Abstract
Objective
To develop modern patient-reported outcome measures that assess pain interference and pain behavior after spinal cord injury (SCI).
Design
Grounded-theory based qualitative item development; large-scale item calibration field-testing; confirmatory factor analyses; graded response model item response theory analyses; statistical linking techniques to transform scores to the Patient Reported Outcome Measurement Information System (PROMIS) metric.
Setting
Five SCI Model Systems centers and one Department of Veterans Affairs medical center in the United States.
Participants
Adults with traumatic SCI.
Interventions
N/A.
Outcome Measures
Spinal Cord Injury - Quality of Life (SCI-QOL) Pain Interference item bank, SCI-QOL Pain Interference short form, and SCI-QOL Pain Behavior scale.
Results
Seven hundred fifty-seven individuals with traumatic SCI completed 58 items addressing various aspects of pain. Items were then separated by whether they assessed pain interference or pain behavior, and poorly functioning items were removed. Confirmatory factor analyses confirmed that each set of items was unidimensional, and item response theory analyses were used to estimate slopes and thresholds for the items. Ultimately, 7 items (4 from PROMIS) comprised the Pain Behavior scale and 25 items (18 from PROMIS) comprised the Pain Interference item bank. Ten of these 25 items were selected to form the Pain Interference short form.
Conclusions
The SCI-QOL Pain Interference item bank and the SCI-QOL Pain Behavior scale demonstrated robust psychometric properties. The Pain Interference item bank is available as a computer adaptive test or short form for research and clinical applications, and scores are transformed to the PROMIS metric.
Keywords: Outcome assessment (health care), Psychometrics, Rehabilitation, Spinal cord injuries
Introduction
Pain after spinal cord injury (SCI) is common, and is often complex, chronic, and severe.1 The reported prevalence of pain after SCI is extremely variable, from 26–96%,2 depending on definition, type, and measurement of pain, as well as other factors.3,4 Among individuals with SCI, pain is associated with worse physical functioning,5 reduced self-efficacy,6 depression7 and other forms of psychological distress,8,9 poor sleep,7 unemployment,10 higher healthcare utilization and expenditures,11 and more difficult delivery of rehabilitation services.12 For many individuals with SCI, pain is chronic and resistant to treatment.1,13 However, individuals vary in the degree to which pain affects their behavior (e.g. displays of anger or sadness) and in the degree to which pain interferes with activities. Pain interference or pain impact describes how pain may limit or interfere with individuals’ physical, mental, and social activities, and may be modifiable when pain itself is not. For example, individuals may develop coping strategies to limit the impact that pain has on their life14 or may learn these skills in psychotherapy.15,16
Pain is a multidimensional construct, and there are many measures that exist to assess each aspect of the pain experience, its correlates, and its consequences. Bryce et al.17 reported on the 2006 National Institute on Disability and Rehabilitation Research (NIDRR) SCI Measures Meeting, which produced a consensus on the best available measures to assess pain phenomena after SCI. As noted by these authors and others,18 assessing pain after SCI is unique because patients’ motor and sensory impairments need to be taken into consideration. For example, many measures developed for non-SCI populations ask about pain interference with walking, which is not likely to be useful and may actually be insulting for a person who uses a wheelchair on a regular basis. Bryce et al.19 recommended using the single pain interference item from the SF-36 as well as pain interference items from the Multidimensional Pain Inventory (MPI) and/or the Brief Pain Inventory (BPI).20 The MPI and BPI have been tailored to better fit an SCI population18,21 than their original versions, but these measures still have limitations. For example, the MPI-SCI version may confound interference from pain with interference from physical impairment.22 Equally problematic as unnecessary or inappropriate material is that measures that were adapted to SCI post-hoc may omit or underemphasize content that is important to pain interference in persons with SCI. Another limitation of existing measures is that they are static instruments, meaning that they require respondents to complete every item regardless of whether the item confers additional information about the respondent's pain interference. Many static instruments are consequently clumsy, inaccurate, and inefficient. In turn, this obscures researchers’ and clinicians’ understanding of SCI-related pain interference and behavior and their responses to interventions.
Recently, a wave of modern patient reported outcomes (PRO) tools that are founded in item response theory (IRT)23,24 have been developed that permit computerized adaptive testing (CAT)—an alternative to static testing.25–27 These tools include the Patient Reported Outcome Measurement Information System (PROMIS),28 the Quality of Life in Neurological Disorders (Neuro-QOL) measurement system,29,30 and the Spinal Cord Injury Quality of Life (SCI-QOL) measurement system.31,32 These are multidimensional sets of item banks that assess health-related quality of life in the domains of physical, mental, and social functioning. This paper is to describe the development and initial validation of two pain assessment tools that are part of the SCI-QOL. An item bank is a comprehensive set of items (i.e. questions) that capture all levels of the trait or dimension being studied (e.g. pain interference). A CAT administration of an item bank strategically selects next items to administer based on the participant's earlier responses. In this way, IRT and CAT permit precise, individually-tailored measurement of a trait or dimension with only a few items. Resulting scores are directly comparable across participants and time, despite the likelihood that participants have completed different items within the bank.26 One particular strength of the PROMIS is that it is has sophisticated normative data that match the demographic makeup of the 2000 U.S. Census.
The PROMIS Pain Interference item bank comprises 41 items that were calibrated with 14,848 people, including 531 individuals with SCI.33 However, even though this measure included individuals with SCI in the calibration sample, it is designed for use with the general population, contains inappropriate items for individuals with SCI (e.g. “how often did pain prevent you from walking more than 1 mile?”), and may omit content that is important to pain interference after SCI specifically.
The Spinal Cord Injury – Quality of Life (SCI-QOL) measurement system is a comprehensive set of instruments to assess health-related quality of life after SCI.31,32 This endeavor began with focus groups and individual interviews with individuals with SCI and clinicians who specialize in SCI to identify important content areas.34 When an existing item bank from PROMIS or Neuro-QOL addressed an important content area (e.g. depression), it was optimized for use with individuals with SCI. This involved removing inappropriate items, writing new items to address missed content, recalibrating the items with an SCI-only sample, and transforming resulting scores to a PROMIS metric for use with the PROMIS normative data. When no existing PROMIS or Neuro-QOL item bank assessed focus group generated content, a new item bank was created. The SCI-QOL consists of 19 calibrated item banks and 3 fixed-length scales in total. Three item banks and one scale were derived from the PROMIS and contain mostly PROMIS items (Depression, Anxiety, Pain Interference, and Pain Behavior). Four item banks were derived from the Neuro-QOL measurement system (Positive Affect and Well-Being, Stigma, Ability to Participate in Social Roles and Activities, and Satisfaction with Social Roles and Activities) and contain mostly Neuro-QOL items. The remaining 12 item banks and 2 fixed-length scales did not derive from PROMIS or Neuro-QOL and assess constructs advanced by SCI stakeholders. These include domains of physical functioning35–38 (basic mobility, ambulation, fine motor functioning, self-care, and wheelchair mobility), physical-medical health (pressure ulcers,39 bladder management difficulties,40 bladder complications, bowel management difficulties), and emotional health (resilience,41 grief/loss,42 self-esteem,43 and psychological trauma44) and Independence. Here, we present the development and psychometric properties of the SCI-QOL Pain Interference item bank and short form, and the SCI-QOL Pain Behavior scale. These were adapted from the PROMIS Pain Interference and Pain Behavior item banks and optimized for individuals with SCI.
