Abstract
Objectives
To determine factors associated with functional status six months following a traumatic cervical and thoracic spinal cord injury (SCI), with a particular interest in factors related to the acute care hospitalization stay.
Design
This is a prospective cohort study. Sixteen potential predictive variables were studied. Univariate regression analyses were first performed to determine the strength of association of each variable independently with the total Spinal Cord Independence Measure (SCIM) score. Significant ones were then included in a General linear model in order to determine the most relevant predictive factors among them. Analyses were carried out separately for tetraplegia and paraplegia.
Setting
A single specialized Level I trauma center.
Participants
One hundred fifty-nine patients hospitalized for an acute traumatic SCI between January 2010 and February 2015.
Interventions
Not applicable.
Main outcome measure
The SCIM (version 3) functional score.
Results
Motor-complete SCI (AIS-A,B) was the main predictive factor associated with decreased total SCIM score in tetraplegia and paraplegia. Longer acute care length of stay and the occurrence of acute medical complications (either pneumonia, urinary tract infections or pressure ulcers) were predictors of decreased functional outcome following tetraplegia, while increased body mass index and higher trauma severity were predictive of decreased functional outcome following paraplegia.
Conclusions
This study supports previous work while adding information regarding the importance of optimizing acute care hospitalization as it may influence chronic functional status following traumatic SCI.
Keywords: Spinal cord injuries, Prediction, Function, Acute, Trauma
Introduction
The occurrence of traumatic spinal cord injury (T-SCI) may be devastating as it is associated with significant permanent functional disabilities. Prediction of function is important after a T-SCI in order to improve patient's care, plan rehabilitation and better optimize resources utilization. However, reliably predicting functional outcome following acute SCI remains difficult. Failure to consider various clinical factors influencing the acute care hospitalization and to underline the most relevant factors among them may contribute to that issue.
Previous studies agree that the severity of the T-SCI at initial presentation is the main factor associated with neurologic and functional outcomes, with complete SCI predicting worse outcome.1–5 The impact of other clinical and socio-demographic characteristics, such as the level of the SCI or age, is debated.1,2,5,6 While most predictive factors of functional recovery following SCI are non-modifiable, potential modifiable predictors, such as clinical events occurring during the course of the acute care hospitalization may be of importance. In addition, the surgical planning,7–11 the development of early spasticity,12,13 the occurrence of medical complications and the acute care length of stay (LOS)14 were suggested to influence the rehabilitation process and/or the neurological recovery. However, there is no study to date that has considered factors related to the acute care hospitalization process in a prediction model of functional outcome.
Previous studies predicting functional recovery are based on general functional outcome scales, such as the Functional Independence Measure (FIM) or the Glasgow Outcome Scale (GOS).1,4,15,16 Unfortunately, these instruments were not designed for evaluating individuals sustaining T-SCI. The Spinal Cord Independence Measure (SCIM) was created to specifically assess functional outcome in individuals with SCI17 and is more sensitive to change as compared to the FIM scale.17 The SCIM scale is now widely used and has demonstrated its consistent reliability, consistency and sensitivity to change.17
The purpose of this study was to determine the impact of various socio-demographic and clinical characteristics collected during the acute care hospitalization on functional recovery after a T-SCI, as measured by the total SCIM score. Because tetraplegia and paraplegia may be associated with distinct outcome predictors, analyses were performed separately.
Methods
Patients
This study consisted in a review of a prospective database collected in a single Level-1 trauma center specialized in spinal cord injury (SCI) care. A total of 159 adult patients with acute T-SCI from C1 to L1 consecutively admitted between January 2010 and February 2015 (126 males and 33 females; 46.2±20.0 years old) were included. Patients without overt spinal instability or central cord syndrome were excluded because these individuals typically present distinct outcome. This study was approved by the institutional review board and all patients were enrolled on a voluntary basis during the acute hospitalization. Patients were included in the study if they were seen at the routine follow-up visit planned 6 months after the trauma. Data collection was performed by researcher assistants not involved in the present study.
Data collection
Information pertaining to the age, sex, body mass index (BMI), trauma severity measured by the Injury Severity Score (ISS), presence of a high velocity trauma (defined as the occurrence of a SCI in the context of any motor vehicle accident), as well as presence of a concomitant traumatic brain injury (TBI) were collected. The ISS is a simple method describing patients with multiple traumatic injuries. It corresponds to an anatomical scoring system where each injury is assigned to a specific score according to its severity and location. The ISS takes values from 0 to 75.18 The presence of moderate and severe TBI was also specifically noted. The severity of the traumatic brain injuries (TBI) was based on the Glasgow Coma Scale (GCS) in the first 48 hours following the injury. A GCS score of 9 to 12 refers to moderate TBI, while a GCS of 3 to 8 refers to severe TBI.
