Synopsis
Barrett’s esophagus (BE) predisposes patients to esophageal adenocarcinoma (EAC). 3 to 6% of individuals with gastro-esophageal reflux disease (GERD) are estimated to have BE but only 20 to 25% of BE patients are currently diagnosed. With effective endoscopic treatment methods for dysplasia and early cancer having shifted the balance in favour of screening an acceptable and affordable screening strategy is now required. The current gold standard for diagnosis of BE is per-oral upper GI endoscopy (EGD). As this is not suitable for large-scale screening, a number of alternative methods are currently being investigated: transnasal and video capsule endoscopy, endomicroscopy, cell collection devices like the cytosponge and biomarkers. Some of these are promising; however, the majority of studies were carried out in BE enriched populations mostly in secondary care, so it is not possible to extrapolate to screening populations. Instead, well powered studies carried out in the relevant populations are needed.
Keywords: Barrett’s esophagus, Esophageal adenocarcinoma, Screening, Endoscopy, Cell collection, Biomarkers, Transnasal endoscopy, Video capsule endoscopy
Introduction
The incidence of gastroesophageal reflux disease (GERD) has increased world-wide in the last 40 years1. One of the complications of GERD is Barrett’s esophagus (BE), where esophageal squamous epithelium is replaced with columnar epithelium (metaplasia)2, a process which can be viewed as a teleological adaptation to reflux. BE predisposes patients to esophageal adenocarcinoma (EAC), a cancer with a very poor prognosis carrying an overall 5-year survival of less than 15%3,4. Furthermore, the incidence of EACs has increased dramatically in high income countries in the last 30 years5–7. Since GERD and obesity, which are the main risk factors linked to BE and EAC8–10, are still increasing, EAC rates have been projected to also further increase6.
More than 50% of EAC cases are diagnosed in patients with GERD, who are presenting with alarm symptoms when the cancer is typically advanced11,12. Furthermore, it is estimated that only 20 to 25% of patients with the pre-malignant condition BE are diagnosed. Hence there is little chance of altering the population mortality from EAC through BE surveillance and endoscopic treatment regimes12,13. For those patients who are diagnosed with BE the impact of surveillance programmes is controversial11,14–16. However, when performed well surveillance of BE patients can significantly improve EAC outcomes including cancer-related mortality, especially more recently using outpatient based endoscopic therapies for early disease, which obviate the requirement for surgical intervention17. The question therefore arises whether screening could reduce mortality from oesophageal cancer. Screening should be aimed at detecting early stage cancer, when it is easier to treat, or precancerous stages, when development of cancer can be prevented by removing the pre-cancerous lesion. Screening programmes have already been implemented for several cancers, for example cervical or breast cancer.
Rationale for Screening
To prevent oesophageal cancer screening would be aiming to detect BE. The evidence would suggest that such a programme would be in line with the Wilson and Jungner criteria for the selection of conditions suitable for screening18:
- There should a recognizable latent stage:
-
○BE is a pre-cancerous stage with a long natural history that can be recognised by endoscopy and biopsy.
-
○
- Early detection should lead to a more favorable prognosis:
- ○
-
○Evidence is currently not conclusive whether individuals diagnosed through surveillance programs experience improved survival12.
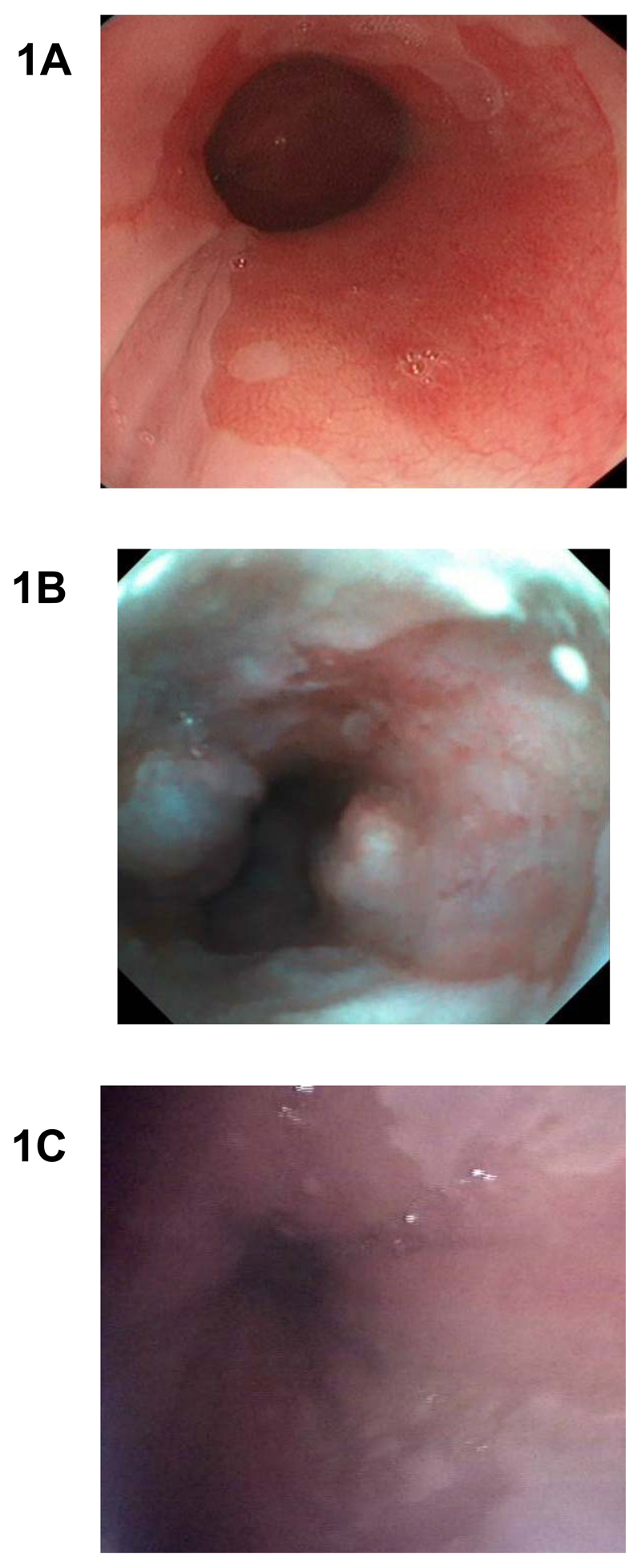
The magnitude of the problem is likely to be large since between 3 and 6% of individuals with GERD are estimated to have BE22. Therefore, in order to identify this number of cases an affordable screening strategy, which is acceptable to the relevant population, is required. Currently diagnosis of BE has been dependent on per-oral upper GI endoscopy (EGD), see Fig. 1A for example diagnostic image. However, this is not suitable for population screening due to the invasive and expensive nature of the test. EGD does not only costs on average £650 or $866 per patient23, but also incurs indirect costs due to patients having to take time off work and require being accompanied24.
