Abstract
Context
Impaired balance function after a spinal cord injury (SCI) hinders performance of daily activities.
Objective
To assess the evidence on the effectiveness of task-specific training on sitting and standing function in individuals with SCI across the continuum of care.
Methods
A systematic search was conducted on literature published to June 2016 using people (acute or chronic SCI), task-specific interventions compared to conventional physical therapy, and outcome (sitting or standing balance function). The PEDro scale was used to investigate the susceptibility to bias and trial quality of the randomized controlled trials (RCTs). A standardized mean difference (SMD) was conducted to investigate the effect size for interventions with sitting or standing balance outcomes.
Results
Nineteen articles were identified; three RCTs, two prospective controlled trials, one cross-over study, nine pre-post studies and four prospective cohort studies. RCT and cross-over studies were rated from 6 to 8 indicating good quality on the PEDro scale. The SMD of task-specific interventions in sitting compared to active and inactive (no training) control groups was –0.09 (95% CI: –0.663 to 0.488) and 0.39 (95% CI: –0.165 to 0.937) respectively, indicating that the addition of task-specific exercises did not affect sit and reach test performance significantly. Similarly, the addition of BWS training did not significantly affect BBS compared to conventional physical therapy –0.36 (95% CI: –0.840 to 0.113). Task-specific interventions reported in uncontrolled trials revealed positive effects on sitting and standing balance function.
Conclusion
Few RCT studies provided balance outcomes, and those that were evaluated indicate negligible effect sizes. Given the importance of balance control underpinning all aspects of daily activities, there is a need for further research to evaluate specific features of training interventions to improve both sitting and standing balance function in SCI.
Keywords: Gait, Postural balance, Rehabilitation, Review, Spinal cord injuries
The ability to keep the center of mass over the base of support is pivotal to any motor task;1,2 indeed, our ability to maintain balance under different postural constraints underlies our fundamental ability to perform functional activities in the home and community.3 In people with spinal cord injury (SCI), the changes in sensation, loss of muscle strength, and the presence of spasticity amongst other pathological changes, can severely impair postural control and balance not only in standing but also in sitting, leading to difficulties with the most basic daily activities such as grooming, dressing, and transfers.4 Therefore, rehabilitation interventions that can promote improvements in sitting and/or standing balance are essential for achieving basic functional goals in people with SCI.
The central nervous system maintains balance by integrating information from visual, vestibular and sensorimotor systems.1 Greater understanding of the principles underlying the neural control of movement has resulted in improved training strategies based on the concept of task-specificity.5–7 This concept has been translated to various rehabilitation interventions, such as those targeting walking outcomes (e.g. body-weight supported treadmill training)8–11 or arm and hand function (e.g. constraint-induced movement therapy).12,13 For balance, task specific rehabilitation similarly focuses on the achievement of the three main functional goals encompassing balance: 1) maintaining an antigravity posture such as sitting and standing, 2) anticipatory postural control during voluntary self-initiated movements and 3) reactive postural control during an unexpected perturbation.14
In terms of mobility, there has been a great deal of focus on the effectiveness of gait training in SCI rehabilitation research,8–11 but there has been relatively little attention on the impact of interventions specifically targeting balance outcomes. Given that postural control and balance are fundamental elements to neuromotor control underlying everyday mobility, it is possible that some of the task-specific interventions designed to improve specific mobility goals, such as walking, may accordingly also improve balance outcomes. One systematic review of studies in people with stroke found moderate evidence that balance could be improved with training techniques that involve exercises challenging static and dynamic balance ability, and practice of balance in different functional tasks, including sitting, standing, walking, and stair climbing.15 However, many of the studies included were of lower quality and the authors did not conduct effect size calculations to quantify the size of the intervention effect to determine the impact of balance training. Core progressive resistance strengthening exercises focusing on posture have also been shown to improve balance outcomes in persons with multiple sclerosis.16,17 Similarly, many of the studies included were susceptible to high risk of bias and the reported difference in means did not include measures of confidence. Over the last several years, the rapid development of advanced technology, such as robotics and virtual reality, in rehabilitation applications has also provided greater options for therapists to retrain balance and mobility.18–20
Our aim here was to provide a systematic review of the literature to investigate the effect of task-based rehabilitation training on postural stability and balance during sitting and standing in people with acute or chronic SCI. We sought to examine the effect of task-based training strategies as compared to conventional physical therapy or inactive control (e.g. education sessions). We also sought to examine whether different task-based training paradigms could be effective in eliciting detectable or meaningful changes in postural stability and balance. We divided the outcomes into sitting and standing balance performance to capture the wide spectrum of balance ability in people with SCI.
Methods
Search strategy for identification of studies
The following databases were searched to June 2016: Cochrane Database of Systematic Reviews (2005 to June 2016), Cumulative Index to Nursing and Allied Health Literature (1982 to June 2016), MEDLINE (PubMed and OVID—1946 to present), EMBASE (Ovid SP—1974 to June 2016), Physiotherapy Evidence Database (PEDro), Web of Science (1900 to June 2016), and Google Scholar.
In the search strategy, all electronic databases were searched with keywords, medical subject heading (MeSH), and search terms suggested by the individual databases. Boolean phrase or was used to search key terms for participants, interventions and outcomes separately; these were then joined with and. Additionally, the references of related clinical trials and systematic reviews were hand searched. Searches were not limited by date or language, but were limited to human trials.
Search terms
The following search terms were used: Participants: spinal cord injury, spinal cord injuries, spinal cord disease, paralysis, paraplegia, quadriplegia, and tetraplegia. Interventions: balance training, balance exercise, rehabilitation intervention. Outcomes: balance, balance ability, postural balance, posture, sitting, standing, sitting function, standing function (Appendix A).
Inclusion of studies
Inclusion criteria were set a priori:
- Participants:
- Participants over the age of 16 with spinal cord injury regardless of method of injury (traumatic, disease, congenital).
- Incomplete or complete spinal cord injury.
- Participants of all times post-injury and any initial sitting or standing capabilities were included.
- Interventions:
- Any task-based training intervention applied over time with more than 1 session for the experimental group (e.g. body-weight supported treadmill training, or technology-assisted task-based training (robot-assisted, virtual reality)).
- Studies making use of more than 1 intervention were included.
- Outcomes
- At least one measure of balance or postural control during sitting or standing (static or dynamic, clinic or laboratory tested).
Randomized controlled trials (RCTs), quasi-RCTs, controlled and uncontrolled trials of Level 4 evidence and higher were included. (Level 4 evidence as described by SCIRE (Spinal Cord Injury Rehabilitation Evidence) includes pre-post, post-test and case series studies.)
Exclusion of studies
Any interventions that measured the effectiveness of external devices such as orthotics, standing frames, seating and chair positions were excluded. Studies were excluded if the control group included some form of gait or balance task-specific training. Examples of task-specific gait activity would include BWS and walking over ground. Outcomes that measured anything other than balance, such as walking (e.g. TUG, etc.) were excluded. Trials that were observational, clinical consensus, case reports, or with n<3 were excluded.
