Abstract
Genetically encoding distinct non-canonical amino acids (ncAAs) into proteins synthesized in cells requires mutually orthogonal aminoacyl-tRNA synthetase (aaRS)/tRNA pairs. The pyrrolysyl-tRNA synthetase/PyltRNA pair from M. mazei (Mm) has been engineered to incorporate diverse ncAAs and is commonly considered an ideal pair for genetic code expansion. However, finding new aaRS/tRNA pairs that share the advantages of the MmPylRS/MmPyltRNA pair and are orthogonal to both endogenous aaRS/tRNA pairs and the MmPylRS/MmPyltRNA pair has proved challenging. Here we demonstrate that several ΔNPylRS/PyltRNACUA pairs, in which PylRS lacks an N-terminal domain, are active, orthogonal and efficiently incorporate ncAAs in E. coli. We create new PylRS/PyltRNA pairs that are mutually orthogonal to the MmPylRS/MmPyltRNA pair and show that transplanting mutations that reprogram the ncAA specificity of MmPylRS into the new PylRS reprograms its substrate specificity. Finally we show that distinct PylRS/PyltRNA derived pairs can function in the same cell, decode distinct codons, and incorporate distinct ncAAs.
Genetically encoding the co-translational incorporation of multiple distinct ncAAs into proteins facilitates strategies for synthesizing proteins bearing combinations of biophysical probes (for example, Förster resonance energy transfer (FRET) probes) to follow protein conformational change1–3, protein stabilization through cyclization1, 4, and decoding the combinatorial effects of post-translational modifications. Moreover, strategies for encoding the co-translational incorporation of multiple ncAAs may provide a foundation for the encoded cellular synthesis of non-canonical biopolymers5.
Encoding multiple ncAAs into proteins synthesized in cells requires (1) the creation of additional codons or the repurposing of triplet codons5, (2) the creation or discovery of aminoacyl-tRNA synthetase/tRNA pairs that are both orthogonal to the synthetases and tRNAs used by the host organism for natural translation, and mutually orthogonal with respect to other orthogonal aminoacyl-tRNA synthetases and tRNAs6, 7, and (3) the reprogramming of these mutually orthogonal pairs to selectively use distinct ncAAs4, 5, 8.
The Methanosarcina mazei (Mm) pyrrolysyl-tRNA synthetase (PylRS encoded by PylS)/MmPyltRNACUA (encoded by MmPylT) pair, along with the homologous pair from Methanosarcina barkeri (Mb), has been extensively developed for the co-translational incorporation of ncAAs into proteins via genetic code expansion9. These orthogonal pairs do not recognize the canonical 20 amino acids, and their active sites have been evolved to accommodate numerous ncAAs, including those used in this study (Fig. 1). Moreover, MmPylRS and MbPylRS do not recognize the anticodon of their cognate PyltRNACUAs10: a feature that facilitates the decoding of diverse codons by these pairs, through mutation of their anticodons1, 11. The Mm or Mb PylRS/PyltRNA pairs have been used in combination with derivatives of the Methanocaldococcus janaschii (Mj) TyrRS/TyrtRNACUA pair, to direct the co-translational incorporation of several pairs of ncAAs into proteins in E. coli1. However, MjTyrRS recognizes the anticodon of its cognate tRNA, which restricts the codons this pair can be easily altered to efficiently decode, and this pair has primarily been used to incorporate aromatic ncAAs related to phenylalanine12. There is a pressing need to discover new highly active and mutually orthogonal synthetase/tRNA pairs with comparable flexibility to the MmPylRS/MmPyltRNACUA pair. Such pairs would provide a foundation for expanding the diversity of chemical structures that can be simultaneously incorporated into proteins and, in combination with emerging advances in creating or repurposing codons4, 13–15, may provide a foundation for increasing the number of monomers that can be simultaneously encoded in cellular translation.
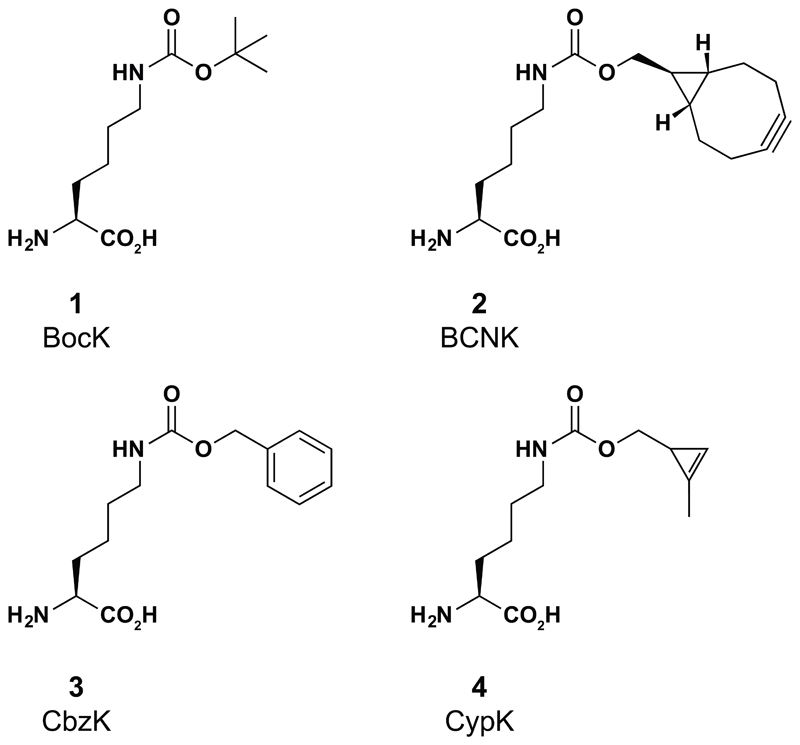
Figure 1. The structures of the amino acids used in this work.
1, Nε-((tertbutoxy)carbonyl)-L-lysine; 2 Nε-(((1R,8S)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy)carbonyl)-L-lysine, exo isomer shown, 4:1 exo/endo mixture used; 3, Nε-benzyloxycarbonyl-L-lysine; 4, Nε-(((2-methylcycloprop-2-en-1-yl)methoxy)carbonyl)-L-lysine.
