* | APH | 3p14.2 | 14.153 | FHIT | Loss or change of FHIT expression associated with common cancers including bladder, esophageal, lung, breast, and prostate cancers (Saldivar et al. 2010) |
| FRA3C | APH | 3q27 | 1.061 | ||
| FRA3D | APH | 3q25 | 0.546 | FS expression linked to schizophrenia (Demirhan et al. 2006) | |
| FRA4A | APH | 4p16.1 | 0.612 | ||
| FRA4C | APH | 4q31.1 | 2.175 | ||
| FRA4D | APH | 4p15 | 0.111 | ||
| FRA4F* | APH | 4q22 | 0.683 | GRID2 | Autosomal recessive cerebellar ataxia associated with retinal dystrophy; ataxia and tonic upgaze (Hills et al. 2013; Van Schil et al. 2015) |
| FRA5C | APH | 5q31.1 | 0.373 | SMAD5 | Copy number gain and increased expression in human hepatocellular carcinoma cells (Zimonjic et al. 2003) |
| FRA5D | APH | 5q15 | 0.834 | Concomitant t(5;21)(q15;q22) and del(5)(q13q33) events in myelodysplastic syndrome (Kasi Loknath Kumar et al. 2016) | |
| FRA5E | APH | 5p14 | 0.293 | Copy number loss observed at 52.4% in early Barrett’s esophagus (Lai et al. 2010) | |
| FRA5F | APH | 5q21 | 0.515 | FS expression significantly higher in patients with rectum cancer and their first-degree relatives (Tunca et al. 2000) | |
| FRA6B | APH | 6p25.1 | 0.985 | Copy number change associated with osteoporotic fractures, gyral pattern anomaly, and speech and language disorder in two dizygotic twins (Bozza et al. 2013; Oei et al. 2014) | |
| FRA6C | APH | 6p22.2 | 0.062 | BAC CITB.564_C_7 | High LOH in cervical tumors; HPV integration site in cervical tumor CC171 (Rader et al. 1996; Thorland et al. 2000) |
| FRA6E* | APH | 6q26 | 2.823 | PARK2 | Parkinson’s; poor outcome in breast cancer (Ambroziak et al. 2015; Letessier et al. 2007) |
| FRA6F* | APH | 6q21 | 0.608 | REV3L, DIF13, FKHRL, etc. | Cancer; schizophrenia (Karayianni et al. 1999; Morelli et al. 2002) |
| FRA6G | APH | 6q15 | 0.115 | Chromosome breakpoints in metastatic melanoma (Limon et al. 1988) | |
| FRA7B* | APH | 7p22 | 0.799 | THSD7A, SDK1, MAD1L1, MIRN589, MIRN339 | Recurrent breakpoint in multiple cancers (Bosco et al. 2010) |
| FRA7C | APH | 7p14.2 | 0.541 | ||
| FRA7D | APH | 7p13 | 0.55 | Highly expressed in a female presenting severe immunodeficiency (Conley et al. 1986) | |
| FRA7E* | APH | 7q21.2 | 0.501 | ||
| FRA7F | APH | 7q22 | 0.107 | Fragile site expression linked to bipolar disorder and schizophrenia (Demirhan et al. 2006, 2009) | |
| FRA7G* | APH | 7q31.2 | 0.111 | Caveolin-1, caveolin-2, TESTIN | Reduced TESTIN expression in 22% of cancer cell lines and 44% of the cell lines derived from hematological malignancies; caveolin-1 gene proposed to be a candidate tumor suppressor (Engelman et al. 1998; Tatarelli et al. 2000) |
| FRA7H* | APH | 7q32.3 | 2.374 | Translocation involving 7q32 found in the RC-K8 cell line derived from a patient with terminal diffuse large B-cell lymphoma (Mishmar et al. 1998; Schneider et al. 2008) | |
| FRA7I* | APH | 7q36 | 0.084 | CNTNAP2 | Implicated in ASD (Rodenas-Cuadrado et al. 2014) |
| FRA7J | APH | 7q11 | 0.83 | LIMK1, EIF4H(WBSCR1) | Breakpoint found between the LIMK1 and EIF4H (WBSCR1) genes in patients with Williams-Beuren syndrome and ASD (Plaja et al. 2015) |
| FRA8B | APH | 8q22.1 | 0.923 | ||
| FRA8C* | APH | 8q24.1 | 0.799 | MYC | Cluster of HPV18 integrations at 8q24 in primary cervical carcinoma; CFS expression frequent in bladder cancer (Ferber et al. 2004; Moriarty and Webster 2003) |
| FRA8D | APH | 8q24.3 | 0.182 | ||
| FRA9D | APH | 9q22.1 | 0.231 | FS expression frequent in bladder cancer (Moriarty and Webster 2003) | |
| FRA9E* | APH | 9q32 | PAPPA | LOH, particularly loss of PAPPA, is linked to ovarian cancer (Callahan et al. 2003) | |
| FRA10D | APH | 10q22.1 | 0.306 | CTNNA3 | Decreased gene expression of CTNNA3 in oropharyngeal squamous cell carcinomas (Gao et al. 2014) |
| FRA10E | APH | 10q25.2 | 0.191 | ||
| FRA10F* | APH | 10q26.1 | 0.408 | FATS | |
| FRA10G* | APH | 10q11.2 | 0.142 | RET, NCOA4 | FS generates oncogenic RET/PTC rearrangements in human thyroid cells (Gandhi et al. 2010) |
| FRA11C | APH | 11p15.1 | 0.51 | FS expression frequent in bladder cancer (Moriarty and Webster 2003) | |
| FRA11D | APH | 11p14.2 | 0.928 | ||
| FRA11E | APH | 11p13 | 0.621 | Translocation breakpoints at 11p13 found in CML patients. | |
| FRA11F | APH | 11q14.2 | 1.771 | Gene amplification linked to FRA11F expression in oral cancer (Reshmi et al. 2007) | |
| FRA11G* | APH | 11q23.3 | 0.098 | Interstitial deletions at 11q23.3 associated with abnormal ultrasound findings during prenatal diagnosis (Fechter et al. 2007; Liu et al. 2014) | |
| FRA11H* | APH | 11q13 | 0.075 | ||
| FRA12B | APH | 12q21.3 | 0.364 | ||
| FRA12E | APH | 12q24 | 0.288 | ||
| FRA13A* | APH | 13q13.2 | 1.061 | NBEA | Neuropsychiatric disorders (Savelyeva et al. 2006a, b) |
| FRA13C | APH | 13q21.2 | 0.124 | HPV16 integration site in the cervical tumor cell line SiHa (Thorland et al. 2000) | |
| FRA13D | APH | 13q32 | 0.178 | Fragile site expression linked to bipolar disorder and schizophrenia (Demirhan et al. 2006, 2009) | |
| FRA14B | APH | 14q23 | 1.429 | Found as a constant FS in bloom syndrome cell lines (Barbi et al. 1984; Shiraishi and Li 1993) | |
| FRA14C | APH | 14q24.1 | 0.382 | Found as frequent HPV integration site in HPV-related cancers (Bodelon et al. 2016) | |
| FRA15A | APH | 15q22 | 0.08 | RORA | RORA gene expression is low in breast, prostate, and ovarian cancers (Zhu et al. 2006) |
| FRA16C | APH | 16q22.1 | 0.488 | High expression at FRA16B/C found in peripheral blood lymphocytes in the male of a couple having trouble conceiving. Chr 16 instability detected in the sperm of the male and in embryos (Martorell et al. 2014) | |
| FRA16D* | APH | 16q23.2 | 7.576 | WWOX/WOX1/FOR | WWOX gene dysregulation associated with multiple cancers, including pancreatic adenocarcinoma, renal cell carcinoma, and endocrine and exocrine carcinomas (Li et al. 2014) |
| FRA17B | APH | 17q23.1 | 0.071 | HPV integration site in cervical tumor CC226 (Thorland et al. 2000) | |
| FRA18A | APH | 18q12.2 | 0.475 | High frequency of LOH on chr 18 in esophageal squamous cell carcinoma (Karkera et al. 2000) | |
| FRA18B | APH | 18q21.3 | 0.182 | DCC | Found as FSs with frequent interstitial deletions in HIV lymphomas (Capello et al. 2010) |
| FRA18C* | APH | 18q22.1–18q22.2 | 0.067 | DOK6 | FS expression in the father of a patient with Beckwith-Wiedemann syndrome and a chromosome truncation 18q22-qter (Debacker et al. 2007) |
| FRA20B | APH | 20p12.2 | |||
| FRA22B | APH | 22q12.2 | 0.275 | Elevated FS expression in bone marrow and peripheral blood of young cigarette smokers (Kao-Shan et al. 1987) | |
| FRAXB* | APH | Xp22.31 | 5.494 | STS, GS1 HDHD1 MIR4767 | Deletions within FRAXB seen in 15% of primary tumors and cell lines examined (Arlt et al. 2002) |
| FRAXC | APH | Xq22.1 | 2.121 | DMD, IL1RAPL1 | |
| FRAXD | APH | Xq27.2 | 0.209 | ||
| FRA1H* | 5-AZ | 1q41–q42.1 | 0.129 | USH2A, ESRRG, MIRN194–1, MIRN215 | |
| FRA1J | 5-AZ | 1q12 | 0.12 | ||
| FRA9F | 5-AZ | 9q12 | 0.453 | ||
| FRA19A | 5-AZ | 19q13 | 0.053 | ||
| FRA4B | BrdU | 4q12 | 0.142 | ||
| FRA5A | BrdU | 5p13 | 0.191 | ||
| FRA5B | BrdU | 5q15 | |||
| FRA6D | BrdU | 6q13 | 0.031 | ||
| FRA9C | BrdU | 9p21 | |||
| FRA10C | BrdU | 10q21 | 0.089 | ||
| FRA13B | BrdU | 13q21 | |||
| FRA4E | UC | 4q27 | 0.382 |
Molecularly mapped fragile sites are indicated by an asterisk
5-AZ, 5-azacytidine, APH aphidicolin, BrdU bromodeoxyuridine, UC unclassified, FS fragile site, LOH loss of heterozygosity, MSI microsatellite instability, ASD autism spectrum disorder
Data derived from Mrasek et al. (2010)
21.2 Early History of Chromosome Fragile Site Discoveries Revisited
21.2.1 Discovery of the First Fragile Site
Early history of chromosome fragile site discoveries makes a captivating read. The term “fragile site” was coined in 1969 by Frederick Hecht to describe hitherto reported spontaneous breaks at a specific site in a human metaphase chromosome. However, the first known fragile site was observed 4 years before the genesis of its terminology (Dekaban 1965). Notably, the definition of fragile site later expanded to include constrictions and gaps in the chromosome as well. In this broader definition, the first chromosomal “secondary constriction,” to be distinguished from the primary constriction or the centromere, was discovered still earlier (Ferguson-Smith et al. 1962).
Nevertheless, the first fragile site in the pedantic sense was observed in a woman who had received multiple X-ray irradiations for eczematous dermatitis and later had borne a malformed child (Dekaban 1965). It was unclear if the fragile site observed on the presumed Chromosome 9 from the woman was linked to the birth of this child. Limited survey of the woman’s family showed normal chromosomes in her abnormal child, her husband, and her father (Dekaban 1965). However, this study raised a question that is still relevant in the research field today. There were two types of chromosome abnormality in the woman’s blood cultures. A relatively more frequent type consisted of a break near the third telomere-proximal portion of the long arm on the presumed Chromosome 9 (24.8%) and a less frequent one involved aberrations such as dicentrics, rings, and deletions on random chromosomes (7.1%). In contrast, her skin culture contained a much lower frequency of breakage on Chromosome 9 (6%), despite a comparable level of other chromosome aberrations (8.8%). Moreover, the break seen in the skin culture occurred at a different location (telomere-distal) on Chromosome 9 from that seen in the blood cultures (telomere-proximal). One theory put forth suggested that the X-ray irradiations had induced a clone of abnormal cells with a telomere-proximal fragile site on Chromosome 9 in the woman’s blood-forming tissues but not in skin tissues. Alternatively, though it could not be ascertained thoroughly due to family members in absentia, there existed tissue-specific formation of chromosome fragile sites. The latter possibility was subsequently more convincingly demonstrated (Kuwano et al. 1990; Murano et al. 1989a). However, the mechanism of tissue-specific fragile site formation still remains a major challenge in the field.
21.2.2 First Report of a Heritable Fragile Site
The first heritable fragile site was demonstrated in a mother-and-daughter case, which involved a fragile site on the long arm of Chromosome 2 near the centromere (Lejeune et al. 1968). The heritability of this fragile site was later confirmed independently (Ferguson-Smith 1973). Curiously, this fragile site gave rise to duplication of the centromere-distal two-thirds of 2q and formation of a three-armed chromosome, which was referred to as qh(2). It was speculated that this endo-duplication event was the result of interruption of a centromere-originated DNA replication signal by the fragile site. Alternatively, it was thought that qh(2) formation was reminiscent of interstitial telomere element-induced endo-duplications elsewhere (Hsu 1963). It was shown that 2q indeed contains interstitial telomere elements, but this fusion site is distinct from the fragile site band 2q11.2 (Ijdo et al. 1991). Finally, it was also surmised that qh(2) formation was due to chromatid breakage followed by mitotic nondisjunction (Ferguson-Smith 1973). To this date, the mechanism for the duplicated q-arms remains a mystery.
Phenotypically the mother and daughter were both of short stature and had intellectual deficiencies, which may or may not be related to the expression of the fragile site. However, subsequent studies demonstrated that the fragility at 2q11.2, which resides in the presumed cytoband responsible for the formation of qh(2), was observed at a significantly higher frequency in “mentally subnormal” school children (Kahkonen et al. 1986) as well as in patients with schizophrenia (Chen et al. 1998). It was not until recent that this fragile site was molecularly characterized as a site with CGG repeat expansion impacting the AFF3 gene and was detected in families with a broad range of neurodevelopmental disorders (Metsu et al. 2014a).
