Abstract
Background
Obesity induces significant changes in lipid mediators, however, the extent to which these changes persist after weight loss has not been investigated.
Subjects/Methods
We fed C57BL6 mice a high fat diet to generate obesity and then switched the diet to a lower fat diet to induce weight loss. We performed a comprehensive metabolic profiling of lipid mediators including oxylipins, endocannabinoids, sphingosines and ceramides in key metabolic tissues including adipose, liver, muscle, hypothalamus and plasma.
Results
We found that changes induced by obesity were largely reversible in most metabolic tissues but the adipose tissue retained a persistent obese metabolic signature. Prostaglandin signaling was perturbed in the obese state and lasting increases in PGD2, downstream metabolites 15-deoxy PGJ2 and delta-12-PGJ2 were observed after weight loss. Furthermore, the enzyme responsible for PGD2 synthesis (hematopoietic prostaglandin D synthase, HPGDS) was increased in obese adipose tissues and remained high after weight loss. We found that inhibition of HPGDS over the course of 5 days resulted in decreased food intake in mice. Increased HPGDS expression was also observed in human adipose tissues compared with lean individuals. We then measured circulating levels of PGD2 in obese patients before and after weight loss and found that while elevated relative to lean subjects, levels of this metabolite did not decrease after significant weight loss.
Conclusions
These results suggest that lasting changes in lipid mediators induced by obesity, still present after weight loss, may play a role in the biological drive to regain weight.
Introduction
In America, an estimated 45 million people attempt to lose weight each year (1) and although many achieve short term success, sustained maintenance of reduced body weight is rarely achieved (2–9). A successful weight loss program incorporating diet and lifestyle changes may lead to 10% reduction in body weight (10), but remarkably 75–95% of people regain this weight (2, 9, 11–14). The driving forces behind weight regain include both biological and psychological factors (4, 15, 16). Weight loss is associated with compensatory decreases in energy expenditure, which opposes the maintenance of the lower weight and promotes weight regain (17–21). Furthermore, changes in circulating levels of peripheral modulators important in appetite regulation, including leptin, peptide PYY, cholecystokinin, insulin, ghrelin gastric inhibitory polypeptide, as well as hunger, remain significantly different from baseline one year after weight loss and play an important role in weight regain (22).
Obesity induces significant changes in the metabolome (23–27), however, the extent to which these changes persist after weight loss has not been investigated. We fed C57BL6 mice a high fat diet (60% calories from fat, HFD) to generate obesity and then switched the diet to a lower fat diet (10% calories from fat, LFD) to induce weight loss (28). In this study we have used targeted metabolomics to study changes in lipid mediators in insulin target tissues (liver, adipose, muscle, hypothalamus) and plasma, in obesity and after weight loss in mice. We performed comprehensive metabolic profiling of lipid mediators including oxylipins, endocannabinoids, sphingosines and ceramides that have been implicated in obesity associated co-morbidities including insulin resistance, diabetes and cardiovascular disease (26, 29–42).
Oxylipins are potent bioactive metabolites that are involved in inflammatory signaling and play important roles in obesity-induced inflammation and insulin resistance (26, 34, 35, 39–41, 43). Oxylipins (eicosanoids, docosanoids, and octadecanoids) are derived from the oxygenation of polyunsaturated fatty acids (PUFAs) by three families of enzymes COX, lipoxygenase (LOX) and cytochrome p450 (CYP) (43). The 12-LOX derived oxylipins are induced in the obese state and studies have shown these oxylipins play an important role in mediating inflammation and result in adipocyte dysfunction (44). The endocannabinoid system plays an important role in energy storage, nutrient transport and insulin sensitivity (29). In the CNS endocannabinoids stimulate food intake through their interactions with the leptin-regulated neurocircuitary and can work as neuromodulators able to directly regulate and release classical neurotransmitters (45, 46). Endocannabinoids act on peripheral tissues including adipose, liver and muscle and overactive endocannabinoid tone is associated with obesity (29, 30, 36–38). Ceramides are bioactive sphingolipids that accumulate in obesity resulting in the development of insulin resistance (31–33, 42, 47). Ceramides are synthesized by a family of six ceramide synthases (CerS) in mammals, which produce ceramides with different N-linked acyl chains of various lengths. Inhibition of ceramide synthesis has been shown to improve systemic metabolism and obesity-induced insulin resistance (33, 42).
While many studies have identified changes in lipid mediators in obesity, the extent to which weight loss reverses these obesity-induced changes has not yet been studied. The mechanisms driving weight rebound are complex and largely unknown, therefore, understanding the barriers to maintaining weight loss are crucial for the prevention of relapse to obesity. We hypothesized that some metabolites induced or repressed in the obese state remain so even after weight loss and these “persistent” metabolites may play a role in driving weight regain. Metabolites that are induced by obesity and not changed upon weight loss will represent therapeutic targets for the maintenance of weight loss.
Materials/Subjects and Methods
In vivo mouse studies
All experiments were approved by and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Diego. Male C57BL6 mice were fed normal chow until 12 weeks of age and then randomly divided into three groups termed LFD (low fat diet, 10% fat; D12450B, Research diets), HFD (high fat diet, 60% fat; D12492, Research Diets) and SWD (switch diet from HFD to LFD). The LFD and HFD group were fed ad libitum for 18 weeks. The SW group were fed HFD for 9 weeks and then switched to LFD for a further 9 weeks after which their body weights had returned to a similar mass as the LF group (n=8 per group). Group sizes were selected based on previous similar studies (28). All groups were sacrificed at 30 wks of age and the tissues (hypothalamus, liver and epididymal adipose tissue) dissected and frozen immediately in liquid nitrogen. Glucose Tolerance tests (GTTs) were performed at 3 and 8 weeks after the diet switch as previously described (48). Briefly, mice were fasted for 6 hrs and then injected with glucose (ip, 1 g/kg dose) and blood samples drawn at 10, 30, 60 and 90 mins to monitor glucose excursion over time. A selective inhibitor of hematopoietic prostaglandin D synthase (HQL-79, 30 mg/kg, (Cayman Chemical, Ann Arbor, MI) or vehicle control (saline) was administered by oral gavage for 5 consecutive days to C57BL6 mice fed HFD 8 wks, from 12 wks of age. Body weight and food intake was measured daily at 10 AM prior to gavage and at the end of the study mice were sacrificed and plasma and adipose tissue collected, n=12 mice per group.
Sample extraction
Oxylipins, endocannabinoids, and ceramides were isolated using a 96-well Ostro™ Pass Through Sample Preparation Plate (Waters Corp, Milford, MA) n=6 samples per tissue/per group. For plasma, 50 µL of sample was added to the plate wells and spiked with a 5 µL anti-oxidant solution (0.2 mg/ml solution BHT/EDTA in 1:1 methanolwater) and 10 µL 1000 nM deuterated surrogates. Next, isopropanol w/ 10 mM ammonium formate and 1% formic acid (150 µL) was added and aspirated three times to mix. Following this step, the mixture was eluted into glass inserts, dried, and reconstituted in the same manner as the tissue samples (as described below). Tissue samples were pulverized (Liver ~20–25 mg, adipose 6–10 mg, muscle 6–10 mg hypothalamus ~0.5–2.5 mg) and added to 2 mL polypropylene tubes spiked with a 5 µL anti-oxidant solution (0.2 mg/ml solution BHT/EDTA in 1:1 methanol:water) and 10 µL of 1000 nM deuterated surrogates in methanol. A total of 50 µL of methanol was added and the tube was placed in a Geno/Grinder 2000 (SPEX Sample Prep, Metuchen, NJ) for 30 sec at 1350 rpm. An additional 550 µL isopropanol w/ 10 mM ammonium formate and 1% formic acid and 100 uL water was added and the tube was placed in a Geno/Grinder for an additional 30 sec before being centrifuged at 10,000g for 5 min at room temp. Supernatants were transferred into Ostro Plate wells and eluted into glass inserts containing 10 µL 20% glycerol in methanol by applying a vacuum at 15 Hg for 10 min. Eluents were dried by vacuum centrifugation in a Genvac EZ-2 (SP Scientific, Stone Ridge, NY) for 35 min at the medium BP setting, before switching to an aqueous setting for an additional 35 min. Once dry, samples were re-constituted with the internal standard 1-cyclohexylureido, 3-dodecanoic acid (Cayman Chemical) and 1-phenylureido, 3-Hexanoic acid (gift from B.D. Hammock, University of California, Davis) at 100 nM (50:50 methanol:acetonitrile), vortexed 1 min, transferred to a spin filter (0.1 µm PVDF membrane, Millipore, Billerica, MA), centrifuged for 3 min at 6°C at <4500 g (rcf), before transfer to 2 mL LC-MS amber vials. Extracts were stored at −20°C until analysis by ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). The internal standards were used to quantify the recovery of surrogate standards.