Selection procedure
The selection procedure was performed according to the Preferred Reporting of Items for Systematic Reviews and Meta-Analysis (PRISMA) 2009 Flow Diagram (Fig. 1). The first author performed the initial identification of retrieved articles. Duplicates were then removed. These were provided to and screened by a second independent reviewer (Spinal Cord Injury Rehabilitation Evidence; SCIRE Research Team). Articles based on the titles, abstracts and inclusion criteria were then screened and removed by 2 reviewers. Full text versions of all relevant articles were then assessed for eligibility. Any disagreements between the reviewers were resolved by consensus.
Figure 1.
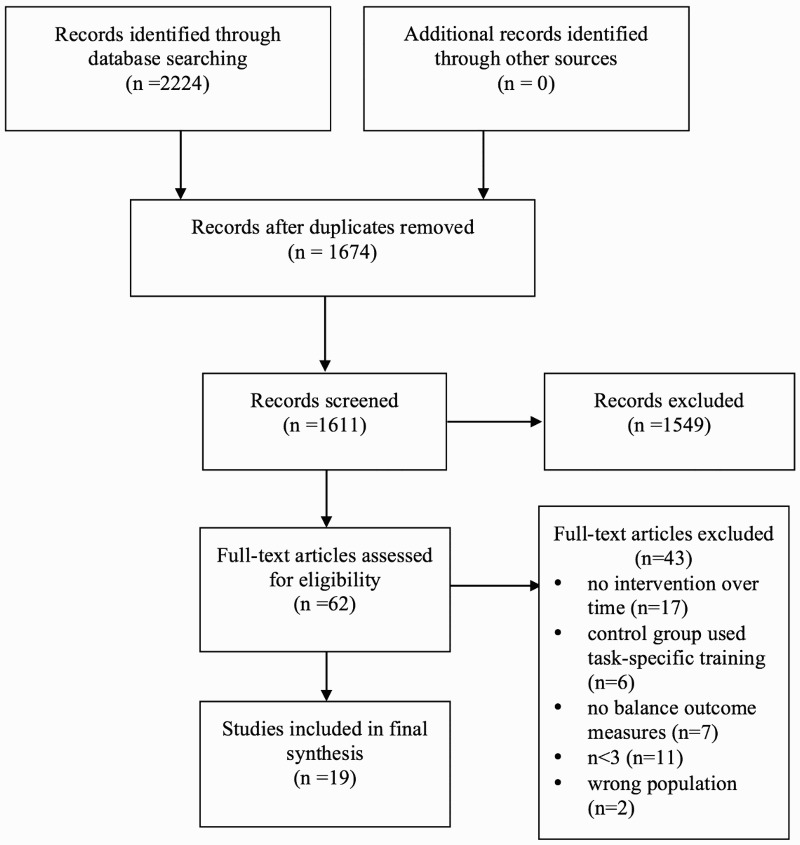
A PRISMA flow diagram of the studies included in the review.
Assessment of risk of bias
Two assessors assessed the risk of bias for the included studies using the PEDro scale21 for RCTs and crossover designs. Prospective cohort and pre-post studies were assessed using the Newcastle-Ottawa Scale (NOS).22 RCTs were considered excellent quality when they were rated 9 or 10, scores of 6 to 8 were considered good quality, scores of 4 or 5 were considered moderate or fair, and scores of less than 4 were considered poor.23 PEDro scores from the included studies were obtained from the PEDro database. Any disagreements between the reviewers were resolved by discussion and consensus.
Data extraction
Data were extracted to a data collection form used by SCIRE. Data included information on participant characteristics (number of participants, time since injury, level of injury, mean age, gender, and American Spinal Cord Injury Association Impairment Scale (AIS) grade), intervention (type of treatment, duration) and balance-related outcomes (balance scales, balance measures in sitting and/or standing) (Table 1). Planned sub-group analysis was performed for trial type (controlled vs. uncontrolled) and intervention type (sitting vs. standing).
Table 1.
Study characteristics on effects of task-specific interventions on sitting and standing balance outcomes.
| Author, Year, n, Age, Sex | Study Design | Dose | Results | ||||
|---|---|---|---|---|---|---|---|
| YPI/Injury Level/AIS | Intervention Type | Time (hr) | Freq (d/wk) | Weeks | |||
| Task-specific standing vs. Active control | |||||||
| Kim et al.,33 2010, n=12, age: 41, M/F: 9/3 | Pro CT | >0.5**/T6-L2/A,B | Exp: conventional PT and goal oriented rockerboard training, Con: conventional PT | 5×10rep | 5 | 4 | Significant increase on functional reach and reduced sway area within experimental group and between groups |
| Harvey et al.,27 2011, n=32, age: 27*, M/F: 30/2 | RCT | 0.2*/T1-L1/A,B,C | Exp: conventional PT and task-specific exercises for unsupported sitting, Con: conventional PT | 0.5 | 3 | 6 | No changes met the minimally worthwhile treatment effect for MLT and MSR |
| Task-specific sitting vs. Inactive control | |||||||
| Boswell-Ruys et al.,34 2010, n=30, age Exp: 42 Con: 48, M/F: 25/5 | RCT | Exp: 10, Con: 19/T1-L2/A,B | Exp: Task-specific exercises for unsupported sitting, Con: no training or therapy | 1 | 3 | 6 | Significant improvement on all outcomes for both groups, and between group mean difference for MBRT, ART, SRT to 45°. |
| Tsang et al.,35 2015 n=19 age: 48 yrs M/F: 11/8 | Pro CT | 16/C6-L1/B,C,D | Exp: Tai Chi, Con: educational talks and social activities | 1.5 | 2 | 12 | Significant group x time interaction, and between group effect for SWS. |
| Task-specific sitting uncontrolled trials | |||||||
| Grigorenko et al.,36 2004, n=24, age: 39, M/F: 9/3 | Pre-Post | 17/T2-L1/A,B,C | Modified kayak on open water | 1 | 2–3 | 8 | Significant decrease in sagittal median frequency. |
| Bjerkefors et al.,37 2006/2007, n=10, age: 38, M/F: 7/3 | Pre-Post | 11.5*/T3-L2/A,B,C | Modified kayak ergometer | 1 | 3 | 10 | Significant increase on Sit and Reach Test. AP angular and linear significantly reduced for all kinematic responses, except AP angular at deceleration. ML angular signficantly decreased at the end of deceleration. Trunk twisting reduce significantly for all kinematic responses. |
| Wall et al.,29 2015, n=5, age: 58.6, M/F: NS | Pre-Post | 7.6/C4-L1/D | Nintendo™ Wii Fit balance games | 1 | 2 | 7 | Significant improvement on forward and lateral functional reach tests. |
| Task-specific standing vs. Active controls | |||||||
| Alexeeva et al.,39 2011, n=35, age: 38.5, M/F: 30/5 | RCT | 4/C2-T10/C,D | Exp: BWST on track or treadmill, Con: conventional PT | 1 | 3 | 13 | The BWST track and structured PT group significanly improved on balance function using TBS |
| Labruyère and Van Hedel,40 2014, n=9, age: 59, M/F: 5/4 | Cross-over | 4.2/C4-T11/D | Exp: BWSTT, Con: resistance training | 0.75 | 4 | 4 | Significant improvement on BBS with resistance training. |
| Task-specifc standing uncontrolled trials | |||||||
| Villiger et al.,42 2013, n=14, age: 53, M/F: 9/5 | Pre-Post | 4/C4-T12/C,D | Virtual reality augmented training for lower limbs while sitting | 0.75 | 4–5 | 4 | Significant improvement on BBS. |
| Sayenko et al.,41 2010, n=6, age: 41, M/F: 5/1 | Pre-Post | 7/C4-T12/C,D | Virtual reality visual feedback training with games while standing on a force platform | 1 | 3 | 4 | Significantly improved root mean square distance and confidence ellipse area for the static test, and increased stability zone for the dynamic test. |
| Fritz et al.,45 2011, n=15, age L:38.5, H: 50.4, M/F: L: 8/2, H: 3/2 | Pre-Post | L: 6.6, H: 5.7/NS/C,D | Intensity mobility training | 3 | 3–5 | 10 | Small effect size for BBS for the higher functioning group |
| Bertolucci et al.,43 2015, n=13, age: 46.3, M/F: 6/7 | Pre-Post | 14.5/NS/NS | BWSTT | 3 | 6 | Significant improvement of BBS. | |