Fig. 1. Endoscopic diagnosis of Barrett’s esophagus with conventional per-oral and office-based transnasal endoscopy.
(A) High resolution white light endoscopy. Barrett’s esophagus appears as salmon red coloured mucosa and normal oesophagus in pale pink. (B) Transnasal EG scan endoscopic view of a short segment of Barrett’s esophagus. (C) Transnasal endosheath endoscopic diagnosis of Barrett’s. This technology also allows biopsies for histological confirmation.
Current Guidelines for Diagnosis of BE
The latest guidelines for the diagnosis of BE by the American College of Gastroenterology (ACG), British Society of Gastroenterology (BSG), Danish Society for Gastroenterology and Hepatology (DSGH), French Society of Digestive Endoscopy (FSDE), and Cancer Council Australia (CCA) all recommend the following:25–29
-
-
EGD is considered as the gold standard.
-
-
Diagnosis should be based on visual evidence of columnar-lined epithelium.
-
-
The length of the BE segment should be recorded using Prague criteria.
-
-
Histological confirmation with biopsies using the Seattle protocol should be performed.
However, their recommendations for screening for BE differ and are summarized in Table 1. None of the national guidelines recommend a population screening program, and, where discussed, it relies on endoscopy and is only recommended for patients with chronic GERD and several risk factors for BE and EAC. Despite general discussions of alternative screening methods, only the ACG guidelines suggest the use of an alternative screening method, which is unsedated transnasal endoscopy (TNE)25.
Table 1.
Recommendations for Barrett’s esophagus screening
| American College of Gastroenterology25 | British Society of Gastroenterology26 | Danish Society for Gastroenterology and Hepatology29 | French Society of Digestive Endoscopy28 | Cancer Council Australian27 | |
|---|---|---|---|---|---|
| TO BE CONSIDERED FOR | Men only with chronic and / or frequent GERD and two or more risk factors | Patients with chronic GERD and multiple risk factors | Patients with chronic, longstanding GERD at increased risk | N/C | N/C |
| PATIENTS WITH FAMILY HISTORY | N/C | Threshold of risk factors can be lowered in presence of family history | N/C | N/C | N/C |
| POPULATION SCREENING | Not recommended | Not recommended | Not recommended | N/C | Not recommended |
| ALTERNATIVE SCREENING METHODS | TNE can be considered as an alternative | N/C | N/C | N/C | N/C |
GERD, Gastro-esophageal reflux disease; N/C, no comment, TNE, transnasal endoscopy
Evaluating Novel Screening Test
A diagnostic screening test for BE should be accurate and cost effective when applied on a population level. Furthermore, it should be simple to administer and acceptable to patients, so would ideally be performed in a non-hospital setting, for example, in a GP surgery.
When evaluating the performance of a novel clinical test, the most commonly used measures are sensitivity, specificity, predictive value, likelihood ratio and under the receiver operating characteristic curve. An ideal screening test would be both highly sensitive, meaning with few false negative results, i.e. few actual cases are missed, and highly specific, meaning producing few false positive results, i.e. resulting in few subjects without the disease having to undergo follow-up procedures30. Suitable sensitivity and specificity should be individually assessed for each test and healthcare setting. For example, a screening test with low specificity would result in many individuals without BE having to undergo endoscopy, which would not be suitable in a healthcare setting with overstretched endoscopy clinics.
It is a common misconception that sensitivity and specificity of a test do not vary with disease prevalence enabling comparison between different study populations. However, a number of studies have shown that test sensitivity and specificity are not as stable as assumed31,32, and do vary with the prevalence and distribution of the disease in the sample population due to an effect called ‘spectrum bias’ or ‘spectrum effect’33. For example, variation in disease prevalence between different study populations may result in differences of symptoms and disease severity, in turn resulting in variations of test sensitivity and specificity. In the case of BE, patients undergoing regular surveillance might, on average, have longer Barrett’s fragments than undiagnosed patients in a screening population, which could impact on test sensitivity and specificity. As the prevalence of BE is low in the screening population of interest, namely patients with chronic GERD, large studies would be required to accurately calculate measures of test accuracy like sensitivity and specificity. As this is often not possible, new tests are commonly validated in populations comprising a large proportion of BE patients undergoing surveillance, as can be seen in the studies cited below. Care must therefore be taken when comparing the performance of tests evaluated in different populations and interpreting test performances.
Novel Screening Methods
A number of alternative methods to EGD are currently being investigated, which are summarized below and in Table 2.
Table 2.
Characteristics on studies assessing alternative methods of diagnosing BE. Were sensitivity and specificity were not calculated as part of the study, we estimated these measures based on the information available.