Lipid mediator analysis
Analytes were separated using a Waters Acquity UPLC (Waters, Milford, MA) with a solvent gradient modified from previously published protocols for oxylipins (40), endocannabinoids (39), and ceramides (49). Samples were held at 10°C. Separated residues were detected in independent injections by negative mode electrospray ionization for oxylipins and positive mode electrospray ionization for endocannabinoids and ceramides using multiple reaction monitoring on an API 4000 QTrap (AB Sciex, Framingham, MA, USA). Analytes were quantified using internal standard methods and 5 to 7 point calibration curves (r2 ≥ 0.997). Calibrants and internal standards were either synthesized [10,11-DHN, 10,11-DHHep, 10(11)-EpHep] or purchased from Cayman Chemical, Avanti Polar Lipids Inc. (Alabaster, AL) or Larodan Fine Lipids (Malmo, Sweden), unless otherwise indicated. Data was processed using AB Sciex MultiQuant version 3.0.2. Lipid mediator data from this study can be accessed from http://www.metabolomicsworkbench.org, data set refs ST000593 and ST000594.
Human samples
Plasma PGD2 levels were determined by ELISA (Cayman chemicals, MI) in samples obtained from obese patients before and after bariatric surgery. Specifically, blood samples were collected at the following time-points: 1) Plasma samples were obtained 3 weeks prior to surgery, after which patients committed to a stringent three week 800 cal/day diet regiment. 2) Plasma samples were collected on the day of surgery, at which time the patients had lost on average 8.7 kg due to the diet regime. 3) Plasma samples were also collected 6 and 12 months after surgery, at which time the patients had lost on average 37.3 kg and 46.7 kg, respectively. These human samples were obtained from a larger study (ClinicalTrials.gov NCT02322073) in agreement with the principal investigator, V. Wallenius. The Regional Ethical Review Board (Gothenburg, Sweden), approved all study procedures (Dnr 682-14) and all patients were enrolled in accordance with the Helsinki Declaration. Written informed consent was obtained from all participants included in this study.
Adipocyte and stromal vascular cell isolation from adipose tissue
Adipocytes and stromal vascular cells were isolated as previously described (50). Briefly, visceral adipose tissue was mechanically chopped and then digested with collagenase II for 15 minutes at 37°C. After passing cells through a 100-µm cell strainer and centrifugation at 1,000 g for 10 minutes, primary adipocytes were collected from the top layer of the supernatant and the pellet containing the stromal vascular fraction (SVC) fraction was then incubated with red blood cell lysis buffer.
RNA isolation and q-PCR
Total RNA was isolated using TRIzol (Invitrogen, CA) according to the manufacturer’s instructions. First-strand cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). For q-PCR, samples were run in a 20-ul reaction (iTaq SYBRgreen supermix; BioRad, CA) using a stepOnePlus Real-Time PCR system (Applied Biosystems). Gene expression levels were calculated after normalization to the standard housekeeping gene Actb and RNA polymerase II, using the ΔΔCt method as described previously (48), and expressed as relative mRNA levels compared with internal control. Primer sequences available upon request.
Statistics
When comparing two groups statistical analysis was performed by a 1 or 2-tailed Student’s t test. A two way ANOVA with repeated measures followed by a Tukey post hoc test was used to compare multiple groups at different time points using GraphPad Prism 6, unless otherwise stated. For the lipid analysis, significance was determined by one-way ANOVA on log10 transformed data and Benjamini and Hochberg was used for false discovery rate adjustment. Significance was defined as q value equal or less than 0.05. All data are expressed as mean ± SEM. “Reversibility” was defined as a significant change between the lean and obese state (q < 0.05 LFD v HFD), which then changes back after weight loss (q < 0.05 HFD v SWD), while the level after weight loss is comparable with the original lean state (q > 0.05 SWD v LFD). The “persistent’ class contains metabolites that change significantly between the lean and obese state (q < 0.05 LFD v HFD), that do not significantly change after weight loss (q > 0.05 HFD v SWD) and the new level after weight loss is also significantly different from the original LFD level (q < 0.05 SWD v LFD). The third class, includes metabolites that are “not fully reversed” after weight loss, where the metabolite is significantly changed in the obese state (LFD v HFD), but after weight loss, the levels are not significantly different from the HFD level (HFD v SWD not significant), and the new level after weight loss is not significantly different from the lean state (SWD v LFD not significant). No blinding was carried out for data analysis.
Results
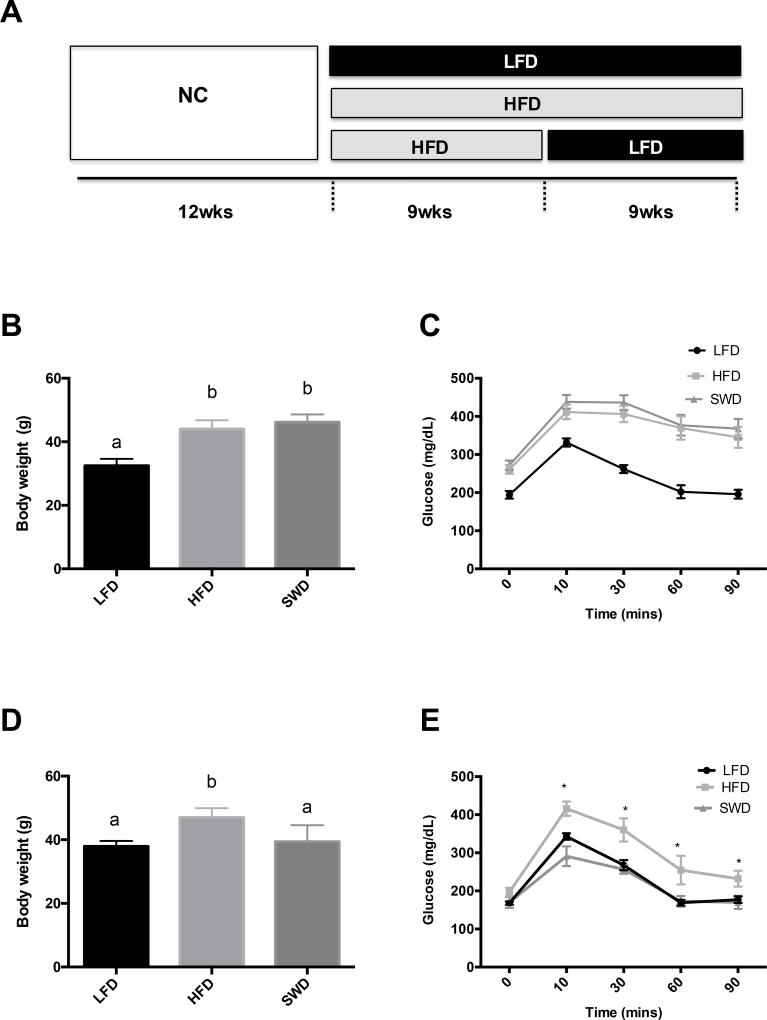
To identify lipid mediators that are regulated by obesity or weight loss we used a model of HFD feeding to induce obesity in C57BL6 mice and then switched the diet to LFD to induce weight loss (Fig 1A). Mice chronically fed HFD developed obesity (Fig 1B) and glucose intolerance (Fig 1C). Switching from HFD to LFD feeding for a further 9 weeks resulted in weight loss (Fig 1D) and improvement in glucose tolerance (Fig 1E) in the SWD group to a similar level as mice that were continuously fed LFD throughout the study.
Figure 1. Model of obesity and weight loss in mice.