Methods
Development of a pain item pool
To learn about the factors that influence health-related quality of life after SCI we conducted semi-structured interviews and focus groups with individuals with SCI and clinicians who specialize in SCI. The details and results of these groups are reported elsewhere.34 Rigorous qualitative analysis45 of the resulting data revealed important content areas. During the focus groups, pain was mentioned in 16% of patient comments and 14% of clinician comments, making pain the most frequently mentioned secondary medical complication of SCI.34 Participants described subtopics of pain such as type of pain (e.g. neurogenic pain, musculoskeletal pain), frequency of pain (e.g. chronic pain), location of pain (e.g. arm/shoulder pain, pain from urinary tract infections), pain interference (e.g. inability to work or socialize due to pain), and pain behavior (e.g. wincing). Eighty-one new items were written based on specific phrases or concepts from the interviews and focus groups. We also identified 34 existing items from the PROMIS Pain Interference item bank and 18 items from the PROMIS Pain Behavior item bank for inclusion in the preliminary item pool. We excluded 6 of the 40 PROMIS Pain Interference items and 21 of the PROMIS Pain Behavior items because they potentially confounded physical functioning with pain. For example, we excluded the PROMIS Pain Interference items “How often did pain prevent you from walking more than one mile” and “How often did pain prevent you from standing for more than 1 hour?”, as well as the PROMIS Pain Behavior item, “Pain caused me to bend over while walking.” In cases where newly written items were redundant with existing PROMIS items, the new items were dropped in favor of the existing items. At this stage of instrument development, it was not yet determined if the different aspects of pain (e.g. pain interference, pain behavior) constituted a unidimensional construct (a requirement of IRT) in individuals with SCI or not. Thus, during the item development phase all items were included in a single item pool related to all aspects of pain.
The preliminary item pool consisted of 133 items—52 PROMIS items and 81 newly written items. These items were then subjected to the same process as PROMIS and Neuro-QOL items.46 Expert Item Review47 was a process whereby project investigators considered items’ relevance and clarity, and suggested revisions and deletions. This winnowed the item pool down to 58 items; including 29 PROMIS items and 29 new items. Given the extensive reviews involved in the PROMIS item development process,48 the 29 PROMIS items did not undergo any further review at this time. The 29 new items, however, then underwent a series of cognitive debriefing interviews49,50 whereby we asked 5 individuals with SCI to answer each item and describe out loud their process of receiving, considering, and answering the question. This was conducted in order to flag items that were unclear, offensive, or otherwise inappropriate. No items were modified or deleted based on the cognitive interviewing. Next, the 29 new items were reviewed for ease of translatability,51 and 6 items were modified to ensure amenability to future translation. For example, the item Pain_15, “I experienced constant pain” was modified to “I had constant pain” due to the difficulty of translating the word “experienced” in this context. Finally, the Lexile framework52 was used to ensure that all items were written at or below a 5th grade reading level.
Calibration sample and analyses
The 58 items in the item pool were presented to persons with SCI at six collaborating sites: Kessler Foundation/Kessler Institute for Rehabilitation, University of Michigan, Rehabilitation Institute of Chicago, University of Washington, Craig Hospital, and the James J. Peters/Bronx Department of Veterans Affairs Medical Center. The pain items were completed along with other preliminary pools of items related to physical/medical health and secondary complications that would become the other domains of the SCI-QOL.31,32,53 All participants provided informed consent according to protocols approved by the institutional review boards of the participating center at which they enrolled. All participants were at least 18 years old, able to read and understand English, and had medically documented traumatic SCI. Participants were stratified by level of injury (paraplegia or tetraplegia), completeness of injury (complete or incomplete), and time since injury (<1 year, 1–3 years, >3 years). We confirmed diagnoses by medical record review; level and completeness was documented by the most recent International Standards for the Neurological Classification of SCI exam.54 To achieve the statistical requirements for graded response model IRT analyses, at least 500 participants were desired to ensure at least 5 participants needed to select each of the 5 response options for each item. Successful stratification was achieved by careful monitoring of enrollment and targeted recruitment of each subgroup (e.g. paraplegia, complete, <1 year). Trained interviewers administered items using a standardized protocol either in person or by telephone, as described elsewhere.31
Data analyses began with a confirmatory factor analysis (CFA) to confirm construct unidimensionality, a psychometric requirement for IRT. Acceptable model fit was defined as Comparative Fit Index (CFI) > 0.90 and Root Mean Square Error of Approximation (RMSEA) < 0.08. Good model fit was defined as CFI > 0.90 and RMSEA < 0.08. Excellent fit was defined as CFI > 0.95 and RMSEA < 0.06. A graded responses IRT model was then used to iteratively identify poorly fitting items, items that displayed differential item functioning (DIF), local item dependence (LID), and significant loadings on the single factor (values > 0.30). DIF occurs when participant responses to a particular item are unduly influenced by characteristics other than the trait of interest.55 In other words, DIF represents “bias” against subsamples of participants, for example, based on age, sex, level of injury, etc. Statistical criteria for possible DIF were a significant χ2 test (P < 0.01) and effect sizes (McFadden's pseudo R2) greater than 0.02—a small but non-negligible effect. A core assumption of IRT is that the items are independent after accounting for the trait being measured.55 LID means that two items are inappropriately correlated, implying that they are redundant and that the inclusion of both would unduly influence the score. Here, LID was defined by residual correlations > |0.20|. Items that were identified as poorly fitting or as displaying DIF were removed from the item pool and the steps were repeated. Items that remained comprised the final item bank. The preliminary IRT parameters that resulted from these analyses were SCI-specific, in that a mean of 50 referenced the calibration sample mean.
Transformation to PROMIS metric
To ensure that the final SCI-QOL Pain items were directly comparable to PROMIS pain scores, a linear transformation was conducted so that SCI-QOL scores reference the PROMIS metric—that is, a sample that represents all United States citizens who completed the 2010 census. Therefore, a T score of 60 (1 standard deviation above the mean) on this measure means that the individual reported more/stronger symptoms than 84% of the general population. This transformation is accomplished by the Stocking-Lord method,31 which uses “anchor items” that are common to both the PROMIS and the SCI-QOL Pain Interference item banks. We examined item-response plots and scatter plots of item parameters, estimated transformation constants, and modified the initial item parameters accordingly. The final calibrations for these items were used to program a CAT on the Assessment Center™ website (www.assessmentcenter.net). A brief, fixed-length form (“short form”) was also assembled from these final items.
Reliability sample and analyses
As also reported elsewhere,31 test-retest reliability was assessed with a separate sample of 244 individuals with SCI at 4 SCI Model Systems centers: University of Michigan, Kessler Foundation/Kessler Institute for Rehabilitation, Rehabilitation Institute of Chicago, and Craig Hospital. Participants were community dwelling individuals with SCI who were more than 4 months post-injury at the time of study enrollment. Participants completed the Pain Interference CAT, as well as item pools related to other domains of functioning, 1–2 weeks apart. Test-retest reliability was assessed with a Pearson's r coefficient and an intraclass correlation coefficient (ICC) between the two assessments.
Results
Participant characteristics
Table 1 summarizes the demographic and injury characteristics of the calibration sample.
Table 1.
Calibration sample characteristics.
| Variable | Calibration Sample |
|---|---|
| (n = 757) | |
| Age (mean ± SD) | 42.9±15.5 years |
| Sex | |
| Male | 79.1% |
| Female | 20.9% |
| Ethnicity | |
| Hispanic | 10.6% |
| Non-Hispanic | 87.8% |
| Not Reported | 1.6% |
| Race | |
| Caucasian | 71.1% |
| Black or African-American | 17.2% |
| Asian | 1.5% |
| American Indian/Alaska Native/Native Hawaiian/Pacific Islander | 0.9% |
| More than one race | 1.5% |
| Other | 6.8% |
| Not Reported | 1.1% |
| Time Since Injury (mean±SD) | 6.7±9.9 years |
| < 1 year post injury | 28.9% |
| 1–3 years post injury | 27.6% |
| > 3 years post injury | 43.5% |
| Diagnosis | |
| Paraplegia Complete | 23.9% |
| Paraplegia Incomplete | 18.5% |
| Tetraplegia Complete | 23.1% |
| Tetraplegia Incomplete | 34.4% |
| Education Level | |
| High school or less | 38.4% |
| Some college | 33.5% |
| Bachelor's degree or more | 28.1% |
| Cause of Injury | |
| Motor Vehicle Accident | 32.4% |
| Fall | 22.3% |
| Gunshot Wound/Violence | 11.8% |
| Diving | 6.6% |
| Other sports | 7.4% |
| Medical/Surgical accident | 3.7% |
| Motorcycle/dirt bike/ ATV accident | 3.9% |
| Other or Not Reported | 11.9% |
| Method(s) of Mobility (not mutually exclusive) | |
| Manual Wheelchair | 54.4% |
| Power Wheelchair | 44.1% |
| Ambulation | 32.7% |
Separation of ‘pain interference’ and ‘pain behavior’
Analyses began with data from the pool of 58 items related to multiple aspects of pain–pain interference, pain behavior, and general aspects of pain; however, as anticipated, the first round of CFA indicated that these three types of items did not measure a single, unidimensional construct. As a consequence, 12 general pain items were removed and the remaining items were split into Pain Interference (28 items) and Pain Behavior (18 items) based on psychometric fit. These items became separate measures and are described below.