21.2.3 First Demonstration of Mendelian Transmission of a Fragile Site
A fragile site on 16q22 was examined in a large family of a boy who had recurrent cold urticaria and immunoglobulin A deficiency (Magenis et al. 1970). Transmission of the fragile site followed a Mendelian pattern. However, later it was demonstrated that this fragile site is distal from the haptoglobin gene whose deficiency is linked to IgA deficiency, suggesting that the IgA condition in the propositus was likely a coincidence (Simmers et al. 1986). Fragility at 16q22 has since been implicated in a wide spectrum of neurological disorders (Demirhan et al. 2006; Kerbeshian et al. 2000) as well as in other conditions such as neutropenia (Glasser et al. 2006; Tassano et al. 2010) and cleft palate (Bettex et al. 1998; Dunner et al. 1983; Janiszewska-Olszowska et al. 2013; McKenzie et al. 2002). A recent study also reported elevated fragile site formation at 16q22.1 in an embryo from a couple who had difficulty achieving pregnancy and in the sperm from the father (Martorell et al. 2014). However, cytogenetic breakage and potential disease-associated gene(s) at this locus are yet to be molecularly characterized.
21.2.4 First Fragile Site Linked to a Clinical Phenotype
The vast majority of fragile sites during the early discoveries appeared innocuous and not associated with any phenotypic abnormalities, even when present in homozygous conditions such as those fragile sites at 10q25.2 and 12q24 (Sutherland 1981; Voiculescu et al. 1991). The only fragile site definitively associated with a clinical phenotype was that which resides on Xq27.3 (FRAXA) and was associated with the Martin-Bell or the fragile X syndrome, an X-linked and most common familial form of mental retardation (Giraud et al. 1976; Harvey et al. 1977; Lubs 1969). Subsequent studies demonstrated that the FRAXA fragility is the result of a CGG repeat expansion and is correlated with hypermethylation, gene silencing, as well as delayed replication at the fragile X mental retardation 1 (FMR1) gene locus (Bell et al. 1991; Dietrich et al. 1991; Hansen et al. 1992, 1993; Heitz et al. 1991; Pieretti et al. 1991). A full mutation (>200 CGG repeats) at the FMR1 locus causes gene silencing and the loss of the protein product, FMRP, which causes the loss of synaptic plasticity and the fragile X pathology. Fragile X biology has been the subject of numerous and up-to-date reviews to which we direct the reader for a comprehensive understanding of the disease etiology, mechanism, and intervention (Ligsay and Hagerman 2016; Lozano et al. 2016; Santoro et al. 2012; Wang et al. 2012; Zhao and Usdin 2016).
21.3 Classification of “Common” vs. “Rare” Fragile Sites
21.3.1 Population Frequencies
The Edinburgh survey estimated that from 3% to 5% of the population contained identifiable chromosomal variants (Court Brown 1966). Subsequent population studies defined two types of fragile sites: those that were present in <1% of the general population and those that were present at a theoretical frequency between 1% and 99% or polymorphic (Hecht 1988). Later, this fine distinction between the two groups was relinquished, and collectively they were referred to as “rare fragile sites” with an observed maximal frequency of 5% (Schmid et al. 1986). This classification was also necessitated by the discovery of what appeared to be common or ubiquitous fragile sites, which were present in all 12 subjects regardless of sex or clinical phenotypes (Glover et al. 1984). Thus, presently, fragile sites are classified as “rare” and “common” fragile sites (RFSs and CFSs, respectively) based on the definitions above.
Both RFSs and CFSs can be further characterized by another important metric—the frequency at which fragile sites are observed/expressed in the cells from a given individual, which is akin to a measurement of penetrance. As current literature makes broad reference to frequencies of fragile sites—i.e., at a population level vs. at a cellular level within an individual—it is important to note the difference between these two metrics. For instance, the frequency of FRAXA expression in fragile X individuals can be as high as 50% (Glover et al. 1984). In contrast, the frequency of aphidicolin-induced CFS expression in individuals can be as low as 0.01% based on a large-scale study (Mrasek et al. 2010). Therefore, arguably a CFS with extremely low penetrance may be better characterized as a RFS because it is conceivable that certain individuals, yet to be identified, might exhibit abnormally high penetrance.
21.3.2 Methods of Induction
It was serendipity that led to the discovery that many RFSs discovered by routine diagnostic cytogenetic screen in early studies were unstable in medium 199, which is deficient for folic acid (Sutherland 1977). This finding thus defined the first group of RFSs, which are sensitive to folate stress induced by folic acid or thymidine deprivation, inhibitors of folate metabolism, inhibitors of thymidylate synthetase, and excess thymidine (Sutherland 1991). Systematic analyses revealed that other culture conditions, such as pH, also impact the frequency of fragile site expression (Sutherland 1979). RFSs can also be induced by a group of chemicals of the non-folate stress type such as a nucleotide analog, bromodeoxyuridine, and a base intercalator, distamycin A. In contrast, CFSs are primarily induced by a DNA polymerase inhibitor, aphidicolin, and to a lesser extent by other chemicals including bromode-oxyuridine and 5′-azacytidine.
It is unclear why RFSs and CFSs show drug-specific induction. Genomic loci may be differentially susceptible to drug-induced replication perturbation. This apparent drug specificity of fragile site expression is likely also due to limitations of the cytological screening methods. Consequently, a given fragile site has been traditionally associated with a specific drug though other drugs could also induce its expression, albeit not as potently. Consistent with this notion, the most frequent aphidicolin-induced CFSs have been shown also inducible by thymidylate stress (Kähkönen et al. 1989). Thus, thymidylate stress can induce not only RFSs but also CFSs, demonstrating effectively “cross-induction” of these two classes of fragile sites. The cross-induction of RFSs by aphidicolin was also confirmed by a genomic scale survey (Mrasek et al. 2010). We further asked if there is a correlation between the expression frequencies (percentage of cells showing fragile sites) of seven RFSs when induced by folate stress (Kähkönen et al. 1989) vs. by aphidicolin (Mrasek et al. 2010). For the study by Käukönen et al., we calculated the expression frequencies only from phenotypically normal individuals for comparison with the study by Mrasek et al. With the exception of a single outlier, FRA22A, which appeared to be highly inducible by aphidicolin, the remaining six RFSs showed a positive correlation (R = 0.8) between the induction frequencies by the two conditions (Fig. 21.1). Nevertheless, the differential mechanisms of drug induction of fragile site expression await further exploration.
Fig. 21.1.
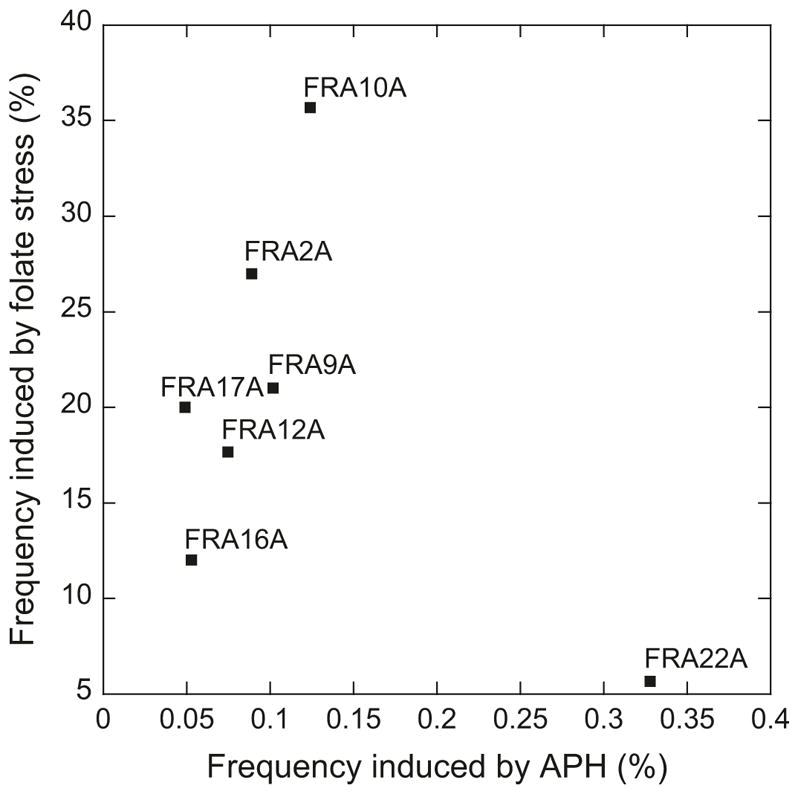
Comparison between RFS expression frequencies induced by folate stress and by aphidicolin (APH). Experimental values obtained from Kähkönen et al. (1989) and Mrasek et al. (2010), respectively
21.3.3 DNA Sequence and Structural Features
Molecular cloning permitted structural distinction between RFSs and CFSs beyond population frequencies and induction methods. RFSs are characterized by repetitive DNA sequences. Of the 30 RFSs, 12 have so far been identified with a repeat motif with 10 containing a CGG trinucleotide repeat tract and 2 containing an AT-rich minisatellite repeat (Table 21.1). Repetitive DNA can generate non-B DNA structures or alternative conformations with unusual secondary structures (Choi and Majima. 2011). For instance, folate-sensitive RFSs are enriched in CGG repeats that can form stable hairpins, slipped strand structures, G-quadruplexes, and i-tetra-plex structures (Fry and Loeb 1994; Usdin and Woodford 1995; Kang et al. 1995). CGG repeats are capable of pausing or stalling replication forks both in vitro and in vivo (Samadashwily et al. 1997). These repeats are polymorphic in normal individuals but undergo dynamic expansion/contraction, resulting in the cytogenic expression of fragile sites under folate stress (Kremer et al. 1991; Verkerk et al. 1991). Non-folate-sensitive RFSs on the other hand consist of expandable AT-rich minisatellite repeats and are specifically induced by AT-dinucleotide binding chemicals such as distamycin A and berenil. For instance, the most frequently observed RFS, FRA16B, contains a polymorphic 33 bp AT-rich minisatellite repeat, which can expand to 2000 copies in certain individuals (Yu et al. 1997). Fourteen copies of this 33 bp AT-rich minisatellite repeat were shown to form DNA secondary structures and cause replication fork stalling, fork regression, and polymerase skipping in vitro (Burrow et al. 2010). Similarly, the BrdU-induced non-folate-sensitive RFS, FRA10B, shares homology with the 33 bp minisatellite in FRA16B and contains an 11 bp inverted repeat that can form hairpins (Handt et al. 2000; Yu et al. 1997).
In contrast, CFSs are not characterized by expandable di- or trinucleotide repetitive sequence. However, some of the cloned CFSs are enriched for short interrupted AT-rich islands with high torsional flexibility and high propensity to form stable secondary structures, similar to the RFSs (Dillon et al. 2013; Zlotorynski et al. 2003). Moreover, a study using a yeast-based genetic assay identified a flexibility peak region (Flex1) of FRA16D with a perfect AT/TA repeat element capable of stalling replication forks (Zhang and Freudenreich 2007). Interestingly, these repeats resemble the AT-rich minisatellites found in non-folate RFSs. The extent of stalling depends on the length of the repeat. Therefore, there appears to be a shared mechanism of repeat-based secondary structure formation and perturbation of the replication fork between RFSs and CFSs, the difference being that RFSs have greater expandability than CFSs (Schwartz et al. 2006). Are there any other cis elements, in addition to DNA secondary structures, that define fragile sites? An RNA/DNA hybrid molecule known as R-loop appears to qualify. R-loops play a wide array of functions in normal cells. For instance, R-loops occur in replication origins in mitochondria as primers for DNA replication (Lee and Clayton 1996). R-loops at CpG islands may also facilitate replication origin specification by generating single-stranded DNA in the nuclear genome (Lombrana et al. 2015). In stimulated B-lymphocytes, R-loop formation at the immunoglobulin heavy chain locus facilitates class switch recombination (Yu et al. 2003). Finally, it was shown that R-loop formation in the guanine-rich transcription pause sites downstream of the poly-A signals is required for transcription termination (Skourti-Stathaki et al. 2011). The genome-wide association of GC skew (asymmetry in guanine distribution between the two strands of DNA), which is conducive to R-loop formation, with transcription termination sites was subsequently validated (Ginno et al. 2013). On the flip side, R-loops are increasingly associated with genome instability and human diseases (Groh and Gromak 2014; Santos-Pereira and Aguilera 2015). In yeast, R-loop formation can sensitize DNA to damaging agents, causing double-strand breaks (Huertas and Aguilera 2003; Li and Manley 2005; Sordet et al. 2009). R-loops at transcription termination sites also require proper resolution by factors such as senataxin, and unresolved R-loops can cause genome instability (Hatchi et al. 2015; Skourti-Stathaki et al. 2011). R-loops can also trigger epigenetic changes in the DNA and bring about the formation of repressive chromatin, which in turn could impede DNA replication (Groh et al. 2014). Evidence for blockage of replication fork by R-loop formation was demonstrated for FRA16D (Madireddy et al. 2016). Finally, R-loop formation can endanger genome stability by incurring replication-transcription conflicts, as exemplified by their association with CFS formation, particularly in large genes such as FHIT (Helmrich et al. 2011). The question becomes what roles do R-loops play in replication stress-induced fragile site formation, the corollary being, is there a genome-wide correlation between R-loop content/density and the probability of fragile site formation?