A) Male C57BL6 mice were fed normal chow (NC) until 12 weeks of age, and then divided into three groups fed either low fat diet (LFD) or high fat diet (HFD) for a further 18 weeks, or HFD for 9 weeks and then switched to LFD for 9 weeks to induce weight loss (SWD). B) Body weights after 9 wks of LFD or HFD feeding. C) GTT after 9 weeks of feeding HFD or LFD. D) Body weights 8 wks after the diet switch E) GTT, 8 weeks after the diet switch. Significance was defined as p-value equal or less that 0.05. *, values labeled with different letters are significantly different.
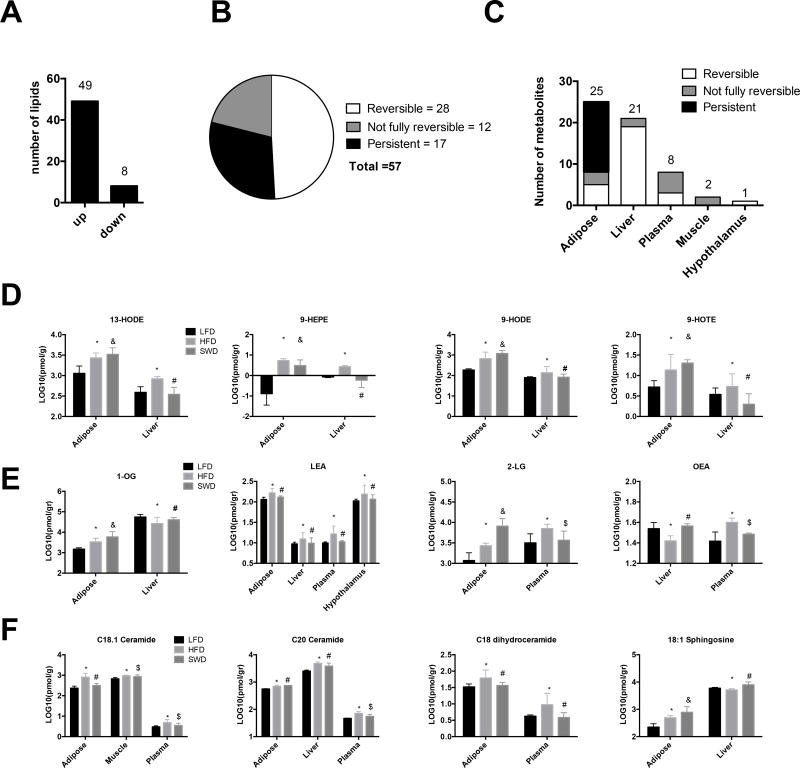
Targeted lipid mediator analysis of endocannabinoids, oxylipins and ceramides in metabolic tissues including liver, adipose tissue, skeletal muscle, hypothalamus and plasma, identified 57 metabolites that were significantly induced or repressed in the obese state compared with lean controls (Fig 2). Of these changes the majority were increased in the HFD fed, obese state while only 8/57 lipids were lower in the HFD fed mice compared with LFD fed mice (Fig 2A). The change in expression levels of metabolites between the lean and obese state were classified as either “reversible”, “persistent”, or “not fully reversed” after weight loss. The majority of these changes (28/57, = 49%) were reversible after weight loss, while 12/57 (= 21% are not fully reversed and (17/57, =30%) are persistent and do not revert back to lean state after weight loss (Fig.2B).
Figure 2. Targeted analysis of lipid mediators: Oxylipins, Endocannabinoids and Ceramides.
A) Changes in lipid mediators observed between the lean (LFD) and obese (HFD) state across all tissues (liver, adipose, skeletal muscle, hypothalamus and plasma). B) The effect of weight loss on lipid mediators that were significantly changed in the obese state versus lean; defined as reversible, persistent and not fully reversible after weight loss induced by LFD feeding. C). Number of metabolites that are reversible, persistent, or not fully reversible within each tissue. Concentration of D) Oxylipins, E) Endocannabinoids and F) Ceramides. Lipid analysis significance was determined by One-Way ANOVA on log10 transformed data and Benjamini and Hochberg false discovery rate was used for multiple comparison adjustments. All data are expressed as mean ± SEM. Significance was defined as q-value equal or less that 0.05. *, Significant change between LFD and HFD, # reversible change after LFD feeding, $, not fully reversible after LFD feeding, & persistent change after diet switch.
The majority of changes in lipid mediators occurred in the adipose (25/57 =43%) and liver tissues (21/57 = 36.8%), while far fewer were observed in the plasma (8/57=14%), muscle (2/57=3.5%) and hypothalamus (1/57=1.8%), (Fig 2C). After weight loss, induced by switching the diet from HFD to LFD, many of the obesity/HFD-induced changes observed in the adipose tissue were persistent and did not return to the equivalent LFD fed levels, while no persistent changes were observed in the liver, muscle, hypothalamus or plasma.
HFD feeding resulted in changes in concentration of lipids across multiple tissues (Fig 2D–F). For example, oxylipins such as 13-HODE, 9-HOTE, 9-HEPE and 9-HODE all increased in both adipose and liver from obese, HFD fed mice compared with lean LFD-fed mice (Fig 2D). Endocannabinoid-like compounds 1-OG, 2-LG and LEA were all increased in HFD fed mice compared with lean, LFD fed mice (Fig 2E). HFD feeding resulted in increased 1-OG in the adipose and liver; increased 2-LG in adipose and plasma; and increased LEA across adipose tissue, liver, hypothalamus and plasma compared with LFD fed mice. HFD feeding resulted in decreased OEA in the liver but increased OEA in the plasma compared with LFD-fed mice (Fig 2E). HFD resulted in increased C18:1 and C20 ceramide in adipose, muscle and plasma; increased C18 dihydroceramide in adipose and plasma and increased 18:1 sphingosine in adipose and liver compared with LFD fed mice (Fig 2F). Some of these changes were reversible upon weight loss, while some persisted at the level observed in the obese state (Fig 2D–F).
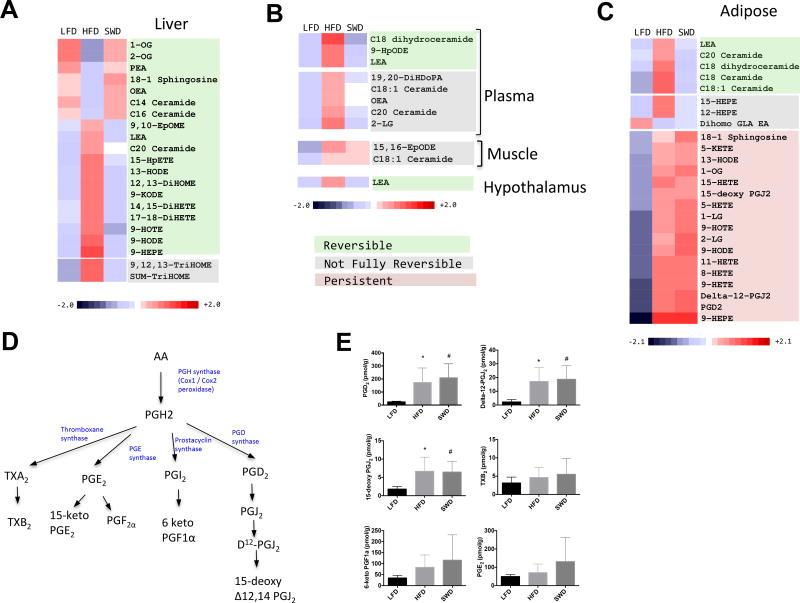
The individual lipids that are significantly different in HFD fed mice compared with LFD fed mice are classified into reversible, not fully reversible and persistent classes (Fig 3). The majority of changes induced by HFD feeding/obesity in the liver (Fig 3A) plasma, muscle and hypothalamus (Fig 3B) are reversible after weight loss. In contrast, the majority of the changes induced by HFD feeding in adipose tissue are persistent after weight loss (Fig 3C). Of the 25 lipids that significantly change in adipose tissue after HFD feeding only 5/25 had fully reversed back to lean levels after weight loss, 3 were partially reversed while 17/25 were persistently high after weight loss. Of these persistent changes, prostaglandin signaling is perturbed with lasting increases in PGD2 and downstream metabolites Delta-12-PGJ2 and 15-deoxy PGJ2 (Fig. 3D & E) in adipose tissue. Notably other cyclooxygenase-dependent metabolites including TXB2, 6-keto PGF1a and PGE2 (Fig 3E) were not induced by HFD feeding.