MmPylRS and MbPylRS are composed of an N-terminal domain, that binds to the T-arm and variable loop of their cognate tRNAs16, 17, linked to a C-terminal catalytic domain (PylRSΔ184 in M. mazei)18. While the catalytic domain can (inefficiently) aminoacylate its cognate tRNA in vitro, the full-length protein is absolutely required for measureable amber suppression activity in cells16, 19. Another group of PylRS enzymes, commonly exemplified by Desulfitobacterium hafniense (Dh), have separate genes encoding the N-terminal domain (DhPylSn) and the C-terminal catalytic domain (DhPylSc) of PylRS as distinct polypeptides. These polypeptides are believed to assemble to create a functional synthetase in vivo. In E. coli the C-terminal protein, DhPylSc, is reported to have less than 1% of the activity of MbPylRS19.
Here we demonstrate that several ΔNPylRS/PyltRNACUA pairs, in which PylRS lacks an N-terminal domain, are active and orthogonal in E. coli and efficiently incorporate ncAAs. We create new PylRS/PyltRNA pairs that are mutually orthogonal to the MmPylRS/MmPyltRNA pair and show that transplanting mutations that reprogram the ncAA specificity of MmPylRS into the new PylRS reprograms its substrate specificity in a predictable manner. Finally we show that two PylRS/PyltRNA derived pairs can function in the same cell, decode distinct codons, and incorporate distinct ncAAs.
Results
Identifying active and orthogonal ΔNPylRS/PyltRNA pairs
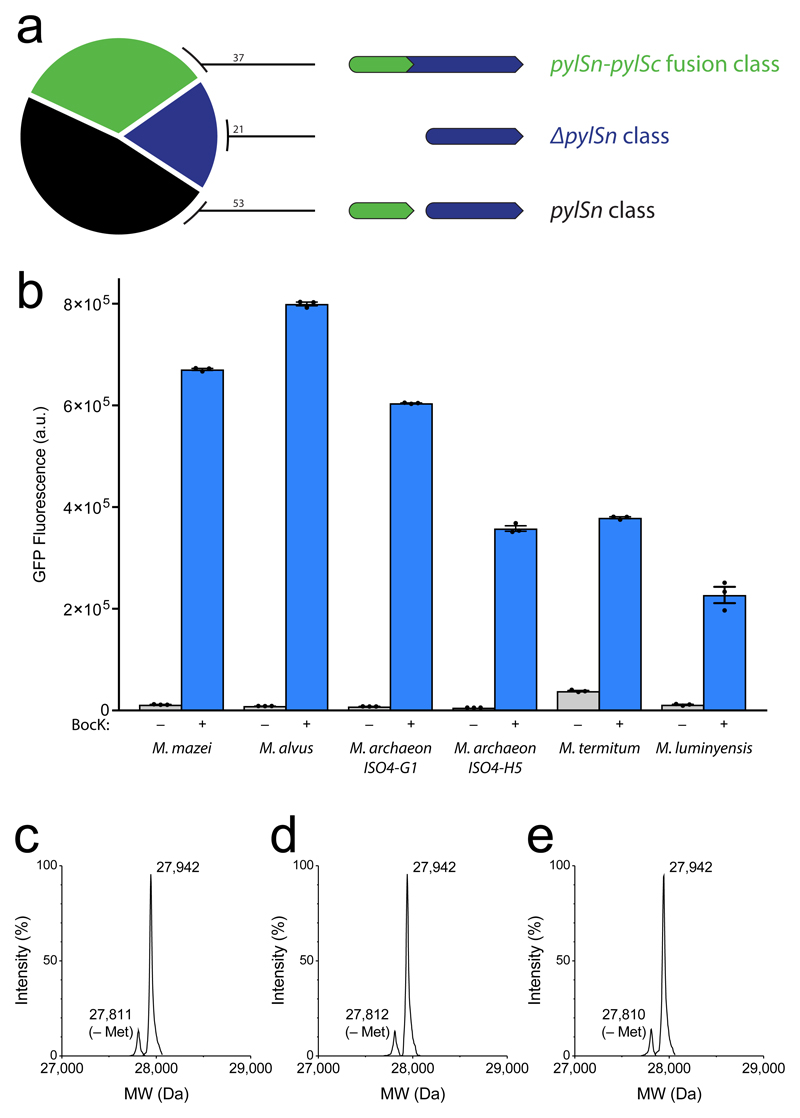
To identify the full complement of PylRS genes we searched genomes for genes that contain the C-terminal region of PylRS, via a BLAST search for sequence similarity to MmPylRSΔ184. We then searched the resulting genomes for sequence similarity to DhPylSn. This search identified genomes that contain both a DhPylSn-like sequence and the C-terminal catalytic domain, either in a single gene (as in M. mazei) or in separate genes (as in D. hafniense). Importantly the search also identified 21 PylRS sequences that contained the C-terminal catalytic domain, but for which we did not identify a DhPylSn-like sequence in the corresponding genome (Fig. 2a); we designated this class of PylRS variants as ΔNPylRS. In some cases the N-terminal domain may not be identified because of an incomplete genome sequence. However, phylogenetic analysis suggested that most of these PylRS sequences that do not contain a DhPylSn-like sequence in their genomes come from organisms that form a distinct clade (Supplementary Fig. 1). Intriguingly, the organisms within this clade are part of a recently described seventh order of methanogens (Methanomassiliicoccales) that have an energy metabolism distinct from other methanogens20 and are divergent from the order of methanogens that contains MmPylRS and MbPylRS (Methanosarcinales)21. For five of the PylRS sequences (Methanomethylophilus alvus (Ma) PylRS, Methanogenic archaeon ISO4-G1 (G1) PylRS, Methanogenic archaeon ISO4-H5 (H5) PylRS, Methanonatronarchaeum termitum (Mt) PylRS, and Methanomassiliicoccus luminyensis (Ml) PylRS) that do not contain a DhPylSn-like sequence in their genome (Supplementary Fig. 2) we were able to identify a PylT-like sequence in the genome. The predicted MaPylT sequence and MlPylT sequence have previously been identified21 and BLAST searches, using these sequences as a reference, allowed us to identify predicted sequences for G1, H5 and Mt PylT in the relevant genomes. The identified tRNAs are predicted to fold into clover-leaf structures with a similar length of D-arm, T-arm, acceptor stem and anticodon stem to MmPyltRNACUA and Dh PyltRNACUA (Supplementary Fig. 3). Interestingly, the majority of tRNA sequences identified contained nucleotide loops or bulges in the anticodon stem; to the best of our knowledge this is a hitherto uncharacterized feature in cellular tRNAs.
Figure 2. Identifying ΔNPylRS/PyltRNA pairs are active and orthogonal in E. coli.
