Abstract
Purpose
Determine whether creatine or vitamin D supplementation improves muscle strength in individuals with spinal cord injury undergoing resistance training.
Methods
Thirteen male and one female with spinal cord injury, from two Portuguese rehabilitation centers, were randomized to creatine (3g daily), vitamin D (25000 IU each two weeks) or placebo group in a double-blind design. All participants performed progressive resistance training during eight weeks. The outcome measures, obtained at baseline and after intervention, included: Sum of four skinfolds; Corrected arm muscle area; Seated medicine ball throw; Handgrip strength with dynamometer; Manual wheelchair slalom test and one repetition maximum for Chest press, Triceps, Pec deck and Lat pulldown. Vitamin D levels were obtained in all participants before and after intervention.
Results
71.4% of participants had deficit values of vitamin D. The corrected arm muscle area improved significantly (p<0.05) in creatine group relatively to the control group. There was a significant correlation (p<0.05) between the one repetition maximum Pec deck and levels of vitamin D.
Conclusions
Supplementation with creatine may improve muscle strength parameters in individuals with spinal cord injury. Vitamin D deficiency is highly prevalent in this population. It is recommended an initial screening of vitamin D levels at the beginning of the physical rehabilitation process.
Keywords: Spinal cord injury, Resistance training, Creatine, Vitamin deficiency, Supplements
Introduction
Spinal cord injury (SCI) leads to muscle strength impairment as a result of profound deconditioning.1 The ability to perform activities of daily living, such as the transfer of body weight and propelling the wheelchair, requires repeated efforts of high intensity from upper limbs.2 Therefore, muscle strength is a highly relevant factor in individuals with SCI since it allows the person to be more independent and have a more active life with a consequent improvement in their quality of life.1 These individuals face considerable constraints to perform their daily activities and would most likely benefit from a resistance training program to increase muscle strength and improve their functional dynamics.
Amongst the most important nutritional guidelines to enhance strength and lean body mass are the combination of resistance training with appropriate energy, protein and micronutrient intake.3 The prevalence of malnutrition amongst patients with spinal cord injuries admitted at SCI centers is common, albeit these recommendations. A multicenter study performed at four British SCI centers concluded that 44.3% of SCI patients, at the time of admission, were malnourished or at risk of undernutrition.4 Thus, a balanced energy intake is fundamental for individuals with SCI who aim to enhance strength and lean body mass. Moreover, supplementation with creatine or vitamin D may be of interest to enhance lean body mass gains.
Creatine is an amino acid derivative that can be synthesized endogenously (1–2 g/day) in the liver, kidneys and pancreas from three amino acids (glycine, arginine and methionine) or obtained from food rich sources. Individuals consuming adequate amounts of meat and fish, will secure some creatine from the diet, which may contribute to the total body creatine pool, with a typical diet supplying approximately 1–2 g of creatine daily.5 Creatine supplementation enhances muscle strength both in younger6 and older7 individuals. In general, enhanced performance is reported in repetitive, high-intensity, short-term exercise bouts. In individuals with SCI, studies regarding creatine supplementation are scarce.8–10 Creatine supplementation showed no influence in muscle strength in individuals with chronic tetraplegia8 and further no improvement in trained athletes with SCI performing 800 meters.9 However, one study reported enhanced exercise performance in individuals with SCI.10 Due to limited data and equivocal outcomes, the benefits from creatine supplementation, in these populations, remains to date unclear.
Vitamin D is classified as a fat-soluble vitamin, albeit acting functionally as a hormone and sharing a similar structure to steroid hormones. Humans acquire vitamin D by two different sources: Endogenous production through sun exposure or directly from the diet (food or supplementation).11 Vitamin D is closely associated with health. A recent systematic review found a moderate to strong inverse association between 25-hydroxyvitamin D (25(OH)D) concentrations and cardiovascular diseases, serum lipid concentrations, inflammation, glucose metabolism disorders, weight gain, infectious diseases, multiple sclerosis, mood disorders, declining cognitive function, impaired physical functioning, and all-cause mortality.12 Vitamin D also influences directly skeletal muscle through the modification of calcium and phosphate balance and by indirect mechanisms purportedly activating the vitamin D receptor in muscle cells and leading to the transcription of genes involved in differentiation and muscle cell proliferation.13 Furthermore, vitamin D seems to influence not only muscle growth and cell differentiation, but also higher muscle contractility through the increase of sarcoplasmic calcium uptake.14 Thus, vitamin D deficiency might lead to muscle weakness with positive effects of vitamin D supplementation being found in muscle function.15–19 The prevalence of vitamin D deficiency is common in able-bodied individuals.20 The same paradigm is found in patients and athletes with SCI, after being admitted in physical rehabilitation centers, where the prevalence of inadequate serum levels of 25(OH)D is estimated between 67 and 93%.21–26 Low vitamin D levels stand as an independent predictor of poor physical function in individuals with SCI.24
The main goal of this study was to clarify whether creatine or vitamin D supplementation might enhance muscle strength in individuals presenting SCI, undergoing resistance training.