Figure 3. Persistent increase in PGD2 levels after weight loss.
Heatmaps compare levels of lipid mediators across the three diet groups (LFD, HFD and SWD). A) Liver. B) Plasma, muscle and hypothalamus. C) Adipose. Lipids shaded green are reversible, grey = not fully reversible, orange = persistent. Fold change is reflected by intensity of color blue (decreased) to red (increased). D) Pathway of prostaglandin synthesis. Cycloxygenase enzymes catalyze the conversion of arachidonic acid to PGH2, which is converted to other species including PGD2, PGE2, PGF2a, prostacyclin (PGI2) and thromboxane (TX) A2 by the action of specific synthases. E) Prostaglandin levels in lean (LFD), obese (HFD fed) and after weight loss (SWD).
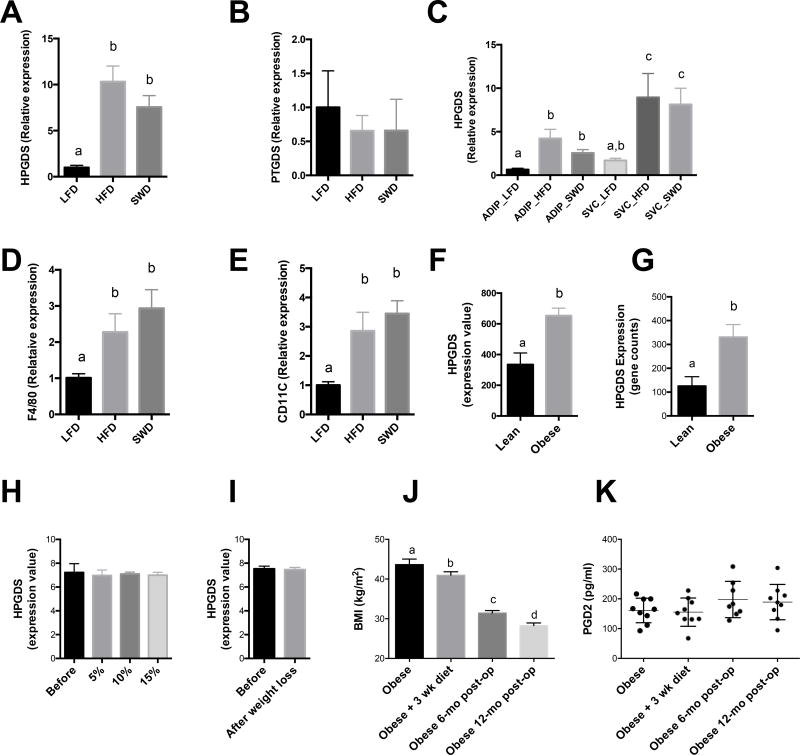
Cyclooxygenase-dependent metabolites, including the prostaglandins (PGs) and thromboxanes (TXs) have broad roles in both healthy and pathological contexts (51). Cycloxygenase enzymes catalyze the conversion of arachidonic acid to PGH2, which is converted to species including PGD2, PGE2, PGF2a, prostacyclin (PGI2) or TXA2 by the action of specific synthases (52). PGD2 is further dehydrated to produce PGJ2, delta 12-PGJ2, and 15-deoxy-delta 12,14-PGJ2. The synthesis of PGD2 from PGH2 can be catalyzed by two synthase enzymes termed prostaglandin D synthase (PTGDS) (53) and hematopoietic prostaglandin D synthase (HPGDS) (54). HPGDS is widely distributed in the peripheral tissues while PTGDS is highly expressed in the central nervous system, but also expressed at lower levels in other peripheral tissues (55). We therefore measured the expression of HPGDS and PTGDS enzymes in adipose tissue from these three diet groups. HPGDS was increased in HFD fed mice by ~10 fold compared with LFD fed mice and the expression level remained high in the SWD group (Fig. 4A), while PTGDS was unchanged between groups (Fig. 4B). To determine the cellular source of HPGDS in adipose tissue we fractionated the adipocytes and SVCs from LFD, HFD and SWD fed mice (Fig 4C). Levels of HPGDS were similar between adipocytes and SVCs from LFD fed mice. However, after HFD feeding the levels of HPGDS increased significantly in both adipocytes and SVCs compared with LFD fed mice and this increase persisted in both adipocyte and SVC fractions after weight loss (Fig 4C). In agreement with other studies (56), we also observed persistent expression of macrophage markers after weight loss (Fig 4 D and E).
Figure 4. Obesity-induced increase in prostaglandin D2 signaling does not decrease after weight loss.
A) Relative expression of HPGDS in adipose tissue from LFD, HFD and SWD mice. B) Relative expression of PTGDS in adipose tissue from LFD, HFD and SWD mice. C) HPGDS expression in adipocyte and stromal vascular cells (SVC) from LFD, HFD and SWD mice. D) F4/80 and E) CD11c expression in SVC from LFD, HFD and SWD mice. F) Expression of H-PGDS in adipose tissue biopsies from lean (n=6, BMI 23.2 ± 0.8) and obese (n= 35, BMI 35.9 ± 1.1) individuals, * q < 0.05 (57). G) Lean and obese omental adipose tissue, NCBI GEO dataset GDS3679 (58), 1 –tailed t-test, q < 0.1. H) Before and after weight loss of 5, 10 and 15% (59). I) Before and after weight loss induced by very low calorie diet for 16wks (60). J) BMI of patients before and after bariatric surgery. K) Plasma PGD2 levels in obese patients before and after gastric bypass surgery. Significance was defined as q-value equal or less that 0.05. *, values labeled with different letters are significantly different.
To determine whether these findings were also conserved in human subjects we measured the levels of these enzymes in human adipose tissue biopsies taken from lean and obese patients. We previously performed microarray analysis on human adipose tissue biopsies (57) and mined this dataset to determine if HPGDS was differentially expressed between lean and obese individuals. HPGDS levels were increased ~1.95 fold compared with lean individuals (BMI 23.2 v 35.9) (Fig. 4F). This increase in HPGDS in adipose tissue from obese patients was also observed when we extracted the expression data from similar published studies (Fig 4G), NCBI GEO dataset GDS3679 (58). We then extracted data from studies comparing expression levels in adipose tissue before and after weight loss (59, 60) and looked at HPGDS levels to see if obesity-induced increase in HPGDS was maintained after weight loss as seen in our mouse studies. In support of the mouse data, diet induced weight loss of up to 15% (Fig 4H) (59), or after a very low calorie diet for 16wks with average weight loss of 27.7 kg (Fig 4I) (60) did not change the expression levels of HPGDS in human adipose tissue. We also measured circulating levels of PGD2 in obese subjects before and after gastric bypass surgery (Fig 4J–K). Circulating levels of PGD2 in obese patients were approximately 2 fold higher than the clinical reference rage for normal individuals (57, 61–64). Furthermore, after substantial weight loss of ~35% (average BMI reduced from 43.6 to 28.2) PGD2 levels were unchanged one year after gastric bypass surgery.