| Stevens et al.,44 2015, n=11, age: 47.7, M/F: 7/4 | Pre-Post | 4.8/C2-L2/C,D | BWS underwater treadmill training | 0.4 | 3 | 8 | Significant improvement of BBS |
| Behrman et al.,32 2012, n=95, age: NS, M/F: 75/20 | Pro Cohort | 1/≥T11/C,D | NRN training - manual facilitated BWS standing and stepping on a treadmill and over-ground | 2 | 4–5 | Significant improvement on BBS. | |
| Buehner et al.,30 2012, n=225, age: 42.5, M/F: 167/58 | Pro Cohort | 2.45/NS/C,D | NRN protocol | 2 | 4–5 | Significant improvement on BBS. | |
| Harkema et al.,11 2012, n=196, age: 41, M/F: 148/48 | Pro Cohort | 0.9/NS/C,D | NRN protocol | 2 | 4–5 | Significant improvement on BBS, and difference between AIS C and D groups. | |
| Lorenz et al.,31 2012, n=337, age: 40, M/F: 255/82 | Pro Cohort | 1/≥T10/C,D | NRN protocol | 2 | 4–5 | Significant improvement on BBS, and difference between AIS level. | |
AP, anterior-posterior; ART, Alternate Reach Test; BBS, Berg Balance Scale; BWSTT, body-weight support treadmill training; Con, control, Exp, experimental; MBRT, Maximal Balance Range Test; ML, medial-lateral; MLT, Maximal Lean Test; MSR, Maximal Sideways Reach; NRN, NeuroRecovery Network; Pro Cohort, prospective cohort; Pro CT, prospective controlled trial; PT, physical therapy; Retro CT, retrospective controlled trial; SRT, Seated Reach Test; SWS, sequential weight shifting; TBS, Tinetti Balance Scale.
Effect sizes
Studies that measured the effect of their intervention on sitting balance (using sit and reach test with a distance measure and AP displacement of the CoP) were analyzed separately from those that measured standing balance using the Berg Balance Scale (BBS). Though the BBS has sitting components, it is primarily used to assess standing balance and was largely used by the studies testing standing balance interventions.
We calculated effect sizes and 95% confidence interval using Cohen’s d estimate with Hedges adjustment for sample size.24 To calculate effect sizes for the controlled studies, the change in balance ability from baseline in both the experimental and control group was calculated. The difference in the mean changes was then divided by the weighted pooled standard deviation between the experimental and control groups. To calculate effect sizes for the pre-post studies, we subtracted the pre-study mean from the post-study mean and divided it by the weighted average standard deviation from baseline to completion. The RCTs and pre-post studies were analyzed separately as effect sizes are larger for pre-post studies due to the lack of a control group to account for non-training related influences.25,26 The effect size and standard error values were used in a fixed-effects model for the sitting and standing balance studies separately (Comprehensive Meta-Analysis software, version 3). Overall Q and I2 values were calculated to test for homogeneity of variance among the effect sizes. The Q value is a measure of variance among the effect sizes, and heterogeneity is indicated by a statistically significant (P < 0.05) sum of the squares of each effect size about the weighted mean (Q). The I2 represents the magnitude of the heterogeneity with large values indicating more heterogeneity.
There was often more than one outcome measure reported among the sitting balance studies (e.g. the Maximal Lean Test and Maximal Sideward Reach Test).27 In this situation, we calculated the effect size of a sit and reach test in the forward direction that used a measure of displacement. An effect size of 0.2–0.5 is considered small, 0.5–0.8 medium and ≥0.8 large.28 When it was not possible to calculate effect sizes from the data presented in the published articles, we contacted the corresponding author directly to request access to the necessary data. Unfortunately, raw BBS scores were unavailable from one study,29 along with functional reach test scores.29 In publications from the same facility with overlapping time frames, we included the Harkema et al.11 study as the main objective focused on the effect of locomotor-based rehabilitation on standing balance.11,30–32
Results
Study selection
The initial electronic database search resulted in a total of 2,224 potentially relevant records. Removal of duplicates within and between databases resulted in 1,674 articles to be reviewed. After reviewing all articles’ titles and abstracts, and removing 1,549 articles that did not meet our criteria, two independent reviewers analyzed 62 full text articles according to the inclusion and exclusion criteria. Seventeen articles did not include an intervention performed over time, six articles used task-specific training as the control group, seven articles did not report any sitting or standing balance outcomes (i.e. only included TUG or other walking outcomes), eleven articles had n<3, and two articles did not study people with SCI. The remaining nineteen articles were included in the review and scrutinized (Fig. 1).
Comparisons and interventions
The effects of task-specific sitting and standing interventions were divided into controlled and uncontrolled trials. Task-specific interventions for improving sitting balance included exercises in unsupported sitting, kayak ergometry, Tai Chi, and sitting on a rocker board while performing various reaching tasks (Table 1). We divided sitting balance into “active control” (conventional physical therapy)27,33 and “inactive control”34,35groups and compared task-specific exercises to these. Of the uncontrolled trials, three were of pre-post design.36–38
Among the studies measuring standing balance there was one RCT,39 and one crossover study40 comparing BWS to conventional physical therapy. There were five pre-post designs,41–44 assessing virtual reality and BWS training. Four prospective cohort trials measuring standing balance were from the same facility11,30–32 (Table 1).