Methods
Subjects
Twenty-two potential participants met the inclusion criteria (adult inpatients with SCI, able to participate in a resistance training program for eight weeks) not presenting simultaneously any of the exclusion criteria (patients that supplemented with creatine or vitamin D or participated in resistance training over the last six months or clinically diagnosed with renal dysfunction). Participants with an incidence of SCI in a time frame less than three months, were also excluded since muscle fibers atrophy is more pronounced in these first three months.27 Fourteen inpatients, thirteen males and one female, mean age 47 ± 10.6 years and mean weight 74.2 ± 11.6 kg, from two Portuguese rehabilitation centers, completed the study successfully. The research was performed between September 2014 and September 2015 in Tocha (40° north latitude) and Vila Nova de Gaia (41° north latitude), Portugal. Six potential participants refused to participate in the study and two participants did not complete the whole study due to medical complications (urinary infection). Participant’s characteristics are shown in Table 1.
Table 1.
Subject’s characteristics.
| Subject | Age (years) | Gender | Weight (kg) | Time injury (months) | Lesional level | ASIA Classification |
|---|---|---|---|---|---|---|
| 1 | 38 | Male | 58.0 | 122 | L-1 | C |
| 2 | 39 | Male | 60.0 | 4 | L-3 | A |
| 3 | 56 | Male | 92.0 | 4 | T-6 | C |
| 4 | 57 | Male | 71.0 | 11 | T-11 | D |
| 5 | 49 | Male | 86.0 | 11 | L-3 | C |
| 6 | 53 | Male | 68.0 | 5 | T-9 | A |
| 7 | 40 | Male | 78.0 | 5 | T-4 | D |
| 8 | 44 | Male | 70.0 | 4 | T-9 | D |
| 9 | 32 | Male | 76.6 | 4 | T-9 | A |
| 10 | 49 | Male | 94.0 | 60 | C-7 | C |
| 11 | 50 | Male | 58.0 | 36 | C-3 | D |
| 12 | 72 | Female | 75.4 | 48 | L-2 | D |
| 13 | 45 | Male | 82.0 | 3 | C-5 | C |
| 14 | 34 | Male | 70.0 | 48 | L-1 | A |
ASIA, American Spinal Injury Association.
The study was approved by the Ethics Committee of the Faculty of Sport from the University of Porto, Portugal (clinicaltrials.gov identifier: NCT02099357). Before enrolling, the purpose and objectives of the study were carefully explained to each participant and a written informed consent was obtained.
Study design
Subjects were randomized, in chronological order according to their date of admission in the SCI center, to creatine, vitamin D or placebo group, in a double-blind manner. Participants were randomly assigned to a number (1, 2 or 3) by the nursing team, who provided each supplement daily. Each number matched a group (creatine, vitamin D or placebo) and the correct sequence was only disclosed at the end of the study. Four subjects were allocated in control group while five subjects were allocated in creatine group and vitamin D group, respectively. Subjects were supplemented (creatine, vitamin D or placebo) for eight weeks while participating in a progressive resistance training. All subjects were evaluated and performed several physical tests at baseline and after eight weeks of training and supplementation. Testing took place at the same time of the day, with replicated food intake and restricting caffeine intake prior to the evaluations. The tests started with an anthropometric evaluation, body mass was assessed using a wheelchair-accessible scale, the sum of four skinfolds (bicipital, tricipital, subscapular and suprailiac) was assessed using an Harpenden® skinfold caliper and the corrected arm muscle area was calculated as per previous research.28 Measurements were obtained in triplicate by the same researcher which is a certified level II anthropometrist by the International Society for the Advancement of Kinanthropometry (ISAK). Participants were allowed three attempts with only the best result being considered after performing the handgrip strength test in a Lafayette® dynamometer,29 the seated medicine ball throw (3kg),30 the manual wheelchair slalom test (MWST)31 and one repetition maximum test (1-RM) for Chest press, Triceps, Pec deck and Lat pulldown in two adapted fitness equipments for people using a wheelchair (HUR® and BH Fitness®). Participants were refrained from participating in strenuous exercise the day before physical testing. Vitamin D levels were assessed in all participants using eletroquimioluminescence and chemiluminescence techniques from venous blood collection both at baseline and after eight weeks of supplementation and resistance training. At baseline, subjects were provided with detailed instructions for the completion of a three-day diet record (three weekdays), with these records being analyzed by the same trained nutritionist, using ESHA Food Processor Plus Software, Version 8.0.