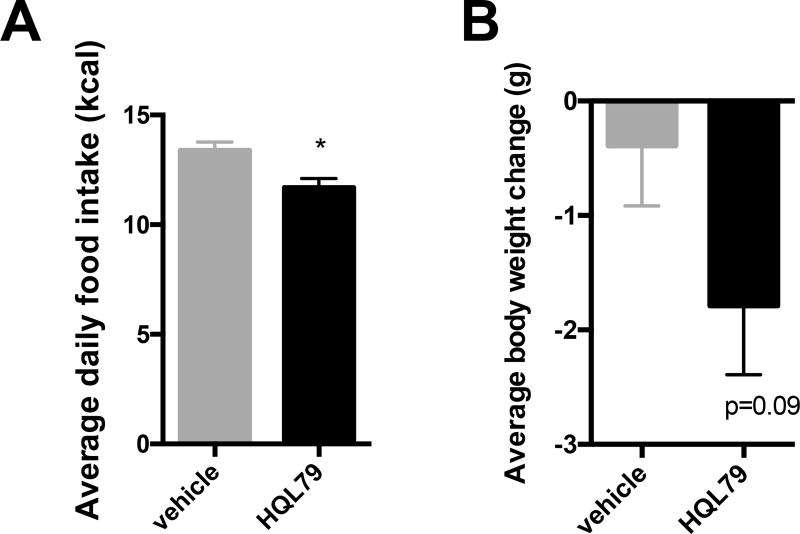
Due to the persistent elevation of PGD2 and HPGDS we hypothesized that PGD2 may play a role in food intake. Central injection of PGD2 has previously been shown to stimulate food intake (65) but whether peripheral levels of PGD2 could play a role in food intake is not yet known. To reduce peripheral levels of PGD2 we treated mice with the selective HPGDS inhibitor HQL-79 (66). Previous studies have shown that HQL-79 selectively inhibits HPGDS while not affecting PTGDS and results in lowering of PGD2 levels (67–69). Treatment of wild type, HFD fed mice with HQL-79 (30mg/kg, oral gavage) for 5 days resulted in a significant reduction in food intake (Fig 5A), with an associated trend in reduction of body weight (p=0.09) (Fig 5A). Whole adipose tissue levels of PGD2 in whole adipose tissue were not significantly different between the control and HQL-79 treatment groups, but the ratios between PGD2 and other connected pathway metabolites indicate a shift toward the production of PGE2 and PGF2a, in addition to their metabolites, instead of PGD2 (Supplemental figure 1) after HQL-79 treatment.
Figure 5. Food intake and body weight changes in mice treated with HPGDS inhibitor.
A) Average daily food intake (kcal) and B) body weight change in HFD fed C57BL6 male mice treated with HPGD2 inhibitor (HQL-79, 30 mg/kg, daily oral gavage) for 5 days (n=12 per group).
Discussion
We hypothesized that lasting changes in the metabolome after weight loss may play a role in feeding behavior and contribute to weight regain. We characterized changes in the levels of oxylipins, endocannabinoid and ceramide lipid mediators in lean, obese and weight-reduced mice. We identified lasting changes in the lipid mediators in the weight-reduced mice and identified many persistent changes specifically in adipose tissue. Of these persistent changes, prostaglandin signaling was perturbed with persistent elevation in PGD2 and downstream metabolites 15-deoxy PGJ2 and delta-12-PGJ2. In addition, levels of the enzyme responsible for PGD2 synthesis (HPGDS) were also increased in adipose tissue in the obese state and did not decrease after weight loss in both mouse and human studies.
PGD2 plays a broad role in many biological processes including sleep (70), analgesia (71) allergic diseases (52, 72) and food intake, where injection of PGD2 directly into the brain increases food intake in mice (65). Prostanoids exert a wide variety of actions in the body, which are mediated by specific receptors on plasma membranes (73). PGD2 acts through two receptors (DP1 and DP2 CRTH2), whereas 15-deoxy PGJ2 can activate some peroxisome proliferator-activated receptors and inhibit proinflammatory pathways (74). Our finding that levels of prostaglandin signaling increased in obesity are in agreement with previous published studies showing elevated PGD2 and HPGDS in mouse adipose tissue (75). Furthermore, transgenic overexpression of H-PGDS in mice results in obesity and increased fat mass (76). We confirm and extend these findings by showing this obesity-induced increase in PGD2 does not decrease after weight loss and may play a role in body weight regulation.
In human studies, prostaglandin signaling has been linked to obesity in correlation studies showing that expression of the prostaglandin synthesis enzyme PTGDS in the cerebrospinal fluid significantly correlated with levels of orexigenic neuropeptides (77). In addition, PTGDS levels in CSF are positively correlated with visceral adipose tissue and negatively correlated with subcutaneous adipose tissue mass (77). In our studies we have mined expression databases of human adipose tissue from lean and obese subjects and found levels of HPGDS were elevated in the obese state and did not decrease after weight loss. In addition circulating levels of PGD2 were elevated compared with the lean reference range, and gastric bypass-induced weight loss did not decrease circulating PGD2 levels.
The lasting changes in prostaglandin signaling in adipose tissue after weight loss suggest a possible role in weight regain. Previous studies have shown that central injection of PGD2 stimulates food intake, (65) but whether peripheral levels of PGD2 could play a role in food intake was not yet known. We show that inhibition of HPGDS (by oral gavage of HQL-79) results in decreased food intake and thus could be a therapeutic strategy in prevention of weight regain after weight loss. After 5 days of HQL-79 administration in HFD fed mice the adipose tissue ratios between PGD2 and other connected pathway metabolites indicate a shift toward PGE2 and PGF2a production instead of PGD2 after HQL-79 treatment (Supplemental fig 1A), however, detected levels of PGD2 were not significantly different in whole adipose tissue. Fractionating the adipocyte and SVF would determine whether either cell type up-regulate PGD2 production in response to HPGDS inhibition (67) but the isolation procedure itself will likely cause alterations in metabolite levels that would no longer reflect the in vivo state (75) due to rapid degradation of PGD2 within a few minutes (78). Furthermore, although we give the H-PGDS inhibitor peripherally, we cannot rule out the possibility that this inhibitor may also act directly on the brain to reduce PGD2 levels which may also play a role in the observed effects on food intake. Future studies are needed to fully understand the role of adipocyte and SVC PGD2 levels on the regulation of food intake and energy expenditure. There are currently specific HPGDS inhibitors being developed for other indications including asthma and chronic obstructive pulmonary disease (79, 80). Our data strongly suggests that inhibition of HPGDS may also be beneficial to maintain weight loss and prevent weight regain.
The role of adipose tissue in the control of food intake is well established, with leptin being the most well known example (81), but how peripheral levels of PGD2 may affect feeding behavior is still unknown. Body weight is controlled centrally by the hypothalamus which integrates signals emanating from the periphery including gastrointestinal, hepatic, and adipose tissue to control food intake and energy expenditure (82) (83). PGD2 from adipose tissue may travel via the systemic circulation, crossing the blood brain barrier and directly reach the brain (82). However, we do not observe a significant increase in PGD2 levels in the plasma of obese mice, which may be due to instability of this metabolite that has a half-life in the blood of less than 2 mins (78), (Supplemental Table 1). Alternatively, the signal from adipose to brain may be transduced by changes in the firing rate of sensory nerve fibers. Viral tracing studies have identified afferent circuits projecting from WAT to the central nervous system, supporting the existence of a sensory WAT pathway to the brain (84, 85). Therefore, further investigation is needed to understand the mechanism by which adipose tissue levels of lipid mediators may regulate food intake.
Despite the persistent metabolic adaptions that act to proportionally counter efforts to reduce body weight (86), some studies report that weight loss maintenance gets easier over time: after individuals have successfully maintained weight loss for 2–5yrs the chances of longer term success greatly increases (9). Human adipocytes turn over very slowly, and on average are only replaced every 10 years (87, 88). Therefore, changes induced in adipocytes by obesity may be longer lasting than in cell types that are replaced more readily. In addition, the obesity-induced increase in macrophage infiltration into adipose tissue does not reverse after weight loss and these cells are still present in adipose tissue after sustained weight loss in mice (56). Therefore, the “metabolic memory” of adipose tissue is difficult to erase even after maintenance of weight loss. In future time course studies, it will be interesting to see if the persistent changes in the adipose metabolome will eventually reverse after a longer time frame.
These studies reveal that obesity induces a lasting metabolic signature that does not resolve after weight loss. This metabolic signature resides predominantly in adipose tissue while other metabolic tissues including liver, muscle and hypothalamus return to pre-obese levels after weight loss. We show that PGD2 signaling is implicated in food intake and that high levels of HPGDS and circulating PGD2 are still present in human subjects despite weight loss. The metabolic characteristics of adipose tissues after weight loss could play an important role in the persistence of a biological drive to regain weight.