| Study | Type of study | Year | Setting | Patient group (n) | Method | BE diagnosis | Se / Sp compared to EGD (%) | No of side-effects | Acceptability measures | Preferred future procedure |
|---|---|---|---|---|---|---|---|---|---|---|
| Transnasal endoscopy (Imaging and cell sampling for pathology) | ||||||||||
| Jobe et al.37 | Randomised crossover | 2006 | Tertiary care; USA | GERD (89) + BE (32) | Unsedated small calibre endoscopy | Based on biopsy for ZAP grades I-III | Se: 84% | 3 complications | Endoscopic tolerability questionnaire | 71% TNE vs 29% EGD |
| Shariff et al.38 | Randomised crossover | 2012 | Tertiary care; UK | BE (49) + controls (46) | Unsedated transnasal endoscopy | Visible BE + IM on biopsy | 98% / 100% | No AEs | STAI + 10 point VAS | 59% TNE vs 33% EGD |
| Shariff et al.39 | Randomized, crossover | 2016 | Tertiary care; UK | BE + controls (21 total) | Transnasal disposable endosheath | Visible BE + biopsies according to 2005 BSG guidelines | 100% / 100% for endoscopic diagnosis; 67% / 100% for histologic diagnosis |
N/R | 10 point VAS | 60% TEE vs 25% EGD |
| Esophageal capsule endoscopy (Imaging only) | ||||||||||
| Koslowsky et al.68 | Prospective single screen | 2006 | Tertiary care, Israel | GERD (42) + BE (8) | Untethered capsule | Visible BE + confirmation on biopsy | 75% / 100% (4 fps); 100% / 100% (14 fps) |
No AEs | N/I | N/I |
| Lin et al.69 | Prospective single screen | 2007 | Tertiary care, USA | GERD (66) + BE (24) | Untethered capsule | Visible BE + IM on biopsy | 67% / 84% | 2AEs | N/I | N/I |
| Galmiche et al.44 | Prospective single screen | 2008 | Tertiary care, USA | GERD (77) | Untethered capsule | Visible BE + IM or GM on biopsy | 71% / 99% | No complications | N/I | N/I |
| Ramirez et al.43 | Prospective single screen | 2008 | Tertiary care, USA | GERD (100) | Tethered capsule | Visible BE + IM on biopsy | 78% / 82% (visual lesions); 93% / 78% (biopsy) |
No complications | 3 point VAS | 81% ECE |
| Sharma et al.70 | Prospective single screen | 2008 | Tertiary care, USA | GERD (41) + BE (53) | Untethered capsule | Visible BE + IM on biopsy | GERD patients: 67% / 87%; BE patients: 79% / 78% |
No SAEs; AEs N/R | N/I | N/I |
| Gralnek et al.71 | Prospective single screen | 2008 | Tertiary care, Israel | Esophageal disease incl. BE (28) | Untethered capsule | Visible BE (ZAP classification) | 100% / 74% | No SAEs; AEs N/R | N/I | N/I |
| Delvaux et al.72 | Prospective single screen | 2008 | Tertiary care, Germany / Fance | GERD (32); BE (5), other esophageal conditions (61) | Untethered capsule | Visible BE ≥2cm length | 45% / 85% | No complication | N/I | N/I |
| Bhardwaj, et al.42 | Meta-analysis | 2009 | N/A | GERD (618, range 20 to 106) | Tethered and untethered | N/A | Pooled Se: 77% Pooled Sp: 86% |
N/A | N/A | N/A |
| Chavalitdhamrong et al.45 | Retrospective review of single screens | 2011 | Tertiary care; USA | GERD (502) | Untethered capsule (Pillcam ESO) | Visible BE + confirmed columnar lining / IM on biopsy | Se: 83% (visual); 50% (IM) | N/A | N/I | N/I |
| Optical coherence tomography (Imaging only) | ||||||||||
| Evans et al.47 | Blinded, prospective trial (single screen) | 2007 | Tertiary care; USA | Patients undergoing routine upper endoscopy including with IM (<1cm) (113) | OCT with diagnostic algorithm (SCJ only) | IM on biopsy | Se for IM at SCJ: 81%; Sp 66% or 57% depending on reader |
N/R | N/I | N/I |
| Volumetric laser endomicroscopy (Imaging only) | ||||||||||
| Gora et al.46 | Single screen pilot study | 2013 | N/R; USA | Healthy (7) and BE (6) | Tethered capsule endomicroscopy | N/R | Se/Sp N/R; high quality endomicroscopic images | No complications | N/I | 92% VLE |
| Wolfsen et al.48 | Prospective, multicentre safety and feasibility study | 2015 | Tertiary care, USA | Known or suspected BE (100) | Nvision VLE system | N/R | Se/Sp N/R; Visualisation of mucosa and submucosa in 87% | 2 AEs | N/I | N/I |
| Trindade et al.49 | Retrospective review of stored VLE images | 2016 | Tertiary care; USA | Slide set of cardia, oesophagus, BE (120) | Nvision VLE system | N/R | 88% / 92% (non-neoplastic) | N/A | N/I | N/I |
| Non-endoscopic cell sampling devices combined with pathology | ||||||||||
| Fennerty el al50 | Single screen pilot study | 1995 | Tertiary care; USA | BE (10) | Balloon cytology | Presence of IM | No goblet cells or definitive columnar cell dysplasia collected | N/R | N/I | N/I |
| Rader et al.51 | Single screen pilot study | 2001 | Tertiary care; USA | BE (11) | Flexible mesh catheter | Visible BE + IM on biopsy | Se 87% | No complications | N/I | N/I |
| Non-endoscopic cell sampling devices combined with biomarkers | ||||||||||
| Kadri et al.56 | Prospective cohort study | 2010 | Primary care, UK | GERD (504) | Cytosponge-TFF3 test | Visible BE (≥C1) + IM on biopsy | 73% / 94% (C≥1); 90% / 94% (C≥2) | No SAEs; AEs N/R | STAI, Impact of events scale, 10 point VAS | N/I |
| Ross-Innes et al.57 | Case-control study | 2015 | Tertiary care; UK | GERD (463) and BE (647) | Cytosponge-TFF3 test | Visible BE (≥C1 or ≥M3) + IM on biopsy | 80% / 92% (C≥1); 87% / 92% (C≥3) |
3 SAEs; AEs N/R | 10 point VAS | N/I |
| Lao-Sirieix et al. (abstract)54 | Prospective single screen | 2015 | Tertiary care; UK | BE (73) | Updated Cytosponge+TFF3 test | Visible BE (≥C1 or ≥M3) + IM on biopsy | Se: 92% | no complications | 10 point VAS | N/I |
| Blood biomarkers | ||||||||||
| Bus et al.65 | Pilot study | 2016 | Tertiary care; Netherlands | Controls (15), BE (41), EAC (59) | Circulating microRNAs | Visible BE + IM on biopsy | 78% / 86% in validation cohort | N/R | N/I | N/I |
AE, adverse event; BE, Barrett’s esophagus; ECE, Esophageal capsules endoscopy; EGD, per-oral upper GI endoscopy; GERD, Gastro-esophageal reflux disease; GM, Gastric metaplasia; IM, Intestinal metaplasia; N/I, not investigated; N/R, not reported; OCT, Optical coherence tomography; SAE, serious adverse event; SCJ, squamo-columnar junction; Se, sensitivity; Sp, specificity; STAI, Spielberger State-Trait Anxiety Inventory; TFF3, trefoil factor 3; VAS, visual analogue scale; VLE, Volumetric laser endomicroscopy.