BWST interventions included those performed on a treadmill, over ground, or underwater. One study utilized intensive mobility therapy, which combines BWST on a treadmill with balance exercises, muscle strengthening, coordination and range of motion in a massed intensive therapy.45 Four trials used the protocol provided by the NeuroRecovery Network (NRN),11,30–32 which consisted of manual facilitated BWST in standing and stepping on a treadmill and over ground as well as community integration. Virtual reality was used as a biofeedback tool during exercises performed in standing on a force platform,41 and balance exercise games played with the Nintendo Wii Fit system.29
Quality of selected studies and risk of bias
Controlled trials
The assessment of the risk of bias is summarized in Table 2 and 3. Overall, the four trials assessed by PEDro were considered good (6 to 8 of a possible total score of 10) (Table 2). The two non-randomized trials assessed by NOS did not define or quantify the balance impairment (outcome) prior to commencement (e.g. BBS less than a certain score indicating impaired balance) (Table 3). Though most trials provided a list of criteria to determine participant eligibility, most did not specify the source of subjects (e.g. hospital, community, clinic, etc.). All included trials did not report participant or therapist blinding.
Table 2.
Detailed PEDro score for RCT and cross-over studies.
| Authors | Harvey27 | Boswell-Ruys34 | Alexeeva39 | Labruyère40 |
|---|---|---|---|---|
| 2011 | 2010 | 2011 | 2014 | |
| Eligibility criteria specified | No | No | Yes | Yes |
| Random allocation | 1 | 1 | 1 | 1 |
| Concealed allocation | 1 | 1 | 1 | 0 |
| Groups similar at baseline | 1 | 1 | 1 | 0 |
| Participants blinding | 0 | 0 | 0 | 0 |
| Therapists blinding | 0 | 0 | 0 | 0 |
| Outcome assessor blinding | 1 | 1 | 1 | 1 |
| Outcomes for 85% of initial participants | 1 | 1 | 1 | 1 |
| Intention to treat analysis | 1 | 1 | 0 | 1 |
| Between group statistical comparison | 1 | 1 | 1 | 1 |
| Point measure and variability data | 1 | 1 | 1 | 1 |
| PEDro Score | 8 | 8 | 7 | 6 |
Items 2–11 are used to calculate the final score.
Table 3.
Detailed Newcastle-Ottawa Scale for non-randomized trials.
| A) Items | Kim33 | Tsang35 |
|---|---|---|
| Selection | ||
| Representativeness of the exposed cohort | * | * |
| Selection of the nonexposed cohort | * | * |
| Ascertainment of exposure | * | * |
| Demonstration that outcome of interest was not present at start of study | NT | NT |
| Comparability | ||
| Comparability of cohorts on the basis of the design or analysis | * | * |
| Outcome | ||
| Assessment of outcome | ND | ND |
| Was follow-up long enough for outcomes to occur | * | * |
| Adequacy of follow-up of cohorts | ND | * |
| Total number of stars | 5 | 6 |
| B) | Items | Behrman32 | Buehner30 | Harkema11 | Lorenz31 |
|---|---|---|---|---|---|
| Selection | |||||
| Representativeness of the exposed cohort | * | * | * | * | |
| Selection of the nonexposed cohort | NA | NA | NA | NA | |
| Ascertainment of exposure | * | * | * | * | |
| Demonstration that outcome of interest was not present at start of study | NT | NT | NT | NT | |
| Comparability | |||||
| Comparability of cohorts on the basis of the design or analysis | NA | NA | NA | NA | |
| Outcome | |||||
| Assessment of outcome | ND | ND | ND | ND | |
| Was follow-up long enough for outcomes to occur | * | * | * | * | |
| Adequacy of follow-up of cohorts | * | * | * | * | |
| Total number of stars | 4 | 4 | 4 | 4 |
| C) | Items | Bertolucci43 | Bjerkefors38 | Fritz45 | Grigorenko36 | Sayenko41 | Stevens44 | Villiger42 | Wall29 |
|---|---|---|---|---|---|---|---|---|---|
| Selection | |||||||||
| Representativeness of the exposed cohort | ND | ND | * | * | ND | * | * | ND | |
| Selection of the nonexposed cohort | NA | NA | NA | NA | NA | NA | NA | NA | |
| Ascertainment of exposure | ND | ND | ND | * | ND | * | * | * | |
| Demonstration that outcome of interest was not present at start of study | NT | NT | NT | NT | NT | NT | NT | NT | |
| Comparability | |||||||||
| Comparability of cohorts on the basis of the design or analysis | NA | NA | NA | NA | NA | NA | NA | NA | |
| Outcome | |||||||||
| Assessment of outcome | ND | ND | ND | ND | ND | ND | ND | ND | |
| Was follow-up long enough for outcomes to occur | * | * | * | * | * | * | * | * | |
| Adequacy of follow-up of cohorts | * | * | ND | * | * | * | * | * | |
| Total number of stars | 2 | 2 | 2 | 4 | 2 | 4 | 4 | 3 |
A) Non-randomized; B) Prospective Cohort; C) Pre-post trials.
Note: Stars are awarded for high quality items such that the highest quality studies are awarded up to nine stars.
NA, not applicable; ND, no data; NT, not tested.
Uncontrolled trials
The assessment of the risk of bias for the four observational prospective cohort studies from the same facilities using BWSTT and overground is summarized in Table 3. The risk of selection bias was considered low because of the representativeness of the cohort from seven outpatient rehabilitation centers located throughout the United States spanning seven years. However, none of the studies demonstrated that balance impairment (did not quantify the impairment) was not present prior to commencement of the study. Detection bias was considered high since none of the assessors was blinded to the intervention. Attrition bias was low for these studies.
The assessment of risk for the eight pre-post studies is summarized in Table 3. All had a high risk of selection bias particularly for the kayak interventions. There was a high risk of reporting bias particularly for the BWS interventions as they relied on self-reports. None of the studies defined the level of balance ability prior to the study. Detection bias risk was high because none of the trials performed an independent blind assessment.
Drop-outs tended to be common usually due to medical conditions and inability to complete the therapy leading to smaller sample sizes, a characteristic not uncommon in SCI clinical research. Adverse events such as low back pain, knee injury, cardiorespiratory distress and spasticity exacerbation were reported27,39,42 Four studies reported no adverse events.35,37,38,40 Eleven studies did not monitor or report adverse events.11,29–33,36,41,43–45
Sitting and standing balance outcome measures
Balance outcomes were reported using clinical and/or biomechanical measures. Clinical measures such as sit and reach tests were used to evaluate dynamic sitting, while the BBS was used to measure dynamic standing balance. Biomechanical tests measuring the displacement of the center of pressure (COP; sway area, mean velocity, angular and linear displacements in the anteroposterior and mediolateral directions) were used to assess both static and dynamic balance in sitting and standing. Data were collected at the final endpoint in situations where more than one endpoint was specified. Negative findings indicate that the experimental treatment was worse than the control treatment.