Data analysis for pain interference items
Preliminary analysis & item removal
After items targeting pain interference were removed from the overall pool of pain items, CFA was performed and 3 items were removed: 1) rPain42: “Neck pain interfered with my ability to do things” was removed due to LID, a low item-total correlation, and DIF for diagnosis; 2) PAININ24: “How often was pain distressing to you?” was removed for misfit (significant χ2 test); and 3) PAININ10, “How much did pain interfere with your enjoyment of recreational activities?” was removed due to LID. For the remaining 25 items, α=0.968 and item-total correlations ranged from 0.47 to 0.86. All of the items had more than 35% of the sample selecting category 1 (“Never” or “Not at all”), and no items had sparse data (i.e. fewer than 5 responses for each level of each item). No additional items were removed at this stage.
Confirmatory factor analysis
CFA results supported good fit to a unidimensional model (CFI = 0.983; RMSEA = 0.063), suggesting that a single dimension underlies the item content. R2 values were greater than 0.400 for 23 of 25 items. The R2 values for the remaining items were rPain24 = 0.390 rPain27=0.307. No item pairs exhibited LID. The first to second eigenvalue ratio was 19.1, indicating that the main factor accounted for a very large proportion of the variance. Collectively, these fit statistics support a unidimensional construct of pain interference.
IRT parameter estimation and model fit
Slopes ranged from 1.26 to 4.44 and thresholds ranged from –0.46 to 2.10. Measurement precision in the theta range between –0.8 and 2.2 is roughly equivalent to a classical reliability of 0.95 or better. Figure 1 shows the Pain Interference bank's test information and precision. We calculated the S-χ2 model fit statistics using the IRTFIT56 macro program. All but 4 items (rPain27, PAININ20, PAININ18, PAININ48) had adequate or better model fit statistics (P > 0.05) with marginal reliability equal to 0.933.
Figure 1.
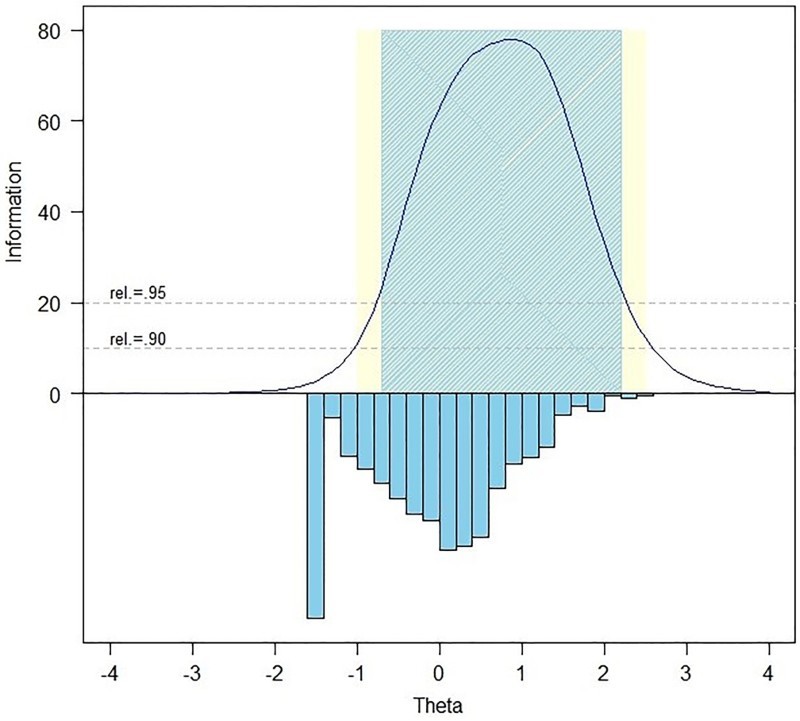
Information and Precision of the SCI-QOL Pain Interference Item Bank. The blue and yellow shaded regions show at what levels of the trait the item bank is highly reliable (.95 and.90, respectively). The inverted columns show the distribution of participant scores at each level of the trait.
Data analysis for pain behavior items
Preliminary analysis & item removal
After the 18 items related to pain behavior were isolated, preliminary analyses with CFA resulted in the removal of 11 items for the following reasons (not mutually exclusive): bimodal distribution (6 items), LID (3 items), misfit (significant χ2 test; 1 item), DIF for sex (1 item, “I had pain so bad it made me cry.”), and DIF for time since injury (1 item, “When I was in pain I called out for someone to help me”). For the 7 retained items, α=0.899 and item-total correlations ranged from 0.59 to 0.81. All of the 5-point items had more than 50% of the sample selecting category 1 (Never or Not at all) and all of the 6-point items (i.e. those PROMIS items that included the option, Had no pain) had more than 30% of the sample selecting category 2 (Never). No items had sparse data and no additional items were removed at this stage. The following summary is based on the final 7-item set. Due to the small number of retained items, the Pain Behavior items are referred to as fixed-length scale rather than an item bank.
Confirmatory factor analysis
CFA results confirmed fit to a unidimensional model, with CFI=0.996 and RMSEA=0.076. R2 values for all 7 items were greater than 0.40. No item pairs were identified for LID and the eigenvalue ratio (first to second) was 9.0.
IRT parameter estimation & model fit
For the 7 items, slopes ranged from 2.08 to 4.97 and thresholds ranged from –1.09 to 2.08. Measurement precision in the theta range of –1.2 to 1.9 was roughly equivalent to a classical reliability of 0.95 or better. Figure 2 shows the Pain Behavior scale's test information and precision. The IRTFIT macro program confirmed that all but 3 items (rPain46, PAINBE23, PAINBE32) had adequate or better fit (P > 0.05) to the S-χ2 model, with marginal reliability equal to 0.920.
Figure 2.
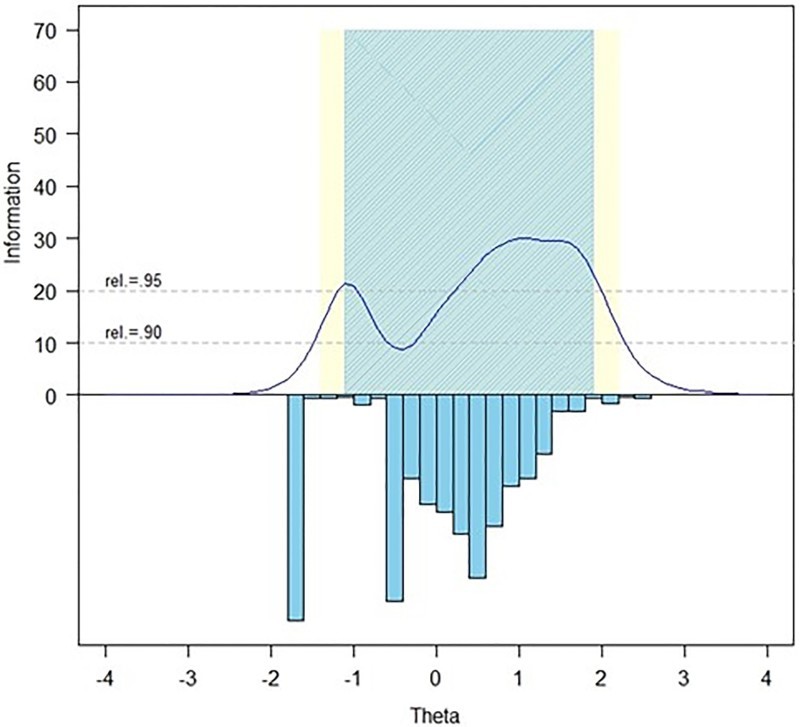
Information and Precision of the SCI-QOL Pain Behavior Scale. The blue and yellow shaded regions show at what levels of the trait the item bank is highly reliable (.95 and.90, respectively). The inverted columns show the distribution of participant scores at each level of the trait.
