Abstract
Background
The greatest challenge to long-term graft survival is the development of chronic lung allograft dysfunction. Th17 responses to collagen type V (colV) predispose lung transplant patients to the severe obstructive form of chronic lung allograft dysfunction, known as bronchiolitis obliterans syndrome (BOS). In a previous study cohort (n = 54), pretransplant colV responses were increased in recipients expressing HLA-DR15, consistent with the high binding avidity of colV (α1) peptides for HLA-DR15, whereas BOS incidence, which was known to be strongly associated with posttransplant autoimmunity to colV, was higher in patients who themselves lacked HLA-DR15, but whose lung donor expressed it.
Methods
To determine if this DR-restricted effect on BOS incidence could be validated in a larger cohort, we performed a retrospective analysis of outcomes for 351 lung transplant recipients transplanted between 1988 and 2008 at the University of Wisconsin. All subjects were followed until graft loss, death, loss to follow-up, or through 2014, with an average follow-up of 7 years. Comparisons were made between recipients who did or did not develop BOS. Grading of BOS followed the recommendations of the international society for heart and lung transplantation.
Results
Donor HLA-DR15 was indeed associated with increased susceptibility to severe BOS in this population. We also discovered that HLA-DR7 expression by the donor or HLA-DR17 expression by the recipient decreased susceptibility.
Conclusions
We show in this retrospective study that specific donor HLA class II types are important in lung transplantation, because they are associated with either protection from or susceptibility to development of severe BOS.
Immunologic tolerance, whereby the alloantigens of the transplant donor trigger anergy, regulation, or clonal deletion in host T and B lymphocytes is the ultimate goal in organ transplantation. In addition, because lung transplants tend to de-stabilize normal “self” tolerance, care must also be taken to restrict Th17-based autoimmunity.1–3 Early events, such as surgical complications or infection, can occur and threaten recipient survival; but the greatest challenge to long-term graft survival is the development of chronic lung allograft dysfunction (CLAD).4,5 CLAD usually manifests itself as obstructive bronchiolitis obliterans syndrome (BOS) but may also take the form of restrictive allograft syndrome.6,7 Classical alloreactivitity may not be the sole driver for the development of BOS,8 but other mechanisms, such as autoimmune response, may be involved.9 Besides being a risk factor for BOS, development of an autoimmune response to collagen type V (colV) was occasionally observed before transplantation and was a significant risk factor for primary graft dysfunction.10 Importantly, analysis of patients with immune responses to colV before transplant revealed that colV responders were more likely to express HLA-DR15,11 suggesting that some DR types might be more autoimmunogenic for colV. After transplantation, colV responders were more likely to express HLA-DR1 or -DR17 (although despite this, the latter became highly BOS-resistant). Remarkably, the donors of those lung transplant patients who became responsive to colV and developed BOS were more likely to express HLA-DR15.11 In line with the influence of the lung donor’s HLA-DR on the recipient’s autoimmune responsiveness, one HLA-DR15neg patient responded to a peptide presented by donor-type HLA-DR15.11 Based on these preliminary data in the relatively small sample tested (n = 54), we examined the relationship of specific HLA-DR antigens to the incidence and severity of CLAD/BOS in a large cohort (n = 351) of lung transplant recipients transplanted at the UW Madison.
MATERIALS AND METHODS
Patient Population
The study population consisted of all primary lung allograft recipients who underwent transplantation at the University of Wisconsin Hospital and Clinics from the time of program inception (1988) through December 31, 2008. Recipients with graft survival less than 40 days or lost to follow-up less than 100 days posttransplant were excluded. Retrospective demographic and outcomes data from 351 recipients were collected via medical record review using University of Wisconsin Institutional Review Board–approved protocol M-2012-0062. Recipients were followed until graft loss (defined as re-transplantation or patient death), date of last follow-up if lost to follow-up, or December 31, 2014; thereby allowing for the potential of at least 6 years of follow-up for each recipient.