Effect of task-specific sitting Interventions
Controlled trials with active control group
There was one good quality RCT of 32 individuals classified as AIS A, B, or C (median time post-injury 2.4 months)27 and one prospective controlled trial of 12 individuals with motor-complete SCI at least 6 months post-injury33 examining the effect of task-specific exercises on sitting balance following standard inpatient rehabilitation (Table 1). Harvey et al. (2011) provided an additional 3 sessions per week of graded task specific exercises in unsupported sitting on top of standard inpatient rehabilitation.27 Kim et al. (2010) examined the effect of task-specific exercises using a rocker board in addition to conventional physical therapy compared to conventional physical therapy alone.42 The pooled effect size from these two studies was –0.09 (95% CI: –0.663 to 0.488, P = 0.766) with no heterogeneity (I2 = 0%), indicating no significant effect of the addition of task-specific exercises on sitting balance outcomes (Fig. 2).
Figure 2.
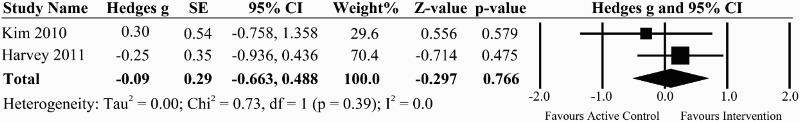
A forest plot of task-specific interventions vs. active control group on the effect size for the sit and reach tests in the forward direction for studies evaluating sitting balance.
Controlled trials with inactive control group
We found one good quality RCT of 30 individuals with chronic SCI34, using the same task specific exercises described in Harvey et al.27 compared to no training and one prospective controlled trial comparing a Tai Chi program compared to educational talks and social activities in chronic SCI35 (Table 1). The pooled effect size from these two studies was 0.39 (95% CI: –0.165 to 0.937, P = 0.17), indicating a beneficial, but non-significant effect of task-specific exercise on sitting balance in individuals with chronic SCI (Fig. 3).
Figure 3.
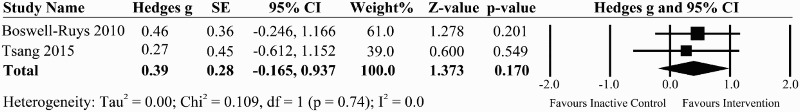
A forest plot of task-specific interventions vs. inactive control group on the effect size for the sit and reach tests in the forward direction for studies evaluating sitting balance.
Uncontrolled trials
There were three pre-post studies that investigated the effects of kayak ergometry on sitting balance36–38 that enrolled a total of 32 individuals, mostly with motor-complete SCI (Table 1). Overall, sitting balance was significantly improved with kayak ergometer training with substantial transfer effects to functional tests in the wheelchair.37,38 The pooled effect size from these three studies was 0.70 (95% CI: 0.202 to 1.193, P = 0.006) indicating a large and significant effect of kayak training on sitting outcomes (Fig. 4). However there were large amounts of heterogeneity within the studies (I2 = 59.6%). Only two studies33,38 met the criteria for minimal detectable change (i.e. greater than 4.63 points) on a forward sit and reach test46 (Table 1).
Figure 4.
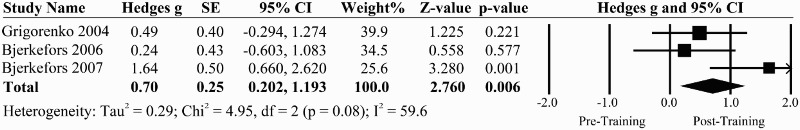
A forest plot evaluating kayak interventions for AP displacement of the CoP in pre-post trials.
Effect of task-specific standing interventions
Controlled trials with active control group
Two good quality trials compared BWS with active conventional physical therapy in a total of 44 individuals classified as AIS C, D (median time since injury 4 years).39,40 Alexeeva et al. (2011) reported that after 8 weeks of training, Tinetti balance scores increased the most for the individualized physical therapy training (control group) followed by BWST on a track and BWST on a treadmill.39 A cross-over trial reported improvements in BBS with BWST with resistance exercises compared to BWST without resistance.40 The pooled effect size from these two trials was –0.36 (95% CI: –0.840 to 0.113, P = 0.14) indicating that active conventional therapy group performed slightly better than BWS therapy though not in a significant way (Fig. 5).
Figure 5.
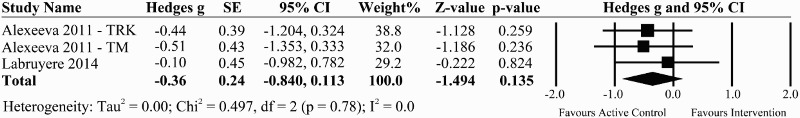
A forest plot of task-specific interventions vs. active control group on the effect size for studies evaluating functional standing balance using the BBS.
Uncontrolled trials
There were three pre-post trials that assessed standing balance in a total of 25 people with chronic incomplete SCI using virtual reality.29,41,42 Interventions included performing exercises using task-specific visual biofeedback while standing or sitting. All trials reported improvements in sitting and standing balance outcomes after training (Table 1).
There were three pre-post trials that measured standing balance in a total of 39 individuals with incomplete SCI following body-weight supported treadmill training either underwater, robotic assisted or in combination with intensive mobility training.43,45 All trials reported improvements in BBS after training (Table 1).
Four pre-post trials from the same clinical setting spanning February 2005 to March 2011 (N=853) reported significant improvements in BBS scores following a program of 3–5 days per week of treadmill- progressing to overground-based BWST11,30–32 (Table 1). Only 3 studies met the criteria for minimal detectable change (i.e. at least 6 points) on the BBS.11,30,44 The pooled effect size for the six pre-post studies for which we could obtain data was 0.45 (95% CI: 0.277 to 0.626, P <0.001) indicating significant improvements post-training on BBS11,42–45,47 (Fig. 6).
Figure 6.
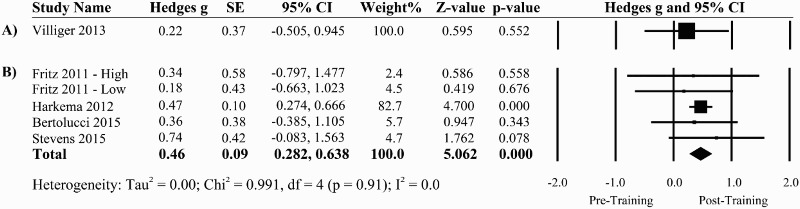
A forest plot of uncontrolled studies using A) virtual reality and B) BWS interventions and their effects on functional standing balance using the BBS.