Differential item functioning
We examined DIF using lordif57 for six characteristics: age (≤49 vs. ≥50), sex (male vs. female), education (some college or less vs. college degree or more), injury level (tetraplegia vs. paraplegia), injury severity (incomplete vs. complete), and time since injury (<1 year vs. >1 year). Eleven remaining Pain Interference items and 3 retained Pain Behavior items produced significant χ2 tests for at least one characteristic. However, the effect sizes associated with these items indicated that the potential DIF was negligible. Descriptive statistics for the final items are presented in Table 2.
Table 2.
Descriptive item statistics.
| Item ID | Item Stem | Mean | SD | % at Min. | % at Max. | |
|---|---|---|---|---|---|---|
| Pain Interference | ||||||
| PAININ1 | P | How difficult was it for you to take in new information because of pain? | 1.52 | 0.96 | 71.3 | 1.8 |
| PAININ12 | P,S | How much did pain interfere with the things you usually do for fun? | 2.08 | 1.31 | 48.0 | 8.4 |
| PAININ13 | P,S | How much did pain interfere with your family life? | 1.67 | 1.09 | 64.9 | 4.0 |
| PAININ16 | P | How often did pain make you feel depressed? | 1.87 | 1.18 | 56.3 | 5.2 |
| PAININ18 | P,S | How much did pain interfere with your ability to work (include work at home)? | 1.98 | 1.33 | 55.2 | 8.7 |
| PAININ19 | P | How much did pain make it difficult to fall asleep? | 2.18 | 1.29 | 44.4 | 7.5 |
| PAININ20 | P | How much did pain feel like a burden to you? | 2.53 | 1.47 | 36.6 | 14.7 |
| PAININ29 | P,S | How often was your pain so severe you could think of nothing else? | 1.87 | 1.16 | 55.4 | 3.7 |
| PAININ3 | P,S | How much did pain interfere with your enjoyment of life? | 2.31 | 1.40 | 40.8 | 12.0 |
| PAININ35 | P | How much did pain interfere with your ability to make trips from home that kept you gone for more than 2 hours? | 1.63 | 1.14 | 70.7 | 4.6 |
| PAININ37 | P | How often did pain make you feel anxious? | 2.03 | 1.20 | 49.0 | 4.0 |
| PAININ39 | P,S | How often did pain make simple tasks hard to complete? | 2.24 | 1.30 | 41.9 | 7.3 |
| PAININ48 | P | How much did pain interfere with your ability to do household chores? | 1.90 | 1.25 | 56.2 | 7.1 |
| PAININ49 | P,S | How much did pain interfere with your ability to remember things? | 1.44 | 0.92 | 76.5 | 2.2 |
| PAININ53 | P,S | PROMIS How often did pain restrict your social life to your home? | 1.83 | 1.16 | 58.0 | 3.7 |
| PAININ56 | P | How irritable did you feel because of pain? | 2.24 | 1.32 | 39.1 | 9.6 |
| PAININ6 | P,S | How much did pain interfere with your close personal relationships? | 1.74 | 1.17 | 63.7 | 4.8 |
| PAININ9 | P,S | How much did pain interfere with your day-to-day activities? | 2.18 | 1.29 | 42.5 | 7.8 |
| rPain24 | Numbness interfered with my ability to do things. | 2.03 | 1.40 | 56.1 | 11.4 | |
| rPain25 | I was not able to accomplish as much as I'd like because of pain. | 2.19 | 1.40 | 48.0 | 11.0 | |
| rPain27 | Shoulder pain interfered with my ability to do things. | 2.13 | 1.34 | 48.5 | 9.5 | |
| rPain39 | Pain interfered with my ability to care for family members. | 1.50 | 1.05 | 77.1 | 4.2 | |
| rPain41 | Muscle pain interfered with my daily activities. | 2.10 | 1.28 | 46.2 | 7.9 | |
| rPain43 | Back pain interfered with my ability to do things. | 2.00 | 1.35 | 55.6 | 8.9 | |
| rPain_Com16 | Pain interfered with my sex life. | 1.54 | 1.17 | 77.6 | 6.7 | |
| Pain Behavior | ||||||
| PAINBE16 | P | When I was in pain I appeared upset or sad. | 2.83 | 1.350 | 16.2 | 3.7 |
| PAINBE23 | P | When I was in pain I asked one or more people to leave me alone. | 2.36 | 1.133 | 16.6 | 2.4 |
| PAINBE32 | P | When I was in pain I became quiet and withdrawn. | 2.85 | 1.393 | 16.2 | 4.5 |
| PAINBE9 | P | When I was in pain I became angry. | 1.87 | 1.184 | 57.7 | 4.5 |
| rPain22 | I was more sensitive to pain than usual. | 1.78 | 1.159 | 61.8 | 4.5 | |
| rPain46 | My pain was so bad that I wanted to give up on everything. | 2.23 | 1.015 | 16.0 | 1.7 | |
| rPain8 | I experienced excruciating pain. | 2.10 | 1.390 | 52.7 | 9.5 | |
P = PROMIS Item; S = Short form 10a item.
Context for all items was: “In the past 7 days…”.
Response sets for Pain Interference were: Not at all/A little bit/Somewhat/Quite a bit/Very much - or - Never/Rarely/Sometimes/Often/Always.
Response Sets for Pain Behavior were: Had No Pain/Never/Rarely/Sometimes/Often/Always or Never/Rarely/Sometimes/Often/Always.
Transformation to PROMIS metric
Stocking-Lord58 techniques were used to calculate the constants, slopes, and intercepts for 17 anchor items (items common to PROMIS and the SCI-QOL). These comprised a linear algebraic formula that transformed the SCI-QOL Pain Interference parameters to the PROMIS Pain Interference metric and the SCI-QOL Pain Behavior parameters to the PROMIS Pain Behavior metric. Final parameters are shown in Table 3. The SCI-QOL Pain Interference calibration sample mean and standard deviation were 48.7 (9.3) before transformation and 53.1 (9.9) after transformation. The calibration sample's mean on the Pain Behavior scale was 49.9 (9.6) before transformation and 53.5 (9.3) after transformation. Because higher scores indicate more pain interference and pain behavior, these results not surprisingly indicate that our SCI sample experienced more pain interference and pain behavior than the general population.
Table 3.
Item response parameters.