(a) Classification of identified PylRS sequences according to the presence of a domain homologous to D. hafniense PylSn either within the same gene (pylSn-pylSc fusion class), present within a separate gene in the same genome (pylSn class), or absent entirely (ΔpylSn class). (b) In vivo amber suppression activity assay using E. coli DH10B bearing pBAD GFP(150TAG)His6 and the corresponding pKW PylRS/PyltRNACUA plasmid in the presence and absence of BocK (1) demonstrates the activity of ΔNPylRS/PyltRNACUA pairs and the orthogonality of several PyltRNAs with respect to E. coli aminoacyl-tRNA sythetases. Each data point shows the mean of three technical replicates that form one biological replicate; the error bars show the mean and SEM of three independent biological replicates. (c), (d), (e) Confirmation of specific BocK incorporation into purified GFPHis6 by MmPylRS/MmPyltRNACUA, MaPylRS/MaPyltRNACUA and G1PylRS/G1PyltRNACUA analysed by electrospray ionisation mass spectrometry (ESI-MS, predicted mass 27,942 Da, observed mass 27,942 Da) reveals the functional orthogonality of each PylRS with respect to E. coli tRNAs. The ESI-MS experiments in (c), (d), (e) were performed once. Raw ESI-MS spectra are provided in Supplementary Fig. 4.
The five (Ma, G1, H5, Mt, Ml) PylRS/PyltRNACUA pairs lack the N-terminal domain commonly considered to be required for in vivo activity, and we were curious whether these pairs are sufficient to support co-translational read-through of the amber codon in vivo. To investigate this possibility we cloned synthetic genes for each synthetase/tRNA pair into a pKW vector for expression in E. coli and tested their ability to produce GFPHis6 from GFP(150TAG)His6 in the presence and absence of Nε-((tert-butoxy)carbonyl)-L-lysine (BocK, 1, Fig. 1). We anticipated that BocK would be a good substrate for the five synthetases because their active sites are homologous to those of MbPylRS and MmPylRS (Supplementary Fig. 2), both of which efficiently incorporate BocK22.
Comparing the GFPHis6 produced by each pair in the presence and absence of BocK to the GFPHis6 produced by the MmPylRS/MmPyltRNACUA pair in the presence and absence of BocK allowed us to assess the activity and orthogonality of the pairs relative to a system commonly used for genetic code expansion (Fig. 2b). The activity of the MaPylRS/MaPyltRNACUA pair with BocK was greater than that of the MmPylRS/MmPyltRNACUA pair with BocK, and the two pairs had comparably low levels of GFP production in the absence of BocK. We conclude that the MaPylRS/MaPyltRNACUA pair is active and that MaPyltRNACUA is functionally orthogonal with respect to the aminoacyl-tRNA sythetases in E. coli.
Electrospray ionization mass spectrometry of GFP-His6 produced from GFP(150TAG)His6 by the MmPylRS/MmPyltRNACUA pair in the presence of BocK reveals a single peak corresponding to the incorporation of BocK in response to the amber stop codon (Fig. 2c, Supplementary Fig. 4), and confirms that MmPylRS is functionally orthogonal with respect to the set of tRNAs in E. coli. Similarly, the MaPylRS/MaPyltRNACUA pair incorporates a single BocK into GFP in response to the amber codon (Fig. 2d, Supplementary Fig. 4) indicating that MaPylRS is functionally orthogonal in E. coli. Taken together our observations reveal that the MaPylRS/MaPyltRNACUA pair is a new extremely active and orthogonal aminoacyl-tRNA synthetase/tRNA pair in E. coli. Similarly, we find that the G1PylRS/G1PyltRNACUA pair is an orthogonal, albeit slightly less active, orthogonal pair in E. coli (Fig. 2b,e, Supplementary Fig. 4). The H5PylRS/ H5PyltRNACUA pair, MlPylRS/MlPyltRNACUA pair and MtPylRS/MtPyltRNACUA pair are less active than the MmPylRS/MmPyltRNACUA pair. The H5PyltRNACUA and MlPyltRNACUA are orthogonal while the MtPylRS/MtPyltRNACUA pair facilitates read through of the amber stop codon in the absence of BocK. Because of the lower activity of these three pairs we did not investigate them further. Our experiments identified the MaPylRS/MaPyltRNACUA pair and G1PylRS/G1PyltRNACUA pair as orthogonal, and very active, pairs for further investigation. In additional experiments we demonstrated that the anticodon of the MaPylRS/MaPyltRNACUA pair can be altered to facilitate the decoding of additional codons (Supplementary Fig. 5).
The high activity of the MaPylRS/MaPyltRNACUA pair and the low activity of the MlPylRS/MlPyltRNACUA pair are consistent with the higher percentage of amber stop codons predicted to be used for termination in the Ml genome (11%) versus the Ma genome (1.6%), the number of amber stop codons in open reading frames predicted to incorporate pyrrolysine in Ml (3) versus Ma (19), and the predicted methylamine availability in the evolutionary history of the two organisms21.
Mutual orthogonality amongst natural PylRS/PyltRNA pairs
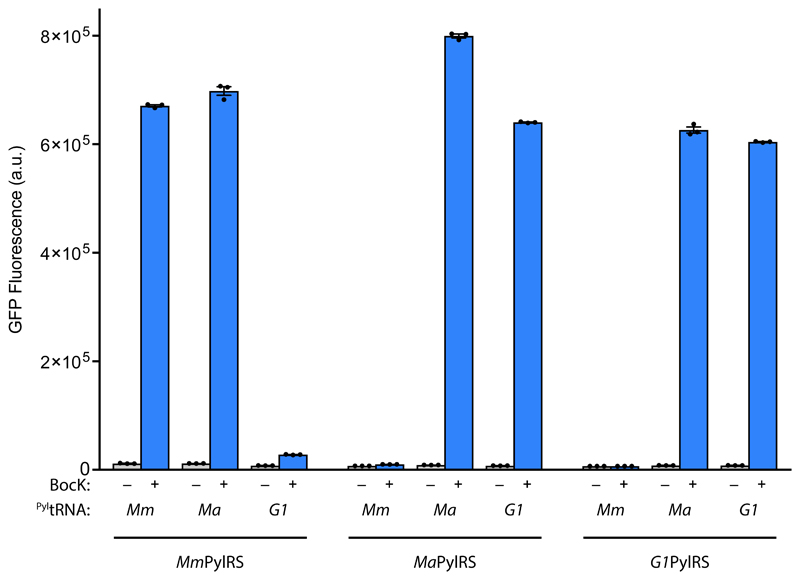
Next we investigated the orthogonality of MmPylRS, MaPylRS, and G1PylRS with respect to MmPyltRNACUA, MaPyltRNACUA, and G1PyltRNACUA by measuring the ability of each of the nine synthetase/tRNA combinations to produce GFPHis6 from GFP(150TAG)His6 in the presence of BocK (Fig. 3). We found that MmPylRS functions with MaPyltRNACUA about as efficiently as with its cognate MmPyltRNACUA. In contrast, MmPylRS functioned 24 times less efficiently with G1PyltRNACUA than with its cognate tRNA. MaPylRS does not function with MmPyltRNACUA but does aminoacylate G1PyltRNACUA. G1PylRS functions with MaPyltRNACUA but not with MmPyltRNACUA.