Resistance training protocol
All participants performed a similar resistance training circuit program with four different type of exercises aiming to strengthen the upper body (Chest press, Triceps, Pec deck and Lat pulldown). These were performed later in the morning, designed and supervised by a certified exercise professional resulting in three training sessions (≈1h) per week, with a 48 h rest period between sessions, for eight weeks. Exercise intensity was calculated based on 1-RM test results performed at baseline. In the first two weeks, exercise intensity was set at 45% 1-RM (3 sets, 15 repetitions). The next two weeks at 60% 1-RM (3 sets, 12 repetitions) with an increase to 65% 1-RM (3 sets, 10 repetitions) for two more weeks. In the last two weeks, exercise intensity was set at 70% 1-RM (3 sets, 8 repetitions).
Nutritional supplementation protocol
The nutritional supplementation protocol occurred over the same eight weeks of the training program, with supplements being ingested at lunch time. Participants that were allocated to the control group ingested 3 g of dextrose daily (placebo powder) with 250 mL of water after lunch and 5 mg of vitamin E (placebo ampoule) every two weeks (comprising a total of four ampoules). Participants in the creatine group ingested 3 g of creatine monohydrate powder daily with 250 mL of water after lunch and 5mg of vitamin E (placebo ampoule) every two weeks (also comprising a total of four ampoules). Participants in the vitamin D group ingested 3 g of dextrose daily (placebo powder) with 250 mL of water after lunch and one vitamin D ampoule (25 000 IU) every two weeks (comprising a total of 4 ampoules). Both the creatine supplement and the dextrose powder were similar in color and texture with same being reported regarding the vitamin D and vitamin E ampoules.
Statistics
Due to the small number of participants in this study the data was analyzed through non-parametric tests. The Wilcoxon test was performed to compare time/trial interactions (before and after training) for each intervention. Mean differences of variables (after and before intervention) between the three groups were analyzed by Kruskal-Wallis test. The Mann-Whitney test was used to compare mean differences between variables (after and before intervention) and also between supplement groups and the control group. The Spearman correlation test was performed to detect associations between variables and 25(OH)D level. The significance level was determined by p<0.05 according to a 95% confidence interval. All data was screened and analyzed by SPSS software 21.0.
Results
Demographic data, following random group assignment, is displayed in Table 2.
Table 2.
Subject’s characteristics by group.
| ASIA Classification | ||||||
|---|---|---|---|---|---|---|
| Group | Sample (n) | Age (years) | Time post injury (months) | A | C | D |
| Control | 4 | 42.3±8.7 | 43.3±54.3 | 1 | 2 | 1 |
| Creatine | 5 | 50.2±13.9 | 4.0 (4.0–48.0) | 1 | 1 | 3 |
| Vitamin D | 5 | 47.6±8.8 | 25.4±26.6 | 2 | 2 | 1 |
Values presented are means±standard deviations and median (range).
Fourteen participants completed successfully both training and supplementation protocols without reporting any adverse effects. The mean value of 25(OH)D from all participants prior to the intervention was 13.3ng/ml (3.0–23.7). 71.4% were deficient in vitamin D (<20ng/ml), 28.6% had insufficient values of vitamin D (20–30ng/ml) with no participant being reported with sufficiency status (Table 3). No statistical differences regarding energy intake, protein, vitamin D and body mass were found at baseline between groups. After the supplementation protocol, as expected, mean values of 25(OH)D improved significantly in the vitamin D group (p<0.05). This improvement was deemed statistically significant when comparing with the control group (p<0.05).
Table 3.