| Item ID | Slope | Threshold | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| PAININ1 | 2.94326 | 1.00559 | 1.52197 | 2.02297 | 2.65759 | -- |
| PAININ12 | 4.27213 | 0.33938 | 0.89954 | 1.36190 | 1.77462 | -- |
| PAININ13 | 3.62569 | 0.78475 | 1.26382 | 1.77684 | 2.18978 | -- |
| PAININ16 | 2.90874 | 0.56811 | 1.07869 | 1.72344 | 2.15765 | -- |
| PAININ18 | 3.68169 | 0.51610 | 0.93308 | 1.39410 | 1.78204 | -- |
| PAININ19 | 2.33926 | 0.19421 | 0.74308 | 1.52047 | 2.08077 | -- |
| PAININ20 | 3.27609 | 0.00225 | 0.55721 | 1.01577 | 1.52601 | -- |
| PAININ29 | 2.57886 | 0.54852 | 1.08582 | 1.77248 | 2.43486 | -- |
| PAININ3 | 3.97897 | 0.15205 | 0.75989 | 1.16018 | 1.58710 | -- |
| PAININ35 | 2.98811 | 0.97986 | 1.32606 | 1.74088 | 2.20347 | -- |
| PAININ37 | 2.62955 | 0.35119 | 0.83253 | 1.59122 | 2.35825 | -- |
| PAININ39 | 3.34840 | 0.16589 | 0.68735 | 1.35501 | 1.91976 | -- |
| PAININ48 | 3.35782 | 0.54239 | 1.03126 | 1.54620 | 1.93442 | -- |
| PAININ49 | 2.70208 | 1.19292 | 1.60108 | 2.23708 | 2.63660 | -- |
| PAININ53 | 3.67835 | 0.60834 | 1.08480 | 1.62202 | 2.23641 | -- |
| PAININ56 | 2.98482 | 0.06140 | 0.84405 | 1.32017 | 1.79485 | -- |
| PAININ6 | 3.66788 | 0.75353 | 1.16351 | 1.64355 | 2.09424 | -- |
| PAININ9 | 4.14230 | 0.20249 | 0.79462 | 1.32543 | 1.81593 | -- |
| rPain24 | 1.42125 | 0.60165 | 1.13721 | 1.84156 | 2.24170 | -- |
| rPain25 | 3.42346 | 0.33929 | 0.76319 | 1.29945 | 1.69083 | -- |
| rPain27 | 1.21526 | 0.26183 | 0.98245 | 1.91654 | 2.55865 | -- |
| rPain39 | 2.80722 | 1.19823 | 1.52511 | 1.93774 | 2.28070 | -- |
| rPain41 | 2.33326 | 0.24769 | 0.90039 | 1.55052 | 2.04096 | -- |
| rPain43 | 1.89948 | 0.53006 | 1.02339 | 1.56041 | 2.10892 | -- |
| rPain_Com16 | 2.56961 | 1.23043 | 1.53021 | 1.80644 | 2.08712 | -- |
| PAINBE16 | 5.25766 | -0.60079 | 0.48878 | 0.90366 | 1.41087 | 1.89856 |
| PAINBE23 | 4.54702 | -0.59437 | 1.00348 | 1.28393 | 1.74683 | 2.09456 |
| PAINBE32 | 4.54279 | -0.61151 | 0.52981 | 0.89017 | 1.39514 | 1.86752 |
| PAINBE9 | 3.27800 | 0.68343 | 1.01218 | 1.60042 | 2.03276 | |
| rPain22 | 2.25605 | 0.80181 | 1.20271 | 1.84087 | 2.31204 | |
| rPain46 | 5.39075 | -0.61851 | 1.16311 | 1.41920 | 1.80285 | 2.15595 |
| rPain8 | 2.41280 | 0.56297 | 0.94877 | 1.37143 | 1.84961 | |
Administration modes
Pain interference
Once the final Pain Interference item parameters were transformed to the PROMIS metric, all items and parameters were programmed into the Assessment Center platform.59 The item bank may be administered as a CAT or as a 10-item fixed-length short form. This form, the SCI-QOL Pain Interference SF10a, may be administered electronically through Assessment Center, by traditional paper-and-pencil or interview methods, or may be administered through alternate data collection platforms such as REDCap.60 Raw scores generated from the SCI-QOL Pain Interference SF10a can be converted to IRT-based T scores using with a lookup table (described below). Participants must complete all 10 items on the short form in order to produce a valid score.
When administering the Pain Interference bank as a CAT, Assessment Center provides the user with options for customized CAT administration. By default, the CAT will administer a minimum of 4 items and a maximum of 12 items and will discontinue when the standard error falls below 0.3. However, in some cases the user may want to maximize reliability by increasing the minimum number of items or reduce respondent burden by decreasing the maximum number of items to be administered. A comparison of the measurement precision of the 25-item bank, 10-item short form, variable length CAT with a minimum of 4 items, and variable length CAT with a minimum of 8 items is presented in Figure 3. Table 4 presents the number of items that were typically administered, as well as their correlation with the total item bank score. Table 5 presents the means and standard errors for the full item bank, CATs, and short form.
Figure 3.
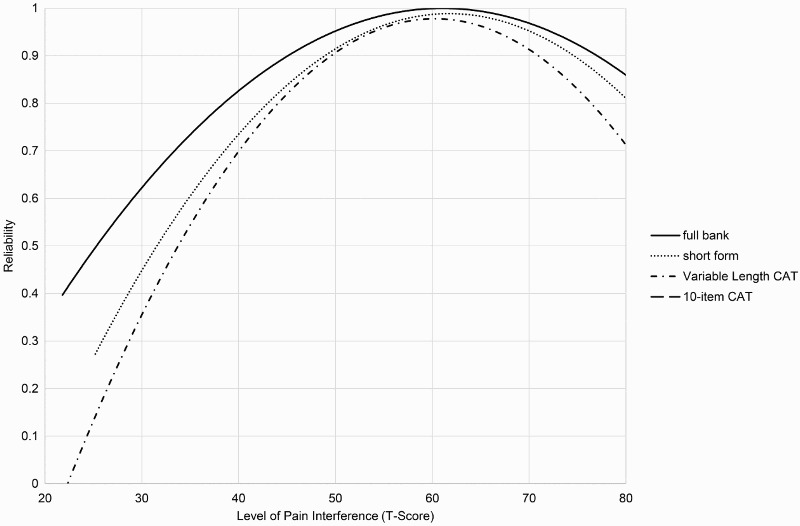
Reliability by Assessment Method and Level of the Pain Interference. This graph shows how reliable is the measurement at each level of the trait and with different assessment methods. CAT = computerized adaptive test.
Table 4.
Modes of administration: Descriptive information and correlations with full-bank score.
| Mode of Administration | N | # Items Admin | %Min | %Max | Corr. w/ Full Bank | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | |||||
| Variable-Length CAT (min 4) | 757 | 6.38 | 3.45 | 4 | 12 | 61.16 | 25.10 | 0.98 |
| Variable-Length CAT (min 8) | 757 | 9.06 | 1.74 | 8 | 12 | 72.26 | 25.10 | 0.99 |
| 10-Item Fixed-Length CAT | 757 | 10 | 0 | 10 | 10 | n/a | n/a | 0.99 |
| 10-Item Short Form | 757 | 10 | 0 | 10 | 10 | n/a | n/a | 0.97 |
* “Corr” = correlation.
Table 5.
Modes of administration: Breadth of coverage.
| Mode of Administration | N | T Score | Standard Error | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | % Ceiling | % Floor | Mean ± SD | Range | ||
| Variable-Length CAT (min 4) | 757 | 52.93±9.70 | 37.23–82.16 | 0.13 | 14.51 | 0.31±0.13 | 0.20–0.58 |
| Variable-Length CAT (min 8) | 757 | 53.09±9.71 | 38.49–80.99 | 0.13 | 19.42 | 0.28±0.16 | 0.15–0.58 |
| 10-Item Fixed-Length CAT | 757 | 53.05±9.79 | 37.79–81.51 | 0.13 | 16.51 | 0.26±0.16 | 0.13–0.58 |
| 10-Item Short Form | 757 | 53.10±9.47 | 40.20–79.60 | 0.26 | 21.93 | 0.31±0.18 | 0.14–0.62 |
| Full Bank | 757 | 53.10±9.89 | 36.80–81.40 | 0.13 | 12.68 | 0.23±0.16 | 0.10–0.58 |
Pain behavior
Due to the small number of initial and retained items, the SCI-QOL Pain Behavior items are only available as a 7-item fixed-length scale. The scale is available either through Assessment Center or as a standalone form.
Scoring
All SCI-QOL scores are reported on the standardized T metric, with a mean of 50 and a standard deviation of 10. Since both the Pain Interference and Pain Behavior scores have been transformed to the PROMIS metric, a score of 50 on either measure reflects the mean of the U.S. population. Also, for both Pain Interference and Pain Behavior, higher scores indicate more severe symptoms, so a T-score of 60 indicates that a participant has 1 standard deviation more pain interference (i.e. their pain interference is worse than) than the general population average. The Pain Interference CAT and Pain Behavior scale are automatically scored by Assessment Center, whereas the Pain Interference SF10a (Table 6) and any paper-and-pencil or other alternate administrations of the Pain Behavior scale must be manually scored and then converted to the T-metric using the lookup tables provided here (Table 7).
Table 6.