The neurologic evaluation was performed based on the recommendation of the American Spinal Cord Injury Association (ASIA) upon admission for all patients and was characterized using the neurologic level of the injury (NLI) defined as the most caudal level with preserved normal sensation and motor function. Then, the NLI was dichotomized for tetraplegia as high (C1 to C4) vs. low cervical (C5 to T1) and for paraplegia as high (T2–T7) vs. low thoracic/lumbar (T8–L1). The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) was used to determine the severity of the SCI and was dichotomized as motor-complete (AIS-A or B) or incomplete (AIS-C or D) injury. The ISNCSCI motor score was also noted, with a higher score designating higher motor strength.19
Clinical factors collected during the course of acute care hospitalization were also collected. First, the occurrence of non-neurological complications (pneumonias, urinary tract infections (UTI) and pressure ulcers (PU)) was noted, since they are the most prevalent complications occurring after a T-SCI.10 Pneumonia was diagnosed using clinical features and confirmed by a radiologist using chest X-rays.20 UTI were diagnosed using criteria from the 2006 Consortium for Spinal Cord Medicine Guidelines for healthcare providers;21 and PU were diagnosed using clinical guidelines defined by the National Pressure Ulcer Advisory Panel (NPUAP).22 The occurrence of any of these complications during the acute care hospitalization as well as the occurrence of multiple complications (two or more) was noted.
Then, the development of spasticity during the course of acute care hospitalization also was noted based on physical findings and symptoms reported by the patient,23,24 and required two of the following three criteria: 1) presence of increased velocity-dependant muscle tone at physical examination (Modified Ashworth scale score >1), 2) spasm and/or clonus noted at physical examination, and 3) spasm and/or clonus reported by the patient. The acute care LOS was defined as the number of days between admission and discharge from the acute care center. Finally, the delay of surgery designated the interval of time between the injury and time of incision (in hours) and was dichotomized into early (<24h post-trauma) and late surgery (≥24h post-trauma).
Outcome variables
The functional outcome corresponds to the primary outcome in this study and was evaluated six months after the trauma using the Spinal Cord Independence Measure Scale (SCIM, version III).17 The SCIM evaluates three different areas of function: self-care (subscore 0–20), respiration and sphincter management (0–40) and mobility and transfers (0–40). The total score can reach 100 points with a higher score corresponding to a higher level of autonomy.
Analysis
IBM SPSS Statistics Version 19 software package (IBM Corp., Armonk, NY, USA) was used for our statistical analyses. Our cohort was described using means ± standard deviation for continuous variables, and proportions or percentages for categorical variables.
All analyses were performed separately for individuals sustaining tetraplegia and paraplegia regardless of the level of the injury. Independent variables initially considered as potential outcome predictors are showed in Table 1. Univariate linear regression analyses were used to determine the strength of association between each independent variable and the total SCIM score (dependant variable), in order to reduce the number of variables to a smaller and relevant subset of outcome predictors to be introduced into the prediction model. Considering the high number of tests performed at this preliminary step, a level of significance was set at 0.1. Considering that the reduced set of independent variables could contain collinear variables, Pearson correlations were used following the univariate regression analyses, and collinearity was confirmed when a level of significance of 0.7 was reached. In the presence of collinearity between two independent variables, the variable with the smallest P-value from the univariate regression analyses was included in the General linear model (GLM) as a potential predictor of the total SCIM score.25 Independent variables that were finally included in each GLMs (for paraplegia and tetraplegia) are indicated by an “x” in Table 1. The association between the independent variables and the total SCIM score in the GLM was expressed in terms of beta (β) coefficients with 95% confidence interval (CI), and the R2 was used as an indicator of the percentage of the variability explained by each model.
Table 1.
Potential predictive variable associated with function six-months posttraumatic SCI.
| Input variable for multivariate analysis | |||
|---|---|---|---|
| Potential predictive variable | Tetraplegia | Paraplegia | Coding |
| 1. Surgical delay | <24h post-trauma | ||
| >24h post-trauma | |||
| 2. Early spasticity | x | x | Presence or not |
| 3. Sex | Male or female | ||
| 4. Age | As continuous data | ||
| 5. Body mass index | x | As continuous data | |
| 6. Smoking status | Active smoker | ||
| Past or non-smoker | |||
| 7. Mechanism of traumatic injury | High-velocity trauma | ||
| Non-high velocity trauma | |||
| 8. Occurrence of medical complications | x | Presence or not | |
| 9. Occurrence of multiple complications | Presence or not | ||
| 10. Initial ASIA Impairment Scale (AIS) grade | x | x | AIS grade A or B; no motor function is preserved in the sacral segments |
| AIS grade C or D; motor function is preserved below the neurological level | |||
| 11. Initial ASIA motor score | As continuous data | ||
| 12. Acute care LOS | x | As continuous data | |
| 13. Presence of TBI | Presence or not | ||
| 14. Presence of moderate or severe TBI | Presence or not | ||
| 15. Initial neurologic level of the injury | High level | ||
| Tetraplegia: C1 to C4 | |||
| Paraplegia: T2 to T7 | |||
| Low level | |||
| Tetraplegia: C4 to T1 | |||
| Paraplegia: T8 to L1 | |||
| 16. Injury severity score (ISS) | x | Continuous data | |
ASIA, American Spinal Injury Association; TBI, traumatic brain injury; LOS, length of stay;
“x” indicates that this variable was included in the multivariate linear analysis.