Discussion
In this systematic review, the effectiveness of various rehabilitation interventions for improving sitting and standing balance in persons with SCI was analyzed. Overall, the addition of task-specific balance training does not appear to result in appreciable improvements in sitting function over that achieved by conventional rehabilitation, as measured by sit and reach tests in the acute stages. However, there is moderate evidence that these exercises may be beneficial in the chronic stages. The small effect sizes observed for standing balance interventions, along with the observation that few studies achieved met the criteria for minimal detectable change in standing balance, suggest that balance ability does not improve significantly with any form of BWST. This is supported by a controlled trial, which found that individualized physical therapy treatment was more effective than BWST for improving balance.39 Evidence from virtual reality interventions show small to large effects for improving standing function through the use of task specific visual biofeedback.41
Most neurologic recovery occurs within the first few months after injury. It is possible that in the acute phase, spontaneous recovery, carry over effects of standard inpatient therapy, and activities of daily living could have masked any significant findings when comparing one intervention with another.27,48 The data from the two RCTs reveal no difference between control and experimental groups suggesting that intensive task-specific balance training in the early phase is not as important as general functional training provided by standard physical therapy. This concept is consistent with systematic reviews of locomotor training in subjects with acute (<1 year)49 or subacute incomplete SCI.10
Balance is primarily assessed according to three different dimensions: maintenance of a position (static balance), anticipatory dynamic postural adjustments to voluntary movements, and reactions to sudden external stresses.14 Among the reviewed studies, static balance was assessed biomechanically by sitting or standing quietly on a force plate33,36,41 and clinically using the upper body sway test.34 Anticipatory dynamic balance was assessed by asking the subject to displace the COP during a limits of stability test41 and clinically using the Maximal Lean Test, Coordinated Stability Test and sit and reach tests.27,33,38,41 The BBS and TBS tests include both static and anticipatory components and were used by the majority of the trials to assess standing balance. Only one trial was able to use predictable and unpredictable perturbations to assess balance reactions after kayak ergometry training.37 Thus, when assessing balance, tests are chosen to reflect both static and anticipatory dynamic dimensions and when applicable, such as in people with high functioning SCI, reactions to sudden perturbations, in order to give a more functionally appropriate understanding of balance capacity in persons with SCI.
There are some drawbacks of measuring balance with only functional outcomes. For instance, in post-stroke patients, the BBS has been shown to be very sensitive to changes in the acute or chronic stage in individuals with an initial score of ≤35. However, in subjects with higher initial scores, it is unclear if further improvements can be made or if the test is insufficiently sensitive to demonstrate change15 as observed by the relatively small effect sizes in studies showing a possible ceiling effect on the BBS.45,47 Nonetheless, functional measures are quick, cost effective and easy to apply in both the research and clinical setting and have the added benefit of being validated for the SCI population.50
The small effect sizes may also be explained by the apparent incongruence between the uses of the BBS to assess balance following gait-training interventions (e.g. BWST). There is evidence that task-specific training promotes recovery in the trained task.51 But training in one task does not necessarily translate to another task. This is supported by a study that reported no transfer to walking function following training to weight bear on the affected leg improved standing balance.52 Similarly, using the BBS to assess walking interventions may not accurately capture balance improvements gained during locomotor training. For example, many of the tasks in the BBS assess balance with the feet stationary while moving the upper body.
On the other hand, biomechanical measurements using force plates and motion analysis systems can be expensive and not easily applied in the clinical setting. Extensive training may be required and patients may not be able to tolerate standing unsupported for the extended periods of time required for reliable biomechanical measures of balance.14 However, COP measures may be more sensitive in detecting postural changes in quiet sitting or standing that may not be evident from functional outcomes.53 Moreover, COP measures have been useful in predicting falls54 whereas clinical measures such as the BBS are unable to predict falls in SCI.55 In light of these limitations, clinical measures have the benefit of assessing functional improvement whereas biomechanical assessment measures may be better able to detect subtle changes in postural control.
Sitting and standing balance is critical to everyday functional activities and impairments in the ability to maintain balance during everyday mobility poses a greater risk of falls, further compromising the health outcomes of this already vulnerable population. The incidence of falls in people with SCI has been reported to be as high as 75% with loss of balance identified as the primary factor contributing to falls in incomplete SCI.56 In stroke, balance problems while performing complex tasks was identified as the strongest predictor of falling.57 It is not known the extent to which deficits in balance may affect people with SCI. Although falls are multifactorial, postural control is an important factor contributing to falls, making rehabilitation targeted to improving balance so important.
Limitations
There were several methodological limitations that could have contributed to the small treatment effects observed. Of the 19 trials reviewed, only six were controlled and of good quality. The remaining 13 trials were uncontrolled and subject to bias and placebo effects. However, this is a common limitation among SCI studies as the implementation of RCTs is challenging due to the relatively small population and clinically heterogeneous patient groups. Differences in chronicity, level of lesion, age and abilities between and within subjects in addition to small sample sizes may have resulted in the small effects observed in the outcome measures. It was suggested that some subgroups might even respond better to treatment though further research was warranted.27 The differences in dose and frequency of the interventions also make it challenging to compare results between studies. The importance of adequate practice intensity has been demonstrated in other areas of neurology.58
Although we decided to include pre-post studies, it is important to note that effect sizes may be inflated with this design.59 There is also a risk of publication bias, however some studies reported negative effects for sitting and standing balance interventions. Finally, while the outcome measures presented in this review are commonly used to assess balance in people with SCI in controlled laboratory/clinical settings, we acknowledge that any changes or improvements do not necessarily reflect the application to real world activity such as would be measured with FIM or SCIM score.
Conclusion
This systematic review shows that task-specific training interventions can provide benefits to sitting and standing balance, but the few randomized controlled trials available indicate that task-specific training does not appear to enhance the effects of standard physical therapy on either sitting or standing balance. Overall effect sizes were small and most of the studies in the literature are non-controlled pre-post trials, which are accompanied by high risk of selection, reporting, and detection bias. Nevertheless, the variety of interventions highlighted in the pre-post studies illustrate the range of interesting training options now available, especially with the increased availability of different rehabilitation technologies. Future research is required in order to clarify the specific features of training interventions essential for improving balance function in SCI.
Disclaimer statements
Contributors None.
Declarations of Interest The authors report no declarations of interest.
Conflicts of interest The authors have no conflicts of interest to declare.
Ethics approval None.