Endoscopic Screening
Transnasal endoscopy
TNE is a less invasive alternative for visualizing the esophagus (Fig. 2B). This procedure uses a thinner caliber scope, less than 6 mm in diameter34. It can be performed using only topical anesthetic, thus avoiding sedation, as gagging is prevented by avoiding contact with the root of the tongue, as would occur during oral intubation. Furthermore, the more compact design of TNE equipment and the use of disposable transnasal endosheeth endoscopy (TEE) (Fig. 2C) obviating the need for sterilization would allow office or mobile unit based screening35,36.
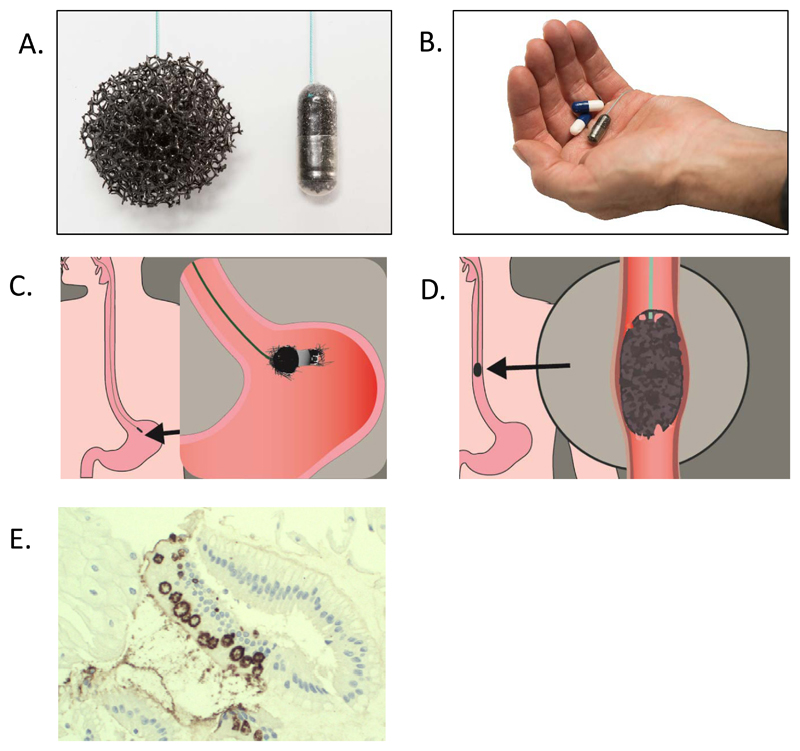
Fig. 2. Use of the Cytosponge™ test.
(A) Expanded Cytosponge (left) and Cytosponge embedded in gelatine capsule (right). (B) The Cytosponge compared to paracetamol capsules in the palm of a hand. (C) The Cytosponge is swallowed and the gelatin capsule dissolves in the stomach within 5 minutes. (D) The Cytosponge is retrieved by a nurse collecting cells as it is pulled up. (E) Immunohistochemical images (20x magnification) illustrating TFF3-positive staining in cells collected with the Cytosponge.
Three randomized, cross-over studies in GERD populations enriched with up to about 50% of BE surveillance patients compared the efficacy of TNE with EGD to detect BE. Jobe and colleagues compared unsedated office based TNE (Olympus, small caliber, diameter 5.1mm) with EGD in a study of 121 individuals, and estimated the sensitivity for diagnosis of BE to be 84%37. The agreement between the two approaches, however, was only moderate (kappa=0.591). Patients undergoing EGD experienced significantly more anxiety, pain, gagging and choking. Using an ultrathin endoscope (EG530N; Fujinon, diameter 5.9mm) Shariff and colleagues found in a study of 95 individuals that TNE was an accurate and well-tolerated method for diagnosing BE with a sensitivity and specificity of 98% and 100% respectively38. Furthermore, they observed high correlation between the two modalities (R2 = 0.97; P<0.001). Shariff and colleagues also investigated TEE in a small pilot study in 25 BE patients and controls39. Compared to EGD, TEE had a sensitivity and specificity of 100% for endoscopy diagnosis, but lower sensitivity (66.7%) for histologic diagnosis. Furthermore, the mean optical quality of EGD was significantly better (Fig.1).
Sami and colleagues carried out a systematic review and meta-analysis to estimate the patient preference and acceptability of unsedated TNE40. The pooled difference in proportion of patients who preferred TNE over EGD was 63% (95% CI, 49.0-76.0, 10 studies) and acceptability was high for TNE (85.2%; 95% CI, 79.1-89.9; 16 studies). This included the two studies by Jobe et al. and Shariff et al. (2012) who found that the majority, 71% and 59% of patients expressed a preference for TNE37. Patients also reported a significantly better experience with TEE, preferring TEE to EGD 38,39. However, a prospective randomised controlled trial in a community population of over 400 patients did not observe increased participation rates for TEE when comparing invitations to EGD, TEE in a mobile van or TEE in a hospital outpatient unit35. Furthermore, the cost of ultrathin devices is comparable to standard endoscopes limiting its use to population screening of high risk patients. In addition, to fully assess the suitability of TEE for population screening the accuracy would have to be assessed in a large screening cohort in a community setting.
Video capsule endoscopy
Esophageal capsules endoscopy (ECE) allows direct non-invasive visualization of the esophagus, which does not require sedation, but also does not allow for taking of biopsies. The majority of studies using this technology for the diagnosis of BE were carried out using an untethered dual-camera wireless capsule endoscope (PillCam ESO), which was approved by the FDA in 200441, however, tethered cameras have also been used (Table 2). A meta-analysis of the diagnostic accuracy of ECE for BE in patients with GERD found nine studies comprising a total of 618 patients42. They estimated the pooled sensitivity and specificity of ECE for the diagnosis of BE overall to be 77% and 86% respectively (no confidence intervals were provided). There was some variation in specificity observed when they either included studies using EGD (90%) or histologically confirmed intestinal metaplasia (IM) (73%) as the reference standard, however, the sensitivity remained the same (78%). The majority of study populations consisted of a combination of screening and BE surveillance populations, however, two of the studies included screening patients only. These two studies of 77 and 100 GERD patients reported sensitivities of 60% and 78% and specificities of 100% and 89% respectively43,44. Chavalitdhamrong and colleagues carried out a retrospective review of 502 ECE Pillcam™ ESO (Given Imaging) video files for patients with GERD to assessing ECE video imaging45. They identified 12 BE patients, which we used to estimate the sensitivity for BE diagnosis as 83% and 50% compared to visual inspection by EGD and histological confirmation with IM respectively. ECE was found to be safe in all studies; however, patient preference was only investigated in one study, where 81% of patients preferred ECE to EGD43.