T-Score lookup table for SCI-QOL Pain Interference Short Form 10a.
| Raw Score | Scaled Score | Standard Error |
|---|---|---|
| 10 | 40.2 | 6.0 |
| 11 | 47.1 | 3.3 |
| 12 | 49.1 | 2.9 |
| 13 | 50.7 | 2.5 |
| 14 | 52.0 | 2.3 |
| 15 | 53.0 | 2.1 |
| 16 | 53.9 | 2.0 |
| 17 | 54.7 | 1.9 |
| 18 | 55.5 | 1.8 |
| 19 | 56.2 | 1.8 |
| 20 | 56.8 | 1.8 |
| 21 | 57.5 | 1.7 |
| 22 | 58.1 | 1.7 |
| 23 | 58.7 | 1.7 |
| 24 | 59.2 | 1.7 |
| 25 | 59.8 | 1.7 |
| 26 | 60.3 | 1.7 |
| 27 | 60.9 | 1.7 |
| 28 | 61.4 | 1.7 |
| 29 | 61.9 | 1.7 |
| 30 | 62.5 | 1.7 |
| 31 | 63.0 | 1.7 |
| 32 | 63.5 | 1.7 |
| 33 | 64.1 | 1.7 |
| 34 | 64.6 | 1.7 |
| 35 | 65.1 | 1.7 |
| 36 | 65.7 | 1.7 |
| 37 | 66.2 | 1.7 |
| 38 | 66.8 | 1.7 |
| 39 | 67.4 | 1.7 |
| 40 | 68.0 | 1.8 |
| 41 | 68.7 | 1.8 |
| 42 | 69.3 | 1.9 |
| 43 | 70.1 | 1.9 |
| 44 | 70.9 | 2.0 |
| 45 | 71.7 | 2.1 |
| 46 | 72.7 | 2.3 |
| 47 | 73.8 | 2.4 |
| 48 | 75.1 | 2.7 |
| 49 | 76.6 | 2.9 |
| 50 | 79.7 | 3.9 |
Table 7.
T-score lookup table for SCI-QOL Pain Behavior Scale.
| Raw Score | Scaled Score | Standard Error |
|---|---|---|
| 7 | 38.2 | 4.0 |
| 8 | 40.8 | 2.7 |
| 9 | 42.8 | 2.5 |
| 10 | 44.7 | 2.4 |
| 11 | 49.1 | 2.3 |
| 12 | 52.1 | 2.3 |
| 13 | 53.1 | 2.2 |
| 14 | 54.1 | 2.2 |
| 15 | 55.7 | 2.1 |
| 16 | 56.9 | 2.0 |
| 17 | 56.9 | 1.9 |
| 18 | 58.5 | 1.9 |
| 19 | 59.1 | 1.9 |
| 20 | 59.6 | 1.8 |
| 21 | 60.1 | 1.8 |
| 22 | 60.9 | 1.8 |
| 23 | 61.7 | 1.7 |
| 24 | 62.7 | 1.7 |
| 25 | 63.5 | 1.7 |
| 26 | 63.7 | 1.7 |
| 27 | 64.5 | 1.6 |
| 28 | 64.9 | 1.6 |
| 29 | 65.8 | 1.6 |
| 30 | 66.4 | 1.6 |
| 31 | 67.6 | 1.7 |
| 32 | 68.2 | 1.7 |
| 33 | 68.8 | 1.8 |
| 34 | 69.4 | 1.8 |
| 35 | 70.1 | 1.9 |
| 36 | 70.8 | 1.9 |
| 37 | 73.3 | 2.0 |
| 38 | 73.4 | 2.2 |
| 39 | 76.1 | 3.3 |
Test-retest reliability
For the community-dwelling reliability sample (n = 244), the default stopping rules for the CAT were used (minimum items = 4; maximum items = 12; maximum SE = 0.3). For Pain Interference, the CAT administration averaged 5.8 items (SD = 3.1). Pearson's r between the CAT scores at the baseline and 1–2 week test-retest assessments, respectively, was 0.84 (P < 0.01) and the intraclass correlation coefficient (ICC) (2,1) was 0.83 (P < 0.01). The test-retest reliability of the Pain Behavior scale was not assessed.
Discussion
Pain after SCI is common and often refractory to treatment; however, individuals vary in the degree to which pain influences behavior and interferes with physical, mental, and social activities. Individuals can learn skills to modify the influence of pain15,16 when pain itself is not modifiable. Existing assessment measures of pain behavior or interference are not optimal for individuals with SCI because they omit important information, confound pain symptoms with physical limitations, contain inappropriate information, and/or are static instruments.
Here, we report that the SCI-QOL Pain Interference measure is a psychometrically sound instrument that has been optimized for individuals with SCI. The item bank and scale capitalize on all of the innovations of PROMIS; they are founded in IRT, can be administered by CAT, and reference high quality normative data from a sample that matches the general U.S. population. The Pain Behavior Scale is a 7-item fixed length scale that contains mostly PROMIS items and references the PROMIS metric. Some other PROMIS Pain Behavior items, when tested in this SCI sample, showed poor psychometric fit (bimodal distributions, local dependence, poor item fit, or differential item functioning) and were removed, resulting in a smaller, fixed length scale.
The advantages of modern patient reported outcome instruments (e.g. PROMIS) have been demonstrated with other instruments and populations.61–65 Instruments that use IRT and CAT are less burdensome to administer, score, and interpret, and can be interpreted alongside other modern patient-reported outcome instruments (e.g. that are also reported on a T metric that reference the general U.S. population).66,67 Furthermore, CAT parameters are highly customizable so administration can be easily tailored for different purposes, for example, in situations where low test burden is more important than precision (e.g. clinical screening), or in the opposite scenario (e.g. studying patient responses to pain intervention). A short form can be administered when internet access is unavailable or respondents have difficulty using a computer. The results presented here demonstrate that all administration modes produce reliable scores and that a 10-item CAT demonstrates precision that is nearly equal to that achieved with administration of the full item bank.
The use of IRT and CAT and optimization of item content and selection algorithms specifically for individuals with SCI likely make the SCI-QOL Pain Interference and Pain Behavior measures more sensitive than traditional measures, although this remains to be studied. Research using traditional measures of pain interference reported that individuals with pain from SCI may experience interference at lower levels of pain, compared to individuals with pain from other causes.68 However, the literature on pain interference after SCI may be obscured because traditional measures may confound interference from pain with interference from physical disability.22 Because the SCI-QOL Pain Interference and Behavior measures were developed specifically for individuals with SCI, we anticipate that they are better able to assess the unique influence of pain, although this remains to be formally studied.
More accurate assessment of these constructs may improve researchers’ and clinicians’ understanding of SCI-related pain interference and behavior and their responses to intervention. For example, future studies may investigate what kind of psychotherapy optimally improves pain interference and behavior in this population, for example, cognitive and behavioral therapy or acceptance and commitment therapy (ACT).69 Among a non-SCI sample at high risk for pain-related disability, four 1-hour sessions of ACT significantly reduced the number of sick days from work and less healthcare utilization than a treatment-as-usual control group for up to the maximum follow-up time point (6 months).70 Future studies may also investigate the interference and behavior caused by different locations and kinds of pain, for example, neuropathic versus nociceptive pain.
Pain is a multidimensional construct and one limitation of this study is that not all dimensions were assessed (e.g. pain intensity). An additional limitation is that types of pain (e.g. musculoskeletal versus neuropathic) were not differentiated. Many of the PROMIS Pain Behavior items did not psychometrically fit our SCI population and were rejected from the final scale. We supplemented the retained PROMIS Pain Behavior items with psychometrically fitting items that were generated from our SCI-specific focus groups, some of which assessed affect. The final SCI-QOL Pain Behavior items fit a unidimensional model in our SCI population, but includes some content that the PROMIS Pain Behavior item bank does not. Finally, although the sample is large and heterogeneous, it was recruited from 5 SCI Model System sites and one VA medical center, and may not be representative of all individuals living with SCI in the United States.
Conclusions
The SCI-QOL Pain Interference item bank and short form and the SCI-QOL Pain Behavior scale are versions of the PROMIS Pain Interference and Pain Behavior item banks, respectively, that have been optimized for individuals with SCI. The research presented here shows these measures to reliably capture pain behavior and interference across a wide range of severity, and with different administration modalities. Because these instruments were developed with modern measurement approaches and designed specifically for individuals with SCI, we anticipate that they will provide more sensitive, valid, and reliable assessment of pain interference and behavior after SCI than traditional measures.