Recent advance in experimental mapping of R-loops capitalized on the utility of the S9.6 antibody, which has a sequence-independent affinity toward the A/B helical RNA/DNA duplex, and coupled the immunoprecipitation of the DNA/RNA hybrid with high-throughput sequencing. Variations of this methodology include DRIPc-seq (DNA/RNA immunoprecipitation followed by cDNA conversion coupled with high-throughput sequencing, an improved version of the previous DRIP-seq method) (Sanz et al. 2016) and RDIP (RNA/DNA immunoprecipitation, implementing key technical modifications in RNase I pretreatment and DNA fragmentation by sonication) (Nadel et al. 2015). DRIPc-seq detected ~70,000 R-loop peaks in the human embryonic carcinoma Ntera2 cells, which is comparable to the number of R-loops detected by RDIP-seq (~64,000 and 39,000 in IMR-90 and HEK 293 T cells, respectively). Consistent with R-loops being co-transcriptional structures, DRIPc-seq peaks were found predominantly in RNA polymerase II-transcribed genes, with two- to three-fold enrichment at the promoter and terminator regions (Sanz et al. 2016). Interestingly, this co-transcriptional model of R-loop formation was contended by the RDIP-seq study, at least at the ribosomal DNA loci (Nadel et al. 2015). Moreover, using a more sensitive readout (than RNA-seq) for transcription, the global run-on sequencing (GRO-seq) method, it was found that only 47.7% of the RNA/DNA hybrids are associated with active transcription (Nadel et al. 2015). Finally, the RDIP-seq study reported a moderate depletion of R-loops at the terminators, in contrast to a twofold enrichment at the promoters (included in a 1.5 kb window downstream from the transcription start site) (Nadel et al. 2015). These studies highlighted the dynamic nature of R-loops and their potential cell type-specific formation. They also necessitate an unbiased approach to identify R-loops in the human genome.
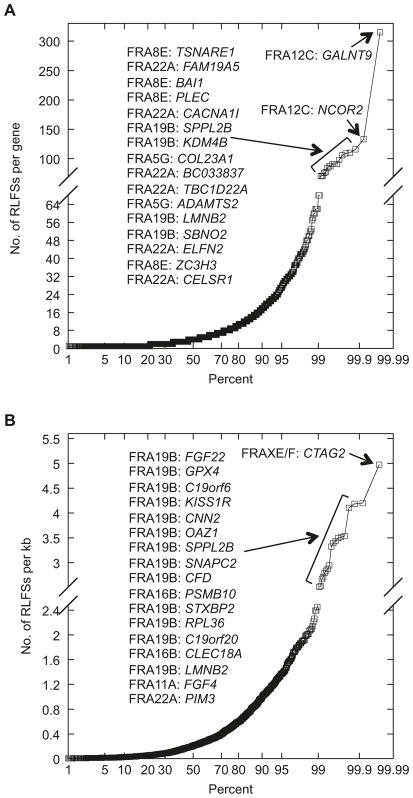
Sequences with high CG content are more conducive to R-loop formation due to high thermostability of the rG·C base pair (Roy and Lieber 2009; Sugimoto et al. 1995; Tracy et al. 2000). Training on previously discovered R-loops, a quantitative model of R-loop forming sequences (RLFSs) has been developed and used to predict RLFSs in the known transcribed regions of the human genome (Wongsurawat et al. 2012). Using the R-loop database (http://rloop.bii.a-star.edu.sg/), we tallied the number of R-loops in each gene of the RFSs as well as calculated their density (number of R-loops per kb in a given gene), as reported in Table 21.1. Our analysis shows that genes residing in RFSs have relatively higher concentration of R-loop forming sequences compared to all genes (Table 21.3). We also queried which RFSs and genes have the highest R-loop content (top 1%), either based on total number or density (Fig. 21.2). The analysis reveals that FRA22A is enriched for genes with the highest number of R-loops (more than 70 R-loops per gene) and that FRA19B is enriched for genes with the highest R-loop density (more than 2.5 R-loops per kb sequence). High R-loop content, together with the fact that many RFSs contain CGG repeat, is intriguing. Because the computational model for R-loop prediction defines an R-loop core sequence as clusters of 3–4 contiguous Gs interspersed by a single nucleotide, CGG trinucleotide repeats per se are not R-loop forming sequences. It would be interesting to test if these two entities have coevolved and that the higher than average R-loop content in the RFSs predisposes CGG repeats to break.
Table 21.3.
Distribution of RLFSs in the genes located in 1973 RFS-associated and 13 cloned CFS-associated genes vs. all annotated genes in the genome
| No. of RLFSs per gene | RFSs | CFSs | Whole genome | |||
|---|---|---|---|---|---|---|
| No. of genes | % | No. of genes | % | No. of genes | % | |
| 1 | 416 | 21.08 | 0 | 16,362 | 41.19 | |
| 2 | 279 | 14.14 | 1 | 7.69 | 7,910 | 19.91 |
| 3 | 219 | 11.10 | 2 | 15.38 | 4,563 | 11.49 |
| 4 | 164 | 8.31 | 1 | 7.69 | 2,880 | 7.25 |
| 5 | 137 | 6.94 | 0 | 1,992 | 5.02 | |
| 6–10 | 366 | 18.55 | 2 | 15.38 | 3,811 | 9.59 |
| 11–50 | 362 | 18.35 | 7 | 53.85 | 2,132 | 5.37 |
| 51–100 | 24 | 1.22 | 0 | 59 | 0.15 | |
| >100 | 6 | 0.31 | 0 | 11 | 0.03 | |
| Total | 1973 | 100 | 13 | 100 | 39,720 | 100 |
Statistics from the whole genome were previously reported (Wongsurawat et al. 2012)
Fig. 21.2.
Probability plots showing the distribution of 1973 genes within RFSs by the number of RLFSs per gene (A) or by the number of RLFSs per kb for a given gene (B). The top 1% of genes in each plot are as shown
We performed similar analysis with a subset (n = 11) of CFSs, which have been cloned, and the associated genes identified (13 total, with FRAXC and FRA10G each housing two genes): FRA1B(DAB1), FRA2F(LRP1B), FRA3B(FHIT), FRA4F(GRID2), FRA6E(PARK2), FRA10D(CTNNA3), FRA10G(RET, NCOA4), FRA15A(RORA), FRA16D(WWOX), and FRAXC(DMD, IL1RAPL1). Seven of these 13 genes (54%) contain 11–50 R-loops, which appeared significantly higher than the reported genomic average (5%) with the same level of R-loop content (Table 21.3). Thus, R-loop formation might also be associated with CFSs, consistent with previous findings that breakage at CFSs harboring large genes can be due to R-loop formation deterring transcription complexes (Helmrich et al. 2011; Wilson et al. 2015).
21.3.4 Mechanisms of Fragile Site Formation
Early studies observed that the fragile X chromosome showed delayed or incomplete replication due to the slowing down or stalling of replication forks (Hansen et al. 1993). This study presented an attractive model for fragile site development as approximately 1% and 20% of the genome in transformed and non-transformed human cells, respectively, undergoes replication even during mitosis (Widrow et al. 1998). Subsequent studies also confirmed late replication timing of a handful of CFSs (Handt et al. 2000; Hellman et al. 2000; Le Beau et al. 1998; Palakodeti et al. 2004; Pelliccia et al. 2008; Wang et al. 1999). However, not all fragile sites appear to replicate late—some are located in the interface of early- and late-replicating regions (El Achkar et al. 2005; Handt et al. 2000), and others are in fact associated with early replication timing (Barlow et al. 2013). Besides replication timing anomalies, CFSs can also be sites of collisions between the replication and the transcription machineries (Helmrich et al. 2011). Additionally, defective processing of CFSs in a Rad52-dependent DNA repair pathway has also been proposed to contribute to fragile site instability (Bhowmick et al. 2016; Sotiriou et al. 2016). However, the requirement for DNA repair is likely linked to a potential underlying defect in DNA replication. Therefore, there appears to be two major factors that underlie fragile site instability: defective DNA initiation/progression and replication-transcription conflicts (Le Tallec et al. 2014; Ozeri-Galai et al. 2014; Sarni and Kerem 2016). Here, we highlight two major areas with competing models, one regarding the replication defects at fragile sites and the other pertaining to the impact of transcriptional status of large genes on chromosome fragility.
While a strong correlation exists between DNA secondary structure formation and fork stalling in both micro- and minisatellites of RFSs, evidences for such direct implication for instability in CFSs are contradictory. Is chromosome fragility the consequence of replication fork stalling at AT-rich sequences in CFSs? Previous studies have shown that replication across CFSs is delayed compared to other regions of the genome, particularly in the presence of aphidicolin (Hellman et al. 2000; Le Beau et al. 1998; Palakodeti et al. 2004; Wang et al. 1999). However, recent studies employing DNA combing have countered that replication fork speed is not different between fragile sites (FRA3B and FRA6E) and non-fragile regions, with or without aphidicolin treatment (Letessier et al. 2011; Palumbo et al. 2010). It was further shown that fragility at FRA3B is the consequence of deficient origin activation within the fragile site when replication fork is slowed down by aphidicolin (Letessier et al. 2011). In contrast, using a similar DNA combing approach, it was shown that replication forks indeed stall at AT-rich sequences in FRA16C (Ozeri-Galai et al. 2011). These contradictory results can be partly explained by significant differences in experimental conditions for replication fork rate measurement, such as the duration of pulse labeling by nucleoside analogs (in DNA combing) and aphidicolin dosage. Considering that fragile site expression level varies significantly with aphidicolin concentrations (Glover et al. 1984), direct comparison between fork rates measured under such different conditions is tenuous. The fact that FRA16C coincides with a RFS, FRA16B, further precludes direct comparison between these studies. Thus, future studies will undoubtedly benefit from standardized experimental conditions. However, both studies agree that paucity of origins in the fragile site region is at least partially responsible for the delayed replication completion of fragile sites (Letessier et al. 2011; Ozeri-Galai et al. 2011; Palumbo et al. 2010).
Another contentious topic is whether the transcriptional status of a large gene impacts fragile site formation. Helmrich et al. suggested that collision between replication and transcription at large genes causes chromosome fragility and, further, breakage frequency is correlated with gene expression level (Helmrich et al. 2012). The authors further posited that R-loop formation as a consequence of transcription along the fragile locus could pose a serious obstacle to the replication fork. However, Le Tallec et al. argued that the expression level of large genes does not correlate with chromosome fragility (Le Tallec et al. 2013). Again, this apparent discrepancy between the two studies is at least attributable to multiple differences in experimental conditions including aphidicolin concentration, definition of large genes, and calculation of break frequency. It further underscores the importance of applying standardized or comparable experimental conditions for effective comparisons between studies.
Finally, an altered epigenetic environment in the fragile loci could be another cause for fragile site instability. The inability to undergo condensation at the time of mitosis could result in DNA strand breakage. Using an in vitro nucleosome reconstitution assay, it was shown that CGG repeats with greater than 50 copies exclude nucleosome and that this exclusion is dependent on the length of the repeat (Wang and Griffith 1996; Wang et al. 1996). It was also shown that the expanded AT-rich minisatellites in FRA16B can exclude nucleosome assembly albeit only in the presence of distamycin A (Hsu and Wang 2002). Finally, it has been shown that hypo-acetylation occurs in CFSs compared to genomic average, indicating a compact chromatin around CFSs (Koch et al. 2007; Savelyeva and Brueckner 2014).
21.3.5 Disease Associations
Fragile sites have been associated with both genetic and epigenetic instability (Smith et al. 2010). Currently there are 11 RFSs that have been molecularly mapped, i.e., the gene(s) that are impacted by fragile site formation have been identified. Many of them are associated with a definitive human disease, predominantly a neurological disorder (Table 21.1). Interestingly, folate-sensitive RFSs are specifically associated with neuropsychiatric disorders including schizophrenia, autism spectrum disorders (ASDs), and mental retardation, while non-folate-sensitive RFSs are associated with a more diverse set of disorders such as infertility and Langer-Giedion syndrome. Schizophrenia, ASDs, and bipolar disorders are all complex neurological disorders that have intricate association with genetics and the environment (Kerner 2014; Miles 2011; Smith et al. 2010). No single gene can account for all the symptoms that characterize these diseases. Therefore, it stands to reason that the genes impacted by chromosome fragility at the folate-sensitive RFSs have a global impact on gene expression and/or protein production in the brain. Many of the CGG repeat-containing genes indeed have high expression in the brain (AFF3, ZNF713, FAM10AC1, FMR1, FMR2) (Uhlen et al. 2010). As the biochemical functions of these RFS-impacted genes become clear, it will help us understand the disease etiology in each of the associated diseases. For instance, the protein product of FMR1, FMRP, is an RNA-binding protein and is estimated to bind 4% of the mRNAs in the brain and regulate their translation (Santoro et al. 2012). Therefore, it remains a challenge to fully understand the genomic impact of FMRP deficiency in the fragile X syndrome.
Moreover, differential expression levels of the disease-associated genes can cause different diseases. For instance, in the case of the FMR1 gene at FRAXA, a full mutation (>200 CGG repeats) induces FMR1 gene silencing and results in the fragile X syndrome, while premutation alleles (55–200 CGG repeats) cause fragile X-associated ataxia/tremor syndrome and fragile X-associated primary ovarian insufficiency [note: premutation alleles do not cause chromosome fragility] (Galloway and Nelson 2009; Garcia-Arocena and Hagerman 2010; Santoro et al. 2012; Usdin et al. 2014). These observations suggest that impaired function of the RFS-associated genes can also impact organs other than the brain. The aforementioned brain-expressed genes associated with RFSs also show high expression in the reproductive organs (Uhlen et al. 2010). In addition, FAM11A gene expression is high in bone marrow, nervous system, and endocrine glands (Unger et al. 2013). Finally, there seems to be a dominance of neurological disorders, as opposed to cancer, associated with RFSs for unknown reasons. CBL2 is the only CGG repeat-associated gene that is both seen in neurological disorders and cancer—while CGG repeat expansion and fragility at CBL2 are linked to Jacobsen syndrome (Jones et al. 1995), CBL2 has also been reported as a proto-oncogene associated with cancer breakpoints in several forms of leukemia and lymphomas (Fu et al. 2003).