Results
From the 159 patients initially enrolled in our study, 71 did not come to their 6-month follow-up or withdrew from the study. Thus, a total of 88 patients were included in our analyses (Fig. 1), including 43 patients with tetraplegia and 45 patients with paraplegia. Table 2 presents the socio-demographic and clinical characteristics of patients with tetraplegia and paraplegia. Considering the high number of patients excluded from the study due to missing 6-month follow-up, comparisons were made between included and excluded patients to ensure that their baseline characteristics were similar, and rule out the presence of a major selection bias (Table 3).
Figure 1.
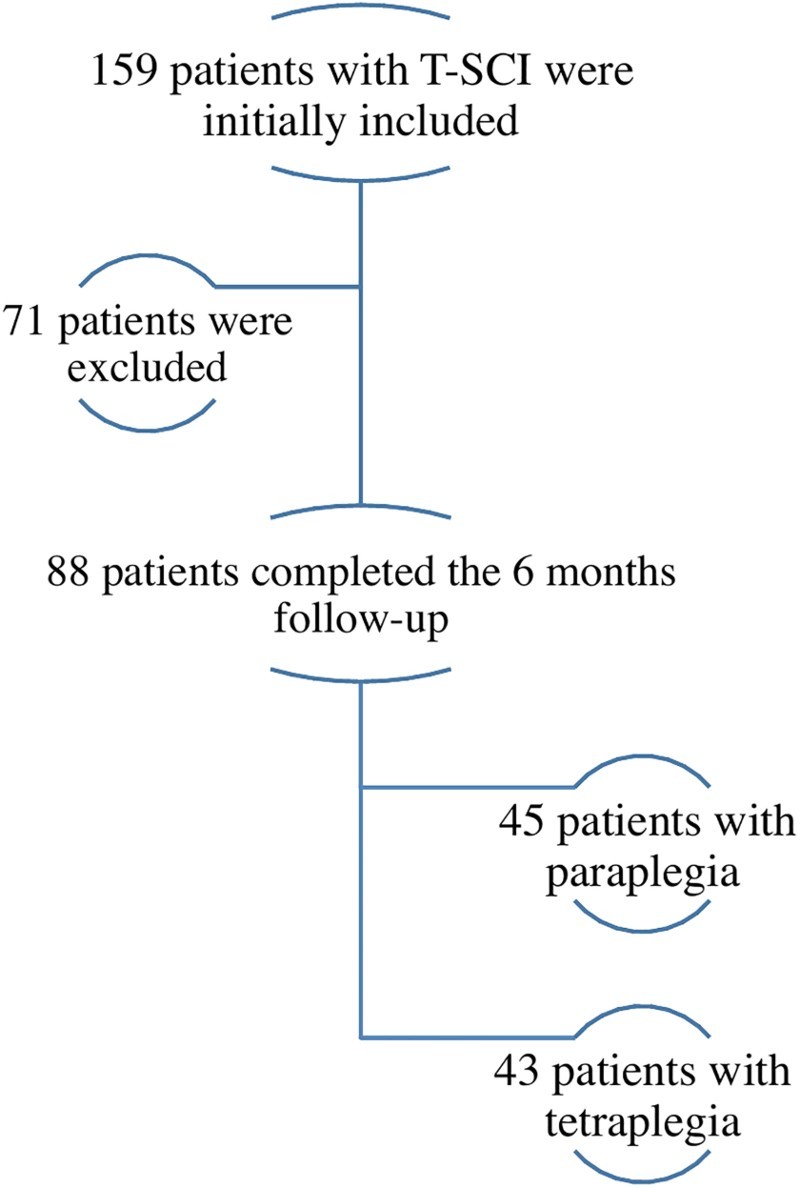
Description of the inclusion process of patients in this prospective study.
Table 2.