When the cost effectiveness of ECE compared to EGD was investigated though, the costs were found to be very similar, and the ability to perform the procedure without sedation is negated by the cost of ECE capsule and equipment24. Despite its attractiveness for screening as it is safe and does not require sedation, its costs currently seem prohibitive. Furthermore, this tool would have to be tested in an appropriate screening population to accurately estimate its accuracy.
Volumetric laser endomicroscopy
Volume laser endomicroscopy (VLE) is a new generation optical coherence tomography which produces high-resolution cross-sectional images of the esophagus. Tearney and colleagues have developed a tethered capsule microendoscopy, which involves swallowing an optomechanically engineered capsule that uses optical frequency domain imaging (OFDI) technology to provide three-dimensional microscopic images of the digestive organs46. OFDI has previously been shown to have capability for the diagnosis of BE47. As the capsule travels through the digestive tract it captures cross-sectional microscopic images at 30 µm (lateral) x 7 µm (axial), which can be used to reconstruct a three-dimensional microscopic representation of the entire organ. In a small, proof of principle study in 7 healthy and 6 BE patients, this technology produced endomicroscopic images of the esophageal mucosa, which could distinguish between patients with and without BE46. Furthermore, 12 of the subjects reported a preference for tethered capsule endomicroscopy over EGD. Once the capsule is withdrawn it can be disinfected for reuse, making it potentially inexpensive and feasible to be used for population screening.
A commercially available OFDI-based imaging system (Nivision VLE System) was evaluated in a safety and feasibility study of 100 patients with BE, where the procedure was shown to be safe. VLE was successfully performed in 87 cases, enabling visualization of the mucosa and submucosa48. 120 stored Nivision VLE images of BE patients with and without dysplasia were evaluated retrospectively blinded to endoscopic and clinical findings49. As OCT and VLE are limited in differentiating between low-grade dysplasia and non-dysplastic BE they were combined in one group (non-dysplastic BE). The overall agreement between users was excellent (kappa = 0.81; 95% CI, 0.79-0.83) when combining non-neoplastic BE and neoplastic BE, however, it was lower for non-dysplastic BE with kappa = 0.66 (95% CI, 0.63-0.69). Compared to EGD, the sensitivity and specificity for non-neoplastic BE (non-dysplastic and low grade dysplasia) was 88% (95%CI, 83%-91%) and 92% (95% CI, 90%-94%) respectively. Even though this is a very promising new technology, the resolution of these images is still poor and the technology requires further development. It also needs to be tested in a number of prospective controlled trials, both in BE and screening populations, to assess suitability for BE screening in a GERD population. For all of these imaging modalities one question that remains is whether an optical diagnosis can suffice without a tissue sample. With the current state of the art it is likely that a tissue biopsy will still be required to confirm the findings from these technologies.
Non-Endoscopic Screening
The non-endoscopic screening methods described below are less invasive than endoscopic methods and can be more readily carried out in primary care, resulting in higher acceptability for patients. In addition, by removing the requirement for a skilled operator, there is also the potential to reduce the cost.
Cell collection devices
A number of relatively simple and low cost non-endoscopic devices, including inflatable balloons and sponges have been developed for collection of esophageal samples. An early study evaluated the use of a cytology balloon for specimen collection from 10 BE patients, however, this device did not collect columnar cells from any of the patients, so was not suitable for BE screening50. Another pilot study investigated the use of a prototype flexible mesh catheter for the diagnosis of BE in patients undergoing surveillance51. Of the 11 BE patients in the study adequate specimens, defined as the presence of at least one glandular cell group, were obtained from 8 patients (73%) and the sensitivity amongst adequate samples was 87.5%. However, even though balloon cytology had a high sensitivity for EAC or BE with high grade dysplasia (80%), the sensitivity was significantly lower for BE with low grade dysplasia or without dysplasia (56%) when tested in a surveillance population52.
The combination of a modified cell collection device with a biomarker has proven more successful. The Cytosponge™ is a cell collection device developed at Cambridge University in the UK. It is composed of a reticulated foam sphere approximately 30 mm in diameter compressed within a gelatin capsule and attached to a string (Fig. 2A and B). The patient swallows the capsule while holding onto the string. The gelatin capsule dissolves after 5 minutes, allowing the sponge to expand and the sponge is pulled up from the stomach to the esophagus and mouth (Figure 2C and D). The cells it collects from the gastro-esophageal junction and the entire length of the esophagus are processed and assesses for the presence of Trefoil Factor 3 (TFF3), a biomarker for BE (Fig. 2E). TFF3 was identified in a gene expression study as a marker specifically for intestinal cells of BE, but not columnar cells derived from the normal gastric cardia or upper airways53.
The Cytosponge™ device combined with the TFF3 test has been tested in 4 clinical studies so far54–57. In an initial cohort study of over 500 GERD patients selected from patients taking acid-suppressants in primary care the procedure was safe and the vast majority of participants (99%) successfully swallowed the device56. Compared to EGD, the sensitivity and specificity of the test were 73.3% (95% CI, 44.9% - 92.2%) and 93.8% (95% CI, 91.3% - 95.8%) respectively for circumferential length of BE of 1cm or more (≥C1) and 90.0% (95% CI, 55.5% - 99.6%) and 93.3% (95% CI, 90.9% - 95.5%) for segments of 2cm or more (≥C2). With 3% (15/501) of the study participants having an endoscopic diagnosis of BE, the sample size was not powered adequately to obtain accurate estimates of the sensitivity and specificity. A large case control study of over 1,000 patients (463 controls with dyspepsia and 647 BE cases) allowed more accurate evaluation of the safety, accuracy and acceptability of this test57. The overall sensitivity of the Cytosponge-TFF3 test in this population was 79.9% (95% CI, 76.4% - 83.0%) for ≥C1, which increased to 87.2% (95% CI, 83.0% - 90.6%) for ≥C3. The specificity was 92.4% (95% CI, 89.5% - 94.7%). The sensitivity increased to 89.7% (95% CI, 82.3% - 94.8%) for patients having a repeat procedure. A commercial version of the Cytosponge™ device in combination with the TFF3 test was found to have a higher overall sensitivity of 91.5% in a smaller prospective study (73 patients)54.