Comparisons were made between recipients who did or did not develop BOS. Grading of BOS followed the International Society of Heart & Lung Transplantation (ISHLT) recommendations4,12 where BOS stage 1 (BOS 1) is defined as a persistent decline in forced expiratory volume in 1 second (FEV1) to between 66 and 80% of maximum posttransplant FEV1 and ≥ BOS stage 2 (BOS ≥ 2) represents a drop to less than 65% of the maximum FEV1 posttransplant value without evidence of other causes of FEV1 decline. We did not differentiate patients with restrictive allograft syndrome from those with an obstructive BOS pattern. Patients with graft loss without BOS or those who died with a functioning organ were censored at the time of graft loss and/or death. Lung allocation score (LAS) was calculated at the time of transplant. For patients transplanted between 1999 and 2005 (before the institution of LAS), an estimated LAS was determined using data from the medical records. No LAS estimates were made for patients transplanted before 1999.
Statistics
Comparisons among groups were performed using the Cox proportional hazard model and life-test Kaplan-Meier (KM) analysis (Breslow). Comparison of demographics among groups were performed using either ANOVA or χ2 analysis. To look for synergy or additive effects of particular HLA-DR antigens on overall DR match/mismatch (MM) effect, we created an interactive term before running the Cox proportional hazard model and Kaplan-Meier (KM) analysis. We did not adjust for multiple comparisons. To exclude the influence of graft losses within the first year, some data were analyzed only on subjects that experienced graft survival, free of BOS 2 for more than 1 year.
RESULTS
Patient Demographics
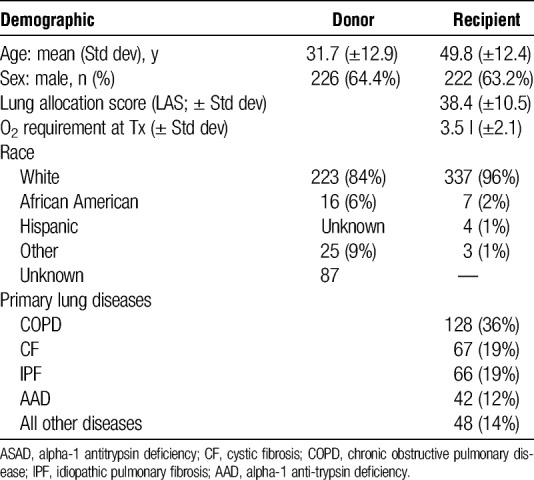
A total of 351 patients met the criteria for inclusion in the analysis, receiving a primary lung transplant at the University of Wisconsin Hospital and Clinics between 1988 and December 31, 2008, with at least 40 days of survival and 100 days of potential follow-up.
The majority of lung transplant recipients were male (Table 1) and white (96%). A majority had received a transplant from an HLA-DR completely MM donor (n = 193 [55%] 2 DR MM), the remaining 45% (n=154), had an HLA-DR partly or fully matched donor with a DR0 or 1 MM. The majority of the transplants were single lung (n = 217, 62%) versus bilateral lungs. The 4 major end-stage lung diseases were chronic obstructive pulmonary disease, cystic fibrosis, idiopathic pulmonary fibrosis and alpha-1 antitrypsin deficiency (Table 1).
TABLE 1.
Patient and donor demographics
Factors Influencing CLAD/BOS Development
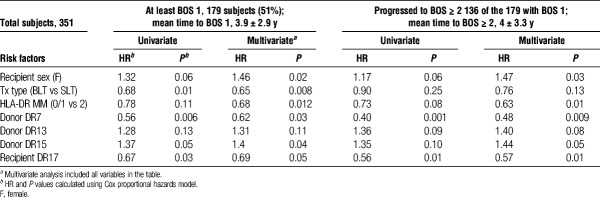
A primary factor limiting long-term lung transplant survival is the development of CLAD. CLAD is defined as a sustained drop in the FEV1 from the posttransplant maximum that cannot be attributed to other causes, such as infection or anastomosis dysfunction. The predominant subset of CLAD is obstructive BOS.5 Determination of levels of BOS was based on the criteria described in Methods and adhered strictly to the recommendations from the ISHLT.4 In our patient population, slightly more than half of the subjects (179, 51%, Table 2) were diagnosed with BOS 1. The majority (n = 136, 76%) of patients with BOS 1 progressed to BOS ≥ 2. Potential risk factors for BOS were first analyzed singly, and those which were either significant, or trending toward significance (P < 0.2), were further explored in a multivariable analysis using the Cox proportional hazards model.
TABLE 2.