Socio-demographic and clinical characteristics at hospital admission for individuals with tetraplegia and paraplegia (N=88).
| Tetraplegia | Paraplegia | |
|---|---|---|
| Characteristics | N=43 | N=45 |
| ASIA grade | ||
| AIS-A,B | 65,1% | 82.2% |
| AIS-C,D | 34.9% | 17.8% |
| Neurologic level | ||
| High tetraplegia (C1-C4) | 39.5% | — |
| Low tetraplegia (C5-T1) | 60.5% | — |
| High paraplegia (T2-T7) | – | 22.2% |
| Low paraplegia (T8-L1) | – | 77.8% |
| ASIA motor score (mean +/-SD) | 38.1 (30.1) | 59.0 (16.7) |
| Age (mean +/-SD) | 44.3 (17.2) | 40.0 (15.6) |
| Sex (% Male) | 74.4% | 86.7% |
| ISS (mean +/-SD) | 25.7 (14.1) | 27.2 (7.7) |
| BMI (mean +/-SD) | 27.4 (10.2) | 25.5 (4.0) |
| Presence of TBI | 53.5% | 37.8% |
| Presence of moderate or severe TBI | 2.3% | 6.7% |
| Early surgery (<24h post-trauma) | 97.7% | 97.8% |
| Acute care LOS (in days) (mean +/-SD) | 32.7 (26.0) | 27.9 (16.8) |
| Presence of medical complications | 58.5% | 40.0% |
| Pneumonia | 37.2% | 20.0% |
| Urinary tract infection | 23.3% | 15.6% |
| Pressure ulcer | 30.2% | 20.0% |
| Presence of multiple complications | 23.3% | 15.6% |
| Presence of early spasticity | 74.4% | 48.9% |
| Smoking status (% active smoker) | 25.6% | 31.1% |
| High-velocity trauma mechanism | 41.9% | 33.3% |
ISS, injury severity score; BMI, body mass index; TBI, traumatic brain injury; LOS, length of stay.
Table 3.
Comparison of socio-demographic and clinical characteristics at hospital admission between individuals that have and have not completed follow-up six-months post injury (N=164).
| Characteristics | Patients with 6 months FU N=88 |
Patients excluded N=71 |
P-value |
|---|---|---|---|
| ASIA grade | |||
| AIS-A,B | 73.9% | 61.4% | 0.12 |
| AIS-C,D | 26.1% | 38.6% | |
| Neurologic level | |||
| High tetraplegia (C1-C4) | 19.3% | 26.8% | 0.34 |
| Low tetraplegia (C5-T1) | 29.5% | 31.0% | 0.86 |
| High paraplegia (T2-T7) | 11.4% | 9.9% | 0.80 |
| Low paraplegia (T8-L1) | 39.8% | 32.4% | 0.41 |
| ASIA motor score (mean +/-SD) | 49.2 (26.0) | 51.1 (26.0) | 0.99 |
| Age (mean +/-SD) | 42.1 (16.5) | 51.2 (22.7) | <10-3* |
| Sex (% Male) | 80.7% | 77.5% | 0.70 |
| ISS (mean +/-SD) | 26.5 (11.1) | 26.3 (10.7) | 0.83 |
| BMI (mean +/-SD) | 26.4 (7.7) | 26.8 (5.8) | 0.99 |
| Presence of TBI | 45.5% | 54.9% | 0.27 |
| Presence of moderate or severe TBI | 4.5% | 1.4% | 0.38 |
| Early surgery (<24h post-trauma) | 100% | 97.7% | 0.50 |
| Acute care LOS (in days) (mean +/-SD) | 30.2 (21.8) | 35.4 (30.1) | 0.07 |
| Presence of medical complications | 53.2% | 46.8% | 0.63 |
| Presence of multiple complications | 19.3% | 16.9% | 0.84 |
| Presence of early spasticity | 61.4% | 67.8% | 0.49 |
| Smoking status (% active smoker) | 31.3% | 22.6% | 0.26 |
| High-velocity trauma mechanism | 37.5% | 29.6% | 0.32 |
ISS, injury severity score; BMI, body mass index; TBI, traumatic brain injury; LOS, length of stay.
Prediction of function for patients with tetraplegia
Four potential predictive factors were included in the GLM (Table 1): AIS grade, occurrence of complications, presence of early spasticity and LOS. The three following variables were excluded from the GLM for collinearity issue: presence of multiple complications, AIS motor score and the ISS. In the end, motor-complete SCI (AIS A or B), the occurrence of complications and longer acute care hospitalization stay were significantly associated with a decreased total SCIM score (Table 4). This model explained 67 percent of the variability of the total SCIM score (R2=0.671).
Table 4.
Factors associated with the total SCIM score six-months post injury for patients with acute traumatic tetraplegia (N=43).
| Total SCIM score | |||
|---|---|---|---|
| Predictive variable | β coefficient | 95%CI | P-value |
| ASIA grade | |||
| AIS A-B | –27.3 | (–42.9; –11.8) | <10-3* |
| AIS C-D | 0d | ||
| Occurrence of complications | –22.7 | (–37.6; –7.8) | <10-3* |
| Acute care LOS | –0.3 | (–0.6; –0.1) | 0.02* |
| Presence of early spasticity | –2.5 | (–19.3; 14.3) | 0.77 |
| R2= 0.671 | |||
0d Reference category.
ASIA, American Spinal Injury Association.
LOS, length of stay.