In contrast, CFSs are clearly associated with cancer breakpoints and their expression linked to carcinogenesis (Arlt et al. 2006; Ma et al. 2012). Interestingly, some CFSs are also implicated in neurological disorders (Parkinson’s, schizophrenia, and intellectual disability) and a variety of diseases associated with immunodeficiency, bone disorders, and infertility (Table 21.2). Twenty-eight of the 90 CFSs have genes associated with schizophrenia (Smith et al. 2010). Two CFSs, FRA7H and FRA7F, also showed high expression in cells from bipolar disorder patients compared to normal individuals when induced with folate-deficient media (Demirhan et al. 2009). These studies indicate that CFSs may trigger neurological symptoms more frequently than previously thought. The latter study also highlights the importance of understanding the cross-induction of fragile sites by chemicals and growth conditions.
Molecular cloning has enabled the identification of disease-associated gene(s) within a broadly defined fragile site. As shown above, R-loops are enriched in both RFSs and CFSs compared to genomic average. R-loops have been associated with neurological disorders such as the dominant juvenile form of amyotrophic lateral sclerosis type 4 (ALS4) and a recessive form of ataxia oculomotor apraxia type 2 (AOA2) (Chen et al. 2004; Moreira et al. 2004). A firm link between R-loop and oncogenesis was also established when it was shown that BRCA2, mutated in breast and ovarian cancer, is required to prevent R-loop accumulation and genome instability (Bhatia et al. 2014). We speculate that RLFSs can serve as a marker for disease-associated genes. Among the genes residing in RFSs, GALNT9 contains the highest number (315) of R-loops (Fig. 21.2). It has been shown that GALNT9 is frequently methylated and silenced in breast to brain metastasis (Pangeni et al. 2015) and its gene expression is a prognostic marker in neuroblastoma patients (Berois et al. 2013). Whether the high R-loop content plays a role in epigenetic regulation of GALNT9 is an interesting question that warrants further investigation. Similarly, CTAG2 shows the highest R-loop density (5 per kb sequence) among RFS-associated genes (Fig. 21.2). In CFSs, the RET gene at FRA10G has the highest number of R-loops and the highest R-loop density (1 every 1.6 kb, Fig. 21.2). RET rearrangements have been observed in several cancerous cell lines (Dillon et al. 2010). Undoubtedly, systematic analysis of R-loop distribution in all known CFSs would be vitally important.
21.4 Genome-Wide Mapping and Analysis of Fragile Sites
Many of the questions regarding population frequency and penetrance of fragile sites can be more effectively addressed by studies with increased scale and resolution. For instance, are there multiple breakage hot spots in a fragile site? How do the breakage spectra vary between cell types and inducing drugs? Mrasek et al. systematically identified aphidicolin-induced CFSs by screening 25,000 metaphase chromosome spreads isolated from lymphocytes of three normal and unrelated individuals (Mrasek et al. 2010). As alluded to above, this study demonstrated that the classically defined RFSs are less dependent on folate stress for induction than previously considered—all but 3 (FRA6A, FRA12D, and FRAXA) of the 30 RFSs were induced by aphidicolin, and only 4 were present in fewer than 3 individuals (total frequency among 3 individuals for each site listed in Table 21.1). However, based on our analysis of the data reported by Mrasek et al., the penetrance of RFS when induced by aphidicolin is significantly lower than that of CFSs, with the median levels being 0.075% and 0.464%, respectively, confirming the biased potency of aphidicolin toward CFSs.
Cytogenetic screens, while discernible and powerful, are not amenable for genome-wide and high-resolution identification of fragile sites. The advent of deep sequencing technology now permits fragile site mapping at a global scale and a faster pace. Currently the following methods have been applied to map DNA double-strand breaks (DSBs) in the human genome: BLESS (direct in situ break labeling, enrichment on streptavidin, and next-generation sequencing) (Crosetto et al. 2013), RAFT (rapid amplification of forum termini involving direct ligation of biotinylated oligonucleotides to DNA DSBs) (Tchurikov et al. 2015), and DSB-seq (using terminal deoxyribonucleotidyl transferase labeling of DSBs with biotinylated nucleotides followed by streptavidin pulladown and library construction) (Baranello et al. 2014). However, only one study applied conditions to induce CFSs, and it identified over 2429 aphidicolin-induced DSBs after correction for copy number variation in HeLa cells (Crosetto et al. 2013). The authors stated “many CFSs were scored as sensitive to aphidicolin following our approach.” Based on our analysis, 190 (8%) and 574 (24%) of these 2429 DSBs overlap with the known rare and common fragile site regions (defined as cytoband coordinates from the UCSC Human Genome Database), respectively. The apparent lack of correlation for these aphidicolin-induced DSBs with RFSs is not unexpected. But the moderate level of overlap with CFSs begs discussion, and we attribute it to the following reasons. First, the moderate level of concordance is most likely the result of cell type (HeLa)-specific fragile site expression with fragile sites primarily defined in lymphocytes. Second, it also highlights the fundamental difference between methodologies. On one hand, cytologically defined chromosome breakage might include also single-stranded DNA breakage which would evade detection by BLESS. On the other hand, computationally predicted micro-fragile chromosomal regions might be missed by cytological screening (Thys et al. 2015). Finally, the usage of growth medium and concentrations of inducing drugs, e.g., aphidicolin, are not standardized across different studies and impinge on fragile site formation. For these reasons, it is at once a necessity and a challenge for future genomic mapping of fragile sites to compare different cell types with standardized inducing conditions.
21.5 Unsolved Mysteries of Chromosome Fragile Sites
So far, we have discussed some of the contentious topics in chromosome fragility in sections above. In this section we highlight three phenomena that still confound researchers and remain not understood. We also note that additional mysteries previously articulated in “Forgotten fragile sites and related phenomena” still remain unsolved (Sutherland and Baker 2003).
21.5.1 What Is the Underlying Cause for Tissue-Specific Fragile Site Formation?
The very first reported case of fragile site already noted cell type-dependent fragile site expression as discussed earlier in this document. Mounting evidence further confirmed this observation (Hosseini et al. 2013; Kuwano et al. 1990; Le Tallec et al. 2013; Letessier et al. 2011; Murano et al. 1989a, 1989b). Since chromosome fragility is enhanced by replication fork instability, the varying locations and activation patterns of origins of replication across tissue types would directly impact fragile site expression. In addition, tissue-specific gene expression would also influence sites of replication-transcription conflict-induced chromosome fragility. Related to this latter point, we have recently postulated that the inducing agent for fragile site expression plays a dual role in simultaneously generating replication stress and untimely gene expression while replication is still incomplete, resulting in replication-transcription conflicts at distinct loci in different cells (Hoffman et al. 2015). This hypothesis was derived from the model organism Saccharomyces cerevisiae, and we are currently testing it in human cell lines. Future studies mapping fragile sites in different cell lines with simultaneous measurements of origin activities and gene expression levels will be ideal for understanding tissue-specific fragility.
21.5.2 Why Are RFSs Preferentially Induced by Folate Stress?
Perhaps one of the foremost interesting questions is how do different classes of drugs define fragile sites. For instance, folic acid deprivation, thymidylate synthase inhibition (e.g., fluorodeoxyuridine), and thymidine deprivation or excess can all induce a class of folate-sensitive RFSs. One of the outstanding features of the folate-sensitive fragile sites is that nearly half of them (10 out of 22) have been found to contain CGG repeats thus far. Does folate deficiency preferentially lead to DNA breaks at CGG repeats? Folic acid is crucial for methyl metabolism, which in turn impacts DNA replication and repair. Deprivation of folic acid blocks the methylation of dUMP to TMP and triggers an increase in dUTP level. Similarly, intra-cellular fluorodeoxyuridine is converted to fluorodeoxyuridine monophosphate, which in turn inhibits thymidylate synthase and also causes an increase of dUTP. Consequently, there is an increase in the incorporation of uracil into DNA. Uracil in DNA is removed by the uracil DNA glycosylase (UDG), which, through a facilitated diffusion mechanism, locates the damaged/modified DNA bases (Schonhoft et al. 2013). It has been proposed that folate-sensitive chromatid breakage is the result of a catastrophic DNA repair cycle where excision of uracil is followed by reincorporation of uracil due to continuous blockage of the dTTP pool (Reidy 1987).
The question then becomes is there a higher level of uracil incorporation occurring at CGG repeat regions than other chromosomal regions, or are CGG repeats more conducive to uracil excision? There seemed to be evidence supporting both arguments. A genome-wide study in S. cerevisiae has demonstrated that the uracil content is relatively lower in early-replicating regions than late-replicating ones (Bryan et al. 2014). RFSs such as FRAXA tend to be late-replicating (Hansen et al. 1993; Subramanian et al. 1996; Webb 1992) and therefore might incorporate uracil at a higher rate. Alternatively but not mutually exclusively, cytosine deamination to uracil at the CGG repeats might be an underlying cause for increased uracil content. On the other hand, incorporated uracil might be easier to recognize by the UDG enzyme in the context of secondary structures due to CGG repeats. Supporting this hypothesis is the observation that a related DNA glycosylase, the human alkylade-nine DNA glycosylase, can capture the site of DNA damage more efficiently on a flexible DNA template containing kinks, bubbles, or gaps than on a continuous B-form DNA duplex (Hedglin et al. 2015). Conceivably, this characteristic can also extend to UDG acting on a DNA template enriched for CGG repeats which readily form hairpin structures.
21.5.3 What Is the Underlying Cause for Sex-Biased Transmission of Autosomal Fragile Sites?
Sherman and Sutherland reported in a population study that folate-sensitive and BrdU-sensitive autosomal fragile site expression was higher when the carrier parent was the mother than if it was the father (Sherman and Sutherland 1986). Similarly, Kähkönen et al. also observed that there was a maternal bias of the transmission of autosomal RFSs (16 out of 19 families were maternal carriers and 1 was a paternal carrier) (Kähkönen et al. 1989). This phenomenon was once again reported much later in a study demonstrating predominantly maternal transmission of ring chromosome 15 (Nikitina et al. 2003). What is the underlying cause for this sex-biased fragile site inheritance? Is the fragile site expression dependent on genomic imprinting of the maternal genes? Or is the maternal transmission of fragile sites a consequence of nonrandom chromosome arrangement in germ lines? Finally, is this phenomenon unique to folate stress? Large-scale population studies are required to confirm these findings. Further genetic and epigenetic characterizations of the autosomal RFSs will also shed new light on this genetic puzzle.
Acknowledgments
We thank Andrew McCulley for assisting data analysis during this work. This work was supported by the National Institutes of Health (5R01 GM118799-01A1 to W.F.).
Contributor Information
Wenyi Feng, Department of Biochemistry and Molecular Biology, SUNY Upstate Medical University, Syracuse, NY, USA.
Arijita Chakraborty, Department of Biochemistry and Molecular Biology, SUNY Upstate Medical University, Syracuse, NY, USA.