Figure 3. Mutual orthogonality amongst natural PylRS/PyltRNA pairs.
In vivo amber suppression activity assay using E. coli DH10B bearing pBAD GFP(150TAG)His6 and the corresponding pKW PylRS/PyltRNACUA plasmid in the presence and absence of BocK (1) demonstrates the activity of the indicated PylRS with cognate and non cognate PyltRNACUA. Each data point shows the mean of three technical replicates that form one biological replicate; the error bars show the mean and SEM of three independent biological replicates.
Thus, the MaPylRS/MaPyltRNACUA pair and the G1PylRS/G1PyltRNACUA pair are not mutually orthogonal, as each synthetase functions with its non-cognate tRNA. And the MmPylRS/MmPyltRNACUA pair and the MaPylRS/MaPyltRNACUA pair are not mutually orthogonal because MmPylRS functions with MaPyltRNACUA.
However, the MmPylRS/MmPyltRNACUA pair and the G1PylRS/G1PyltRNACUA pairs are mutually orthogonal, though there is clearly a low level of activity of MmPylRS with G1PyltRNACUA. This surprising observation reveals that there is sufficient divergence between PylRS/PyltRNACUA recognition within methanogenic archea to generate mutually orthogonal pairs. We are not aware of any prior examples of natural mutual orthogonality between synthetase/tRNA pairs used for the same amino acid within a domain of life.
Evolving mutually orthogonal PylRS/PyltRNACUA pairs
Next we wanted to create a pair that was optimized for both activity and mutual orthogonality with respect to the MmPylRS/MmPyltRNACUA pair. We considered either improving both the orthogonality and the activity of the G1 pair, or improving the orthogonality of the MaPyltRNACUA with respect to MmPylRS, while maintaining the activity of the MaPylRS/MaPyltRNACUA pair. We decided to address the later challenge as we had a clear hypothesis about how to improve the orthogonality of MaPyltRNACUA with minimal effect on the activity of the MaPylRS/MaPyltRNACUA pair. The N-terminal domain of MmPylRS, which is absent in MaPylRS, binds to the T-arm and variable loop regions of its cognate tRNA16, 17. We therefore reasoned that it might be possible to create variants of MaPyltRNACUA that are not recognized by MmPylRS or endogenous synthetases but still function with MaPylRS, by altering the variable loop of MaPyltRNACUA.
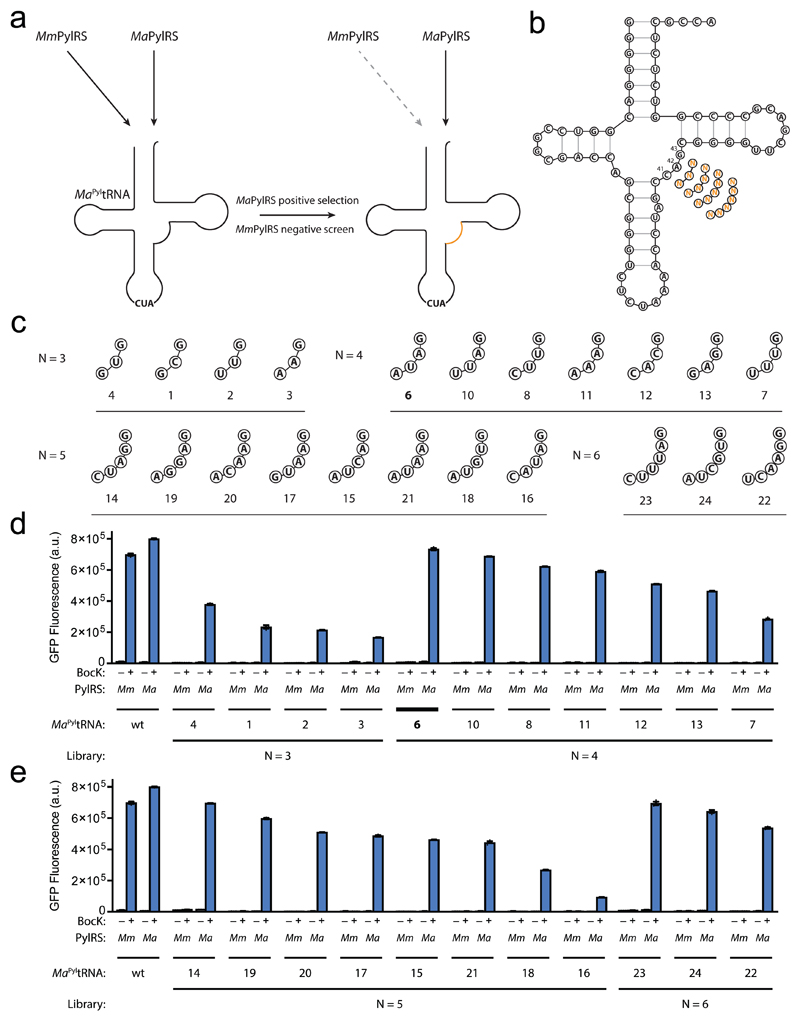
To discover variants of MaPyltRNACUA that are orthogonal to MmPylRS and function efficiently with MaPylRS we preformed a positive selection followed by a negative screen on MaPyltRNACUA libraries (Fig. 4a). We first created a library of 64 MaPyltRNACUA mutants by randomising nucleotide positions 41, 42 and 43 in its variable loop to all possible combinations of the four common bases (Fig. 4b). We selected MaPyltRNACUA variants that functioned with MaPylRS and enabled cells to grow on 100 μg ml-1 of chloramphenicol in the presence of BocK, by facilitating read through of an amber codon at position 111 of a chloramphenicol acetyl transferase reporter, cat(111TAG). Next we performed a negative screen on the selected MaPyltRNACUA variants, to identify tRNAs that do not function with MmPylRS or any endogenous synthetases. Cells bearing GFP(150TAG)His6, MmPylRS and each MaPyltRNACUA variant were provided with BocK and screened for the absence of GFPHis6 expression.