The acceptability of the Cytosponge™-TFF3 test was high with 82% of participants reporting low levels of anxiety before the test. Furthermore, the Cytosponge™ was rated favorably compared to endoscopy (p<0.001). In a qualitative study investigating the acceptability of the Cytosponge™ using interviews and focus groups the acceptability was found to be high, and participants perceived the test to be more comfortable and practical than endoscopy58.
It is noteworthy that the BEST1 and 2 trials have evaluated the accuracy and acceptability of the Cytosponge™-TFF3 test in large, prospective trials; namely a large GERD patient screening population (504 participants) to test feasibility and acceptability in primary care and a large BE enriched population in tertiary care (1110 participants) to obtain more accurate estimates of the test accuracy. Furthermore, a microsimulation of costs was carried out to compare the health benefits and cost effectiveness of screening for BE by either Cytosponge™ or EGD vs no screening which suggested that the Cytosponge™ test could be cost-effective when combined with endoscopic therapy59. This test therefore has the characteristics for a clinically acceptable screening tool: suitability for primary care, high acceptability and tolerability, low cost, and high accuracy. To further assess the suitability of the Cytosponge™-TFF3 test the BEST3 trial (Trial ID ISRCTN68382401) aiming to assess whether invitation to a Cytosponge™–TFF3 test for patients with reflux symptoms will be effective in increasing the detection of BE in primary care and to evaluate its cost effectiveness is due to start in 2017. Patient acceptability will also be evaluated in the BEST3 trial.
Circulating molecular markers
A blood based screening test would be an appealing alternative, as these tests are less invasive, pose minimal risk to patients, and can be carried out in a primary care setting, all of which would increase patient acceptability and appeal. One example is detection of circulating microRNAs (miRNAs) as a method for diagnosing BE. miRNAs are approximately 21 to 25 nucleotides in length, are stable and can be detected in circulating plasma60. They regulate numerous cellular processes and dysregulation of their function has been associated with the pathogenesis of many diseases, including cancer61,62. Eleven studies, seven of which specifically compared normal epithelium and non-dysplastic BE, investigated microRNAs with high biomarker potential for screening and disease monitoring in the esophageal epithelium63. Overall, five biomarkers were identified as promising tissues markers for diagnosing BE. Russo and colleagues confirmed that circulating miRNA levels of two of these markers, miR-145 and miR-215, were significantly increased in BE compared to esophagitis controls64, however, these were not further validated in a larger population. In another pilot study, a different combination of two miRNAs, miR-194-5p and miR-451a, were significantly increased and one, miR136, significantly decreased in BE compared to controls65. These were further investigated in a larger validation study and a combination of four miRNAs were found to be the most informative panel in distinguishing controls (15 patients) from BE (41 patients) with sensitivity and specificity of 78.4% (95% CI, 61.8 – 90.2%) and 85.7% (95% CI, 57.2% – 98.2%) respectively. A limitation of this study, however, was the fact that control patients did not have reflux symptoms, so the changes of miRNAs levels identified could simply be due to GERD. It seems feasible to detect BE using these blood based mi-RNA markers, however, validation studies in larger cohorts are required.
A number of different circulating auto-antibodies, both alone and in combination, have also been investigated for early detection of esophageal cancer66. However, even though studies reported positive associations, the test sensitivities were too low and variability between studies was high. Breath markers also represent an attractive method for cancer screening as it is non-invasive, provides results quickly and is relatively cheap, however, studies are currently limited. One study identified a panel of breath volatile organic compounds (VOCs) that could be used to distinguish esophageal cancer from BE and begin conditions of the upper gastro-intestinal tract, but these VOCs have not been investigated for diagnosis of BE yet67.
Conclusions
BE is a condition which fulfills the criteria for a screening test in order to reduce population mortality from EAC. Endoscopy is the gold standard diagnostic tool, however, less invasive and more cost effective alternatives are required. A number of technologies have been studied some of which are promising; however the majority of studies were carried out in high prevalence populations mostly in secondary care. As discussed it is not appropriate to extrapolate the sensitivity and specificity of these new diagnostic tests developed and validated in high prevalence secondary care populations to a screening scenario in a population with low prevalence as this could result in a decrease in sensitivity and increase in specificity31. Instead, well powered studies carried out in the relevant populations are needed and hence careful consideration should be given to whether the test should be given to an enriched population according to their level of risk. In addition, studies should include evaluation of the acceptability and health economics before decisions can be made about implementing a new test in standard clinical care.
Key Points.
Currently diagnosis of BE is dependent on endoscopy, however this is not suitable for large-scale screening due to the invasive and expensive nature of the test
Less invasive tools such as transnasal and video capsule endoscopy are promising alternatives, but high costs are prohibitive for large-scale screening at the moment.
Non-endoscopic screening methods are less invasive than endoscopic methods and can be more readily carried out in primary care, resulting in higher acceptability for patients.
Large, randomised trials in the primary care setting are required to determine whether screening for Barrett’s oesophagus is feasible and effective
Footnotes
Conflicts of interest
The authors disclose the following: R.C.F. holds patents on the Cytosponge technology, which has been licensed by MRC Technology to Covidien GI Solutions (now Medtronic). R.C.F. has no direct financial arrangement with Metronic. J.O. has no conflict of interest to declare.
References
- 1.Boeckxstaens G, El-Serag HB, Smout AJPM, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63(7):1185–1193. doi: 10.1136/gutjnl-2013-306393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald SA, Lavery D, Wright NA, Jansen M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol. 2015;12(1):50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973-1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope[quest] The American journal of gastroenterology. 2003;98(7):1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Research UK. Oesophageal cancer statistics. [Accessed 25/11/2015];2015 http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer.