References
- Amarose AP, Huttenlocher PR, Sprudzs RM, Laitsch TJ, Pettenati MJ. A heritable fragile 12q24.13 segregating in a family with the fragile X chromosome. Hum Genet. 1987;75:4–6. doi: 10.1007/BF00273829. [DOI] [PubMed] [Google Scholar]
- Ambroziak W, Koziorowski D, Duszyc K, Gorka-Skoczylas P, Potulska-Chromik A, Slawek J, Hoffman-Zacharska D. Genomic instability in the PARK2 locus is associated with Parkinson’s disease. J Appl Genet. 2015;56:451–461. doi: 10.1007/s13353-015-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt MF, Miller DE, Beer DG, Glover TW. Molecular characterization of FRAXB and comparative common fragile site instability in cancer cells. Genes Chromosom Cancer. 2002;33:82–92. doi: 10.1002/gcc.10000. [DOI] [PubMed] [Google Scholar]
- Arlt MF, Durkin SG, Ragland RL, Glover TW. Common fragile sites as targets for chromosome rearrangements. DNA Repair. 2006;5:1126–1135. doi: 10.1016/j.dnarep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Baranello L, Kouzine F, Wojtowicz D, Cui K, Przytycka TM, Zhao K, Levens D. DNA break mapping reveals topoisomerase II activity genome-wide. Int J Mol Sci. 2014;15:13111–13122. doi: 10.3390/ijms150713111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbi G, Steinbach P, Vogel W. Nonrandom distribution of methotrexate-induced aberrations on human chromosomes. Detection of further folic acid sensitive fragile sites. Human Genetics. 1984;68:290–294. doi: 10.1007/BF00292586. [DOI] [PubMed] [Google Scholar]
- Barletta C, Ragusa RM, Garofalo G, Scillato F, Ruggeri M. Segregation analysis of autosomal fragile sites in three families with the fragile X chromosome. Ann Genet. 1991;34:111–114. [PubMed] [Google Scholar]
- Barlow JH, Faryabi RB, Callen E, Wong N, Malhowski A, Chen HT, Gutierrez-Cruz G, Sun HW, McKinnon P, Wright G, et al. Identification of early replicating fragile sites that contribute to genome instability. Cell. 2013;152:620–632. doi: 10.1016/j.cell.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MV, Hirst MC, Nakahori Y, MacKinnon RN, Roche A, Flint TJ, Jacobs PA, Tommerup N, Tranebjaerg L, Froster-Iskenius U, et al. Physical mapping across the fragile X: hyper-methylation and clinical expression of the fragile X syndrome. Cell. 1991;64:861–866. doi: 10.1016/0092-8674(91)90514-y. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Melko M, Bechara EG, Davidovic L, Berretta A, Catania MV, Gecz J, Lalli E, Bardoni B. FRAXE-associated mental retardation protein (FMR2) is an RNA-binding protein with high affinity for G-quartet RNA forming structure. Nucleic Acids Res. 2009;37:1269–1279. doi: 10.1093/nar/gkn1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berois N, Gattolliat CH, Barrios E, Capandeguy L, Douc-Rasy S, Valteau-Couanet D, Benard J, Osinaga E. GALNT9 gene expression is a prognostic marker in neuroblastoma patients. Clin Chem. 2013;59:225–233. doi: 10.1373/clinchem.2012.192328. [DOI] [PubMed] [Google Scholar]
- Bettex M, Graf B, Winkler B, Gerber-Huber S. Oro-palatal dysplasia Bettex-Graf–a new syndrome. Eur J Pediatr Surg. 1998;8:4–8. doi: 10.1055/s-2008-1071109. [DOI] [PubMed] [Google Scholar]
- Bharadwaj R, Jiang Y, Mao W, Jakovcevski M, Dincer A, Krueger W, Garbett K, Whittle C, Tushir JS, Liu J, et al. Conserved chromosome 2q31 conformations are associated with transcriptional regulation of GAD1 GABA synthesis enzyme and altered in prefrontal cortex of subjects with schizophrenia. J Neurosci. 2013;33:11839–11851. doi: 10.1523/JNEUROSCI.1252-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, Barroso SI, Garcia-Rubio ML, Tumini E, Herrera-Moyano E, Aguilera A. BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature. 2014;511:362–365. doi: 10.1038/nature13374. [DOI] [PubMed] [Google Scholar]
- Bhowmick R, Minocherhomji S, Hickson ID. RAD52 facilitates mitotic DNA synthesis following replication stress. Mol Cell. 2016;64:1117–1126. doi: 10.1016/j.molcel.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Blumrich A, Zapatka M, Brueckner LM, Zheglo D, Schwab M, Savelyeva L. The FRA2C common fragile site maps to the borders of MYCN amplicons in neuroblastoma and is associated with gross chromosomal rearrangements in different cancers. Hum Mol Genet. 2011;20:1488–1501. doi: 10.1093/hmg/ddr027. [DOI] [PubMed] [Google Scholar]
- Bodelon C, Untereiner ME, Machiela MJ, Vinokurova S, Wentzensen N. Genomic characterization of viral integration sites in HPV-related cancers. Int J Cancer. 2016;139:2001–2011. doi: 10.1002/ijc.30243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco N, Pelliccia F, Rocchi A. Characterization of FRA7B, a human common fragile site mapped at the 7p chromosome terminal region. Cancer Genet Cytogenet. 2010;202:47–52. doi: 10.1016/j.cancergencyto.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Bozza M, Bernardini L, Novelli A, Brovedani P, Moretti E, Canapicchi R, Doccini V, Filippi T, Battaglia A. 6p25 interstitial deletion in two dizygotic twins with gyral pattern anomaly and speech and language disorder. Eur J Paediatr Neurol. 2013;17:225–231. doi: 10.1016/j.ejpn.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Bryan DS, Ransom M, Adane B, York K, Hesselberth JR. High resolution mapping of modified DNA nucleobases using excision repair enzymes. Genome Res. 2014;24:1534–1542. doi: 10.1101/gr.174052.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys M, Migdalska-Sek M, Pastuszak-Lewandoska D, Forma E, Czarnecka K, Domanska D, Nawrot E, Wilkosz J, Rozanski W, Brzezianska E. Diagnostic value of DNA alteration: loss of heterozygosity or allelic imbalance-promising for molecular staging of prostate cancers. Med Oncol. 2013;30:391. doi: 10.1007/s12032-012-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrow AA, Marullo A, Holder LR, Wang YH. Secondary structure formation and DNA instability at fragile site FRA16B. Nucleic Acids Res. 2010;38:2865–2877. doi: 10.1093/nar/gkp1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan G, Denison SR, Phillips LA, Shridhar V, Smith DI. Characterization of the common fragile site FRA9E and its potential role in ovarian cancer. Oncogene. 2003;22:590–601. doi: 10.1038/sj.onc.1206171. [DOI] [PubMed] [Google Scholar]
- Capello D, Scandurra M, Poretti G, Rancoita PM, Mian M, Gloghini A, Deambrogi C, Martini M, Rossi D, Greiner TC, et al. Genome wide DNA-profiling of HIV-related B-cell lymphomas. Br J Haematol. 2010;148:245–255. doi: 10.1111/j.1365-2141.2009.07943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Shih HH, Wang-Wuu S, Tai JJ, Wuu KD. Chromosomal fragile site expression in lymphocytes from patients with schizophrenia. Hum Genet. 1998;103:702–706. doi: 10.1007/s004390050894. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A, Kennerson ML, Rabin BA, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4) Am J Hum Genet. 2004;74:1128–1135. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Majima T. Conformational changes of non-B DNA. Chem Soc Rev. 2011;40:5893–5909. doi: 10.1039/c1cs15153c. [DOI] [PubMed] [Google Scholar]
- Conley ME, Spinner NB, Emanuel BS, Nowell PC, Nichols WW. A chromosomal breakage syndrome with profound immunodeficiency. Blood. 1986;67:1251–1256. [PubMed] [Google Scholar]
- Court Brown WM. Chromosome studies on adults. Cambridge U.P: Galton Laboratory, University of London, Cambridge; 1966. [Google Scholar]
- Crosetto N, Mitra A, Silva MJ, Bienko M, Dojer N, Wang Q, Karaca E, Chiarle R, Skrzypczak M, Ginalski K, et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods. 2013;10:361–365. doi: 10.1038/nmeth.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo CS, Moller Dos Santos MF, Alonso LG, Koiffmann CP. Two new cases of 1p21.3 deletions and an unbalanced translocation t(8;12) among individuals with syndromic obesity. Mol Syndromology. 2015;6:63–70. doi: 10.1159/000371600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacker K, Kooy RF. Fragile sites and human disease. Hum Mol Genet. 2007;16(Spec No 2):R150–R158. doi: 10.1093/hmg/ddm136. [DOI] [PubMed] [Google Scholar]
- Debacker K, Winnepenninckx B, Ben-Porat N, FitzPatrick D, Van Luijk R, Scheers S, Kerem B, Frank Kooy R. FRA18C: a new aphidicolin-inducible fragile site on chromosome 18q22, possibly associated with in vivo chromosome breakage. J Med Genet. 2007;44:347–352. doi: 10.1136/jmg.2006.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaban A. Persisting clone of cells with an abnormal chromosome in a woman previously irradiated. J Nucl Med. 1965;6:740–746. [PubMed] [Google Scholar]
- Demirhan O, Tastemir D, Sertdemir Y. Chromosomal fragile sites in schizophrenic patients. Genetika. 2006;42:985–992. [PubMed] [Google Scholar]
- Demirhan O, Tastemir D, Sertdemir Y. The expression of folate sensitive fragile sites in patients with bipolar disorder. Yonsei Med J. 2009;50:137–141. doi: 10.3349/ymj.2009.50.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Kioschis P, Monaco AP, Gross B, Korn B, Williams SV, Sheer D, Heitz D, Oberle I, Toniolo D, et al. Molecular cloning and analysis of the fragile X region in man. Nucleic Acids Res. 1991;19:2567–2572. doi: 10.1093/nar/19.10.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon LW, Burrow AA, Wang YH. DNA instability at chromosomal fragile sites in cancer. Curr Genomics. 2010;11:326–337. doi: 10.2174/138920210791616699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon LW, Pierce LC, Ng MC, Wang YH. Role of DNA secondary structures in fragile site breakage along human chromosome 10. Hum Mol Genet. 2013;22:1443–1456. doi: 10.1093/hmg/dds561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley TP, Mitchison HM, Munroe PB, Probst P, Neal M, Siciliano MJ, Deng Z, Doggett NA, Callen DF, Gardiner RM, et al. Mapping of two phenol sulphotransferase genes, STP and STM, to 16p: candidate genes for Batten disease. Biochem Biophys Res Commun. 1994;205:482–489. doi: 10.1006/bbrc.1994.2691. [DOI] [PubMed] [Google Scholar]
- Dunner JA, Martin AO, Traisman ES, Traisman HS, Elias S. Enhancement of a fra(16)(q22) with distamycin A: a family ascertained through an abnormal proposita. Am J Med Genet. 1983;16:277–284. doi: 10.1002/ajmg.1320160216. [DOI] [PubMed] [Google Scholar]
- Durkin SG, Glover TW. Chromosome fragile sites. Annu Rev Genet. 2007;41:169–192. doi: 10.1146/annurev.genet.41.042007.165900. [DOI] [PubMed] [Google Scholar]
- El Achkar E, Gerbault-Seureau M, Muleris M, Dutrillaux B, Debatisse M. Premature condensation induces breaks at the interface of early and late replicating chromosome bands bearing common fragile sites. Proc Natl Acad Sci U S A. 2005;102:18069–18074. doi: 10.1073/pnas.0506497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Lisanti MP. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998;436:403–410. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- Fechter A, Buettel I, Kuehnel E, Savelyeva L, Schwab M. Common fragile site FRA11G and rare fragile site FRA11B at 11q23.3 encompass distinct genomic regions. Genes Chromosom Cancer. 2007;46:98–106. doi: 10.1002/gcc.20389. [DOI] [PubMed] [Google Scholar]
- Ferber MJ, Eilers P, Schuuring E, Fenton JA, Fleuren GJ, Kenter G, Szuhai K, Smith DI, Raap AK, Brink AA. Positioning of cervical carcinoma and Burkitt lymphoma translocation breakpoints with respect to the human papillomavirus integration cluster in FRA8C at 8q24.13. Cancer Genet Cytogenet. 2004;154:1–9. doi: 10.1016/j.cancergencyto.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Ferguson-Smith MA. Inherited constriction fragility of chromosome 2. Ann Genet. 1973;16:29–34. [PubMed] [Google Scholar]
- Ferguson-Smith MA, Ferguson-Smith ME, Ellis PM, Dickson M. The sites and relative frequencies of secondary constrictions in human somatic chromosomes. Cytogenetics. 1962;1:325–343. doi: 10.1159/000129743. [DOI] [PubMed] [Google Scholar]
- Fry M, Loeb LA. The fragile X syndrome d(CGG)n nucleotide repeats form a stable tetra-helical structure. Proc Natl Acad Sci U S A. 1994;91:4950–4954. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JF, Hsu JJ, Tang TC, Shih LY. Identification of CBL, a proto-oncogene at 11q23.3, as a novel MLL fusion partner in a patient with de novo acute myeloid leukemia. Genes Chromosom Cancer. 2003;37:214–219. doi: 10.1002/gcc.10204. [DOI] [PubMed] [Google Scholar]
- Fungtammasan A, Walsh E, Chiaromonte F, Eckert KA, Makova KD. A genome-wide analysis of common fragile sites: what features determine chromosomal instability in the human genome? Genome Res. 2012;22:993–1005. doi: 10.1101/gr.134395.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JN, Nelson DL. Evidence for RNA-mediated toxicity in the fragile X-associated tremor/ataxia syndrome. Futur Neurol. 2009;4:785. doi: 10.2217/fnl.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Dillon LW, Pramanik S, Nikiforov YE, Wang YH. DNA breaks at fragile sites generate oncogenic RET/PTC rearrangements in human thyroid cells. Oncogene. 2010;29:2272–2280. doi: 10.1038/onc.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Kasperbauer JL, Tombers NM, Wang V, Mayer K, Smith DI. A selected group of large common fragile site genes have decreased expression in oropharyngeal squamous cell carcinomas. Genes Chromosom Cancer. 2014;53:392–401. doi: 10.1002/gcc.22150. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginno PA, Lim YW, Lott PL, Korf I, Chedin F. GC skew at the 5′ and 3′ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 2013;23:1590–1600. doi: 10.1101/gr.158436.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud F, Ayme S, Mattei JF, Mattei MG. Constitutional chromosomal breakage. Hum Genet. 1976;34:125–136. doi: 10.1007/BF00278880. [DOI] [PubMed] [Google Scholar]