Vitamin D levels before and after intervention.
| Control | Creatine | Vitamin D | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| 25(OH)D (ng/ml) | 17.3±6.1 | 14.8±4.3 | 9.8±4.6 | 10.4±5.6 | 13.6±9.1 | 26.2±7.8 *# |
All values are means±standard errors.
*Statistically different after nutrition supplementation (P<0.05).
# Statistically different from Control group after intervention (P<0.05).
The resistance training program improved most variables, as displayed in Table 4. In the control group, there were no statistical improvements in any variable. In creatine group, an improvement was detected (p<0.05) in all variables, except for the MWST. In the vitamin D group, improvements were also observed in which concerns (p<0.05) the corrected arm muscle area, seated medicine ball throw and 1-RM Chest press. The corrected arm muscle area improved significantly (p<0.05) in the creatine group comparing with the control group (Table 4).
Table 4.
Variables values before and after intervention.
| Control | Creatine | Vitamin D | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Sum four skinfolds (mm) | 29.4 (24.7–82.3) | 29.1 (23.5–75.9) | 61.3±16.3 | 55.0±12.0* | 61.3±16.0 | 56.3±12.6 |
| Arm muscle area (cm2) | 55.3±19.3 | 58.3±17.8 | 47.0±9.6 | 57.4±10.2*# | 50.5±4.7 | 54.8±5.1* |
| MWST (s) | 17.8±5.2 | 15.5±5.0 | 21.7±4.5 | 17.9±3.1 | 19.3±4.6 | 16.7±4.0 |
| Medicine ball throw (m) | 3.92±1.5 | 4.26±1.5 | 3.40±0.5 | 4.28±0.6* | 2.63±1.2 | 3.09±1.2* |
| Handgrip strength (kg) | 36.5±6.5 | 38.4±6.8 | 21.0±10.2 | 23.0±10.9* | 25.0 (24.5–25.5) | 27.0 (27.0–27.5) |
| Chest press (kg) | 22.1±3.4 | 35.3±4.4 | 18.3 (15.0–43.0) | 30.0 (21.5–55.0)* | 32.3±34.2 | 47.8±44.3* |
| Triceps (kg) | 6.9±3.8 | 10.9±5.1 | 6.7 (5.0–20.0) | 11.0 (10.0–30.0)* | 9.5 (6.7–22.5) | 13.7 (10.4–35.0) |
| Pec deck (kg) | 21.7±6.7 | 28.6±11.5 | 22.2±3.8 | 29.0±5.7* | 31.2±16.0 | 40.1±16.6 |
| Lat pulldown (kg) | 20.5±3.4 | 27.2±6.7 | 16.7 (11.7–48.0) | 23.3 (20.0–52.0)* | 20.4 (13.3–48.0) | 24.2 (20.0–88.0) |
Values presented are means±standard deviations and median (range). *Change score statistically different after intervention (P<0.05).
# Change score statistically different from Control group after intervention (P<0.05).
Some participants were not able to perform all the physical measures due to loss of hand control. Three participants, from different groups, did not perform the MWST. Two participants from the vitamin D group were unable to comply with the handgrip strength test. One vitamin D group participant failed the 1-RM evaluation of the Triceps, Pec deck and Lat pulldown exercises. One control group participant was unable to comply with the evaluation for the Lat pulldown 1-RM. Individual outcomes for both the MWST and handgrip strength test are displayed in figure 1 and 2.
Figure 1.
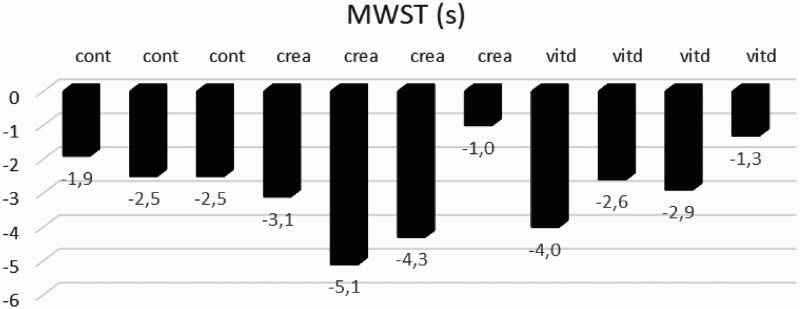
Individual differences of MWST after intervention
Cont: Control group; crea: Creatine group; vitd: Vitamin D group.
Figure 2.