Factors affecting BOS 1 or BOS ≥ 2
Recipient age, LAS, O2 requirement at transplant, donor age, donor sex, donor DR types 1, 4, 11, or 17, recipient DR types 1, 4, 7, 11, or 15 were found to be neither significant (P < 0.05) or trending toward (P < 0.2) significance. In contrast, transplant type, and certain HLA-DR types of donor (DR7 and 15) and recipient (DR17) were significantly associated with development of BOS 1 (Table 2). Recipient sex (female) and HLA-DR MM (2 DR MM) trended toward significance for BOS 1. In multivariate analysis (including all variables with P < 0.2 in univariate analysis, that is, recipient sex, single vs bilateral transplant, HLA-DR MM (0/1 vs 2MM), donor DR7, donor DR13, donor DR15, or recipient DR17), recipient sex, transplant type and HLA-DR MM all were significantly associated with development of BOS 1 (Table 2). Similar results were seen in risk factors for BOS ≥ 2; however, transplant type lost significance. This seems to be due to the fact that although patients with a bilateral lung transplant develop BOS 1 more slowly than do those with a single lung transplant, once they experience BOS 1, they quickly developed BOS ≥ 2. Although the patients were transplanted over a considerable period (1988-2008), the majority were transplanted after 2001 (214 of the 351). To determine if there was an “era” effect, we created variables for interaction effect of era (transplantation before 2001 vs after 2001) and the variables included in the previous multivariable analysis. We then reran the multivariable analyses, including the era and the interaction effects. The results suggested that there was no era effect.
Not Only HLA-DR MM, But Also HLA-DR15 in the Donor Lung Increases Risk of CLAD/BOS
Superimposed on the degree of HLA-DR MM, whose association with development of BOS was relatively weak (P = .021, Figure 1), was the influence of particular HLA-DR types. In the Cox analysis of the major donor HLA-DR types in our Upper Midwest US population (Table 2 and Table S1, SDC, http://links.lww.com/TP/B526), we found that patients receiving a lung transplant from a donor that expressed HLA-DR15, had a 40% increased risk for BOS 1 (P = 0.04). The incidence of severe BOS (≥2) for those receiving a DR15pos lung transplant was similar in magnitude and statistical significance (KM P = 0.03, Figure 2A). Note that little difference was seen in the incidence of severe BOS for subjects who have or have not received lungs expressing HLA-DR15 until 2 years posttransplant (Figure 2A). However, beyond that time point, the rate of development of severe BOS was significantly greater for the subjects with a DR15pos donor.
FIGURE 1.
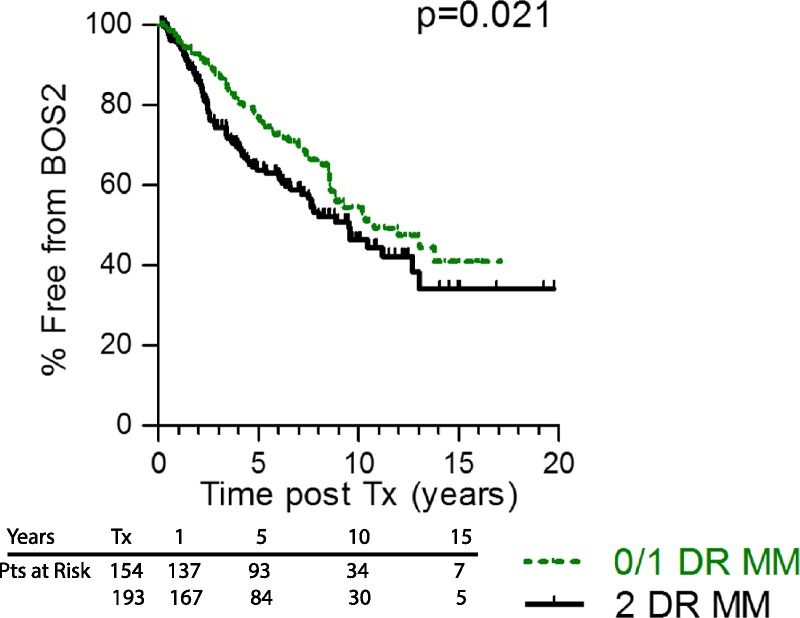
Poor DR MM is associated with increased severe BOS. Patients with higher degree of HLA-DR MM (2 DR antigen MM, black line) experience severe BOS earlier than did individuals that received lungs from a better HLA-DR matched donor (0 or 1 DR MM, green line), P = 0.021. Numbers of subjects at risk at transplant (Tx), 1, 5, 10, and 15 years posttransplant is on the graphic.