- Glasser L, Meloni-Ehrig A, Joseph P, Mendiola J. Benign chronic neutropenia with abnormalities involving 16q22, affecting mother and daughter. Am J Hematol. 2006;81:262–270. doi: 10.1002/ajh.20550. [DOI] [PubMed] [Google Scholar]
- Glover TW, Berger C, Coyle J, Echo B. DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet. 1984;67:136–142. doi: 10.1007/BF00272988. [DOI] [PubMed] [Google Scholar]
- Groh M, Gromak N. Out of balance: R-loops in human disease. PLoS Genet. 2014;10:e1004630. doi: 10.1371/journal.pgen.1004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh M, Lufino MM, Wade-Martins R, Gromak N. R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet. 2014;10:e1004318. doi: 10.1371/journal.pgen.1004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handt O, Sutherland GR, Richards RI. Fragile sites and minisatellite repeat instability. Mol Genet Metab. 2000;70:99–105. doi: 10.1006/mgme.2000.2996. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Gartler SM, Scott CR, Chen SH, Laird CD. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum Mol Genet. 1992;1:571–578. doi: 10.1093/hmg/1.8.571. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Canfield TK, Lamb MM, Gartler SM, Laird CD. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993;73:1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- Harvey J, Judge C, Wiener S. Familial X-linked mental retardation with an X chromosome abnormality. J Med Genet. 1977;14:46–50. doi: 10.1136/jmg.14.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchi E, Skourti-Stathaki K, Ventz S, Pinello L, Yen A, Kamieniarz-Gdula K, Dimitrov S, Pathania S, McKinney KM, Eaton ML, et al. BRCA1 recruitment to transcriptional pause sites is required for R-loop-driven DNA damage repair. Mol Cell. 2015;57:636–647. doi: 10.1016/j.molcel.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht F. Rare and polymorphic fragile sites and cancer. Cancer Genet Cytogenet. 1988;34:195–199. doi: 10.1016/0165-4608(88)90259-2. [DOI] [PubMed] [Google Scholar]
- Hedglin M, Zhang Y, O’Brien PJ. Probing the DNA structural requirements for facilitated diffusion. Biochemistry. 2015;54:557–566. doi: 10.1021/bi5013707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz D, Rousseau F, Devys D, Saccone S, Abderrahim H, Le Paslier D, Cohen D, Vincent A, Toniolo D, Della Valle G, et al. Isolation of sequences that span the fragile X and identification of a fragile X-related CpG island. Science. 1991;251:1236–1239. doi: 10.1126/science.2006411. [DOI] [PubMed] [Google Scholar]
- Hellman A, Rahat A, Scherer SW, Darvasi A, Tsui LC, Kerem B. Replication delay along FRA7H, a common fragile site on human chromosome 7, leads to chromosomal instability. Mol Cell Biol. 2000;20:4420–4427. doi: 10.1128/mcb.20.12.4420-4427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmrich A, Ballarino M, Tora L. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol Cell. 2011;44:966–977. doi: 10.1016/j.molcel.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Helmrich U, Marsano A, Melly L, Wolff T, Christ L, Heberer M, Scherberich A, Martin I, Banfi A. Generation of human adult mesenchymal stromal/stem cells expressing defined xeno-genic vascular endothelial growth factor levels by optimized transduction and flow cytometry purification. Tissue Eng Part C Methods. 2012;18:283–292. doi: 10.1089/ten.tec.2011.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Harada Y, Takahashi E, Hou J, Wagner MJ, Wells DE. Assignment of fragile site 8E (FRA8E) to human chromosome band 8q24.11 adjacent to the hereditary multiple exostoses 1 gene and two overlapping Langer-Giedion syndrome deletion endpoints. Cytogenet Cell Genet. 1997;78:56–57. doi: 10.1159/000134628. [DOI] [PubMed] [Google Scholar]
- Hills LB, Masri A, Konno K, Kakegawa W, Lam AT, Lim-Melia E, Chandy N, Hill RS, Partlow JN, Al-Saffar M, et al. Deletions in GRID2 lead to a recessive syndrome of cerebellar ataxia and tonic upgaze in humans. Neurology. 2013;81:1378–1386. doi: 10.1212/WNL.0b013e3182a841a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, McCulley A, Haarer B, Arnak R, Feng W. Break-seq reveals hydroxyurea-induced chromosome fragility as a result of unscheduled conflict between DNA replication and transcription. Genome Res. 2015;25:402–412. doi: 10.1101/gr.180497.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JJ, Walker M, Chalifoux M, White BN. Trinucleotide repeats at the FRAXF locus: frequency and distribution in the general population. Am J Med Genet. 1996;64:424–427. doi: 10.1002/(SICI)1096-8628(19960809)64:2<424::AID-AJMG38>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Hormozian F, Schmitt JG, Sagulenko E, Schwab M, Savelyeva L. FRA1E common fragile site breaks map within a 370kilobase pair region and disrupt the dihydropyrimidine dehydrogenase gene (DPYD) Cancer Lett. 2007;246:82–91. doi: 10.1016/j.canlet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Hosseini SA, Horton S, Saldivar JC, Miuma S, Stampfer MR, Heerema NA, Huebner K. Common chromosome fragile sites in human and murine epithelial cells and FHIT/FRA3B loss-induced global genome instability. Genes Chromosom Cancer. 2013;52:1017–1029. doi: 10.1002/gcc.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Du L, Lei B, Pang CP, Zhang M, Zhuang W, Zhang M, Huang L, Gong B, Wang M, et al. Genome-wide association analysis of Vogt-Koyanagi-Harada syndrome identifies two new susceptibility loci at 1p31.2 and 10q21.3. Nat Genet. 2014;46:1007–1011. doi: 10.1038/ng.3061. [DOI] [PubMed] [Google Scholar]
- Howell RT, McDermott A, Evans JL. A new apparently folate sensitive fragile site, 5q35. J Med Genet. 1990;27:527–528. doi: 10.1136/jmg.27.8.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TC. Longitudinal differentiation of chromosomes and the possibility of interstitial telomeres. Exp Cell Res. 1963;24(SUPPL9):73–85. doi: 10.1016/0014-4827(63)90246-5. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Wang YH. Human fragile site FRA16B DNA excludes nucleosomes in the presence of distamycin. J Biol Chem. 2002;277:17315–17319. doi: 10.1074/jbc.M200901200. [DOI] [PubMed] [Google Scholar]
- Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. 2003;12:711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Ijdo JW, Wells RA, Baldini A, Reeders ST. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res. 1991;19:4780. doi: 10.1093/nar/19.17.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewska-Olszowska J, Gawrych E, Dydyk A, Studniak E, Biadun-Poplawska A, Zajaczek S. Oro-palatal dysplasia Bettex-Graf – clinical findings, genetic background, treatment. J Craniomaxillofac Surg. 2013;41:e29–e32. doi: 10.1016/j.jcms.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Jones C, Slijepcevic P, Marsh S, Baker E, Langdon WY, Richards RI, Tunnacliffe A. Physical linkage of the fragile site FRA11B and a Jacobsen syndrome chromosome deletion breakpoint in 11q23.3. Hum Mol Genet. 1994;3:2123–2130. doi: 10.1093/hmg/3.12.2123. [DOI] [PubMed] [Google Scholar]
- Jones C, Penny L, Mattina T, Yu S, Baker E, Voullaire L, Langdon WY, Sutherland GR, Richards RI, Tunnacliffe A. Association of a chromosome deletion syndrome with a fragile site within the proto-oncogene CBL2. Nature. 1995;376:145–149. doi: 10.1038/376145a0. [DOI] [PubMed] [Google Scholar]
- Kahkonen M, Leisti J, Thoden CJ, Autio S. Frequency of rare fragile sites among mentally subnormal schoolchildren. Clin Genet. 1986;30:234–238. doi: 10.1111/j.1399-0004.1986.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Kähkönen M, Tengstrom C, Alitalo T, Matilainen R, Kaski M, Airaksinen E. Population cytogenetics of folate-sensitive fragile sites. II. Autosomal rare fragile sites. Hum Genet. 1989;82:3–8. doi: 10.1007/BF00288261. [DOI] [PubMed] [Google Scholar]
- Kang S, Ohshima K, Shimizu M, Amirhaeri S, Wells RD. Pausing of DNA synthesis in vitro at specific loci in CTG and CGG triplet repeats from human hereditary disease genes. J Biol Chem. 1995;270:27014–27021. doi: 10.1074/jbc.270.45.27014. [DOI] [PubMed] [Google Scholar]
- Kao-Shan CS, Fine RL, Whang-Peng J, Lee EC, Chabner BA. Increased fragile sites and sister chromatid exchanges in bone marrow and peripheral blood of young cigarette smokers. Cancer Res. 1987;47:6278–6282. [PubMed] [Google Scholar]
- Karayianni E, Magnanini C, Orphanos V, Negrini M, Maniatis GM, Spathas DH, Barbanti-Brodano G, Morelli C. Transcriptional map of chromosome region 6q16 – >q21. Cytogenet Cell Genet. 1999;86:263–266. doi: 10.1159/000015356. [DOI] [PubMed] [Google Scholar]
- Karkera JD, Ayache S, Ransome RJ, Jr, Jackson MA, Elsayem AF, Sridhar R, Detera-Wadleigh SD, Wadleigh RG. Refinement of regions with allelic loss on chromosome 18p11.2 and 18q12.2 in esophageal squamous cell carcinoma. Clin Cancer Res. 2000;6:3565–3569. [PubMed] [Google Scholar]
- Kasi Loknath Kumar A, Weckbaugh B, Sirridge C, Woodroof J, Persons D, Kambhampati S. Myelodysplastic syndrome with concomitant t(5;21)(q15;q22) and del(5)(q13q33): case report and review of literature. Stem Cell Inv. 2016;3:3. doi: 10.3978/j.issn.2306-9759.2016.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbeshian J, Severud R, Burd L, Larson L. Peek-a-boo fragile site at 16d associated with Tourette syndrome, bipolar disorder, autistic disorder, and mental retardation. Am J Med Genet. 2000;96:69–73. doi: 10.1002/(sici)1096-8628(20000207)96:1<69::aid-ajmg14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kerner B. Genetics of bipolar disorder. Appl Clin Genet. 2014;7:33–42. doi: 10.2147/TACG.S39297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CM, Andrews RM, Flicek P, Dillon SC, Karaoz U, Clelland GK, Wilcox S, Beare DM, Fowler JC, Couttet P, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17:691–707. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EJ, Pritchard M, Lynch M, Yu S, Holman K, Baker E, Warren ST, Schlessinger D, Sutherland GR, Richards RI. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991;252:1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Kuwano A, Murano I, Kajii T. Cell type-dependent difference in the distribution and frequency of excess thymidine-induced common fragile sites: T lymphocytes and skin fibroblasts. Hum Genet. 1990;84:527–531. doi: 10.1007/BF00210803. [DOI] [PubMed] [Google Scholar]
- Lai LA, Kostadinov R, Barrett MT, Peiffer DA, Pokholok D, Odze R, Sanchez CA, Maley CC, Reid BJ, Gunderson KL, et al. Deletion at fragile sites is a common and early event in Barrett’s esophagus. Mol Cancer Res. 2010;8:1084–1094. doi: 10.1158/1541-7786.MCR-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau MM, Rassool FV, Neilly ME, Espinosa R, 3rd, Glover TW, Smith DI, McKeithan TW. Replication of a common fragile site, FRA3B, occurs late in S phase and is delayed further upon induction: implications for the mechanism of fragile site induction. Hum Mol Genet. 1998;7:755–761. doi: 10.1093/hmg/7.4.755. [DOI] [PubMed] [Google Scholar]
- Le Tallec B, Millot GA, Blin ME, Brison O, Dutrillaux B, Debatisse M. Common fragile site profiling in epithelial and erythroid cells reveals that most recurrent cancer deletions lie in fragile sites hosting large genes. Cell Rep. 2013;4:420–428. doi: 10.1016/j.celrep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Le Tallec B, Koundrioukoff S, Wilhelm T, Letessier A, Brison O, Debatisse M. Updating the mechanisms of common fragile site instability: how to reconcile the different views? Cell Mol Life Sci. 2014;71:4489–4494. doi: 10.1007/s00018-014-1720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Clayton DA. Properties of a primer RNA-DNA hybrid at the mouse mitochondrial DNA leading-strand origin of replication. J Biol Chem. 1996;271:24262–24269. doi: 10.1074/jbc.271.39.24262. [DOI] [PubMed] [Google Scholar]
- Lejeune J, Dutrillaux B, Lafourcade J, Berger R, Abonyi D, Rethore MO. Selective endo-reduplication of the long arm of the 2 chromosome in a woman and her daughter. C R Acad Sci Hebd Seances Acad Sci D. 1968;266:24–26. [PubMed] [Google Scholar]
- Letessier A, Garrido-Urbani S, Ginestier C, Fournier G, Esterni B, Monville F, Adelaide J, Geneix J, Xerri L, Dubreuil P, et al. Correlated break at PARK2/FRA6E and loss of AF-6/Afadin protein expression are associated with poor outcome in breast cancer. Oncogene. 2007;26:298–307. doi: 10.1038/sj.onc.1209772. [DOI] [PubMed] [Google Scholar]
- Letessier A, Millot GA, Koundrioukoff S, Lachages AM, Vogt N, Hansen RS, Malfoy B, Brison O, Debatisse M. Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature. 2011;470:120–123. doi: 10.1038/nature09745. [DOI] [PubMed] [Google Scholar]