Supplementary Material
Supplemental Figure 1. A) Lipid mediators in adipose from HFD fed mice after treatment with HQL-79 or vehicle for 5 days. B) Plasma levels of PGD2 from HFD fed mice after treatment with HQL-79 or vehicle for 5 days. Significant change between vehicle and HQL79 treatment and HFD, * p < 0.05, # p< 0.1
Supplemental Table 1. lipid mediator data for adipose, liver, muscle hypothalamus and plasma of LFD, HFD and SWD fed mice.
Acknowledgments
This study was supported by UCSD/UCLA NIDDK Diabetes Research Center P30 DK063491 (OO) and a Pilot and Feasibility grant (OO) from the UC Davis NIH West Coast Metabolomics Center U24 DK097154. Angelina Hernandez-Carretero is an IRACDA fellow and was supported by NIGMS/NIH award K12GM068524. Additional funding was provided by USDA intramural project #2032-51530-022-00D (JWN). The USDA is an equal opportunity provider and employer. We thank Johannes Fahrmann for his contribution to the statistical analysis. The authors have no competing financial interests in relation to the work described.
References
- 1.BMC. Nutrition & Weight Management. 2015 Available from: https://http://www.bmc.org/nutritionweight/services/weightmanagement.htm.
- 2.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. The American journal of clinical nutrition. 2001 Nov;74(5):579–84. doi: 10.1093/ajcn/74.5.579. PubMed PMID: 11684524. [DOI] [PubMed] [Google Scholar]
- 3.Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. American journal of public health. 2015 Sep;105(9):e54–9. doi: 10.2105/AJPH.2015.302773. PubMed PMID: 26180980. Pubmed Central PMCID: 4539812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix MC, Caillol M, Durieux D, Monnerie R, Grebert D, Pellerin L, et al. Long-Lasting Metabolic Imbalance Related to Obesity Alters Olfactory Tissue Homeostasis and Impairs Olfactory-Driven Behaviors. Chemical senses. 2015 Oct;40(8):537–56. doi: 10.1093/chemse/bjv039. PubMed PMID: 26209545. [DOI] [PubMed] [Google Scholar]
- 5.Pasman WJ, Saris WH, Westerterp-Plantenga MS. Predictors of weight maintenance. Obesity research. 1999 Jan;7(1):43–50. doi: 10.1002/j.1550-8528.1999.tb00389.x. PubMed PMID: 10023729. [DOI] [PubMed] [Google Scholar]
- 6.Stunkard A, Mc L-HM. The results of treatment for obesity: a review of the literature and report of a series. AMA archives of internal medicine. 1959 Jan;103(1):79–85. doi: 10.1001/archinte.1959.00270010085011. PubMed PMID: 13605305. [DOI] [PubMed] [Google Scholar]
- 7.Vogels N, Diepvens K, Westerterp-Plantenga MS. Predictors of long-term weight maintenance. Obesity research. 2005 Dec;13(12):2162–8. doi: 10.1038/oby.2005.268. PubMed PMID: 16421351. [DOI] [PubMed] [Google Scholar]
- 8.Westerterp-Plantenga MS, Kempen KP, Saris WH. Determinants of weight maintenance in women after diet-induced weight reduction. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1998 Jan;22(1):1–6. doi: 10.1038/sj.ijo.0800536. PubMed PMID: 9481593. [DOI] [PubMed] [Google Scholar]
- 9.Wing RR, Phelan S. Long-term weight loss maintenance. The American journal of clinical nutrition. 2005 Jul;82(1 Suppl):222S–5S. doi: 10.1093/ajcn/82.1.222S. PubMed PMID: 16002825. [DOI] [PubMed] [Google Scholar]
- 10.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. The New England journal of medicine. 2005 Nov 17;353(20):2111–20. doi: 10.1056/NEJMoa050156. PubMed PMID: 16291981. [DOI] [PubMed] [Google Scholar]
- 11.Methods for voluntary weight loss and control. NIH Technology Assessment Conference Panel. Annals of internal medicine. 1992 Jun 1;116(11):942–9. doi: 10.7326/0003-4819-116-11-942. PubMed PMID: 1580453. [DOI] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Program Research G. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009 Nov 14;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. PubMed PMID: 19878986. Pubmed Central PMCID: 3135022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look ARG, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Archives of internal medicine. 2010 Sep 27;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. PubMed PMID: 20876408. Pubmed Central PMCID: 3084497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Annals of internal medicine. 2005 Jan 4;142(1):56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. PubMed PMID: 15630109. [DOI] [PubMed] [Google Scholar]
- 15.Reilly S. The role of the gustatory thalamus in taste-guided behavior. Neuroscience and biobehavioral reviews. 1998 Oct;22(6):883–901. doi: 10.1016/s0149-7634(98)00015-3. PubMed PMID: 9809317. [DOI] [PubMed] [Google Scholar]
- 16.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002 Oct 10;36(2):199–211. doi: 10.1016/s0896-6273(02)00969-8. PubMed PMID: 12383777. [DOI] [PubMed] [Google Scholar]
- 17.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity. 2016 May 2; doi: 10.1002/oby.21538. PubMed PMID: 27136388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England journal of medicine. 1995 Mar 9;332(10):621–8. doi: 10.1056/NEJM199503093321001. PubMed PMID: 7632212. [DOI] [PubMed] [Google Scholar]
- 19.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. American journal of physiology Regulatory, integrative and comparative physiology. 2006 Jun;290(6):R1577–88. doi: 10.1152/ajpregu.00810.2005. PubMed PMID: 16455763. [DOI] [PubMed] [Google Scholar]
- 20.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, et al. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. American journal of physiology Regulatory, integrative and comparative physiology. 2004 Dec;287(6):R1306–15. doi: 10.1152/ajpregu.00463.2004. PubMed PMID: 15331386. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, Foster GD, Letizia KA, Mullen JL. Long-term effects of dieting on resting metabolic rate in obese outpatients. Jama. 1990 Aug 8;264(6):707–11. PubMed PMID: 2374273. [PubMed] [Google Scholar]
- 22.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. The New England journal of medicine. 2011 Oct 27;365(17):1597–604. doi: 10.1056/NEJMoa1105816. PubMed PMID: 22029981. [DOI] [PubMed] [Google Scholar]
- 23.Connor SC, Hansen MK, Corner A, Smith RF, Ryan TE. Integration of metabolomics and transcriptomics data to aid biomarker discovery in type 2 diabetes. Molecular bioSystems. 2010 May;6(5):909–21. doi: 10.1039/b914182k. PubMed PMID: 20567778. [DOI] [PubMed] [Google Scholar]
- 24.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PloS one. 2010;5(12):e15234. doi: 10.1371/journal.pone.0015234. PubMed PMID: 21170321. Pubmed Central PMCID: 3000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013 Feb;62(2):639–48. doi: 10.2337/db12-0495. PubMed PMID: 23043162. Pubmed Central PMCID: 3554384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grapov D, Adams SH, Pedersen TL, Garvey WT, Newman JW. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PloS one. 2012;7(11):e48852. doi: 10.1371/journal.pone.0048852. PubMed PMID: 23144998. Pubmed Central PMCID: 3493609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suhre K, Meisinger C, Doring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PloS one. 2010;5(11):e13953. doi: 10.1371/journal.pone.0013953. PubMed PMID: 21085649. Pubmed Central PMCID: 2978704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. The Journal of biological chemistry. 2010 May 14;285(20):15333–45. doi: 10.1074/jbc.M110.100263. PubMed PMID: 20308074. Pubmed Central PMCID: 2865288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. Journal of neuroendocrinology. 2008 Jun;20(6):850–7. doi: 10.1111/j.1365-2826.2008.01728.x. PubMed PMID: 18601709. [DOI] [PubMed] [Google Scholar]
- 30.Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes' bow. Journal of neuroendocrinology. 2008 May;20(Suppl 1):130–8. doi: 10.1111/j.1365-2826.2008.01682.x. PubMed PMID: 18426512. [DOI] [PubMed] [Google Scholar]