FIGURE 2.
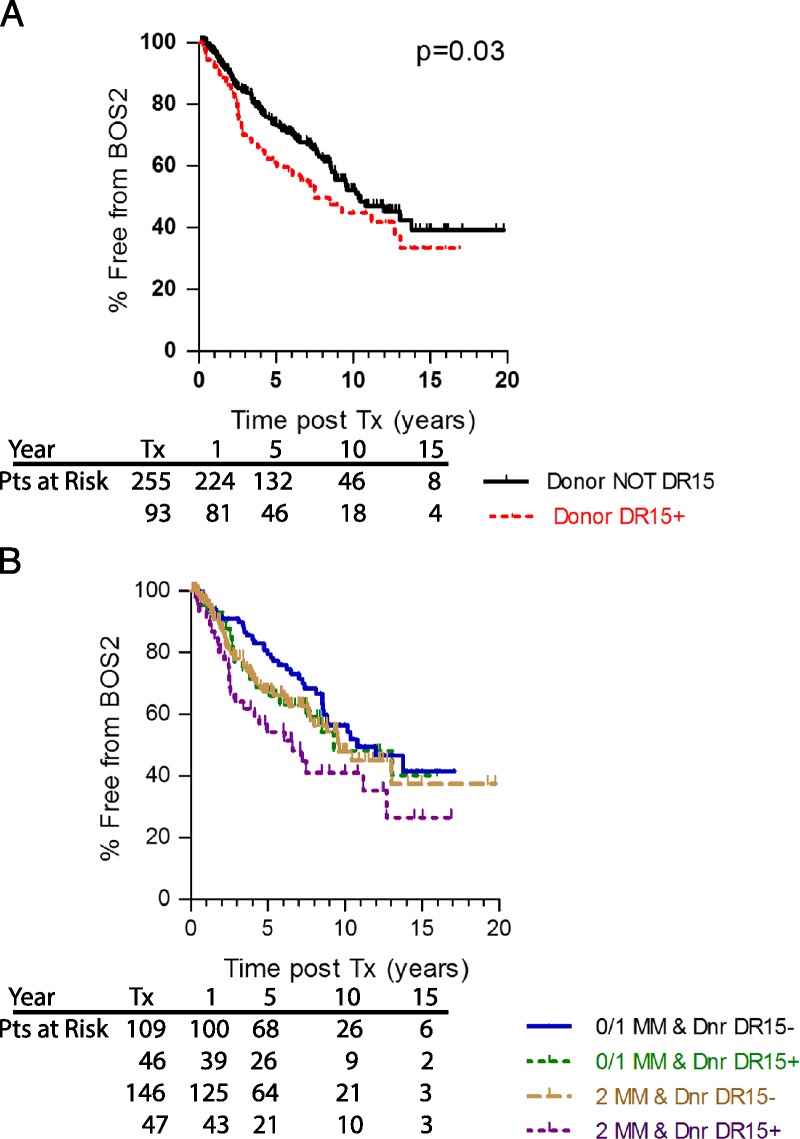
Donor HLA-DR15 is a risk for BOS. Patients who received lungs from a donor expressing HLA-DR15 were at higher risk for developing BOS 1 (P = 0.02, data not shown) and severe BOS (red dashed line, panel A, P = 0.03) than were those who received lungs that did not express HLA-DR15 (black line). Patients were divided into those that received well matched (DR MM of 0 or 1) vs poorly matched (2 DR MM) lungs where the donor did or did not express DR15 (panel B). Here, the best outcomes were in patients that received well matched, but DR15neg lungs (solid blue line), and the worst outcome was in patients that received poorly matched, DR15pos lungs (dotted purple line). Cox proportional hazard, P = 0.012; HR, 1.89 for DR15pos, 2 DR MM donors). Numbers of subjects at risk at Tx, 1, 5, 10 and 15 years posttransplant is on the graphic.