- Li X, Manley JL. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Li J, Liu J, Ren Y, Yang J, Liu P. Common chromosomal fragile site gene WWOX in metabolic disorders and tumors. Int J Biol Sci. 2014;10:142–148. doi: 10.7150/ijbs.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QS, Parrado AR, Samtani MN, Narayan VA Alzheimer’s Disease Neuroimaging, I. Variations in the FRA10AC1 fragile site and 15q21 are associated with cerebrospinal fluid Abeta1–42 level. PLoS One. 2015;10:e0134000. doi: 10.1371/journal.pone.0134000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligsay A, Hagerman RJ. Review of targeted treatments in fragile X syndrome. Intractable Rare Dis Res. 2016;5:158–167. doi: 10.5582/irdr.2016.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon J, Dal Cin P, Sait SN, Karakousis C, Sandberg AA. Chromosome changes in meta-static human melanoma. Cancer Genet Cytogenet. 1988;30:201–211. doi: 10.1016/0165-4608(88)90186-0. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Liu X, Chang JS, Chien WK, Chen JH, Tsang H, Hung CH, Lin TH, Huang SM, Liao CC, et al. Coronary artery aneurysms occurrence risk analysis between Kawasaki disease and LRP1B gene in Taiwanese children. Biomedicine. 2014;4:10. doi: 10.7603/s40681-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S, Splitt M, Edney S, Berney TP, Knight SJ, Davies KE, O’Brien O, Gale M, Burn J. PPM-X: a new X-linked mental retardation syndrome with psychosis, pyramidal signs, and macroorchidism maps to Xq28. Am J Hum Genet. 1996;58:1120–1126. [PMC free article] [PubMed] [Google Scholar]
- Lipska BS, Koczkowska M, Wierzba J, Ploszynska A, Iliszko M, Izycka-Swieszewska E, Adamkiewicz-Drozynska E, Limon J. On the significance of germline cytogenetic rearrangements at MYCN locus in neuroblastoma. Mol Cytogenet. 2013;6:43. doi: 10.1186/1755-8166-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Yan J, Chen X, Song J, Wang B, Yao Y. Prenatal diagnosis of a de novo interstitial deletion of 11q (11q22.3 –> q23.3) associated with abnormal ultrasound findings by array comparative genomic hybridization. Mol Cytogenet. 2014;7:62. doi: 10.1186/s13039-014-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombrana R, Almeida R, Alvarez A, Gomez M. R-loops and initiation of DNA replication in human cells: a missing link? Front Genet. 2015;6:158. doi: 10.3389/fgene.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Azarang A, Wilaisakditipakorn T, Hagerman RJ. Fragile X syndrome: a review of clinical management. Intractable Rare Dis Res. 2016;5:145–157. doi: 10.5582/irdr.2016.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubs HA. A marker X chromosome. Am J Hum Genet. 1969;21:231–244. [PMC free article] [PubMed] [Google Scholar]
- Lukusa T, Fryns JP. Human chromosome fragility. Biochim Biophys Acta. 2008;1779:3–16. doi: 10.1016/j.bbagrm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Ma K, Qiu L, Mrasek K, Zhang J, Liehr T, Quintana LG, Li Z. Common fragile sites: genomic hotspots of DNA damage and carcinogenesis. Int J Mol Sci. 2012;13:11974–11999. doi: 10.3390/ijms130911974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madireddy A, Kosiyatrakul ST, Boisvert RA, Herrera-Moyano E, Garcia-Rubio ML, Gerhardt J, Vuono EA, Owen N, Yan Z, Olson S, et al. FANCD2 facilitates replication through common fragile sites. Mol Cell. 2016;64:388–404. doi: 10.1016/j.molcel.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis RE, Hecht F, Lovrien EW. Heritable fragile site on chromosome 16: probable localization of haptoglobin locus in man. Science. 1970;170:85–87. doi: 10.1126/science.170.3953.85. [DOI] [PubMed] [Google Scholar]
- Maltby EL, Higgins S. Folate sensitive site at 10q23 and its expression as a deletion. J Med Genet. 1987;24:299. doi: 10.1136/jmg.24.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell MR, Martinez-Pasarell O, Lopez O, Polo A, Sandalinas M, Garcia-Guixe E, Bassas L. Chromosome 16 abnormalities in embryos and in sperm from a male with a fragile site at 16q22.1. Cytogenet Genome Res. 2014;142:134–139. doi: 10.1159/000357411. [DOI] [PubMed] [Google Scholar]
- McAvoy S, Zhu Y, Perez DS, James CD, Smith DI. Disabled-1 is a large common fragile site gene, inactivated in multiple cancers. Genes Chromosom Cancer. 2008;47:165–174. doi: 10.1002/gcc.20519. [DOI] [PubMed] [Google Scholar]
- McKenzie F, Turner A, Withers S, Dalzell P, McGlynn M, Kirk EP. Dominant inheritance of cleft palate, microstomia and micrognathia–possible linkage to the fragile site at 16q22 (FRA16B) Clin Dysmorphol. 2002;11:237–241. doi: 10.1097/00019605-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Metsu S, Rooms L, Rainger J, Taylor MS, Bengani H, Wilson DI, Chilamakuri CS, Morrison H, Vandeweyer G, Reyniers E, et al. FRA2A is a CGG repeat expansion associated with silencing of AFF3. PLoS Genet. 2014a;10:e1004242. doi: 10.1371/journal.pgen.1004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsu S, Rainger JK, Debacker K, Bernhard B, Rooms L, Grafodatskaya D, Weksberg R, Fombonne E, Taylor MS, Scherer SW, et al. A CGG-repeat expansion mutation in ZNF713 causes FRA7A: association with autistic spectrum disorder in two families. Hum Mutat. 2014b;35:1295–1300. doi: 10.1002/humu.22683. [DOI] [PubMed] [Google Scholar]
- Michaelis RC, Velagaleti GV, Jones C, Pivnick EK, Phelan MC, Boyd E, Tarleton J, Wilroy RS, Tunnacliffe A, Tharapel AT. Most Jacobsen syndrome deletion breakpoints occur distal to FRA11B. Am J Med Genet. 1998;76:222–228. [PubMed] [Google Scholar]
- Miles JH. Autism spectrum disorders–a genetics review. Genet Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- Mirkin SM. DNA structures, repeat expansions and human hereditary disorders. Curr Opin Struct Biol. 2006;16:351–358. doi: 10.1016/j.sbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Mishmar D, Rahat A, Scherer SW, Nyakatura G, Hinzmann B, Kohwi Y, Mandel-Gutfroind Y, Lee JR, Drescher B, Sas DE, et al. Molecular characterization of a common fragile site (FRA7H) on human chromosome 7 by the cloning of a simian virus 40 integration site. Proc Natl Acad Sci U S A. 1998;95:8141–8146. doi: 10.1073/pnas.95.14.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira MC, Klur S, Watanabe M, Nemeth AH, Le Ber I, Moniz JC, Tranchant C, Aubourg P, Tazir M, Schols L, et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat Genet. 2004;36:225–227. doi: 10.1038/ng1303. [DOI] [PubMed] [Google Scholar]
- Morelli C, Karayianni E, Magnanini C, Mungall AJ, Thorland E, Negrini M, Smith DI, Barbanti-Brodano G. Cloning and characterization of the common fragile site FRA6F harboring a replicative senescence gene and frequently deleted in human tumors. Oncogene. 2002;21:7266–7276. doi: 10.1038/sj.onc.1205573. [DOI] [PubMed] [Google Scholar]
- Moriarty HT, Webster LR. Fragile sites and bladder cancer. Cancer Genet Cytogenet. 2003;140:89–98. doi: 10.1016/s0165-4608(02)00650-7. [DOI] [PubMed] [Google Scholar]
- Mrasek K, Schoder C, Teichmann AC, Behr K, Franze B, Wilhelm K, Blaurock N, Claussen U, Liehr T, Weise A. Global screening and extended nomenclature for 230 aphidicolin-inducible fragile sites, including 61 yet unreported ones. Int J Oncol. 2010;36:929–940. doi: 10.3892/ijo_00000572. [DOI] [PubMed] [Google Scholar]
- Mulatinho MV, de Carvalho Serao CL, Scalco F, Hardekopf D, Pekova S, Mrasek K, Liehr T, Weise A, Rao N, Llerena JC., Jr Severe intellectual disability, omphalocele, hypospadia and high blood pressure associated to a deletion at 2q22.1q22.3: case report. Mol Cytogenet. 2012;5:30. doi: 10.1186/1755-8166-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano I, Kuwano A, Kajii T. Fibroblast-specific common fragile sites induced by aphidi-colin. Hum Genet. 1989a;83:45–48. doi: 10.1007/BF00274145. [DOI] [PubMed] [Google Scholar]
- Murano I, Kuwano A, Kajii T. Cell type-dependent difference in the distribution and frequency of aphidicolin-induced fragile sites: T and B lymphocytes and bone marrow cells. Hum Genet. 1989b;84:71–74. doi: 10.1007/BF00210675. [DOI] [PubMed] [Google Scholar]
- Nadel J, Athanasiadou R, Lemetre C, Wijetunga NA, O Broin P, Sato H, Zhang Z, Jeddeloh J, Montagna C, Golden A, et al. RNA:DNA hybrids in the human genome have distinctive nucleotide characteristics, chromatin composition, and transcriptional relationships. Epigenetics Chromatin. 2015;8:46. doi: 10.1186/s13072-015-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina NV, Bushueva OA, Nikolaeva EB, Pavlov GV, Lurie IW. Maternal transmission of a ring chromosome 15. Genet Couns. 2003;14:181–186. [PubMed] [Google Scholar]
- Oei L, Hsu YH, Styrkarsdottir U, Eussen BH, de Klein A, Peters MJ, Halldorsson B, Liu CT, Alonso N, Kaptoge SK, et al. A genome-wide copy number association study of osteoporotic fractures points to the 6p25.1 locus. J Med Genet. 2014;51:122–131. doi: 10.1136/jmedgenet-2013-102064. [DOI] [PubMed] [Google Scholar]
- Olavesen MG, Davies AF, Broxholme SJ, Wixon JL, Senger G, Nizetic D, Campbell RD, Ragoussis J. An integrated map of human chromosome 6p23. Genome Res. 1995;5:342–358. doi: 10.1101/gr.5.4.342. [DOI] [PubMed] [Google Scholar]
- Ozeri-Galai E, Lebofsky R, Rahat A, Bester AC, Bensimon A, Kerem B. Failure of origin activation in response to fork stalling leads to chromosomal instability at fragile sites. Mol Cell. 2011;43:122–131. doi: 10.1016/j.molcel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Ozeri-Galai E, Tur-Sinai M, Bester AC, Kerem B. Interplay between genetic and epigenetic factors governs common fragile site instability in cancer. Cell Mol Life Sci. 2014;71:4495–4506. doi: 10.1007/s00018-014-1719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palakodeti A, Han Y, Jiang Y, Le Beau MM. The role of late/slow replication of the FRA16D in common fragile site induction. Genes Chromosom Cancer. 2004;39:71–76. doi: 10.1002/gcc.10290. [DOI] [PubMed] [Google Scholar]
- Palumbo E, Matricardi L, Tosoni E, Bensimon A, Russo A. Replication dynamics at common fragile site FRA6E. Chromosoma. 2010;119:575–587. doi: 10.1007/s00412-010-0279-4. [DOI] [PubMed] [Google Scholar]
- Pangeni RP, Channathodiyil P, Huen DS, Eagles LW, Johal BK, Pasha D, Hadjistephanou N, Nevell O, Davies CL, Adewumi AI, et al. The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin Epigenetics. 2015;7:57. doi: 10.1186/s13148-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliccia F, Bosco N, Curatolo A, Rocchi A. Replication timing of two human common fragile sites: FRA1H and FRA2G. Cytogenet Genome Res. 2008;121:196–200. doi: 10.1159/000138885. [DOI] [PubMed] [Google Scholar]
- Pelliccia F, Bosco N, Rocchi A. Breakages at common fragile sites set boundaries of amplified regions in two leukemia cell lines K562 – molecular characterization of FRA2H and localization of a new CFS FRA2S. Cancer Lett. 2010;299:37–44. doi: 10.1016/j.canlet.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Plaja A, Castells N, Cueto-Gonzalez AM, del Campo M, Vendrell T, Lloveras E, Izquierdo L, Borregan M, Rodriguez-Santiago B, Carrio A, et al. A novel recurrent breakpoint responsible for rearrangements in the Williams-Beuren region. Cytogenet Genome Res. 2015;146:181–186. doi: 10.1159/000439463. [DOI] [PubMed] [Google Scholar]
- Rader JS, Kamarasova T, Huettner PC, Li L, Li Y, Gerhard DS. Allelotyping of all chromosomal arms in invasive cervical cancer. Oncogene. 1996;13:2737–2741. [PubMed] [Google Scholar]
- Reidy JA. Folate- and deoxyuridine-sensitive chromatid breakage may result from DNA repair during G2. Mutat Res. 1987;192:217–219. doi: 10.1016/0165-7992(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Reshmi SC, Huang X, Schoppy DW, Black RC, Saunders WS, Smith DI, Gollin SM. Relationship between FRA11F and 11q13 gene amplification in oral cancer. Genes Chromosom Cancer. 2007;46:143–154. doi: 10.1002/gcc.20394. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Capello D, Scandurra M, Greiner TC, Chan WC, Bhagat G, Rossi D, Morra E, Paulli M, Rambaldi A, et al. Single nucleotide polymorphism-arrays provide new insights in the pathogenesis of post-transplant diffuse large B-cell lymphoma. Br J Haematol. 2010;149:569–577. doi: 10.1111/j.1365-2141.2010.08125.x. [DOI] [PubMed] [Google Scholar]
- Rodenas-Cuadrado P, Ho J, Vernes SC. Shining a light on CNTNAP2: complex functions to complex disorders. Eur J Hum Genet. 2014;22:171–178. doi: 10.1038/ejhg.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Lieber MR. G clustering is important for the initiation of transcription-induced R-loops in vitro, whereas high G density without clustering is sufficient thereafter. Mol Cell Biol. 2009;29:3124–3133. doi: 10.1128/MCB.00139-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldivar JC, Shibata H, Huebner K. Pathology and biology associated with the fragile FHIT gene and gene product. J Cell Biochem. 2010;109:858–865. doi: 10.1002/jcb.22481. [DOI] [PubMed] [Google Scholar]
- Samadashwily GM, Raca G, Mirkin SM. Trinucleotide repeats affect DNA replication in vivo. Nat Genet. 1997;17:298–304. doi: 10.1038/ng1197-298. [DOI] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Santos-Pereira JM, Aguilera A. R loops: new modulators of genome dynamics and function. Nat Rev Genet. 2015;16:583–597. doi: 10.1038/nrg3961. [DOI] [PubMed] [Google Scholar]