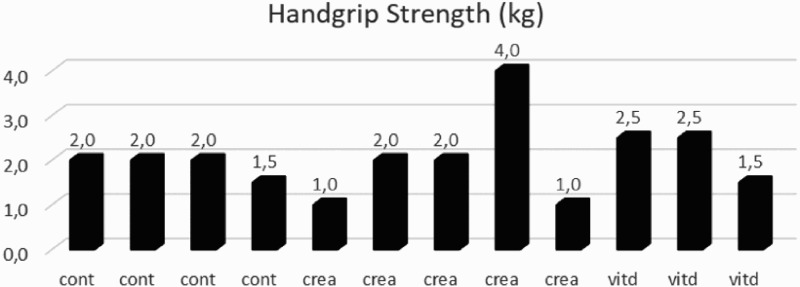
Individual differences of handgrip strength test after intervention
Cont: Control group; crea: Creatine group; vitd: Vitamin D group.
An association was detected, with moderate positive and statistically significant effect (r=0.604; P= 0.029) between Pec deck 1-RM and vitamin D levels. No other association between vitamin D levels and other variables was detected.
Discussion
To date, few studies have been designed, involving individuals with SCI, and the effects of nutritional supplementation with resistance training in muscle strength gains. This type of research is scarce and conditioned to the small number of available participants. It should also be noted that this is a very heterogeneous population, due to the type and level of injury which may, ultimately, lead to different potential in which regards strength gains.
In this study, significant improvements were observed in nearly all variables concerning the creatine group and in some variables regarding the vitamin D group. In which concerns the control group, no significant improvements were observed after the intervention. However, when directly comparing outcomes from the creatine and vitamin D group, only one improvement is statistically significant — The corrected arm muscle area, in the creatine group.
Vitamin D
Vitamin D has gained increasing attention in recent years for its actions, beyond bone health. The discovery of the vitamin D receptor in human skeletal muscle led to an increasing body of evidence regarding a direct effect in skeletal muscle activity.32 It has been long-recognized that vitamin D deficiency is associated with muscle weakness, particularly proximal muscle weakness, and that this may be resolved by restoring vitamin D levels.33 A review performed on healthy adults reported a positive association between 25(OH)D levels and muscle strength34 with a recent meta-analysis also reporting that muscle strength was significantly lower in individuals with levels below 12ng/ml.15 Therefore, deficiency levels of vitamin D may compromise muscle strength gains. In individuals with SCI, muscle strength is already impaired due to neurological injury, this adds extra importance in avoiding strength decrements from a possible vitamin D deficiency. A recent study, performed in athletes with SCI, displayed unclear results in which concerns muscle strength, when supplementing 6000 IU of vitamin D per day over twelve weeks.25
Although a consensus in which concerns optimal serum levels of 25(OH)D as not been reached amongst experts, vitamin D deficiency is mainly described in the literature as levels below 20 ng/ml. Vitamin D insufficiency is, additionally classified, by levels between 20 to 31 ng/mL, with values above 32 ng/mL indicating sufficiency.35 As expected, this study found a high prevalence (71.4%) of deficiency in which not a single participant reached levels of sufficiency. Serum values of 25(OH)D increased significantly in the vitamin D group, from mean 13.6 ± 9.1 ng/ml to 26.2 ± 7.8 ng/ml. In the vitamin D group, at baseline, three patients had vitamin D deficiency and two patients had had insufficient levels. After eight weeks of supplementation, no participants with vitamin D deficiency were found. Similar results had already been reported in another study, in which supplementing 2000 IU of vitamin D for three months, were able to reverse vitamin D deficiency in seven individuals with SCI.36 Reported mean changes in this study were very significant, with levels increasing from 14 ± 2ng/ml to 48 ± 17ng/ml after three months.
Some studies have reported association between vitamin D levels and handgrip strength values in the general population37–39 while others failed.40 In this study, a positive association between vitamin D levels and handgrip strength was not found, most likely due to only three participants being able to perform the test, while other two could not comply because of impaired hand control.
In our research, vitamin D supplementation showed a positive statistically significant association, with the Pec deck 1-RM outcome. However, this study was unable to demonstrate beneficial improvements in other muscle strength variables.