- 5.Edgren G, Adami H-O, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62(10):1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- 6.Kong CY, Kroep S, Curtius K, et al. Exploring the recent trend in esophageal adenocarcinoma incidence and mortality using comparative simulation modeling. Cancer Epidemiol Biomarkers Prev. 2014;23(6):997–1006. doi: 10.1158/1055-9965.EPI-13-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otterstatter MC, Brierley JD, De P, et al. Esophageal cancer in Canada: Trends according to morphology and anatomical location. Canadian Journal of Gastroenterology. 2012;26(10):723–727. doi: 10.1155/2012/649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness-Jensen E, Gottlieb-Vedi E, Wahlin K, Lagergren J. All-cause and cancer-specific mortality in GORD in a population-based cohort study (the HUNT study) Gut. 2016 doi: 10.1136/gutjnl-2016-312514. [DOI] [PubMed] [Google Scholar]
- 9.Kubo A, Corley DA. Body Mass Index and Adenocarcinomas of the Esophagus or Gastric Cardia: A Systematic Review and Meta-analysis. Cancer Epidem Biomar. 2006;15(5):872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y. Epidemiology of esophageal cancer. World journal of gastroenterology : WJG. 2013;19(34):5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett's oesophagus: a population-based study. Gut. 2015;64(1):20–25. doi: 10.1136/gutjnl-2013-305506. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan TL, Fitzgerald RC. Precision prevention of oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(4):243–248. doi: 10.1038/nrgastro.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310(6):627–636. doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 14.Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145(2):312–319 e311. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's Esophagus and Mortality from Esophageal Adenocarcinoma: A Population-Based Cohort Study. The American journal of gastroenterology. 2014;109(8):1215–1222. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 16.Whiteman DC. Does a prior diagnosis of Barrett's oesophagus influence risk of dying from oesophageal adenocarcinoma? Gut. 2015;64(1):5–6. doi: 10.1136/gutjnl-2014-307171. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Naik AD, Duan Z, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett's oesophagus. Gut. 2016;65(8):1252–1260. doi: 10.1136/gutjnl-2014-308865. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JMG, J G. Principles and practice of screening for disease. Geneva: WHO; 1968. [Google Scholar]
- 19.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency Ablation in Barrett's Esophagus with Dysplasia. New England Journal of Medicine. 2009;360(22):2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 20.Phoa K, van Vilsteren FI, Weusten BM, et al. Radiofrequency ablation vs endoscopic surveillance for patients with barrett esophagus and low-grade dysplasia: A randomized clinical trial. JAMA. 2014;311(12):1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 21.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119(6):1149–1158. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99(4):918–922. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 23.Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett's esophagus. Gastroenterology. 2009;136(7):2101–2114 e2101–2106. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenstein JH, Inadomi JM, Brill JV, Eisen GM. Cost Utility of Screening for Barrett's Esophagus With Esophageal Capsule Endoscopy Versus Conventional Upper Endoscopy. Clinical Gastroenterology and Hepatology. 5(3):312–318. doi: 10.1016/j.cgh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett/'s Esophagus. The American journal of gastroenterology. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2013 doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman DC, Appleyard M, Bahin FF, et al. Australian clinical practice guidelines for the diagnosis and management of Barrett's esophagus and early esophageal adenocarcinoma. Journal of Gastroenterology and Hepatology. 2015;30(5):804–820. doi: 10.1111/jgh.12913. [DOI] [PubMed] [Google Scholar]
- 28.Boyer J, Laugier R, Chemali M, et al. French Society of Digestive Endoscopy SFED guideline: monitoring of patients with Barrett’s esophagus. Endoscopy. 2007;39(09):840–842. doi: 10.1055/s-2007-966653. [DOI] [PubMed] [Google Scholar]
- 29.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointestinal endoscopy. 2006;63(4):570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. Inhalation toxicology. 2014;26(13):811–828. doi: 10.3109/08958378.2014.955932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usher-Smith JA, Sharp SJ, Griffin SJ. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ. 2016;353:i3139. doi: 10.1136/bmj.i3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2013;185(11):E537–544. doi: 10.1503/cmaj.121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299(17):926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 34.Atar M, Kadayifci A. Transnasal endoscopy: Technical considerations, advantages and limitations. World Journal of Gastrointestinal Endoscopy. 2014;6(2):41–48. doi: 10.4253/wjge.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sami SS, Dunagan KT, Johnson ML, et al. A Randomized Comparative Effectiveness Trial of Novel Endoscopic Techniques and Approaches for Barrett's Esophagus Screening in the Community. The American journal of gastroenterology. 2015;110(1):148–158. doi: 10.1038/ajg.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peery AF, Hoppo T, Garman KS, et al. Feasibility, Safety, Acceptability and Yield of Office-based, Screening Transnasal Esophagoscopy. Gastrointestinal endoscopy. 2012;75(5):945–953.e942. doi: 10.1016/j.gie.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett's esophagus: a randomized and blinded comparison. The American journal of gastroenterology. 2006;101(12):2693–2703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 38.Shariff MK, Bird-Lieberman EL, O'Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointestinal endoscopy. 2012;75(5):954–961. doi: 10.1016/j.gie.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Shariff MK, Varghese S, O’Donovan M, et al. Pilot randomized crossover study comparing the efficacy of transnasal disposable endosheath with standard endoscopy to detect Barrett’s esophagus. Endoscopy. 2016;48(02):110–116. doi: 10.1055/s-0034-1393310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sami SS, Subramanian V, Ortiz-Fernández-Sordo J, et al. Performance characteristics of unsedated ultrathin video endoscopy in the assessment of the upper GI tract: systematic review and meta-analysis. Gastrointestinal endoscopy. 2015;82(5):782–792. doi: 10.1016/j.gie.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Sharma VK, Eliakim R, Sharma P, Faigel D. ICCE Consensus for Esophageal Capsule Endoscopy. Endoscopy. 2005;37(10):1060–1064. doi: 10.1055/s-2005-870311. [DOI] [PubMed] [Google Scholar]