To exclude the influence of early graft loss, the KM analyses were performed on the subset of subjects with longer than 1 year survival. For most of the risk factors impacting the rate of severe BOS 2, there was minimal effect of excluding the early loss patients; however, the development of severe BOS was significantly worse for those that received a DR15 pos lung (P = 0.014).
The donor DR15 effect on BOS development became clearer when lung transplants were divided according to degree of HLA-DR MM. We created a combined variable including both MM and a specific donor or recipient HLA-DR expression, and used both Cox proportional hazard regression and KM analysis to evaluate their association with development of severe BOS (≥2). The best outcomes were seen in patients that received a well matched, but DR15neg lung (Figure 2B; blue line), and the worst outcomes in patients that received a poorly matched, DR15pos lung (purple line, Cox P = 0.012; hazard ratio [HR], 1.89 for DR15 pos, 2 DR MM donors). Between these 2 extremes, the outcomes for those with poor match (2 DR MM), but without DR15 and those with DR15pos donor, but better match (0 or 1 DR MM) were nearly identical.
HLA-DR7 in the Donor Lung Decreased the Risk of CLAD/BOS Outcomes
An unexpected result of the analysis of donor DR types was that recipients who received a HLA-DR7pos lung experienced a dramatically lower incidence of BOS when compared to those whose donors did not express DR7 (Table 2). Although recipient DR7 was not associated with freedom from BOS ≥ 2 (data not shown), the benefit of freedom from BOS ≥ 2 for those receiving a lung from a donor expressing HLA-DR7 was profound, and was evident in all patients, regardless of degree of DR MM (Figure 3A, KM P = 0.006). For example, in patients receiving a lung from a HLA-DR7pos donor, those that received a 0 or 1 DR MM lung experienced a lower incidence of severe BOS than did those with a 2 DR MM (green vs purple lines, KM P = 0.04), but those receiving a well matched (0 or 1 DR MM) lung that also expressed DR7 experienced significantly less severe BOS than did those with similar match grade, but without DR7 (panel B, green vs blue; KM, P = 0.006; Cox HR, 0.241). Even in the 2 DR MM recipients (purple line vs tan line, P = 0.021; HR, 0.485), receiving a lung with a DR7 was still beneficial, with the recipients of a DR 2MM lung expressing DR7 having equivalent BOS 2 timelines through year 5 to those without a donor DR7 expressing lung, but a better match. During the first 2 to 3 years posttransplant, the survival curves for all subjects except the well matched individuals with a DR7 donor were very similar. Long term, the survival curves for those lucky enough to receive a DR7pos donor lung were better, regardless of match, indicating that receiving a DR7pos lung at our center outweighed HLA-DR matching in promoting freedom from severe BOS.