Creatine
Creatine enhances the ability of the muscle to maintain power output during brief periods of high-intensity exercise.41 Creatine supplementation has already shown benefits in healthy students during rehabilitation, due to a faster recovery rate from muscle atrophy.42
Some studies performed in individuals with SCI have shown no efficacy of creatine supplementation to promote muscle strength gains8,9 while others have shown interest.10 These inconsistent results may be partially explained by differences at the nutritional status level, exercise capacity, neurological damage, and methods used to evaluate muscle strength gains. Furthermore, all these studies used creatine supplementation without concomitant resistance training which might reduce changes in muscle strength.43 In this study, participants in the creatine group had significant improvements in almost all variables tested. However only one variable (corrected arm muscle area) reached statistical significance (p<0.05). It is likely that due to the small sample size, some variables could not reach statistical significance.
Most studies have used a “loading” protocol of 20 g of creatine per day for 4 to 6 days. However, more recent research suggests, in able-bodies, that a lower dose of 3 g creatine per day might be as effective as the “loading” protocol after 30 days.43 Nevertheless, in SCI populations it is unclear whether creatine supplementation results in increased intramuscular concentration. As previously addressed,8 it is possible that weaker muscles from SCI present impaired creatine uptake.
Our research also demonstrated, in the creatine group, a statistically significant improvement (p<0.05) in which concerns the seated medicine ball throw test when comparing to baseline, however statistical significance was not reached when comparing these outcomes with the control group. A recent study, conducted in women without SCI, revealed that creatine supplementation (0.05 g/kg body weight) combined with eight weeks of a resistance training program, could lead to improvements in the medicine ball throw. However authors also failed to detect statistically significant differences when comparing with the control group.44
Despite several allegations in which concerns detrimental effects of oral creatine supplementation in liver function, research in humans has not shown significant increases in plasma urea or liver enzyme activity even after five years of creatine supplementation. Creatine supplementation also does not seem to modify glomerular filtration rate or increase microalbuminuria.5 Considering the relative safety of creatine supplementation, more research in these populations is recommended in order to identify any potential benefits from creatine in regard to muscular strength.
Further research is warranted to understand the impact of creatine or vitamin D supplementation regarding muscle strength gains in individuals with SCI. From this research study, it is not clear whether a positive association exists.
Limitations
The sample size was limited to a small number of participants due to the stringent inclusion and exclusion criteria. It would be advisable to perform this study with only one intervention group, either creatine or vitamin D, since it was not possible to extend its duration. Due to the small sample size, this study is most likely underpowered regarding some variables which does not allow us to draw definite conclusions.
The heterogeneity of the sample, type and time of injury, is another limitation regarding this study, since the potential for muscular strength gain can differ between individuals. Only three women accepted to participate in this study with unfortunately the two dropouts being females. The only female participant was allocated in the creatine group, which might have influenced the results in this group.
Also, some participants were not able to perform all the physical tests and assessments due to specific limitations, which probably impacted the results of the groups.
Conclusion
Supplementation with creatine might improve muscle strength parameters in individuals with SCI. Vitamin D deficiency was found to be highly prevalent in individuals with SCI. Thus, it is recommended that a pre-screening for this vitamin is undertaken before the beginning of the rehabilitation therapy.
Acknowledgements