Prediction of function for patients with paraplegia
Four independent variables were included in the GLM (Table 1): the AIS grade, BMI, trauma severity (ISS) and presence of early spasticity based on the simple regression linear analyses. The AIS motor score was excluded because of its collinearity with the AIS grade. Motor-complete SCI (AIS A or B), higher BMI and ISS were significantly associated with a decreased total SCIM score (Table 5). This model explained nearly 55 percent of the variability of the total SCIM score (R2=0.548).
Table 5.
Factors associated with the total SCIM score six-months post injury for patients with acute traumatic paraplegia (N=45).
| Total SCIM score | |||
|---|---|---|---|
| Predictive variable | β coefficient | 95%CI | P-value |
| ASIA grade | |||
| AIS A-B | –19.1 | (–31.3; –6.9) | <10-3* |
| AIS C-D | 0d | ||
| BMI | –1.3 | (–2.3; –0.4) | <10-3* |
| ISS | –0.8 | (–1.4; –0.2) | 0.01* |
| Presence of early spasticity | –6.3 | (–13.9; 1.4) | 0.11 |
| R2= 0.548 | |||
0d Reference category.
ASIA, American Spinal Injury Association.
BMI, body mass index.
ISS, injury severity score.
Discussion
Health professionals working with individuals sustaining SCI should benefit from early identification of predictors of mid to long-term function to allow better communication with the patient and its relatives, promote efficient coordinated care and optimize resources utilization. This study identified relevant acute clinical factors associated with function six-months after a T-SCI, accounting for various factors specific to individuals sustaining tetraplegia and paraplegia during acute care hospitalization.
The severity of the SCI remains the most important acute factor associated with chronic functional outcome following a cervical or thoracic SCI (Tables 4 and 5). The association of motor-complete SCI with total SCIM score was particularly strong, as shown by the beta coefficients in both models. This finding further supports previous work1,5,16 suggesting that a motor-complete SCI predicts limited neurological recovery,26 thereby leading to worst functional outcome.2,3
The occurrence of medical complications most frequently associated with T-SCI (pneumonia, UTI and PU) during the course of acute care hospitalization was also strongly associated with functional outcome six-months following tetraplegia. According to Table 2, pneumonias were the most frequent complication in this group. The occurrence of pneumonia may prolonged the intensive care stay, interfere the rehabilitation process and delay the mechanical weaning process. It is recognized that the occurrence these complications in chronic SCI may interfere with the physical and social well-being.27 But this study also suggests that the occurrence of medical complications during the acute phase may still influence the functional outcome as far as six-months post injury. Delay of the rehabilitation process and community reintegration may be possible consequences of acute care complications occurrence,28 particularly given that it also predisposes individuals with SCI at higher risk of chronic recurrences.29 However, it was not revealed as a predictive factor of function following paraplegia. Two hypotheses may be proposed to explain this. First, previous studies have suggested that individuals sustaining tetraplegia may suffer from a higher number and increased severity of complications compared to patients with paraplegia,30–33 which could further limit their functional recovery. However, although severity of complications was not assessed in this study, additional analysis did not revealed any difference between in the number of complications between the two groups (P-values of 0.1, 0.4 and 0.3 for pneumonia, urinary tract infection and pressure ulcer respectively). Then, it is possible that the timing of follow-up may explain our results. Indeed, as individuals with tetraplegia generally required longer acute care and inpatient rehabilitation hospitalization stay compared to paraplegic patients,34,35 any significant delay in the process (such as the occurrence of medical complications) could therefore have underestimate functional results collected only six-months post-injury. It is therefore possible that a prolonged follow-up up to a point where the functional rehabilitation would be completed for all patients with tetraplegia (e.g. at one year post-injury) would negate the impact of acute care medical complications on function. Nevertheless, early pro-active management towards the prevention of secondary conditions following SCI should not be overlooked. As acute care specialized SCI-centers were showed to decrease the number and severity of complications,36 prompt transfer to SCI-centers, particularly following motor-complete tetraplegia, is recommended.
Longer acute care LOS was revealed as a significant factor associated with decreased total SCIM score following tetraplegia. However, describing the causal effect of longer acute care hospitalization is tenuous as many confounding factors may interfere. Indeed, various variables such as the severity of the SCI, age, trauma severity, the occurrence of medical complications and surgical timing are some of the factors influencing the acute care LOS.37–39 However, since these variables showed a weak correlation with the LOS, we might suggest that efficient transfer to inpatient rehabilitation facility following tetraplegia may optimize the long-term functional recovery independently of the factors studied in the present study, except for the trauma severity (ISS) which was significantly correlated (collinear) to the acute care LOS. But trauma severity was excluded from the general linear model because of its smaller significance with the outcome variable following the simple linear regression analysis. Therefore, higher trauma severity (ISS score) should be also considered as a potential factor associated with prolonged acute care LOS. Again, one efficient way to optimize the acute care LOS is early referral to a specialized SCI acute care center as shown in previous studies.36,40
While it is assumed that spasticity can alter functional outcome, it remains unproven.13 Spasticity could potentially compensate for muscle weakness and ease mobility, but it can also interfere with movement, posture, sleeping, may be associated to pain and/or fatigue. Development of spasticity during the acute care stay was significantly associated with decreasing SCIM score in the univariate regression analyses, but it was not associated with the functional outcome when accounting for other covariates in our multivariate regression analyses, as showed in Table 4 and 5. However, the severity of the spasticity was not taken into account in this study, and investigating the association between the severity of spasticity and function should be addressed in a future study.