Appendix A
Search strategy: MEDLINE (OVID)
01. spinal cord injury
02. spinal cord injuries
03. spinal cord disease
04. paralysis
05. paraplegia
06. quadriplegia
07. tetraplegia
08. 1 or 2 or 3 or 4 or 5 or 6 or 7
09. balance training
10. balance exercise
11. rehabilitation intervention
12. 9 or 10 or 11
13. balance
14. balance ability
15. postural balance
16. posture
17. sitting
18. standing
19. sitting function
20. standing function
21. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22. 8 and 12 and 21
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
ORCID
Cynthia M. Tse http://orcid.org/0000-0001-8363-5869
Janice J. Eng http://orcid.org/0000-0002-2093-0788
References
- 1.Horak FB, Macpherson JM.. Postural Orientation and Equilibrium. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2011. [Google Scholar]
- 2.Winter DA. Human balance and posture control during standing and walking. Gait Posture 1995;3(4):193–214. doi: 10.1016/0966-6362(96)82849-9 [DOI] [Google Scholar]
- 3.Huxham FE, Goldie PA, Patla AE.. Theoretical considerations in balance assessment. Aust J Physiother 2001;47(2):89–100. doi: 10.1016/S0004-9514(14)60300-7 [DOI] [PubMed] [Google Scholar]
- 4.Chen CL, Yeung KT, Bih LI, Wang CH, Chen MI, Chien JC.. The relationship between sitting stability and functional performance in patients with paraplegia. Arch Phys Med Rehabil 2003;84(9):1276–81. doi: 10.1016/S0003-9993(03)00200-4 [DOI] [PubMed] [Google Scholar]
- 5.Wolpaw JR, Tennissen AM.. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 2001;24(1):807–43. doi: 10.1146/annurev.neuro.24.1.807 [DOI] [PubMed] [Google Scholar]
- 6.Behrman AL, Harkema SJ.. Physical Rehabilitation as an Agent for Recovery After Spinal Cord Injury. Phys Med Rehabil Clin N Am 2007;18(2):183–202. doi: 10.1016/j.pmr.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Fouad K, Tetzlaff W.. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol 2012;235(1):91–9. doi: 10.1016/j.expneurol.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Mehrholz J, Kugler J, Pohl M.. Locomotor training for walking after spinal cord injury. The Cochrane Collaboration , editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd; 2008. [Google Scholar]
- 9.Van Hedel HJA, Dietz V.. Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neurosci 2010;28(1):123–34. [DOI] [PubMed] [Google Scholar]
- 10.Wessels M, Lucas C, Eriks I, de Groot S.. Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J Rehabil Med 2010;42(6):513–9. doi: 10.2340/16501977-0525 [DOI] [PubMed] [Google Scholar]
- 11.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL.. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 2012;93(9):1508–17. doi: 10.1016/j.apmr.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 12.Taub E, Uswatte G, Pidikiti R.. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation-a clinical review. J Rehabil Res Dev 1999;36(3):237–51. [PubMed] [Google Scholar]
- 13.Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ.. Repetitive task practice: A critical review of constraint-induced movement therapy in stroke. Neurologist 2002;8(6):325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg K. Balance and its measure in the elderly: a review. Physiother Can 1989;41(5):240–6. doi: 10.3138/ptc.41.5.240 [DOI] [Google Scholar]
- 15.Lubetzky-Vilnai A, Kartin D.. The effect of balance training on balance performance in individuals poststroke. J Neurol Phys Ther 2010;34(3):127–37. doi: 10.1097/NPT.0b013e3181ef764d [DOI] [PubMed] [Google Scholar]
- 16.Freeman J, Allison R.. Group exercise classes in people with multiple sclerosis: a pilot study. Physiother Res Int 2004;9(2):104–7. doi: 10.1002/pri.307 [DOI] [PubMed] [Google Scholar]
- 17.Kjolhede T, Vissing K, Dalgas U.. Multiple sclerosis and progressive resistance training: a systematic review. Mult Scler J 2012;18(9):1215–28. doi: 10.1177/1352458512437418 [DOI] [PubMed] [Google Scholar]
- 18.Holden MK. Virtual environments for motor rehabilitation: review. Cyberpsychol Behav 2005;8(3):187–211. doi: 10.1089/cpb.2005.8.187 [DOI] [PubMed] [Google Scholar]
- 19.Tefertiller C, Pharo B, Evans N, Winchester P.. Efficacy of rehabilitation robotics for walking training in neurological disorders: a review. J Rehabil Res Dev 2011;48(4):387–416. doi: 10.1682/JRRD.2010.04.0055 [DOI] [PubMed] [Google Scholar]
- 20.Diaz I, Gil JJ, Sanchez E.. Lower-limb robotic rehabilitation: literature review and challenges. J Robot 2011;(6263):1–11. [Google Scholar]
- 21.PEDro-Physiotherapy Evidence Database. PEDro Scale 1999. Available at: http://www.pedro.org.au/english/downloads/pedro-scale/. Accessed October 1, 2013.
- 22.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Quality Assessment Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed June 29, 2017.
- 23.Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Aubut JA, et al Spinal cord injury rehabilitation evidence: method of the SCIRE systematic review. Top Spinal Cord Inj Rehabil 2007;13(1):1–10. doi: 10.1310/sci1301-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner HM, Bernard RM.. Calculating and synthesizing effect sizes. Contemp Issues Commun Sci Disord 2006;33:42–55. [Google Scholar]
- 25.Carlson KD, Schmidt FL.. Impact of experimental design on effect size: Findings from the research literature on training. J App Psych 1999;84(6):851–62. doi: 10.1037/0021-9010.84.6.851 [DOI] [Google Scholar]
- 26.Lipsey MW, Wilson DB.. The efficacy of psychological, educational, and behavioral treatment. Confirmation from meta-analysis. Am Psychol 1993;48(12):1181–209. doi: 10.1037/0003-066X.48.12.1181 [DOI] [PubMed] [Google Scholar]
- 27.Harvey LA, Ristev D, Hossain MS, Hossain MA, Bowden JL, Boswell-Ruys CL, et al Training unsupported sitting does not improve ability to sit in people with recently acquired paraplegia: a randomised trial. J Physiother 2011;57(2):83–90. doi: 10.1016/S1836-9553(11)70018-2 [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical power analysis for the behavioral sciences. 1988, Hillsdale, NJ: L. Lawrence Earlbaum Associates. [Google Scholar]
- 29.Wall T, Feinn R, Chui K, Cheng MS.. The effects of the Nintendo™ Wii Fit on gait, balance, and quality of life in individuals with incomplete spinal cord injury. J Spinal Cord Med 2015;38(6):777–83. doi: 10.1179/2045772314Y.0000000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buehner JJ, Forrest GF, Schmidt-Read M, White S, Tansey K, Basso DM.. Relationship between ASIA examination and functional outcomes in the NeuroRecovery Network Locomotor Training Program. Arch Phys Med Rehabil 2012;93(9):1530–40. doi: 10.1016/j.apmr.2012.02.035 [DOI] [PubMed] [Google Scholar]