Figure 4. Evolution of MaPyltRNA to create mutually orthogonal PylRS/PyltRNA pairs.
(a) Evolution MaPyltRNA to abolish its function with MmPylRS while preserving its function with MaPylRS, via positive selection in the presence of MaPylRS followed by a negative screen in the presence of MmPylRS. (b) Libraries of MaPyltRNA created by randomising or expanding the length of the variable loop to 4, 5 or 6 randomised nucleotides. (c) Variable loop sequences for the MaPyltRNA hits identified. Hits are grouped according to the library from which they were identified. Hits 6, 8, 10, 12, 15, 16, 17, 18 and 20 also contained a G55A mutation; hits 14, 19, 22, 23 and 24 also contained a G55T mutation; hits 11, 13 and 21 also contained a G55C mutation; hit 7 also contained a C15T mutation. (d), (e) In vivo amber suppression activity assay (libraries N = 3 and 4 (d) and libraries N = 5 and 6 (e)). Each data point shows the mean of three technical replicates that form one biological replicate; the error bars show the mean and SEM of three independent biological replicates. wt, wild-type
This serial positive selection and negative screen identified four unique sequences, which vary nucleotides 41 and 42 but conserve G43 (Fig. 4c). Expression of GFP(150TAG)His6 in the presence of each evolved MaPyltRNACUA variant, BocK, and either MmPylRS or MaPylRS revealed that the selected sequences do not function with MmPylRS but do function with MaPylRS (Fig. 4d). However, the activity of these evolved MaPylRS/MaPyltRNACUA pairs did not exceed 47% that of the parent MaPylRS/MaPyltRNACUA pair.
Next we investigated whether MaPylRS/MaPyltRNACUA pairs that are more active but still mutually orthogonal to the MmPylRS/MmPyltRNACUA pair can be created by introducing further diversity in the variable loop of MaPyltRNACUA. We generated libraries in which the length of the variable loop was expanded from 3 nucleotides to 4, 5 or 6 randomised nucleotides (Fig. 4b). We performed the serial positive selection and negative screen for each of the expanded libraries and characterized the resulting MaPyltRNACUA clones. From the expanded libraries we obtained evolved MaPyltRNACUA sequences with expanded variable loops and non-programmed mutations in either the T-loop or D-loop (e.g. MaPyltRNA(6)CUA, MaPyltRNA(10)CUA, MaPyltRNA(14)CUA and MaPyltRNA(23)CUA (Fig. 4c)). These tRNAs form MaPylRS/MaPyltRNACUA pairs that are as active as the MmPylRS/MmPyltRNACUA pair, and do not function with MmPylRS (Fig. 4d,e). These experiments demonstrated that the MmPylRS/MmPyltRNACUA pair and several of the evolved MaPylRS/MaPyltRNACUA pairs, with expanded variable loops and high activity, are mutually orthogonal. The activity of the evolved MaPylRS/MaPyltRNA(6)CUA pair is 91% of the parent MaPylRS/MaPyltRNACUA and is slightly greater than that of the G1PylRS/G1PyltRNACUA pair. The evolved MaPylRS/MaPyltRNACUA pairs are more orthogonal than the G1PylRS/G1PyltRNACUA pair, with respect to the MmPylRS/MmPyltRNACUA pair, since G1PyltRNACUA shows a low level of activity with MmPylRS (Fig. 3).
Engineering MaPylRS for selective ncAA incorporation
MmPylRS and MbPylRS have been used extensively for genetic code expansion and the active sites of these enzymes naturally accept several ncAA substrates, but exclude others9, 12. Structure-guided mutagenesis and directed evolution of the active site of MbPylRS and MmPylRS has expanded the repertoire of ncAAs that can be incorporated23, 24, and created enzymes that are selective for some ncAAs with respect to others25. Mutants discovered in MbPylRS can commonly be transplanted to MmPylRS and vice versa.
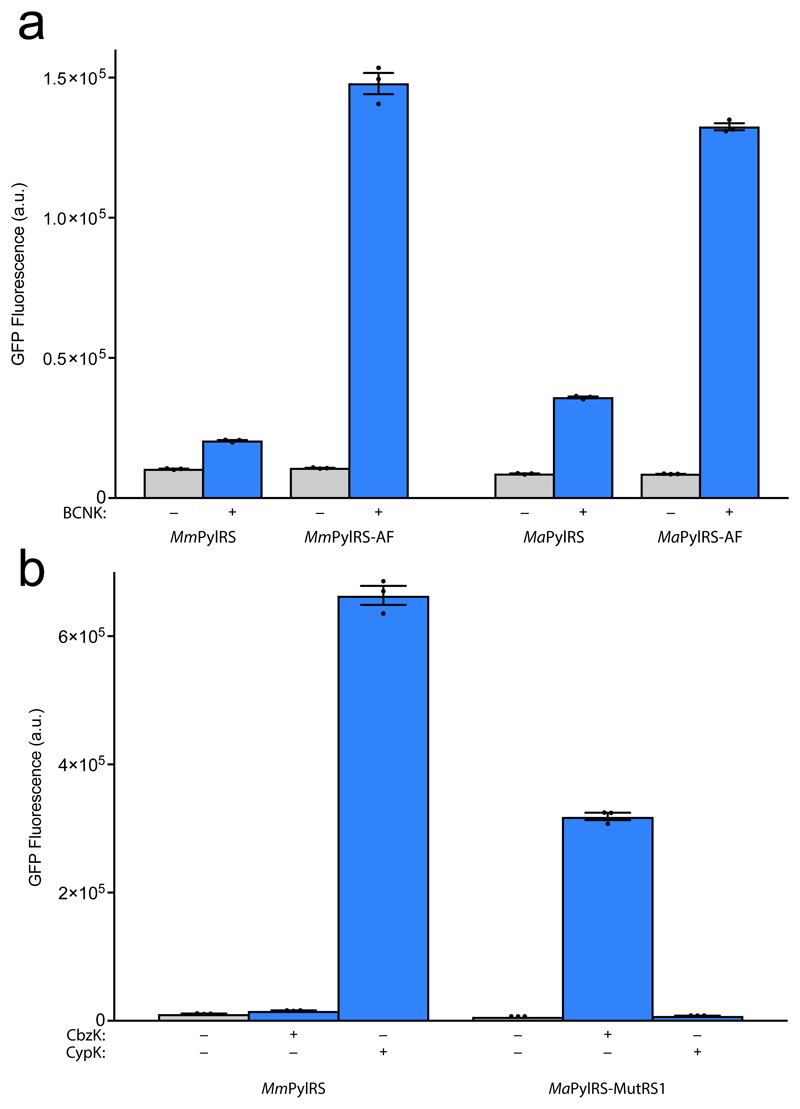
In light of the extensive homology in the amino acid-binding pockets of MmPylRS and MaPylRS (Supplementary Fig. 2), we investigated whether the substrate specificity of MaPylRS could be expanded through the introduction of mutations identified in the active sites of MmPylRS or MbPylRS. The Y306A, Y384F double mutant of MmPylRS (MmPylRS-AF) expands its substrate specificity to facilitate the incorporation of several additional ncAAs23. Using an alignment of MmPylRS and MaPylRS (Supplementary Fig. 2) we identified Y126 and Y206 in MaPylRS as the residues corresponding to Y306 and Y384 in MmPylRS, and created the corresponding Y126A and Y206F mutations in MaPylRS (MaPylRS-AF). The MmPylRS-AF/MmPyltRNACUA pair facilitates the incorporation of a bicyclononyne-containing amino acid (BCNK, 2), which is poorly incorporated by MmPylRS/MmPyltRNACUA pair (Fig. 5a)26, 27. Similarly we find that the MaPylRS/MaPyltRNACUA pair inefficiently incorporates BCNK, and that the efficiency of BCNK incorporation is substantially improved by using the MaPylRS-AF/MaPyltRNACUA pair (Fig. 5a). These experiments demonstrated that the mutations identified in MmPylRS can be transferred to MaPylRS to expand its substrate specificity.