Increased BMI significantly decreased functional recovery in paraplegia, but not in tetraplegia (Tables 4 and 5). Overweight or obesity may represent an additional challenge for mobility and accomplishing activities of daily living. It is possible that BMI affects functional outcome specifically in patients with paraplegia as an increased body weight could limit the optimal use of upper extremities in tasks such as transfers, wheelchair propulsion or the use of technical aids. Moreover, obesity may increase respiratory dysfunction associated with SCI by aggravating restrictive pulmonary syndrome,41 which in turn can alter general function. However, this variable had only a lower impact on the model as shown by its beta coefficient.
Finally, higher trauma severity (increased ISS) was significantly associated with decreased total SCIM score following paraplegia. Associated injuries may be associated with additional invasive treatments and functional limitations, which can delay rehabilitation and alter the functional recovery 6 months after the injury. Since the beta coefficient associated with trauma severity was relatively low for paraplegia and non significant for tetraplegia, it would also be interesting to assess the impact of ISS on function at later stage (1 year or more after injury), once all associated injuries have reached a chronic phase.
Study limitations
There are recognized limitations associated with this study. First, there was a significant loss to follow-up at 6 months. However, as shown in Table 3, baseline characteristics of patients lost to follow-up were similar to those completing the study, except for age. In addition to the SCI, older age is typically associated with decreased mobility, which may explain the difficulty to comply with scheduled postoperative visits for patients not seen at the 6-month follow-up. However, an interim analyses of 41 patients of the missing patients at 6 months but seen later at one year post-injury showed that the results were similar, suggesting that there was no significant selection bias in the current study. The interval of six months was chosen in the present study as the vast majority of the recovery was shown to occur within the first three months following tetraplegia3 and generally reaches a plateau around six months post-injury to slow down thereafter2,3,42 and subsequently, the intensive functional rehabilitation is generally advanced or completed at this time.43 However, a future study evaluating predictors of functional outcome 12 months post injury will be done as soon as follow-up of patients will be completed.
Then, criteria used in the present study to define the occurrence of spasticity can be debated. Because the definition of spasticity and the agreement on clinical scales of spasticity vary widely, there is no reliable instrument to measure spasticity available. Although our criteria were based on the recent spasticity literature in terms of clinical measurement of spasticity23,44 and the importance of patient's perception,24 strong validation studies are still lacking. Types of medical complications considered in this study are relatively small. Authors recognized that other complications and secondary conditions related or not to the SCI may have also influence outcome following SCI. Finally, a future study should investigate factors associated with functional outcome in individuals with central cord syndrome and without spinal instability since they were excluded from this study.
Conclusions
By using a specific functional outcome scale (SCIM scale) and by including various acute clinical factors potentially influencing the outcome, this study identifies relevant clinical predicting factors of functional outcome 6 months after the T-SCI causing tetraplegia and paraplegia. The severity of the SCI (ISNCSCI grade) remains the main predictive factor of global function six-months post injury regardless of the neurological level. Higher body mass index and increased burden of associated injuries (trauma severity) were predictive factors of worst functional outcome following paraplegia, while the occurrence of acute medical complications and longer acute care stay were significantly associated with worst functional outcome following tetraplegia. The optimization of acute care hospitalization may therefore significantly influence mid to long-term functional recovery and this might underline the importance of early referral to specialized SCI-centers particularly following acute traumatic cervical SCI.
Acknowledgement of presentation: This research was presented at the American Spinal Injury Association (ASIA) Annual Meeting in April 2016.
Disclaimer statements
Contributors None.
Conflict of interest None.
Ethics approval None.
Funding Statement
Department of the Army – United States Army Medical Research Acquisition Activity; MENTOR program of the Canadian Institutes of Health Research, Rick Hansen Spinal Cord Registry; Fonds de Recherche du Québec – Santé.