- 31.Borg ML, Omran SF, Weir J, Meikle PJ, Watt MJ. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. The Journal of physiology. 2012 Sep 1;590(Pt 17):4377–89. doi: 10.1113/jphysiol.2012.233288. PubMed PMID: 22674717. Pubmed Central PMCID: 3473292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. The Journal of clinical investigation. 2011 May;121(5):1858–70. doi: 10.1172/JCI43378. PubMed PMID: 21490391. Pubmed Central PMCID: 3083776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell metabolism. 2007 Mar;5(3):167–79. doi: 10.1016/j.cmet.2007.01.002. PubMed PMID: 17339025. [DOI] [PubMed] [Google Scholar]
- 34.Moller K, Ostermann AI, Rund K, Thoms S, Blume C, Stahl F, et al. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and oxylipins in obese subjects. Prostaglandins, leukotrienes, and essential fatty acids. 2016 Mar;106:39–49. doi: 10.1016/j.plefa.2015.12.001. PubMed PMID: 26751601. [DOI] [PubMed] [Google Scholar]
- 35.Newman JW, Pedersen TL, Brandenburg VR, Harris WS, Shearer GC. Effect of omega-3 fatty acid ethyl esters on the oxylipin composition of lipoproteins in hypertriglyceridemic, statin-treated subjects. PloS one. 2014;9(11):e111471. doi: 10.1371/journal.pone.0111471. PubMed PMID: 25393536. Pubmed Central PMCID: 4230929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. The Journal of clinical investigation. 2005 May;115(5):1298–305. doi: 10.1172/JCI23057. PubMed PMID: 15864349. Pubmed Central PMCID: 1087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. The Journal of clinical investigation. 2008 Sep;118(9):3160–9. doi: 10.1172/JCI34827. PubMed PMID: 18677409. Pubmed Central PMCID: 2491458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero-Zerbo SY, Bermudez-Silva FJ. Cannabinoids, eating behaviour, and energy homeostasis. Drug testing and analysis. 2014 Jan-Feb;6(1–2):52–8. doi: 10.1002/dta.1594. PubMed PMID: 24375977. [DOI] [PubMed] [Google Scholar]
- 39.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. Journal of lipid research. 2010 Aug;51(8):2074–81. doi: 10.1194/jlr.M900193-JLR200. PubMed PMID: 19671931. Pubmed Central PMCID: 2903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strassburg K, Huijbrechts AM, Kortekaas KA, Lindeman JH, Pedersen TL, Dane A, et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Analytical and bioanalytical chemistry. 2012 Sep;404(5):1413–26. doi: 10.1007/s00216-012-6226-x. PubMed PMID: 22814969. Pubmed Central PMCID: 3426673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tourdot BE, Ahmed I, Holinstat M. The emerging role of oxylipins in thrombosis and diabetes. Frontiers in pharmacology. 2014 Jan 7;4:176. doi: 10.3389/fphar.2013.00176. PubMed PMID: 24432004. Pubmed Central PMCID: 3882718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, et al. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell metabolism. 2015 Aug 4;22(2):266–78. doi: 10.1016/j.cmet.2015.06.007. PubMed PMID: 26190650. Pubmed Central PMCID: 4527941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massey KA, Nicolaou A. Lipidomics of oxidized polyunsaturated fatty acids. Free radical biology & medicine. 2013 Jun;59:45–55. doi: 10.1016/j.freeradbiomed.2012.08.565. PubMed PMID: 22940496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. American journal of physiology Endocrinology and metabolism. 2011 Jan;300(1):E175–87. doi: 10.1152/ajpendo.00203.2010. PubMed PMID: 20978234. Pubmed Central PMCID: 3023204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001 Apr 12;410(6830):822–5. doi: 10.1038/35071088. PubMed PMID: 11298451. [DOI] [PubMed] [Google Scholar]
- 46.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. British journal of pharmacology. 2002 Jun;136(4):550–7. doi: 10.1038/sj.bjp.0704767. PubMed PMID: 12055133. Pubmed Central PMCID: 1573386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao S, Zhu G, Gao X, Wu D, Carrasco P, Casals N, et al. Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jun 7;108(23):9691–6. doi: 10.1073/pnas.1103267108. PubMed PMID: 21593415. Pubmed Central PMCID: 3111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osborn O, Oh DY, McNelis J, Sanchez-Alavez M, Talukdar S, Lu M, et al. G protein-coupled receptor 21 deletion improves insulin sensitivity in diet-induced obese mice. The Journal of clinical investigation. 2012 Jul 2;122(7):2444–53. doi: 10.1172/JCI61953. PubMed PMID: 22653059. Pubmed Central PMCID: 3386820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods in molecular biology. 2009;579:443–67. doi: 10.1007/978-1-60761-322-0_22. PubMed PMID: 19763489. [DOI] [PubMed] [Google Scholar]
- 50.Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. The Journal of clinical investigation. 2017 Mar 01;127(3):1019–30. doi: 10.1172/JCI90350. PubMed PMID: 28192375. Pubmed Central PMCID: 5330737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halushka PV, Mais DE, Mayeux PR, Morinelli TA. Thromboxane, prostaglandin and leukotriene receptors. Annual review of pharmacology and toxicology. 1989;29:213–39. doi: 10.1146/annurev.pa.29.040189.001241. PubMed PMID: 2543270. [DOI] [PubMed] [Google Scholar]
- 52.Joo M, Sadikot RT. PGD synthase and PGD2 in immune resposne. Mediators of inflammation. 2012;2012:503128. doi: 10.1155/2012/503128. PubMed PMID: 22791937. Pubmed Central PMCID: 3389719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urade Y, Hayaishi O. Biochemical, structural, genetic, physiological, and pathophysiological features of lipocalin-type prostaglandin D synthase. Biochimica et biophysica acta. 2000 Oct 18;1482(1–2):259–71. doi: 10.1016/s0167-4838(00)00161-8. PubMed PMID: 11058767. [DOI] [PubMed] [Google Scholar]
- 54.Kanaoka Y, Urade Y. Hematopoietic prostaglandin D synthase. Prostaglandins, leukotrienes, and essential fatty acids. 2003 Aug-Sep;69(2–3):163–7. doi: 10.1016/s0952-3278(03)00077-2. PubMed PMID: 12895599. [DOI] [PubMed] [Google Scholar]
- 55.Urade Y, Hayaishi O. Prostaglandin D synthase: structure and function. Vitamins and hormones. 2000;58:89–120. doi: 10.1016/s0083-6729(00)58022-4. PubMed PMID: 10668396. [DOI] [PubMed] [Google Scholar]
- 56.Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, et al. Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice. Diabetes. 2017 Feb;66(2):392–406. doi: 10.2337/db16-0500. PubMed PMID: 28108608. Pubmed Central PMCID: 5248991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, et al. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2009 Nov 3;106(44):18745–50. doi: 10.1073/pnas.0903032106. PubMed PMID: 19841271. Pubmed Central PMCID: 2763882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacLaren RE, Cui W, Lu H, Simard S, Cianflone K. Association of adipocyte genes with ASP expression: a microarray analysis of subcutaneous and omental adipose tissue in morbidly obese subjects. BMC medical genomics. 2010 Jan 27;3:3. doi: 10.1186/1755-8794-3-3. PubMed PMID: 20105310. Pubmed Central PMCID: 2843642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell metabolism. 2016 Apr 12;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. PubMed PMID: 26916363. Pubmed Central PMCID: 4833627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nookaew I, Svensson PA, Jacobson P, Jernas M, Taube M, Larsson I, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. The Journal of clinical endocrinology and metabolism. 2013 Feb;98(2):E370–8. doi: 10.1210/jc.2012-2764. PubMed PMID: 23264395. Pubmed Central PMCID: 3633773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PloS one. 2011 Feb 16;6(2):e16957. doi: 10.1371/journal.pone.0016957. PubMed PMID: 21359215. Pubmed Central PMCID: 3040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silva CA, Webb K, Andre BG, Marques MA, de Carvalho FM, de Macedo CS, et al. Type 1 reaction in leprosy patients corresponds with a decrease in pro-resolving and an increase in pro-inflammatory lipid mediators. The Journal of infectious diseases. 2016 Dec 08; doi: 10.1093/infdis/jiw541. PubMed PMID: 27932613. [DOI] [PubMed] [Google Scholar]