Funding Statement
This study was supported by National Institutes of Health grant number 5R01HD054569 (Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Center for Medical Rehabilitation Research and the National Institute of Neurological Disorders and Stroke) and National Institute on Disability, Independent Living, and Rehabilitation Research grant number H133N060022. The study was also supported by NIH grant number U01AR057929 (National Institute of Arthritis and Musculoskeletal and Skin Diseases). Salary support for Dr. Cohen was also provided by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod).
Disclaimer statements
Contributors None.
Conflict of interest None.
Ethics approval None.
ORCID
Trevor A. Dyson-Hudson http://orcid.org/0000-0002-0252-2764
References
- 1.Finnerup NB. Pain in patients with spinal cord injury. Pain 2013;154 (Suppl 1):S71–6. doi: 10.1016/j.pain.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 2.Dijkers M, Bryce T, Zanca J.. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev 2009;46(1):13–29. doi: 10.1682/JRRD.2008.04.0053 [DOI] [PubMed] [Google Scholar]
- 3.van Gorp S, Kessels AG, Joosten EA, van Kleef M, Patijn J.. Pain prevalence and its determinants after spinal cord injury: a systematic review. Eur J Pain 2015;19(1):5–14. doi: 10.1002/ejp.522 [DOI] [PubMed] [Google Scholar]
- 4.Putzke JD, Richards JS, Hicken BL, DeVivo MJ.. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain 2002;100(3):231–42. doi: 10.1016/S0304-3959(02)00069-6 [DOI] [PubMed] [Google Scholar]
- 5.Alschuler KN, Jensen MP, Sullivan-Singh SJ, Borson S, Smith AE, Molton IR.. The association of age, pain, and fatigue with physical functioning and depressive symptoms in persons with spinal cord injury. J Spinal Cord Med 2013;36(5):483–91. doi: 10.1179/2045772312Y.0000000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang MY, Eng JJ, Lin KH, Tang PF, Hung C, Wang YH.. Association of depression and pain interference with disease-management self-efficacy in community-dwelling individuals with spinal cord injury. J Rehabil Med 2009;41(13):1068–73. doi: 10.2340/16501977-0455 [DOI] [PubMed] [Google Scholar]
- 7.Avluk OC, Gurcay E, Gurcay AG, Karaahmet OZ, Tamkan U, Cakci A.. Effects of chronic pain on function, depression, and sleep among patients with traumatic spinal cord injury. Ann Saudi Med 2014;34(3):211–6. doi: 10.5144/0256-4947.2014.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues D, Tran Y, Wijesuriya N, Guest R, Middleton J, Craig A.. Pain intensity and its association with negative mood states in patients with spinal cord injury. Pain Ther 2013;2(2):113–9. doi: 10.1007/s40122-013-0017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullrich PM, Smith BM, Poggensee L, Evans CT, Stroupe KT, Weaver FM, et al. Pain and post-traumatic stress disorder symptoms during inpatient rehabilitation among Operation Enduring Freedom/Operation Iraqi Freedom veterans with spinal cord injury. Arch Phys Med Rehabil 2013;94(1):80–5. doi: 10.1016/j.apmr.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 10.Goossens D, Dousse M, Ventura M, Fattal C.. Chronic neuropathic pain in spinal cord injury patients: what is the impact of social and environmental factors on care management? Ann Phys Rehabil Med 2009;52(2):173–9. doi: 10.1016/j.rehab.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 11.Margolis JM, Juneau P, Sadosky A, Cappelleri JC, Bryce TN, Nieshoff EC.. Health care utilization and expenditures among Medicaid beneficiaries with neuropathic pain following spinal cord injury. J Pain Res 2014;7:379–87. doi: 10.2147/JPR.S63796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanca JM, Dijkers MP, Hammond FM, Horn SD.. Pain and its impact on inpatient rehabilitation for acute traumatic spinal cord injury: analysis of observational data collected in the SCIRehab study. Arch Phys Med Rehabil 2013;94(4 Suppl):S137–44. doi: 10.1016/j.apmr.2012.10.035 [DOI] [PubMed] [Google Scholar]
- 13.Lofgren M, Norrbrink C. “But I know what works”—patients’ experience of spinal cord injury neuropathic pain management. Disabil Rehabil 2012;34(25):2139–47. doi: 10.3109/09638288.2012.676146 [DOI] [PubMed] [Google Scholar]
- 14.Taylor J, Huelbes S, Albu S, Gomez-Soriano J, Penacoba C, Poole HM.. Neuropathic pain intensity, unpleasantness, coping strategies, and psychosocial factors after spinal cord injury: an exploratory longitudinal study during the first year. Pain Med 2012;13(11):1457–68. doi: 10.1111/j.1526-4637.2012.01483.x [DOI] [PubMed] [Google Scholar]
- 15.Heutink M, Post MW, Luthart P, Schuitemaker M, Slangen S, Sweers J, et al. Long-term outcomes of a multidisciplinary cognitive behavioural programme for coping with chronic neuropathic spinal cord injury pain. J Rehabil Med 2014;46(6):540–5. doi: 10.2340/16501977-1798 [DOI] [PubMed] [Google Scholar]
- 16.Burns AS, Delparte JJ, Ballantyne EC, Boschen KA.. Evaluation of an interdisciplinary program for chronic pain after spinal cord injury. PM R 2013;5(10):832–8. doi: 10.1016/j.pmrj.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J Spinal Cord Med 2007;30(5):421–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raichle KA, Osborne TL, Jensen MP, Cardenas D.. The reliability and validity of pain interference measures in persons with spinal cord injury. J Pain 2006;7(3):179–86. doi: 10.1016/j.jpain.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 19.Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research: report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J Spinal Cord Med 2007;30(5):421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleeland CS, Ryan KM.. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23(2):129–38. [PubMed] [Google Scholar]
- 21.Widerstrom-Noga EG, Cruz-Almeida Y, Martinez-Arizala A, Turk DC.. Internal consistency, stability, and validity of the spinal cord injury version of the multidimensional pain inventory. Arch Phys Med Rehabil 2006;87(4):516–23. doi: 10.1016/j.apmr.2005.12.036 [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Almeida Y, Alameda G, Widerstrom-Noga EG.. Differentiation between pain-related interference and interference caused by the functional impairments of spinal cord injury. Spinal Cord 2009;47(5):390–5. doi: 10.1038/sc.2008.150 [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Chang CH.. A discussion of item response theory and its applications in health status assessment. Med Care 2000;38(9 Suppl):II66–72. [DOI] [PubMed] [Google Scholar]
- 24.Fries JF, Bruce B, Cella D.. The promise of PROMIS: using item response theory to improve assessment of patient-reported outcomes. Clin Exp Rheumatol 2005;23(5 Suppl 39):S53–7. [PubMed] [Google Scholar]
- 25.Bjorner JB, Chang CH, Thissen D, Reeve BB.. Developing tailored instruments: item banking and computerized adaptive assessment. Qual Life Res 2007;16 (Suppl 1):95–108. doi: 10.1007/s11136-007-9168-6 [DOI] [PubMed] [Google Scholar]
- 26.Chakravarty EF, Bjorner JB, Fries JF.. Improving patient reported outcomes using item response theory and computerized adaptive testing. J Rheumatol 2007;34(6):1426–31. [PubMed] [Google Scholar]
- 27.Thissen D, Reeve BB, Bjorner JB, Chang CH.. Methodological issues for building item banks and computerized adaptive scales. Qual Life Res 2007;16 (Suppl 1):109–19. doi: 10.1007/s11136-007-9169-5 [DOI] [PubMed] [Google Scholar]