References
- 1.Abdul-Sattar AB. Predictors of functional outcome in patients with traumatic spinal cord injury after inpatient rehabilitation: in Saudi Arabia. NeuroRehabilitation 2014;35(2):341–7. [DOI] [PubMed] [Google Scholar]
- 2.Al-Habib AF, Attabib N, Ball J, Bajammal S, Casha S, Hurlbert RJ.. Clinical predictors of recovery after blunt spinal cord trauma: systematic review. J Neurotrauma. 2011;28(8):1431–43. doi: 10.1089/neu.2009.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. . Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007;45(3):190–205. doi: 10.1038/sj.sc.3102007 [DOI] [PubMed] [Google Scholar]
- 4.Saboe LA, Darrah JM, Pain KS, Guthrie J.. Early predictors of functional independence 2 years after spinal cord injury. Arch Phys Med Rehabil 1997;78(6):644–50. doi: 10.1016/S0003-9993(97)90431-7 [DOI] [PubMed] [Google Scholar]
- 5.Wilson JR, Cadotte DW, Fehlings MG.. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J Neurosurg Spine 2012;17(1 Suppl):11–26. doi: 10.3171/2012.4.AOSPINE1245 [DOI] [PubMed] [Google Scholar]
- 6.Furlan JC, Bracken MB, Fehlings MG.. Is age a key determinant of mortality and neurological outcome after acute traumatic spinal cord injury? Neurobiol Aging 2010;31(3):434–46. doi: 10.1016/j.neurobiolaging.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 7.Bourassa-Moreau E, Mac-Thiong JM, Li A, Ehrmann Feldman D, Gagnon DH, Thompson C, et al. . Do patients with complete spinal cord injury benefit from early surgical decompression? Analysis of neurological improvement in a prospective cohort study. J Neurotrauma 2016;33(3):301–6. [DOI] [PubMed] [Google Scholar]
- 8.van Middendorp JJ, Hosman AJ, Doi SA.. The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J Neurotrauma 2013;30(21):1781–94. doi: 10.1089/neu.2013.2932 [DOI] [PubMed] [Google Scholar]
- 9.Del Curto D, Tamaoki MJ, Martins DE, Puertas EB, Belloti JC.. Surgical approaches for cervical spine facet dislocations in adults. Cochrane Database Syst Rev 2014;10:CD008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourassa-Moreau E, Mac-Thiong JM, Feldman DE, Thompson C, Parent S.. Non-neurological outcomes after complete traumatic spinal cord injury: the impact of surgical timing. J Neurotrauma 2013;30(18):1596–601. doi: 10.1089/neu.2013.2957 [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Cao K, Huang S, Li L, Yu T, Cao C, et al. . Comparison of anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy. Eur Spine J 2015;24(8):1621–30. doi: 10.1007/s00586-015-3911-4 [DOI] [PubMed] [Google Scholar]
- 12.Bhimani R, Anderson L.. Clinical understanding of spasticity: implications for practice. Rehabil Res Pract 2014;2014:279175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. . Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005;27(1–2):2–6. doi: 10.1080/09638280400014576 [DOI] [PubMed] [Google Scholar]
- 14.Street JT, Noonan VK, Cheung A, Fisher CG, Dvorak MF.. Incidence of acute care adverse events and long-term health-related quality of life in patients with TSCI. Spine J 2015;15(5):923–32. doi: 10.1016/j.spinee.2013.06.051 [DOI] [PubMed] [Google Scholar]
- 15.Tee JW, Chan PC, Fitzgerald MC, Liew SM, Rosenfeld JV.. Early predictors of functional disability after spine trauma: a level 1 trauma center study. Spine (Phila Pa 1976) 2013;38(12):999–1007. doi: 10.1097/BRS.0b013e31828432a3 [DOI] [PubMed] [Google Scholar]
- 16.Wilson JR, Grossman RG, Frankowski RF, Kiss A, Davis AM, Kulkarni AV, et al. . A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma 2012;29(13):2263–71. doi: 10.1089/neu.2012.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A.. SCIM—spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord 1997;35(12):850–6. doi: 10.1038/sj.sc.3100504 [DOI] [PubMed] [Google Scholar]
- 18.Baker SP, O'Neill B, Haddon W Jr., Long WB.. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974;14(3):187–96. doi: 10.1097/00005373-197403000-00001 [DOI] [PubMed] [Google Scholar]
- 19.Kirshblum SC, Burns SP, Biering-Sørensen F, Donovan W, Graves DE, Jha A, et al. . International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medicine CfSC Respiratory management following spinal cord injury: a clinical practive guideline for health-care professionals. J Spinal Cord Med 2005;28:259–93. doi: 10.1080/10790268.2005.11753821 [DOI] [PubMed] [Google Scholar]
- 21.Medicine CfSC Bladder management for adults with adults with spinal cord injury: a clinical practive guideline for health-care providers. J Spinal Cord Med 2006;29(5):527–73. [PMC free article] [PubMed] [Google Scholar]
- 22.NPUAP-EPUAP I NPUAP pressure ulcer stages/categories. 2007.