Figure 5. Engineering the active site of MaPylRS for selective ncAA incorporation.
(a) In vivo amber suppression activity assay using E. coli DH10B bearing pBAD GFP(150TAG)His6 and the corresponding pKW PylRS/PyltRNACUA plasmid in the presence and absence of BCNK (2) demonstrates the transferability of the MmPylRS-AF mutations into MaPylRS to facilitate improved incorporation of BCNK using MaPylRS-AF. Each data point shows the mean of three technical replicates that form one biological replicate; the error bars show the mean and SEM of three independent biological replicates. (b) In vivo amber suppression activity assay using E. coli DH10B bearing pBAD GFP(150TAG)His6 and the corresponding pKW PylRS/PyltRNACUA plasmid in the presence and absence of CbzK (3) and CypK (4) demonstrates the selective incorporation of CypK by MmPylRS and the selective incorporation of CbzK by MaPylRS-MutRS1. Each data point shows the mean of three technical replicates that form one biological replicate; the error bars show the mean and SEM of three independent biological replicates.
Next we asked whether we could create MaPylRS and MmPylRS variants with mutually orthogonal ncAA substrate specificity. We recently demonstrated that parallel positive selections on a MbPylRS library in the presence of either Nε - benzyloxycarbonyl-L-lysine (CbzK, 3) or Nε-(((2-methylcycloprop-2-en-1-yl)methoxy)carbonyl)-L-lysine (CypK, 4) or in the absence of ncAA, coupled to deep sequencing and comparative sequence analysis, enables the direct identification of MbPylRS variants that incorporate CbzK, but not CypK or any natural amino acids, and vice versa25. MbPylRS-MutRS1 (Y271M, L274G, C313T) selectively incorporates CbzK, but not CypK; we introduced the corresponding mutations into MaPylRS, creating MaPylRS-MutRS1 (Y126M, M129G,V168T). We found that the MaPylRS-MutRS1/MaPyltRNACUA pair directs the incorporation of CbzK but not CypK (Fig. 5b). MbPylRS-MutRS2 (A267S) selectively incorporates CypK, but not CbzK. We found that the MmPylRS-MutRS2 (A302S)/MmPyltRNACUA pair directs the incorporation of CypK but not CbzK (Supplementary Fig. 6); we subsequently found that the wild-type MmPylRS/MmPyltRNACUA pair directs the incorporation of CypK but not CbzK with comparable efficiency and specificity to the mutant, and used this pair for further experiments (Fig. 5b). These experiments demonstrated that active sites of MaPylRS and MmPylRS can be diverged for mutually orthogonal ncAA incorporation.
Encoding distinct ncAAs with mutually orthogonal PylRS/PyltRNA pairs
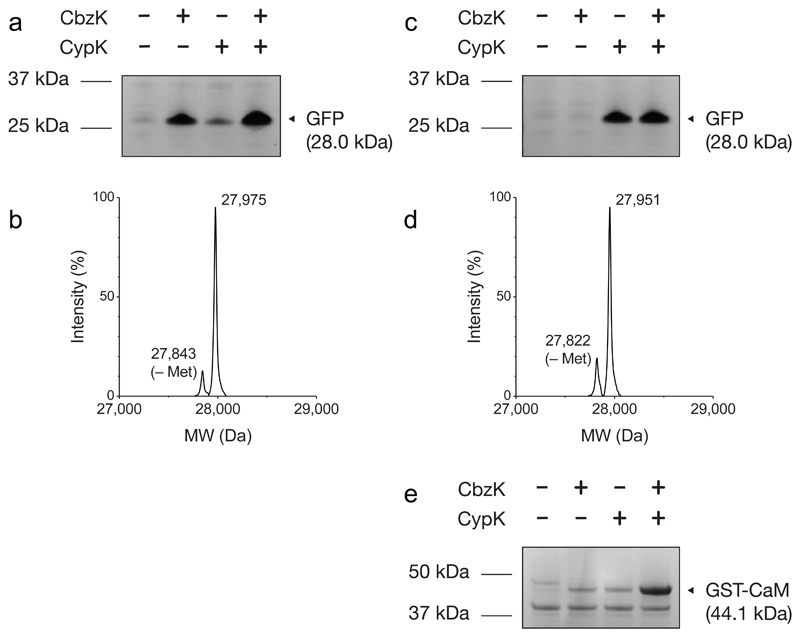
Next we demonstrated that the MaPylRS-MutRS1/MaPyltRNA(6) and MmPylRS/MmPyltRNA pairs can function as mutually orthogonal pairs in the same cell. We created a single vector encoding both the MaPylRS-MutRS1/MaPyltRNA(6)CUA pair and the MmPylRS/MmPyltRNAUCCU pair, and introduced this into cells along with GFP(150TAG)His6. Addition of the MaPylRS-MutRS1 substrate, CbzK, led to robust GFPHis6 synthesis, while addition of the MmPylRS substrate, CypK, led to minimal synthesis of GFPHis6, and addition of both CbzK and CypK led to robust GFPHis6 synthesis (Fig. 6a, Supplementary Fig. 7). Mass spectrometry demonstrates that GFPHis6 produced from GFP(150TAG)His6 in the presence of the MaPylRS-MutRS1/MaPyltRNA(6)CUA and MmPylRS/MmPyltRNAUCCU pairs and both CbzK and CypK incorporates CbzK but not CypK (Fig 6b, Supplementary Fig. 8). In complementary experiments we introduced the MaPylRS-MutRS1/MaPyltRNA(6)UACU and MmPylRS/MmPyltRNACUA pairs, along with GFP(150TAG)His6, into cells. Addition of the MmPylRS substrate, CypK, led to robust GFPHis6 synthesis, while addition of the MaPylRS-MutRS1 substrate, CbzK, led to minimal synthesis of GFPHis6, and addition of both CbzK and CypK led to robust GFPHis6 synthesis (Fig. 6c, Supplementary Fig. 7). Mass spectrometry demonstrates that GFPHis6 produced from GFP(150TAG)His6 in the presence of the MaPylRS-MutRS1/MaPyltRNA(6)UACU and MmPylRS/MmPyltRNACUA pairs and both CbzK and CypK incorporates CypK, but not CbzK (Fig 6d, Supplementary Fig. 8).