- 42.Bhardwaj A, Hollenbeak CS, Pooran N, Mathew A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett's esophagus in patients with gastroesophageal reflux disease. The American journal of gastroenterology. 2009;104(6):1533–1539. doi: 10.1038/ajg.2009.86. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez FC, Akins R, Shaukat M. Screening of Barrett's esophagus with string-capsule endoscopy: a prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointestinal endoscopy. 2008;68(1):25–31. doi: 10.1016/j.gie.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 44.Galmiche JP, Sacher-Huvelin S, Coron E, et al. Screening for esophagitis and Barrett's esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. The American journal of gastroenterology. 2008;103(3):538–545. doi: 10.1111/j.1572-0241.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- 45.Chavalitdhamrong D, Chen GC, Roth BE, Goltzer O, Sul J, Jutabha R. Esophageal capsule endoscopy for evaluation of patients with chronic gastroesophageal reflux symptoms: findings and its image quality. Diseases of the Esophagus. 2011;24(5):295–298. doi: 10.1111/j.1442-2050.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- 46.Gora MJ, Sauk JS, Carruth RW, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013;19(2):238–240. doi: 10.1038/nm.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JA, Bouma BE, Bressner J, et al. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointestinal endoscopy. 2007;65(1):50–56. doi: 10.1016/j.gie.2006.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfsen HC, Sharma P, Wallace MB, Leggett C, Tearney G, Wang KK. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett’s esophagus (with videos) Gastrointestinal endoscopy. 2015;82(4):631–640. doi: 10.1016/j.gie.2015.03.1968. [DOI] [PubMed] [Google Scholar]
- 49.Trindade AJ, Inamdar S, Smith MS, et al. Volumetric laser endomicroscopy in Barrett’s esophagus: interobserver agreement for interpretation of Barrett’s esophagus and associated neoplasia among high-frequency users. Gastrointestinal endoscopy. doi: 10.1016/j.gie.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 50.Fennerty MB, DiTomasso J, Morales TG, et al. Screening for Barrett's esophagus by balloon cytology. The American journal of gastroenterology. 1995;90(8):1230–1232. [PubMed] [Google Scholar]
- 51.Rader AE, Faigel DO, Ditomasso J, Magaret N, Burm M, Fennerty MB. Cytological screening for Barrett's esophagus using a prototype flexible mesh catheter. Dig Dis Sci. 2001;46(12):2681–2686. doi: 10.1023/a:1012771328187. [DOI] [PubMed] [Google Scholar]
- 52.Falk GW, Chittajallu R, Goldblum JR, et al. Surveillance of patients with Barrett's esophagus for dysplasia and cancer with balloon cytology. Gastroenterology. 1997;112(6):1787–1797. doi: 10.1053/gast.1997.v112.pm9178668. [DOI] [PubMed] [Google Scholar]
- 53.Lao-Sirieix P, Boussioutas A, Kadri SR, et al. Non-endoscopic screening biomarkers for Barrett’s oesophagus: from microarray analysis to the clinic. Gut. 2009;58(11):1451–1459. doi: 10.1136/gut.2009.180281. [DOI] [PubMed] [Google Scholar]
- 54.Lao-Sirieix P, Debiram -Beecham I, Sarah K, et al. 54 Evaluation of a Minimally-Invasive Cytosponge Esophageal Cell Collection System in Patients With Barrett's Esophagus. Gastroenterology. 2015;148(4):S–16. [Google Scholar]
- 55.Lao-Sirieix P, Rous B, O’Donovan M, Hardwick RH, Debiram I, Fitzgerald RC. Non-endoscopic immunocytological screening test for Barrett’s oesophagus. Gut. 2007;56(7):1033–1034. doi: 10.1136/gut.2007.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. 2010:341. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross-Innes CS, Debiram-Beecham I, O'Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS medicine. 2015;12(1):e1001780. doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freeman M, Offman J, Walter FM, Sasieni P, Smith SG. Acceptability of the Cytosponge procedure for detecting Barrett’s oesophagus: A qualitative study. doi: 10.1136/bmjopen-2016-013901. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health Benefits and Cost Effectiveness of Endoscopic and Nonendoscopic Cytosponge Screening for Barrett's Esophagus. Gastroenterology. 2013;144(1):62–73.e66. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 62.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in Human Cancer. In: Schmitz U, Wolkenhauer O, Vera J, editors. MicroRNA Cancer Regulation: Advanced Concepts, Bioinformatics and Systems Biology Tools. Dordrecht: Springer Netherlands; 2013. pp. 1–20. [Google Scholar]
- 63.Mallick R, Patnaik SK, Wani S, Bansal A. A Systematic Review of Esophageal MicroRNA Markers for Diagnosis and Monitoring of Barrett’s Esophagus. Dig Dis Sci. 2016;61(4):1039–1050. doi: 10.1007/s10620-015-3959-3. [DOI] [PubMed] [Google Scholar]
- 64.Cabibi D, Caruso S, Bazan V, et al. Analysis of tissue and circulating microRNA expression during metaplastic transformation of the esophagus. Oncotarget. 2016;7(30):47821–47830. doi: 10.18632/oncotarget.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bus P, Kestens C, Ten Kate FJW, et al. Profiling of circulating microRNAs in patients with Barrett’s esophagus and esophageal adenocarcinoma. Journal of Gastroenterology. 2016;51(6):560–570. doi: 10.1007/s00535-015-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yazbeck R, Jaenisch SE, Watson DI. From blood to breath: New horizons for esophageal cancer biomarkers. World Journal of Gastroenterology. 2016;22(46):10077–10083. doi: 10.3748/wjg.v22.i46.10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann Surg. 2015;262(6):981–990. doi: 10.1097/SLA.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 68.Koslowsky B, Jacob H, Eliakim R, Adler SN. PillCam ESO in esophageal studies: improved diagnostic yield of 14 frames per second (fps) compared with 4 fps. Endoscopy. 2006;38(1):27–30. doi: 10.1055/s-2005-921034. [DOI] [PubMed] [Google Scholar]
- 69.Lin OS, Schembre DB, Mergener K, et al. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett's esophagus in patients with chronic gastroesophageal reflux. Gastrointestinal endoscopy. 2007;65(4):577–583. doi: 10.1016/j.gie.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 70.Sharma P, Wani S, Rastogi A, et al. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett's esophagus: a blinded, prospective study. The American journal of gastroenterology. 2008;103(3):525–532. doi: 10.1111/j.1572-0241.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 71.Gralnek IM, Adler SN, Yassin K, Koslowsky B, Metzger Y, Eliakim R. Detecting esophageal disease with second-generation capsule endoscopy: initial evaluation of the PillCam ESO 2. Endoscopy. 2008;40(04):275–279. doi: 10.1055/s-2007-995645. [DOI] [PubMed] [Google Scholar]
- 72.Delvaux M, Papanikolaou IS, Fassler I, et al. Esophageal capsule endoscopy in patients with suspected esophageal disease: double blinded comparison with esophagogastroduodenoscopy and assessment of interobserver variability. Endoscopy. 2008;40(01):16–22. doi: 10.1055/s-2007-966935. [DOI] [PubMed] [Google Scholar]