FIGURE 3.
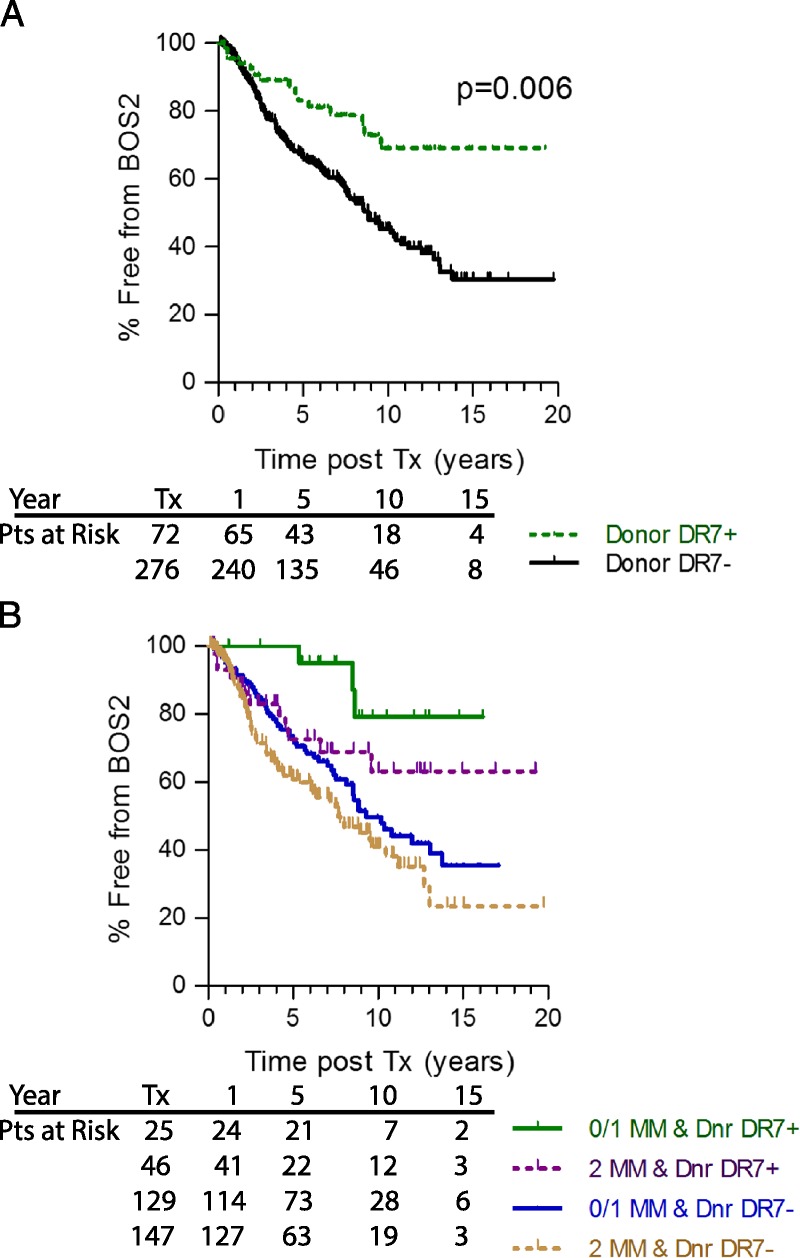
Donor HLA-DR7 is protective for BOS ≥ 2 and DR MM plays an additive or synergistic role. Lung transplant recipients receiving a transplant from a donor expressing HLADR7 experienced significantly less BOS (panel A, green dashed line) than those recipients who received lungs from a donor that did not express HLA-DR7 (black line, P = 0.006). Patients were divided by those that received well matched (0 or 1 DR MM, solid lines) or poorly matched (2DR MM, dashed lines) lungs where the donor did or did not express DR7 (panel B). Patients with the best match and DR7 expressing donor (green line) experienced the best outcome, followed by those with poor match, but DR7 expressing donor (purple line, P = 0.04), then the better matched recipients where the donor did not express DR7 (blue line, P = 0.006 compared to 0 or 1 MM DR7pos donors) and finally the worst outcome was in the patients with poor match and donor without DR7 (tan line). Numbers of subjects at risk at Tx, 1, 5, 10, and 15 years posttransplant is on the graphic.
On the recipient side, only one HLA-DR type (of the 7 major DR alleles analyzed) was associated with freedom from BOS. Confirming our previous study,11 individuals expressing HLA-DR17 were less likely to experience BOS ≥ 2 than were those without DR17 (Table 2, P = 0.01). HLA-DR17pos recipients experienced a considerably lower incidence of BOS ≥ 2 than did those without HLA-DR17 (Figure 4A, P = 0.003). As with the beneficial impact of combining good HLA-DR match with donor DR7 expression, those patients who are HLA-DR17 and receive a better DR matched lung have superior BOS free survival than do those without DR17 who received a poorly matched lung (Figure 4B, green vs tan line, Cox; P = 0.01; HR, 0.396). Expression of the DR17 seemed to be protective in those with a 0 or 1 DR MM lung (green line), and those that received a 2 DR MM lung (purple line) as the survival in these 2 groups were superior to those who did not express HLA-DR17 (DR 0 or 1 MM, blue line and 2 DR MM tan line). Even in the 2 DR MM patients, patients with DR17 had significantly better protection from severe BOS (P = 0.025; HR, 0.534) than did those without DR17.
FIGURE 4.