- 23.Bhimani RH, Anderson LC, Henly SJ, Stoddard SA.. Clinical measurement of limb spasticity in adults: state of the science. J Neurosci Nurs 2011;43(2):104–15. doi: 10.1097/JNN.0b013e31820b5f9f [DOI] [PubMed] [Google Scholar]
- 24.Bhimani RH, McAlpine CP, Henly SJ.. Understanding spasticity from patients' perspectives over time. J Adv Nurs 2012;68(11):2504–14. doi: 10.1111/j.1365-2648.2012.05949.x [DOI] [PubMed] [Google Scholar]
- 25.Tabachnick BG, Fidell Linda S.. Using Multivariate Statistics (6th Edition): Pearson; 2012. 1024 p. [Google Scholar]
- 26.Kirshblum S, Botticello A, Lammertse DP, Marino RJ, Chiodo AE, Jha A.. The impact of sacral sensory sparing in motor complete spinal cord injury. Arch Phys Med Rehabil 2011;92(3):376–83. doi: 10.1016/j.apmr.2010.07.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Consortium for Spinal Cord Medicine Clinical Practice G Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2001;24 Suppl 1:S40–101. [DOI] [PubMed] [Google Scholar]
- 28.Houghton PE CKaCP Canadian Best Practice Guidelines for the Prevention and Management of Pressure Ulcers in People with Spinal Cord Injury. A resource handbook for Clinicians. http://www.onf.org2013.
- 29.Salzberg CA, Byrne DW, Cayten CG, van Niewerburgh P, Murphy JG, Viehbeck M.. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil 1996;75(2):96–104. doi: 10.1097/00002060-199603000-00004 [DOI] [PubMed] [Google Scholar]
- 30.Grossman RG, Frankowski RF, Burau KD, Toups EG, Crommett JW, Johnson MM, et al. . Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine 2012;17(1 Suppl):119–28. doi: 10.3171/2012.5.AOSPINE12127 [DOI] [PubMed] [Google Scholar]
- 31.Hagen EM. Acute complications of spinal cord injuries. World J Orthop 2015;6(1):17–23. doi: 10.5312/wjo.v6.i1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Consortium for Spinal Cord M Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2008;31(4):403–79. doi: 10.1080/10790268.2008.11760744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ropper AE, Neal MT, Theodore N.. Acute management of traumatic cervical spinal cord injury. Pract Neurol 2015;15(4):266–72. doi: 10.1136/practneurol-2015-001094 [DOI] [PubMed] [Google Scholar]
- 34.Information CIfH Life after traumatic spinal cord injury: From inpatient rehabilitation back to the community. Analysis in Brief 2006.
- 35.Information CIfH Inpatient rehabilitation in Canada 2004–2005. 2006.
- 36.Parent S, Barchi S, LeBreton M, Casha S, Fehlings MG.. The impact of specialized centers of care for spinal cord injury on length of stay, complications, and mortality: a systematic review of the literature. J Neurotrauma 2011;28(8):1363–70. doi: 10.1089/neu.2009.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radhakrishna M, Makriyianni I, Marcoux J, Zhang X.. Effects of injury level and severity on direct costs of care for acute spinal cord injury. Int J Rehabil Res 2014;37(4):349–53. doi: 10.1097/MRR.0000000000000081 [DOI] [PubMed] [Google Scholar]
- 38.Mac-Thiong JM, Feldman DE, Thompson C, Bourassa-Moreau E, Parent S.. Does timing of surgery affect hospitalization costs and length of stay for acute care following a traumatic spinal cord injury? J Neurotrauma 2012;29(18):2816–22. doi: 10.1089/neu.2012.2503 [DOI] [PubMed] [Google Scholar]
- 39.Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF.. Complications and costs of management of acute spinal cord injury. Paraplegia 1993;31(11):700–14. [DOI] [PubMed] [Google Scholar]
- 40.Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF.. Neurological recovery, mortality and length of stay after acute spinal cord injury associated with changes in management. Paraplegia 1995;33(5):254–62. [DOI] [PubMed] [Google Scholar]
- 41.Gater DR., Jr Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18(2):333–51, vii. doi: 10.1016/j.pmr.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 42.Ditunno JF., Jr The John Stanley Coulter Lecture. Predicting recovery after spinal cord injury: a rehabilitation imperative. Arch Phys Med Rehabil 1999;80(4):361–4. doi: 10.1016/S0003-9993(99)90270-8 [DOI] [PubMed] [Google Scholar]
- 43.Eastwood EA, Hagglund KJ, Ragnarsson KT, Gordon WA, Marino RJ.. Medical rehabilitation length of stay and outcomes for persons with traumatic spinal cord injury—1990–1997. Arch Phys Med Rehabil 1990;80(11):1457–63. doi: 10.1016/S0003-9993(99)90258-7 [DOI] [PubMed] [Google Scholar]
- 44.Fleuren JF, Voerman GE, Erren-Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, et al. . Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 2010;81(1):46–52. doi: 10.1136/jnnp.2009.177071 [DOI] [PubMed] [Google Scholar]