- 31.Lorenz D, Datta S, Harkema S.. Longitudinal patterns of functional recovery in patients with incomplete spinal cord injury receiving activity-based rehabilitation. Arch Phys Med Rehabil 2012;93(9):1541–52. doi: 10.1016/j.apmr.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 32.Behrman AL, Ardolino E, VanHiel LR, Kern M, Atkinson D, Lorenz DJ, et al Assessment of functional improvement without compensation reduces variability of outcome measures after human spinal cord injury. Arch Phys Med Rehabil 2012;93(9):1518–29. doi: 10.1016/j.apmr.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Chung YJ, Shin HK.. Effects of balance training on patients with spinal cord injury. J Phys Ther Sci 2010;22(3):311–6. doi: 10.1589/jpts.22.311 [DOI] [Google Scholar]
- 34.Boswell-Ruys C, Harvey LA, Barker JJ, Ben M, Middleton JW, Lord SR.. Training unsupported sitting in people with chronic spinal cord injuries: a randomized controlled trial. Spinal Cord 2010;48(2):138–43. doi: 10.1038/sc.2009.88 [DOI] [PubMed] [Google Scholar]
- 35.Tsang WWN, Gao KL, Chan KM, Purves S, Macfarlane DJ, Fong SSM.. Sitting Tai Chi improves the balance control and muscle strength of community-dwelling persons with spinal cord injuries: a pilot study. Evid Based Complement Alternat Med 2015;2015(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigorenko A, Bjerkefors A, Rosdahl H.. Sitting balance and effects of kayak training in paraplegics. J Rehabil Med 2004;36(3):110–6. doi: 10.1080/16501970310020401 [DOI] [PubMed] [Google Scholar]
- 37.Bjerkefors A, Carpenter MG, Thorstensson A.. Dynamic trunk stability is improved in paraplegics following kayak ergometer training. Scand J Med Sci Sports 2007;17(6):672–9. doi: 10.1111/j.1600-0838.2006.00621.x [DOI] [PubMed] [Google Scholar]
- 38.Bjerkefors A, Thorstensson A.. Effects of kayak ergometer training on motor performance in paraplegics. Int J Sports Med 2006;27(10):824–9. doi: 10.1055/s-2005-872970 [DOI] [PubMed] [Google Scholar]
- 39.Alexeeva N, Sames C, Jacobs PL, Hobday L, Distasio MM, Mitchell SA, et al Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. J Spinal Cord Med 2011;34(4):362–79. doi: 10.1179/2045772311Y.0000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labruyère R, Van Hedel HJA.. Strength training versus robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study in patients depending on walking assistance. J NeuroEng Rehabil 2014;11(1):4. doi: 10.1186/1743-0003-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayenko DG, Alekhina MI, Masani K, Vette AH, Obata H, Popovic MR, et al Positive effect of balance training with visual feedback on standing balance abilities in people with incomplete spinal cord injury. Spinal Cord 2010;48(12):886–93. doi: 10.1038/sc.2010.41 [DOI] [PubMed] [Google Scholar]
- 42.Villiger M, Bohli D, Kiper D, Pyk P, Spillmann J, Meilick B, et al Virtual reality–augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair 2013;27(8):675–83. doi: 10.1177/1545968313490999 [DOI] [PubMed] [Google Scholar]
- 43.Bertolucci F, Di Martino S, Orsucci D, Ienco EC, Siciliano G, Rossi B, et al Robotic gait training improves motor skills and quality of life in hereditary spastic paraplegia. NeuroRehabil 2015;36(1):93–9. [DOI] [PubMed] [Google Scholar]
- 44.Stevens SL, Caputo JL, Fuller DK, Morgan DW.. Effects of underwater treadmill training on leg strength, balance, and walking performance in adults with incomplete spinal cord injury. J Spinal Cord Med 2015;38(1):91–101. doi: 10.1179/2045772314Y.0000000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fritz SL, Merlo-Rains AM, Rivers ED, Peters DM, Goodman A, Watson ET, et al An intensive intervention for improving gait, balance, and mobility in individuals with chronic incomplete spinal cord injury: a pilot study of activity tolerance and benefits. Arch Phys Med Rehabil 2011;92(11):1776–84. doi: 10.1016/j.apmr.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 46.Lynch SM, Leahy P, Barker SP.. Reliability of measurements obtained with a modified functional reach test in subjects with spinal cord injury. Phys Ther 1998;78(2):128–33. doi: 10.1093/ptj/78.2.128 [DOI] [PubMed] [Google Scholar]
- 47.Wu M, Landry J, Schmit B, Hornby TG, Yen SC.. Robotic resistance treadmill training improves locomotor function in human spinal cord injury: a pilot study. Arch Phys Med Rehabil 2012;93(5):782–9. doi: 10.1016/j.apmr.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 48.Field-Fote EC. Spinal cord control of movement: implications for locomotor rehabilitation following spinal cord injury. Phys Ther 2000;80(5):477–84. [PubMed] [Google Scholar]
- 49.Morawietz C, Moffat F.. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 2013;94(11):2297–308. doi: 10.1016/j.apmr.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 50.Lemay JF, Nadeau S.. Standing balance assessment in ASIA D paraplegic and tetraplegic participants: concurrent validity of the Berg Balance Scale. Spinal Cord 2010;48(3):245–50. doi: 10.1038/sc.2009.119 [DOI] [PubMed] [Google Scholar]
- 51.Girgis J, Merrett D, Kirkland S, Metz GAS, Verge V, Fouad K.. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 2007;130(11):2993–3003. doi: 10.1093/brain/awm245 [DOI] [PubMed] [Google Scholar]
- 52.Dickstein R, Nissan M, Pillar T, Scheer D.. Foot-ground pressure pattern of standing hemiplegic patients. Major characteristics and patterns of improvement. Phys Ther 1984;64(1):19–23. doi: 10.1093/ptj/64.1.19 [DOI] [PubMed] [Google Scholar]
- 53.Pajala S, Era P, Koskenvuo M, Kaprio J, Törmäkangas T, Rantanen T.. Force platform balance measures as predictors of indoor and outdoor falls in community-dwelling women aged 63–76 years. J Gerontol A Biol Sci Med Sci 2008;63(2):171–8. doi: 10.1093/gerona/63.2.171 [DOI] [PubMed] [Google Scholar]
- 54.Maki BE, Holliday PJ, Topper AK.. A prospective-study of postural balance and risk of falling in an ambulatory and independent elderly population. J of Gerontol 1994;49(2):M72–84. doi: 10.1093/geronj/49.2.M72 [DOI] [PubMed] [Google Scholar]
- 55.Wirz M, Müller R, Bastiaenen C.. Falls in persons with spinal cord injury: validity and reliability of the Berg Balance Scale. Neurorehabil Neural Repair 2010;24:70–7. doi: 10.1177/1545968309341059 [DOI] [PubMed] [Google Scholar]
- 56.Brotherton SS, Krause JS, Nietert PJ.. Falls in individuals with incomplete spinal cord injury. Spinal Cord 2006;45(1):37–40. doi: 10.1038/sj.sc.3101909 [DOI] [PubMed] [Google Scholar]
- 57.Lamb SE, Ferrucci L, Volapto S, Fried LP, Guralnik JM.. Risk factors for falling in home-dwelling older women with stroke: the Women's Health and Aging Study. Stroke 2003;34(2):494–501. doi: 10.1161/01.STR.0000053444.00582.B7 [DOI] [PubMed] [Google Scholar]
- 58.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil Rehabil 2006;28(13–14):823–30. doi: 10.1080/09638280500534861 [DOI] [PubMed] [Google Scholar]
- 59.Morris SB, DeShon RP.. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods 2002;7(1):105–25. doi: 10.1037/1082-989X.7.1.105 [DOI] [PubMed] [Google Scholar]