- Sanz LA, Hartono SR, Lim YW, Steyaert S, Rajpurkar A, Ginno PA, Xu X, Chedin F. Prevalent, dynamic, and conserved R-loop structures associate with specific epigenomic signatures in mammals. Mol Cell. 2016;63:167–178. doi: 10.1016/j.molcel.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarni D, Kerem B. The complex nature of fragile site plasticity and its importance in cancer. Curr Opin Cell Biol. 2016;40:131–136. doi: 10.1016/j.ceb.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Savelyeva L, Brueckner LM. Molecular characterization of common fragile sites as a strategy to discover cancer susceptibility genes. Cell Mol Life Sci. 2014;71:4561–4575. doi: 10.1007/s00018-014-1723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelyeva L, Sagulenko E, Schmitt JG, Schwab M. Low-frequency common fragile sites: link to neuropsychiatric disorders? Cancer Lett. 2006a;232:58–69. doi: 10.1016/j.canlet.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Savelyeva L, Sagulenko E, Schmitt JG, Schwab M. The neurobeachin gene spans the common fragile site FRA13A. Hum Genet. 2006b;118:551–558. doi: 10.1007/s00439-005-0083-z. [DOI] [PubMed] [Google Scholar]
- Schmid M, Feichtinger W, Jessberger A, Kohler J, Lange R. The fragile site (16) (q22). I. Induction by AT-specific DNA-ligands and population frequency. Hum Genet. 1986;74:67–73. doi: 10.1007/BF00278788. [DOI] [PubMed] [Google Scholar]
- Schneider B, Nagel S, Kaufmann M, Winkelmann S, Bode J, Drexler HG, MacLeod RA. T(3;7)(q27;q32) fuses BCL6 to a non-coding region at FRA7H near miR-29. Leukemia. 2008;22:1262–1266. doi: 10.1038/sj.leu.2405025. [DOI] [PubMed] [Google Scholar]
- Schonhoft JD, Kosowicz JG, Stivers JT. DNA translocation by human uracil DNA glycosylase: role of DNA phosphate charge. Biochemistry. 2013;52:2526–2535. doi: 10.1021/bi301561d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Zlotorynski E, Kerem B. The molecular basis of common and rare fragile sites. Cancer Lett. 2006;232:13–26. doi: 10.1016/j.canlet.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Seki N, Sumiya H, Shimazaki J, Toyama Y, Takahashi E, Murata M, Hori T. Chromosome abnormalities and rare fragile sites detected in azoospermia patients. Jpn J Hum Genet. 1992;37:215–222. doi: 10.1007/BF01900715. [DOI] [PubMed] [Google Scholar]
- Shabtai F, Klar D, Halbrecht I. Chromosome 17 has a real fragile site at p12. Hum Genet. 1982;61:177–179. doi: 10.1007/BF00274215. [DOI] [PubMed] [Google Scholar]
- Shang Z, Lv H, Zhang M, Duan L, Wang S, Li J, Liu G, Ruijie Z, Jiang Y. Genome-wide haplotype association study identify TNFRSF1A, CASP7, LRP1B, CDH1 and TG genes associated with Alzheimer’s disease in Caribbean Hispanic individuals. Oncotarget. 2015;6:42504–42514. doi: 10.18632/oncotarget.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL, Sutherland GR. Segregation analysis of rare autosomal fragile sites. Hum Genet. 1986;72:123–128. doi: 10.1007/BF00283929. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Li MJ. Uncorrected SCE levels of Bloom syndrome cells by cell hybridization with malignant cells with 14q32 structural abnormalities. Cancer Genet Cytogenet. 1993;69:45–50. doi: 10.1016/0165-4608(93)90112-y. [DOI] [PubMed] [Google Scholar]
- Simmers RN, Stupans I, Sutherland GR. Localization of the human haptoglobin genes distal to the fragile site at 16q22 using in situ hybridization. Cytogenet Cell Genet. 1986;41:38–41. doi: 10.1159/000132193. [DOI] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Proudfoot NJ, Gromak N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell. 2011;42:794–805. doi: 10.1016/j.molcel.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Bolton A, Nguyen G. Genomic and epigenomic instability, fragile sites, schizophrenia and autism. Curr Genomics. 2010;11:447–469. doi: 10.2174/138920210793176001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordet O, Redon CE, Guirouilh-Barbat J, Smith S, Solier S, Douarre C, Conti C, Nakamura AJ, Das BB, Nicolas E, et al. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 2009;10:887–893. doi: 10.1038/embor.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou SK, Kamileri I, Lugli N, Evangelou K, Da-Re C, Huber F, Padayachy L, Tardy S, Nicati NL, Barriot S, et al. Mammalian RAD52 functions in break-induced replication repair of collapsed DNA replication forks. Mol Cell. 2016;64:1127–1134. doi: 10.1016/j.molcel.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian PS, Nelson DL, Chinault AC. Large domains of apparent delayed replication timing associated with triplet repeat expansion at FRAXA and FRAXE. Am J Hum Genet. 1996;59:407–416. [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Nakano S, Katoh M, Matsumura A, Nakamuta H, Ohmichi T, Yoneyama M, Sasaki M. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry. 1995;34:11211–11216. doi: 10.1021/bi00035a029. [DOI] [PubMed] [Google Scholar]
- Sutherland GR. Fragile sites on human chromosomes: demonstration of their dependence on the type of tissue culture medium. Science. 1977;197:265–266. doi: 10.1126/science.877551. [DOI] [PubMed] [Google Scholar]
- Sutherland GR. Heritable fragile sites on human chromosomes I. Factors affecting expression in lymphocyte culture. Am J Hum Genet. 1979;31:125–135. [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR. Heritable fragile sites on human chromosomes. VII. Children homozygous for the BrdU-requiring fra(10)(q25) are phenotypically normal. Am J Hum Genet. 1981;33:946–949. [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR. Chromosomal fragile sites. Genet Anal Tech Appl. 1991;8:161–166. doi: 10.1016/1050-3862(91)90056-w. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Baker E. Unusual behaviour of a human autosome having two rare folate sensitive fragile sites. Ann Genet. 1993;36:159–162. [PubMed] [Google Scholar]
- Sutherland GR, Baker E. Forgotten fragile sites and related phenomena. Cytogenet Genome Res. 2003;100:89–91. doi: 10.1159/000072842. [DOI] [PubMed] [Google Scholar]
- Tassano E, Tavella E, Micalizzi C, Panarello C, Morerio C. Refractory cytopenia of childhood with monosomy 7 presenting as isolated neutropenia in a patient with fragile site at 16q22. Cancer Genet Cytogenet. 2010;201:70–71. doi: 10.1016/j.cancergencyto.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Tatarelli C, Linnenbach A, Mimori K, Croce CM. Characterization of the human TESTIN gene localized in the FRA7G region at 7q31.2. Genomics. 2000;68:1–12. doi: 10.1006/geno.2000.6272. [DOI] [PubMed] [Google Scholar]
- Tchurikov NA, Kretova OV, Fedoseeva DM, Chechetkin VR, Gorbacheva MA, Snezhkina AV, Alembekov IR, Kravatskaya GI, Kravatsky YV. Genome-wide mapping of hot spots of DNA double-strand breaks in human cells as a tool for epigenetic studies and cancer genomics. Genomics Data. 2015;5:89–93. doi: 10.1016/j.gdata.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorland EC, Myers SL, Persing DH, Sarkar G, McGovern RM, Gostout BS, Smith DI. Human papillomavirus type 16 integrations in cervical tumors frequently occur in common fragile sites. Cancer Res. 2000;60:5916–5921. [PubMed] [Google Scholar]
- Thys RG, Lehman CE, Pierce LC, Wang YH. DNA secondary structure at chromosomal fragile sites in human disease. Curr Genomics. 2015;16:60–70. doi: 10.2174/1389202916666150114223205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy RB, Hsieh CL, Lieber MR. Stable RNA/DNA hybrids in the mammalian genome: inducible intermediates in immunoglobulin class switch recombination. Science. 2000;288:1058–1061. doi: 10.1126/science.288.5468.1058. [DOI] [PubMed] [Google Scholar]
- Tunca B, Egeli U, Zorluoglu A, Yilmazlar T, Yerci O, Kizil A. The expression of fragile sites in lymphocytes of patients with rectum cancer and their first-degree relatives. Cancer Lett. 2000;152:201–209. doi: 10.1016/s0304-3835(00)00334-7. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Unger S, Gorna MW, Le Bechec A, Do Vale-Pereira S, Bedeschi MF, Geiberger S, Grigelioniene G, Horemuzova E, Lalatta F, Lausch E, et al. FAM111A mutations result in hypoparathyroidism and impaired skeletal development. Am J Hum Genet. 2013;92:990–995. doi: 10.1016/j.ajhg.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin K, Woodford KJ. CGG repeats associated with DNA instability and chromosome fragility form structures that block DNA synthesis in vitro. Nucleic Acids Res. 1995;23:4202–4209. doi: 10.1093/nar/23.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin K, Hayward BE, Kumari D, Lokanga RA, Sciascia N, Zhao XN. Repeat-mediated genetic and epigenetic changes at the FMR1 locus in the Fragile X-related disorders. Front Genet. 2014;5:226. doi: 10.3389/fgene.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schil K, Meire F, Karlstetter M, Bauwens M, Verdin H, Coppieters F, Scheiffert E, Van Nechel C, Langmann T, Deconinck N, et al. Early-onset autosomal recessive cerebellar ataxia associated with retinal dystrophy: new human hotfoot phenotype caused by homozygous GRID2 deletion. Genet Med. 2015;17:291–299. doi: 10.1038/gim.2014.95. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vianna-Morgante AM, Mingroni-Netto RC, Barbosa AC, Otto PA, Rosenberg C. FRAXF in a patient with chromosome 8 duplication. J Med Genet. 1996;33:611–614. doi: 10.1136/jmg.33.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiculescu I, Back E, Schempp W. Homozygous condition for a BrdU-requiring fragile site on chromosome 12. Hum Genet. 1991;86:416–417. doi: 10.1007/BF00201849. [DOI] [PubMed] [Google Scholar]
- Wang YH, Griffith J. Methylation of expanded CCG triplet repeat DNA from fragile X syndrome patients enhances nucleosome exclusion. J Biol Chem. 1996;271:22937–22940. [PubMed] [Google Scholar]
- Wang YH, Gellibolian R, Shimizu M, Wells RD, Griffith J. Long CCG triplet repeat blocks exclude nucleosomes: a possible mechanism for the nature of fragile sites in chromosomes. J Mol Biol. 1996;263:511–516. doi: 10.1006/jmbi.1996.0593. [DOI] [PubMed] [Google Scholar]
- Wang L, Darling J, Zhang JS, Huang H, Liu W, Smith DI. Allele-specific late replication and fragility of the most active common fragile site, FRA3B. Hum Mol Genet. 1999;8:431–437. doi: 10.1093/hmg/8.3.431. [DOI] [PubMed] [Google Scholar]
- Wang T, Bray SM, Warren ST. New perspectives on the biology of fragile X syndrome. Curr Opin Genet Dev. 2012;22:256–263. doi: 10.1016/j.gde.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T. Delayed replication of Xq27 in individuals with the fragile X syndrome. Am J Med Genet. 1992;43:1057–1062. doi: 10.1002/ajmg.1320430633. [DOI] [PubMed] [Google Scholar]
- Webb T, Thake A. Fragile 22q13 segregating in a family. Clin Genet. 1984;26:125–128. doi: 10.1111/j.1399-0004.1984.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Widrow RJ, Hansen RS, Kawame H, Gartler SM, Laird CD. Very late DNA replication in the human cell cycle. Proc Natl Acad Sci U S A. 1998;95:11246–11250. doi: 10.1073/pnas.95.19.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Arlt MF, Park SH, Rajendran S, Paulsen M, Ljungman M, Glover TW. Large transcription units unify copy number variants and common fragile sites arising under replication stress. Genome Res. 2015;25:189–200. doi: 10.1101/gr.177121.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnepenninckx B, Debacker K, Ramsay J, Smeets D, Smits A, FitzPatrick DR, Kooy RF. CGG-repeat expansion in the DIP2B gene is associated with the fragile site FRA12A on chromosome 12q13.1. Am J Hum Genet. 2007;80:221–231. doi: 10.1086/510800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsurawat T, Jenjaroenpun P, Kwoh CK, Kuznetsov V. Quantitative model of R-loop forming structures reveals a novel level of RNA-DNA interactome complexity. Nucleic Acids Res. 2012;40:e16. doi: 10.1093/nar/gkr1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Li LY, He XX, Xiao JY. Fragile site 1q44 involved in nasopharyngeal carcinoma. A study of a marker chromosome der(1)t(1;3)(q44;p11) Cancer Genet Cytogenet. 1988;35:135–140. doi: 10.1016/0165-4608(88)90133-1. [DOI] [PubMed] [Google Scholar]
- Yu S, Mangelsdorf M, Hewett D, Hobson L, Baker E, Eyre HJ, Lapsys N, Le Paslier D, Doggett NA, Sutherland GR, et al. Human chromosomal fragile site FRA16B is an amplified AT-rich minisatellite repeat. Cell. 1997;88:367–374. doi: 10.1016/s0092-8674(00)81875-9. [DOI] [PubMed] [Google Scholar]
- Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- Zhang H, Freudenreich CH. An AT-rich sequence in human common fragile site FRA16D causes fork stalling and chromosome breakage in S. cerevisiae. Mol Cell. 2007;27:367–379. doi: 10.1016/j.molcel.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XN, Usdin K. Ups and downs: mechanisms of repeat instability in the fragile X-related disorders. Genes. 2016:7. doi: 10.3390/genes7090070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, McAvoy S, Kuhn R, Smith DI. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene. 2006;25:2901–2908. doi: 10.1038/sj.onc.1209314. [DOI] [PubMed] [Google Scholar]
- Zimonjic DB, Durkin ME, Keck-Waggoner CL, Park SW, Thorgeirsson SS, Popescu NC. SMAD5 gene expression, rearrangements, copy number, and amplification at fragile site FRA5C in human hepatocellular carcinoma. Neoplasia. 2003;5:390–396. doi: 10.1016/s1476-5586(03)80041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotorynski E, Rahat A, Skaug J, Ben-Porat N, Ozeri E, Hershberg R, Levi A, Scherer SW, Margalit H, Kerem B. Molecular basis for expression of common and rare fragile sites. Mol Cell Biol. 2003;23:7143–7151. doi: 10.1128/MCB.23.20.7143-7151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]