- 28.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012;78(23):1860–7. doi: 10.1212/WNL.0b013e318258f744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gershon RC, Lai JS, Bode R, Choi S, Moy C, Bleck T, et al. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual Life Res 2012;21(3):475–86. doi: 10.1007/s11136-011-9958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tulsky DS, Kisala PA, Victorson D, Choi SW, Gershon R, Heinemann AW, et al. Methodology for the development and calibration of the SCI-QOL item banks. J Spinal Cord Med 2015;38(3):270–87. doi: 10.1179/2045772315Y.0000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulsky DS, Kisala PA.. The Spinal Cord Injury - Quality of Life (SCI-QOL) measurement system: development, psychometrics, and item bank calibration. J Spinal Cord Med 2015;38(3):251–6. doi: 10.1179/2045772315Y.0000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150(1):173–82. doi: 10.1016/j.pain.2010.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulsky DS, Kisala PA, Victorson D, Tate D, Heinemann AW, Amtmann D, et al. Developing a contemporary patient-reported outcomes measure for spinal cord injury. Arch Phys Med Rehabil 2011;92(10 Suppl):S44–51. doi: 10.1016/j.apmr.2011.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tulsky DS, Jette AM, Kisala PA, Kalpakjian C, Dijkers MP, Whiteneck G, et al. Spinal cord injury-functional index: item banks to measure physical functioning in individuals with spinal cord injury. Arch Phys Med Rehabil 2012;93(10):1722–32. doi: 10.1016/j.apmr.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jette AM, Slavin MD, Ni P, Kisala PA, Tulsky DS, Heinemann AW, et al. Development and initial evaluation of the SCI-FI/AT. J Spinal Cord Med 2015;38(3):409–18. doi: 10.1179/2045772315Y.0000000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinemann AW, Dijkers MP, Ni P, Tulsky DS, Jette A.. Measurement properties of the Spinal Cord Injury-Functional Index (SCI-FI) short forms. Arch Phys Med Rehabil 2014;95(7):1289–97 e5. doi: 10.1016/j.apmr.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jette AM, Tulsky DS, Ni P, Kisala PA, Slavin MD, Dijkers MP, et al. Development and initial evaluation of the spinal cord injury-functional index. Arch Phys Med Rehabil 2012;93(10):1733–50. doi: 10.1016/j.apmr.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 39.Kisala PA, Tulsky DS, Choi SW, Kirshblum SC.. Development and psychometric characteristics of the SCI-QOL Pressure Ulcers scale and short form. J Spinal Cord Med 2015;38(3):303–14. doi: 10.1179/2045772315Y.0000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tulsky DS, Kisala PA, Tate DG, Spungen AM, Kirshblum SC.. Development and psychometric characteristics of the SCI-QOL Bladder Management Difficulties and Bowel Management Difficulties item banks and short forms and the SCI-QOL Bladder Complications scale. J Spinal Cord Med 2015;38(3):288–302. doi: 10.1179/2045772315Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Victorson D, Tulsky DS, Kisala PA, Kalpakjian CZ, Weiland B, Choi SW.. Measuring resilience after spinal cord injury: development, validation and psychometric characteristics of the SCI-QOL Resilience item bank and short form. J Spinal Cord Med 2015;38(3):366–76. doi: 10.1179/2045772315Y.0000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalpakjian CZ, Tulsky DS, Kisala PA, Bombardier CH.. Measuring grief and loss after spinal cord injury: development, validation and psychometric characteristics of the SCI-QOL Grief and Loss item bank and short form. J Spinal Cord Med 2015;38(3):347–55. doi: 10.1179/2045772315Y.0000000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalpakjian CZ, Tate DG, Kisala PA, Tulsky DS.. Measuring self-esteem after spinal cord injury: development, validation and psychometric characteristics of the SCI-QOL Self-esteem item bank and short form. J Spinal Cord Med 2015;38(3):377–85. doi: 10.1179/2045772315Y.0000000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kisala PA, Victorson D, Pace N, Heinemann AW, Choi SW, Tulsky DS.. Measuring psychological trauma after spinal cord injury: development and psychometric characteristics of the SCI-QOL Psychological Trauma item bank and short form. J Spinal Cord Med 2015;38(3):326–34. doi: 10.1179/2045772315Y.0000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kisala PA, Tulsky DS.. Opportunities for CAT applications in medical rehabilitation: development of targeted item banks. J Appl Meas 2010;11(3):315–30. [PMC free article] [PubMed] [Google Scholar]
- 46.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cella D, Nowinski C, Peterman A, Victorson D, Miller D, Lai JS, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil 2011;92(10 Suppl):S28–36. doi: 10.1016/j.apmr.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amtmann D, Cook KF, Johnson KL, Cella D.. The PROMIS initiative: involvement of rehabilitation stakeholders in development and examples of applications in rehabilitation research. Arch Phys Med Rehabil 2011;92(10 Suppl):S12–9. doi: 10.1016/j.apmr.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willis GB. Cognitive interviewing: a “how to” guide. Research Triangle Park, NC: Research Triangle Institute; 1999. [Google Scholar]
- 50.Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks, CA: Sage; 2005. doi: 10.4135/9781412983655 [DOI] [Google Scholar]
- 51.Eremenco SL, Cella D, Arnold BJ.. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof 2005;28(2):212–32. doi: 10.1177/0163278705275342 [DOI] [PubMed] [Google Scholar]
- 52.MetaMetrics The LEXILE framework for reading. Durham, NC: MetaMetrics, Inc.; 1995. [Google Scholar]
- 53.Tulsky DS, Kisala PA, Victorson D, Tate DG, Heinemann AW, Charlifue S, et al. Overview of the Spinal Cord Injury - Quality of Life (SCI-QOL) measurement system. J Spinal Cord Med 2015;38(3):257–69. doi: 10.1179/2045772315Y.0000000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baylor C, Hula W, Donovan NJ, Doyle PJ, Kendall D, Yorkston K.. An introduction to item response theory and Rasch models for speech-language pathologists. Am J Speech Lang Pathol 2011;20:243–259. doi: 10.1044/1058-0360(2011/10-0079) [DOI] [PubMed] [Google Scholar]
- 56.Bjorner JB, Smith K, Stone C, Sun X.. IRTFIT: A Macro for Item Fit and Local Dependence Tests under IRT Models. 2007. [Google Scholar]
- 57.Choi SW, Gibbons LE, Crane PK.. lordif: an R package for detecting differential item functioning using iterative hybrid ordinal logistic regression/item response theory and Monte Carlo simulations. J Stat Softw 2011;39(8):1–30. doi: 10.18637/jss.v039.i08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stocking ML, Lord FM.. Developing a common metric in item response theory. Appl Psychol Meas 1983;7(2):201–10. doi: 10.1177/014662168300700208 [DOI] [Google Scholar]
- 59.Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W.. The development of a clinical outcomes survey research application: assessment center. Qual Life Res 2010;19(5):677–85. doi: 10.1007/s11136-010-9634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi SW, Reise SP, Pilkonis PA, Hays RD, Cella D.. Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Qual Life Res 2010;19(1):125–36. doi: 10.1007/s11136-009-9560-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haley SM, Ni P, Hambleton RK, Slavin MD, Jette AM.. Computer adaptive testing improved accuracy and precision of scores over random item selection in a physical functioning item bank. J Clin Epidemiol 2006;59(11):1174–82. doi: 10.1016/j.jclinepi.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 63.Hays RD, Morales LS, Reise SP.. Item response theory and health outcomes measurement in the 21st century. Med Care 2000;38(9 Suppl):II28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Revicki D, Cella D.. Health status assessment for the twenty-first century: item response theory, item banking and computer adaptive testing. Qual Life Res 1997;6(6):595–600. doi: 10.1023/A:1018420418455 [DOI] [PubMed] [Google Scholar]
- 65.Khanna D, Maranian P, Rothrock N, Cella D, Gershon R, Khanna PP, et al. Feasibility and construct validity of PROMIS and “legacy” instruments in an academic scleroderma clinic. Value Health 2012;15(1):128–34. doi: 10.1016/j.jval.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senders A, Hanes D, Bourdette D, Whitham R, Shinto L.. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Mult Scler 2014;20(8):1102–11. doi: 10.1177/1352458513517279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN.. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma 2014;28(8):439–43. doi: 10.1097/BOT.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 68.Hanley MA, Masedo A, Jensen MP, Cardenas D, Turner JA.. Pain interference in persons with spinal cord injury: classification of mild, moderate, and severe pain. J Pain 2006;7(2):129–33. doi: 10.1016/j.jpain.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 69.Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain 2011;152(9):2098–107. doi: 10.1016/j.pain.2011.05.016 [DOI] [PubMed] [Google Scholar]
- 70.Dahl J, Wilson KG, Nilsson A.. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: a preliminary randomized trial. Behav Ther 2004;35(4):785–801. doi: 10.1016/S0005-7894(04)80020-0 [DOI] [Google Scholar]