Figure 6. Encoding distinct ncAAs using mutually orthogonal PylRS/PyltRNA pairs.
(a) GFPHis6 purified from E. coli containing GFP(150TAG)His6, MmPylRS/MmPyltRNAUCCU and MaPylRS-MutRS1/MaPyltRNA(6)CUA and analyzed by SDS-PAGE. Two independent experiments were performed with similar results. (b) Electrospray ionization mass spectrometry of GFPHis6 purified from E. coli containing GFP(150TAG)His6, MmPylRS/MmPyltRNAUCCU, MaPylRS-MutRS1/MaPyltRNA(6)CUA in the presence of both CbzK and CypK. The peak corresponds to CbzK incorporation (predicted mass 27,976 Da, observed mass 27,975 Da). ESI-MS was performed once. (c) GFPHis6 purified from E. coli containing GFP(150TAG)His6, MmPylRS/MmPyltRNACUA, MaPylRS-MutRS1/MaPyltRNA(6)UACU and analyzed by SDS-PAGE. Two independent experiments were performed with similar results. (d) Electrospray ionization mass spectrometry of GFPHis6 purified from E. coli containing GFP(150TAG)His6, MmPylRS/MmPyltRNACUA, MaPylRS-MutRS1/MaPyltRNA(6)UACU in the presence of both CbzK and CypK. The peak corresponds to CypK incorporation (predicted mass 27,952 Da, observed mass 27,951 Da). ESI-MS was performed once. (e) GST-CaM purifications from E. coli containing ribo-Q1, o-GST-CaM(1TAG, 40AGGA), MmPylRS/MmPyltRNAUCCU, MaPylRS-MutRS1/MaPyltRNA(6)CUA grown in presence and absence of the indicated ncAAs. Samples were analyzed by SDS-PAGE. Two independent experiments were performed with similar results.
These experiments demonstrated the modular combination of mutations in MaPyltRNA(6)CUA, that confer orthogonality with respect to MmPylRS, with the mutations in MaPylRS-MutRS1, that re-program the ncAA specificity of this enzyme, to produce a pair that combines the properties conferred by each set of mutations. Moreover, these experiments demonstrated that the MaPylRS-MutRS1/MaPyltRNA(6) and MmPylRS/MmPyltRNA pairs can function independently in the same cell.
Finally we demonstrated that we can encode distinct ncAAs into a single polypeptide using the mutually orthogonal PylRS/PyltRNA pairs. We introduced the MaPylRS-MutRS1/MaPyltRNA(6)CUA pair and MmPylRS/MmPyltRNAUCCU pair into cells that produce ribo-Q, an orthogonal ribosome that is directed to an orthogonal message and facilitates decoding of quadruplet and amber codons by cognate tRNAs4. Cells also contained o-GST-CaM(1UAG, 40AGGA), an orthogonal message for a glutathione-S-transferase-calmodulin fusion that is read by ribo-Q1 and contains amber and quadruplet codons that are complementary to the anticodons of MaPyltRNA(6)CUA and MmPyltRNAUCCU. Addition of both CbzK and CypK to cells was required for efficient full length GST-CaM synthesis, with an unoptimized yield of approximately 0.5 mg per L of culture, from the orthogonal message (Fig. 6e, Supplementary Fig. 7). Additional experiments and mass spectrometry confirmed the programmed incorporation of both ncAAs into a single polypeptide (Supplementary Fig. 9). These experiments demonstrated that the MaPylRS-MutRS1/MaPyltRNA(6)CUA and MmPylRS/MmPyltRNAUCCU pairs can function in the same cell to incorporate distinct substrates into a single polypeptide in response to distinct codons.
Discussion
The N-terminal domain of PylRS is commonly believed to be essential for robust activity in vivo. We have discovered PylRS variants that lack the N-terminal domain but are exceptionally active and orthogonal in E. coli and can be used to direct the incorporation of ncAAs. These single domain PylRS variants may provide an improved starting point for engineering new substrate specificity. We have shown that the G1PylRS/G1PyltRNA pair is mutually orthogonal with the MmPylRS/MmPyltRNA pair. Our results reveal that there is sufficient divergence between PylRS/PyltRNACUA recognition within methanogenic archea to generate mutually orthogonal pairs. We are not aware of any prior examples of natural mutual orthogonality between synthetase/tRNA pairs used for the same amino acid within a domain of life and our results suggest it will be interesting to further explore the mutual orthogonality of aaRS/tRNA pairs from distinct archeal orders.
We have created exceptionally active variants of the MaPylRS/MaPyltRNA pair that have a high level of mutual orthogonality with respect to the MmPylRS/MmPyltRNA pair. Moreover we have shown that the active sites of the MaPylRS/MaPyltRNA and MmPylRS/MmPyltRNA pairs can be diverged to recognize distinct substrates, and that the pairs can be used together to program distinct ncAAs into proteins in E. coli. We anticipate that the MaPylRS/MaPyltRNA(6) pair and its derivatives may be used to further expand the combinations of ncAAs that can be encoded into polypeptides synthesized in E. coli. It will be interesting to explore whether the new PylRS/tRNA pairs reported herein are also orthogonal in eukaryotic cells and organisms.
Supplementary Material
Summary.
Pyrrolysyl-tRNA synthetase(PylRS)/PyltRNACUA pairs that lack the N-terminal domain but are active and orthogonal are discovered, and pairs that are mutually orthogonal to existing PylRS/PyltRNACUA pairs are developed. Mutually orthogonal PylRS/PyltRNA pairs are combined to genetically encode the incorporation of distinct ncAAs into proteins synthesized in E. coli.