- 63.Thongdee P, Kuesap J, Wisedpanichkij R, Na-Bangchang K. Possible role of PGD2 in malaria infections. Asian Pacific journal of tropical medicine. 2016 Sep;9(9):856–9. doi: 10.1016/j.apjtm.2016.07.006. PubMed PMID: 27633298. [DOI] [PubMed] [Google Scholar]
- 64.Zhai L, Guo X, Zhang H, Jin Q, Zeng Q, Tang X, et al. Non-ionic iodinated contrast media related immediate reactions: A mechanism study of 27 patients. Legal medicine. 2017 Jan;24:56–62. doi: 10.1016/j.legalmed.2016.11.006. PubMed PMID: 28081790. [DOI] [PubMed] [Google Scholar]
- 65.Ohinata K, Takagi K, Biyajima K, Fujiwara Y, Fukumoto S, Eguchi N, et al. Central prostaglandin D(2) stimulates food intake via the neuropeptide Y system in mice. FEBS letters. 2008 Mar 5;582(5):679–84. doi: 10.1016/j.febslet.2008.01.050. PubMed PMID: 18258196. [DOI] [PubMed] [Google Scholar]
- 66.Aritake K, Kado Y, Inoue T, Miyano M, Urade Y. Structural and functional characterization of HQL-79, an orally selective inhibitor of human hematopoietic prostaglandin D synthase. The Journal of biological chemistry. 2006 Jun 2;281(22):15277–86. doi: 10.1074/jbc.M506431200. PubMed PMID: 16547010. [DOI] [PubMed] [Google Scholar]
- 67.Farhat A, Philibert P, Sultan C, Poulat F, Boizet-Bonhoure B. Hematopoietic-Prostaglandin D2 synthase through PGD2 production is involved in the adult ovarian physiology. Journal of ovarian research. 2011 Feb 25;4:3. doi: 10.1186/1757-2215-4-3. PubMed PMID: 21352547. Pubmed Central PMCID: 3050850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsushita N, Aritake K, Takada A, Hizue M, Hayashi K, Mitsui K, et al. Pharmacological studies on the novel antiallergic drug HQL-79: II. Elucidation of mechanisms for antiallergic and antiasthmatic effects. Japanese journal of pharmacology. 1998 Sep;78(1):11–22. doi: 10.1254/jjp.78.11. PubMed PMID: 9804057. [DOI] [PubMed] [Google Scholar]
- 69.Matsushita N, Hizue M, Aritake K, Hayashi K, Takada A, Mitsui K, et al. Pharmacological studies on the novel antiallergic drug HQL-79: I. Antiallergic and antiasthmatic effects in various experimental models. Japanese journal of pharmacology. 1998 Sep;78(1):1–10. doi: 10.1254/jjp.78.1. PubMed PMID: 9804056. [DOI] [PubMed] [Google Scholar]
- 70.Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Current opinion in pharmacology. 2007 Feb;7(1):33–8. doi: 10.1016/j.coph.2006.09.004. PubMed PMID: 17129762. [DOI] [PubMed] [Google Scholar]
- 71.Popp L, Haussler A, Olliges A, Nusing R, Narumiya S, Geisslinger G, et al. Comparison of nociceptive behavior in prostaglandin E, F, D, prostacyclin and thromboxane receptor knockout mice. European journal of pain. 2009 Aug;13(7):691–703. doi: 10.1016/j.ejpain.2008.09.001. PubMed PMID: 18938093. [DOI] [PubMed] [Google Scholar]
- 72.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. The Journal of experimental medicine. 2001 Jan 15;193(2):255–61. doi: 10.1084/jem.193.2.255. PubMed PMID: 11208866. Pubmed Central PMCID: 2193345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugimoto Y, Narumiya S, Ichikawa A. Distribution and function of prostanoid receptors: studies from knockout mice. Progress in lipid research. 2000 Jul;39(4):289–314. doi: 10.1016/s0163-7827(00)00008-4. PubMed PMID: 10856600. [DOI] [PubMed] [Google Scholar]
- 74.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998 Jan 1;391(6662):79–82. doi: 10.1038/34178. PubMed PMID: 9422508. [DOI] [PubMed] [Google Scholar]
- 75.Virtue S, Masoodi M, de Weijer BA, van Eijk M, Mok CY, Eiden M, et al. Prostaglandin profiling reveals a role for haematopoietic prostaglandin D synthase in adipose tissue macrophage polarisation in mice and humans. International journal of obesity. 2015 Jul;39(7):1151–60. doi: 10.1038/ijo.2015.34. PubMed PMID: 25801691. Pubmed Central PMCID: 4486370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujitani Y, Aritake K, Kanaoka Y, Goto T, Takahashi N, Fujimori K, et al. Pronounced adipogenesis and increased insulin sensitivity caused by overproduction of prostaglandin D2 in vivo. The FEBS journal. 2010 Mar;277(6):1410–9. doi: 10.1111/j.1742-4658.2010.07565.x. PubMed PMID: 20136655. [DOI] [PubMed] [Google Scholar]
- 77.Elias E, Benrick A, Behre CJ, Ekman R, Zetterberg H, Stenlof K, et al. Central nervous system lipocalin-type prostaglandin D2-synthase is correlated with orexigenic neuropeptides, visceral adiposity and markers of the hypothalamic-pituitary-adrenal axis in obese humans. Journal of neuroendocrinology. 2011 Jun;23(6):501–7. doi: 10.1111/j.1365-2826.2011.02128.x. PubMed PMID: 21438929. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki F, Hayashi H, Hayaishi O. Transport of prostaglandin D2 into brain. Brain research. 1986 Oct 22;385(2):321–8. doi: 10.1016/0006-8993(86)91079-6. PubMed PMID: 3465420. [DOI] [PubMed] [Google Scholar]
- 79.Carron CP, Trujillo JI, Olson KL, Huang W, Hamper BC, Dice T, et al. Discovery of an Oral Potent Selective Inhibitor of Hematopoietic Prostaglandin D Synthase (HPGDS) ACS medicinal chemistry letters. 2010 May 13;1(2):59–63. doi: 10.1021/ml900025z. PubMed PMID: 24900177. Pubmed Central PMCID: 4007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazari AM, Hegazy UM, Mannervik B. Identification of new inhibitors for human hematopoietic prostaglandin D2 synthase among FDA-approved drugs and other compounds. Chemico-biological interactions. 2015 Mar 05;229:91–9. doi: 10.1016/j.cbi.2015.01.014. PubMed PMID: 25603235. [DOI] [PubMed] [Google Scholar]
- 81.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998 Oct 22;395(6704):763–70. doi: 10.1038/27376. PubMed PMID: 9796811. [DOI] [PubMed] [Google Scholar]
- 82.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006 Sep 21;443(7109):289–95. doi: 10.1038/nature05026. PubMed PMID: 16988703. [DOI] [PubMed] [Google Scholar]
- 83.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012 Mar;18(3):363–74. doi: 10.1038/nm.2627. PubMed PMID: 22395709. [DOI] [PubMed] [Google Scholar]
- 84.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and cellular endocrinology. 2010 Apr 29;318(1–2):34–43. doi: 10.1016/j.mce.2009.08.031. PubMed PMID: 19747957. Pubmed Central PMCID: 2826518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. American journal of physiology Regulatory, integrative and comparative physiology. 2009 Mar;296(3):R501–11. doi: 10.1152/ajpregu.90786.2008. PubMed PMID: 19109367. Pubmed Central PMCID: 2665851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity. 2016 Aug;24(8):1612–9. doi: 10.1002/oby.21538. PubMed PMID: 27136388. Pubmed Central PMCID: 4989512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010 Jan;59(1):105–9. doi: 10.2337/db09-0942. PubMed PMID: 19846802. Pubmed Central PMCID: 2797910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011 Oct 6;478(7367):110–3. doi: 10.1038/nature10426. PubMed PMID: 21947005. Pubmed Central PMCID: 3773935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A) Lipid mediators in adipose from HFD fed mice after treatment with HQL-79 or vehicle for 5 days. B) Plasma levels of PGD2 from HFD fed mice after treatment with HQL-79 or vehicle for 5 days. Significant change between vehicle and HQL79 treatment and HFD, * p < 0.05, # p< 0.1
Supplemental Table 1. lipid mediator data for adipose, liver, muscle hypothalamus and plasma of LFD, HFD and SWD fed mice.