We would like to thank Laboratoires SMB S.A. for providing the ampoules D-CURE (vitamin D solubilized in olive oil) commercialized in Portugal under the brand Dmed Azevedos and placebo ampoules as well to Grupo Azevedos for financial participation in vitamin D levels testing. We also thank to AlzChem AG for providing Creapure®, which was the creatine used in this study.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL.. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord. 2011;49(11):1103–27. doi: 10.1038/sc.2011.62 [DOI] [PubMed] [Google Scholar]
- 2.Jacobs PL. Effects of resistance and endurance training in persons with paraplegia. Med Sci Sports Exerc. 2009;41(5):992–7. doi: 10.1249/MSS.0b013e318191757f [DOI] [PubMed] [Google Scholar]
- 3.Tipton KD, Phillips SM.. Dietary protein for muscle hypertrophy. Nestle Nutr Inst Workshop Ser. 2013;76:73–84. doi: 10.1159/000350259 [DOI] [PubMed] [Google Scholar]
- 4.Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A.. The prevalence of malnutrition in spinal cord injuries patients: a UK multicentre study. Br J Nutr. 2012;108(5):918–23. doi: 10.1017/S0007114511006234 [DOI] [PubMed] [Google Scholar]
- 5.Poortmans JR, Rawson ES, Burke LM, Stear SJ, Castell LM.. A-Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance Part 11. Br J Sports Med. 2010;44(10):765–6. doi: 10.1136/bjsm.2010.076117 [DOI] [PubMed] [Google Scholar]
- 6.Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003;13(2):198–226. doi: 10.1123/ijsnem.13.2.198 [DOI] [PubMed] [Google Scholar]
- 7.Devries MC, Phillips SM.. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc. 2014;46(6):1194–203. doi: 10.1249/MSS.0000000000000220 [DOI] [PubMed] [Google Scholar]
- 8.Kendall RW, Jacquemin G, Frost R, Burns SP.. Creatine supplementation for weak muscles in persons with chronic tetraplegia: a randomized double-blind placebo-controlled crossover trial. J Spinal Cord Med.. 2005;28(3):208–13. doi: 10.1080/10790268.2005.11753814 [DOI] [PubMed] [Google Scholar]
- 9.Perret C, Mueller G, Knecht H.. Influence of creatine supplementation on 800 m wheelchair performance: a pilot study. Spinal Cord. 2006;44(5):275–9. doi: 10.1038/sj.sc.3101840 [DOI] [PubMed] [Google Scholar]
- 10.Jacobs PL, Mahoney ET, Cohn KA, Sheradsky LF, Green BA.. Oral creatine supplementation enhances upper extremity work capacity in persons with cervical-level spinal cord injury. Arch Phys Med Rehabil. 2002;83(1):19–23. doi: 10.1053/apmr.2002.26829 [DOI] [PubMed] [Google Scholar]
- 11.Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int. 2013;92(2):163–76. doi: 10.1007/s00223-012-9634-1 [DOI] [PubMed] [Google Scholar]
- 12.Autier P, Boniol M, Pizot C, Mullie P.. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7 [DOI] [PubMed] [Google Scholar]
- 13.Ceglia L, Harris SS.. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013;92(2):151–62. doi: 10.1007/s00223-012-9645-y [DOI] [PubMed] [Google Scholar]
- 14.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE.. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocrine reviews. 2013;34(1):33–83. doi: 10.1210/er.2012-1012 [DOI] [PubMed] [Google Scholar]
- 15.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742 [DOI] [PubMed] [Google Scholar]
- 16.Muir SW, Montero-Odasso M.. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59(12):2291–300. doi: 10.1111/j.1532-5415.2011.03733.x [DOI] [PubMed] [Google Scholar]
- 17.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL.. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–71. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson PB, Joseph C, Angioi M.. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J Sci Med Sport. 2015;18(5):575–80. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CM, Ismaeel A, Griffis RB, Weems S.. Effects of Vitamin D Supplementation on Muscle Strength in Athletes: A Systematic Review. J Strength Cond Res. 2017;31(2):566–74. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Chen TC.. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080s–6s. doi: 10.1093/ajcn/87.4.1080S [DOI] [PubMed] [Google Scholar]
- 21.Nemunaitis GA, Mejia M, Nagy JA, Johnson T, Chae J, Roach MJ.. A descriptive study on vitamin D levels in individuals with spinal cord injury in an acute inpatient rehabilitation setting. PM R. 2010;2(3):202–8; quiz 28. doi: 10.1016/j.pmrj.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 22.Pellicane AJ, Wysocki NM, Schnitzer TJ.. Prevalence of 25-hydroxyvitamin D deficiency in the outpatient rehabilitation population. Am J Phys Med Rehabil. 2010;89(11):899–904. doi: 10.1097/PHM.0b013e3181f71112 [DOI] [PubMed] [Google Scholar]
- 23.Smith EM, Comiskey CM, Carroll AM.. A study of bone mineral density in adults with disability. Arch Phys Med Rehabil. 2009;90(7):1127–35. doi: 10.1016/j.apmr.2008.09.578 [DOI] [PubMed] [Google Scholar]