Acknowledgements
This work was supported by the Medical Research Council, UK (MC_U105181009 and MC_UP_A024_1008), BBSRC (BB/M000842/1, for automation) and the ERC Advanced Grant (SGCR), all to J.W.C. J.C.W.W. was supported by the EPSRC Nanoscience and Nanotechnology CDT at Cambridge University.
Footnotes
Data availability statement
Source data for all Figures are available from the corresponding author upon reasonable request.
Author contributions
J.C.W.W. performed all experiments. J.C.W.W and J.W.C. conceived and designed experiments, analyzed the data and wrote the paper.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Wang K, et al. Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double-labelling and FRET. Nature chemistry. 2014;6:393–403. doi: 10.1038/nchem.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachdeva A, Wang K, Elliott T, Chin JW. Concerted, rapid, quantitative, and site-specific dual labeling of proteins. Journal of the American Chemical Society. 2014;136:7785–7788. doi: 10.1021/ja4129789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee A, Sun SB, Furman JL, Xiao H, Schultz PG. A Versatile Platform for Single- and Multiple-Unnatural Amino Acid Mutagenesis in Escherichia coli. Biochemistry. 2013 doi: 10.1021/bi4000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann H, Wang K, Davis L, Garcia-Alai M, Chin JW. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature. 2010;464:441–444. doi: 10.1038/nature08817. [DOI] [PubMed] [Google Scholar]
- 5.Chin JW. Expanding and reprogramming the genetic code. Nature. 2017;550:53–60. doi: 10.1038/nature24031. [DOI] [PubMed] [Google Scholar]
- 6.Neumann H, Slusarczyk AL, Chin JW. De NovoGeneration of Mutually Orthogonal Aminoacyl-tRNA Synthetase/tRNA Pairs. Journal of the American Chemical Society. 2010;132:2142–2144. doi: 10.1021/ja9068722. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee A, Xiao H, Schultz PG. Evolution of multiple, mutually orthogonal prolyl-tRNA synthetase/tRNA pairs for unnatural amino acid mutagenesis in Escherichia coli. P Natl Acad Sci USA. 2012;109:14841–14846. doi: 10.1073/pnas.1212454109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JC, et al. An expanded genetic code with a functional quadruplet codon. P Natl Acad Sci USA. 2004;101:7566–7571. doi: 10.1073/pnas.0401517101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin JW. Expanding and Reprogramming the Genetic Code of Cells and Animals. Annu Rev Biochem. 2014;83:379–408. doi: 10.1146/annurev-biochem-060713-035737. [DOI] [PubMed] [Google Scholar]
- 10.Ambrogelly A, et al. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc Natl Acad Sci U S A. 2007;104:3141–3146. doi: 10.1073/pnas.0611634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott TS, et al. Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nature biotechnology. 2014;32:465–472. doi: 10.1038/nbt.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumas A, Lercher L, Spicer CD, Davis BG. Designing logical codon reassignment - Expanding the chemistry in biology. Chem Sci. 2015;6:50–69. doi: 10.1039/c4sc01534g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, et al. Defining synonymous codon compression schemes by genome recoding. Nature. 2016;539:59–64. doi: 10.1038/nature20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrov N, et al. Design, synthesis, and testing toward a 57-codon genome. Science. 2016;353:819–822. doi: 10.1126/science.aaf3639. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. A semi-synthetic organism that stores and retrieves increased genetic information. Nature. 2017;551:644–647. doi: 10.1038/nature24659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang R, Krzycki JA. PylSn and the homologous N-terminal domain of pyrrolysyl-tRNA synthetase bind the tRNA that is essential for the genetic encoding of pyrrolysine. J Biol Chem. 2012;287:32738–32746. doi: 10.1074/jbc.M112.396754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, et al. Crystal structures reveal an elusive functional domain of pyrrolysyl-tRNA synthetase. Nat Chem Biol. 2017;13:1261–1266. doi: 10.1038/nchembio.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavran JM, et al. Structure of pyrrolysyl-tRNA synthetase, an archaeal enzyme for genetic code innovation. Proc Natl Acad Sci U S A. 2007;104:11268–11273. doi: 10.1073/pnas.0704769104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herring S, et al. The amino-terminal domain of pyrrolysyl-tRNA synthetase is dispensable in vitro but required for in vivo activity. FEBS letters. 2007;581:3197–3203. doi: 10.1016/j.febslet.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sollinger A, et al. Phylogenetic and genomic analysis of Methanomassiliicoccales in wetlands and animal intestinal tracts reveals clade-specific habitat preferences. FEMS Microbiol Ecol. 2016;92 doi: 10.1093/femsec/fiv149. [DOI] [PubMed] [Google Scholar]
- 21.Borrel G, et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genomics. 2014;15:679. doi: 10.1186/1471-2164-15-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virdee S, Ye Y, Nguyen DP, Komander D, Chin JW. Engineered diubiquitin synthesis reveals Lys29-isopeptide specificity of an OTU deubiquitinase. Nat Chem Biol. 2010;6:750–757. doi: 10.1038/nchembio.426. [DOI] [PubMed] [Google Scholar]
- 23.Yanagisawa T, et al. Multistep Engineering of Pyrrolysyl-tRNA Synthetase to Genetically Encode Nε-(o-Azidobenzyloxycarbonyl) lysine for Site-Specific Protein Modification. Chemistry & Biology. 2008;15:1187–1197. doi: 10.1016/j.chembiol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Neumann H, Peak-Chew SY, Chin JW. Genetically encoding Nε-acetyllysine in recombinant proteins. Nat Chem Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 25.Zhang MS, et al. Biosynthesis and genetic encoding of phosphothreonine through parallel selection and deep sequencing. Nat Methods. 2017;14:729–736. doi: 10.1038/nmeth.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrmann A, et al. Genetic Encoding of a Bicyclo[6.1.0]nonyne-Charged Amino Acid Enables Fast Cellular Protein Imaging by Metal-Free Ligation. ChemBioChem. 2012;13:2094–2099. doi: 10.1002/cbic.201200407. [DOI] [PubMed] [Google Scholar]
- 27.Lang K, et al. Genetic Encoding of Bicyclononynes and trans-Cyclooctenes for Site-Specific Protein Labeling in Vitro and in Live Mammalian Cells via Rapid Fluorogenic Diels–Alder Reactions. Journal of the American Chemical Society. 2012;134:10317–10320. doi: 10.1021/ja302832g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.