- 24.Barbonetti A, Sperandio A, Micillo A, D'Andrea S, Pacca F, Felzani G, et al. Independent Association of Vitamin D With Physical Function in People With Chronic Spinal Cord Injury. Arch Phys Med Rehabil. 2016. [DOI] [PubMed] [Google Scholar]
- 25.Flueck JL, Hartmann K, Strupler M, Perret C.. Vitamin D deficiency in Swiss elite wheelchair athletes. Spinal cord. 2016;54(11):991–5. doi: 10.1038/sc.2016.33 [DOI] [PubMed] [Google Scholar]
- 26.Pritchett K, Pritchett R, Ogan D, Bishop P, Broad E, LaCroix M.. 25(OH)D Status of Elite Athletes with Spinal Cord Injury Relative to Lifestyle Factors. Nutrients. 2016;8(6). doi: 10.3390/nu8060374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro MJ, Apple DF Jr., Staron RS, Campos GE, Dudley GA.. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol (1985). 1999;86(1):350–8. doi: 10.1152/jappl.1999.86.1.350 [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW.. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36(4):680–90. doi: 10.1093/ajcn/36.4.680 [DOI] [PubMed] [Google Scholar]
- 29.Sisto SA, Dyson-Hudson T.. Dynamometry testing in spinal cord injury. J Rehabil Res Dev. 2007;44(1):123–36. doi: 10.1682/JRRD.2005.11.0172 [DOI] [PubMed] [Google Scholar]
- 30.Harris C, Wattles AP, DeBeliso M, Sevene-Adams PG, Berning JM, Adams KJ.. The seated medicine ball throw as a test of upper body power in older adults. J Strength Cond Res. 2011;25(8):2344–8. doi: 10.1519/JSC.0b013e3181ecd27b [DOI] [PubMed] [Google Scholar]
- 31.Gagnon D, Decary S, Charbonneau MF.. The timed manual wheelchair slalom test: a reliable and accurate performance-based outcome measure for individuals with spinal cord injury. Arch Phys Med Rehabil. 2011;92(8):1339–43. doi: 10.1016/j.apmr.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 32.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. [DOI] [PubMed] [Google Scholar]
- 33.Gunton JE, Girgis CM, Baldock PA, Lips P.. Bone muscle interactions and vitamin D. Bone. 2015;80:89–94. doi: 10.1016/j.bone.2015.02.029 [DOI] [PubMed] [Google Scholar]
- 34.Redzic M, Lewis RM, Thomas DT.. Relationship between 25-hydoxyvitamin D, muscle strength, and incidence of injury in healthy adults: a systematic review. Nutrition research (New York, NY). 2013;33(4):251–8. doi: 10.1016/j.nutres.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 35.Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni OR, Hadioonzadeh R, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports medicine (Auckland, NZ). 2015;45(3):365–78. doi: 10.1007/s40279-014-0267-6 [DOI] [PubMed] [Google Scholar]
- 36.Bauman WA, Emmons RR, Cirnigliaro CM, Kirshblum SC, Spungen AM.. An effective oral vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med. 2011;34(5):455–60. doi: 10.1179/2045772311Y.0000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhanwal DK, Dharmshaktu P, Gautam VK, Gupta N, Saxena A.. Hand grip strength and its correlation with vitamin D in Indian patients with hip fracture. Arch Osteoporos. 2013;8:158. doi: 10.1007/s11657-013-0158-8 [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Gong HS, Song CH, Lee JE, Lee YH, Baek GH.. Evaluation of vitamin D level and grip strength recovery in women with a distal radius fracture. J Hand Surg Am. 2013;38(3):519–25. doi: 10.1016/j.jhsa.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 39.von Hurst PR, Conlon C, Foskett A.. Vitamin D status predicts hand-grip strength in young adult women living in Auckland, New Zealand. J Steroid Biochem Mol Biol. 2013;136:330–2. doi: 10.1016/j.jsbmb.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 40.Ceglia L, Chiu GR, Harris SS, Araujo AB.. Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin Endocrinol (Oxf). 2011;74(3):370–6. doi: 10.1111/j.1365-2265.2010.03926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bemben MG, Lamont HS.. Creatine supplementation and exercise performance: recent findings. Sports Med. 2005;35(2):107–25. doi: 10.2165/00007256-200535020-00002 [DOI] [PubMed] [Google Scholar]
- 42.Hespel P, Op't Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, et al. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536(Pt 2):625–33. doi: 10.1111/j.1469-7793.2001.0625c.xd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, et al. American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32(3):706–17. doi: 10.1097/00005768-200003000-00024 [DOI] [PubMed] [Google Scholar]
- 44.Stastny SN, Christensen BK, Hilgers Greterman S, Okamatsu H, Manikowske TL, Youd L, et al. The effect of creatine supplementation with milk combined with resistance training on strength and power in women. Gazz Med Ital - Arch Sci Med 2015 May;174(5):209–24. [Google Scholar]