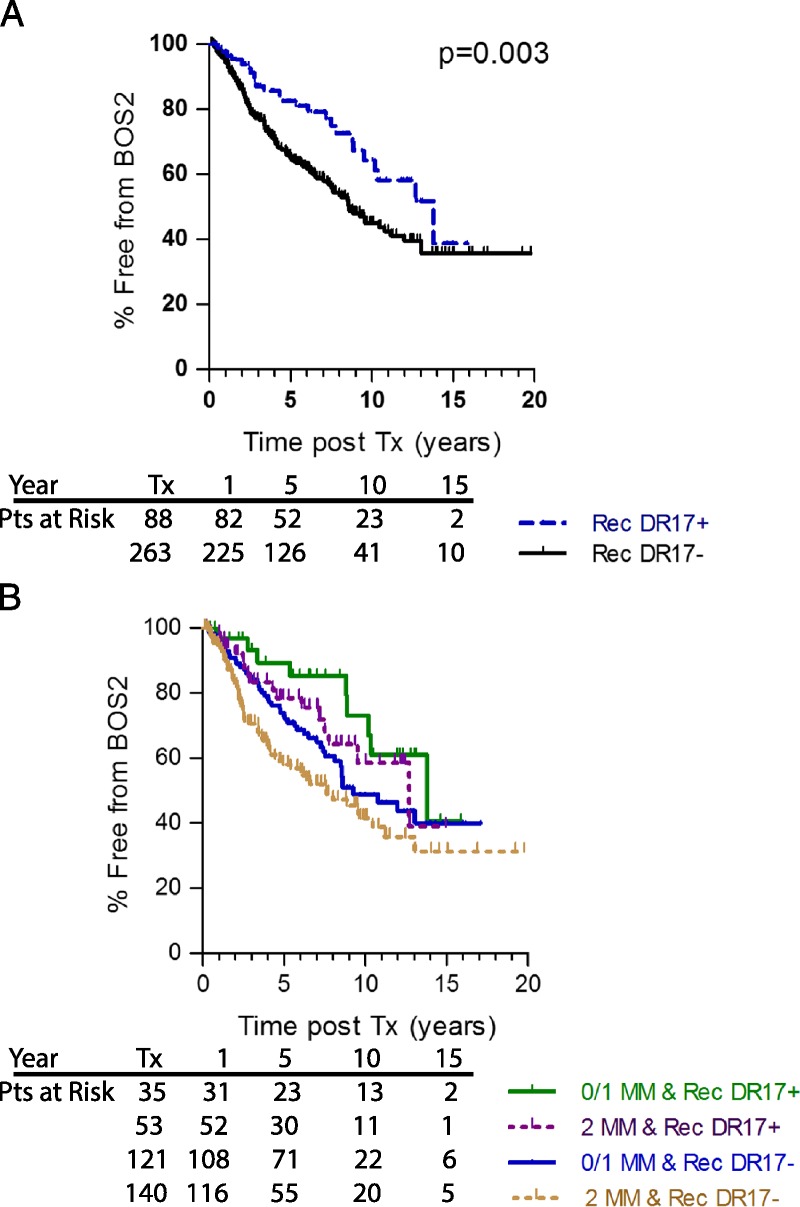
Recipient HLA-DR17 is protective for BOS ≥2 and DR MM is additive or synergistic. Lung recipients expressing HLA-DR17 (blue dashed line) were at a lower risk for BOS 1 (data not shown) and severe BOS (≥2, panel A, P = 0.003) then were recipients that did not express HLA-DR17 (black solid line). Patients were divided by those that received well matched (DR MM of 0 or 1, solid lines) or poorly matched (2DR MM, dashed lines) lungs where the recipient did or did not express DR17 (panel B). DR17 expressing patients with the best match (green line) experienced the best outcome, followed by those with poor match (purple line, P = 0.04), then the recipients that do not express HLA-DR17, but received better matched lungs (blue line) and finally the worst outcome was in the HLA-DR17 negative patients with poor match (tan line). Numbers of subjects at risk at Tx, 1, 5, 10 and 15 years posttransplant is on the graphic.
Finally, we compared outcomes in patients who received a lung from a donor expressing both DR15 (an allele which increases risk of BOS) and DR7 (an allele which protects from BOS). As shown in Figure 5, panel A, the outcome was better in patients who received a donor DR7pos lung (green and purple lines) even if that lung also expressed DR15 (purple line). The worst outcome was again in patients who received a donor DR15-expressing lung without DR7 as the other DR allele (tan line). Interestingly, as shown in panel B, when the recipient expressed HLA-DR17, the BOS survival benefit was only seen if the donor did not express DR15 (green line). In all other cases, the outcomes were almost identical.
FIGURE 5.
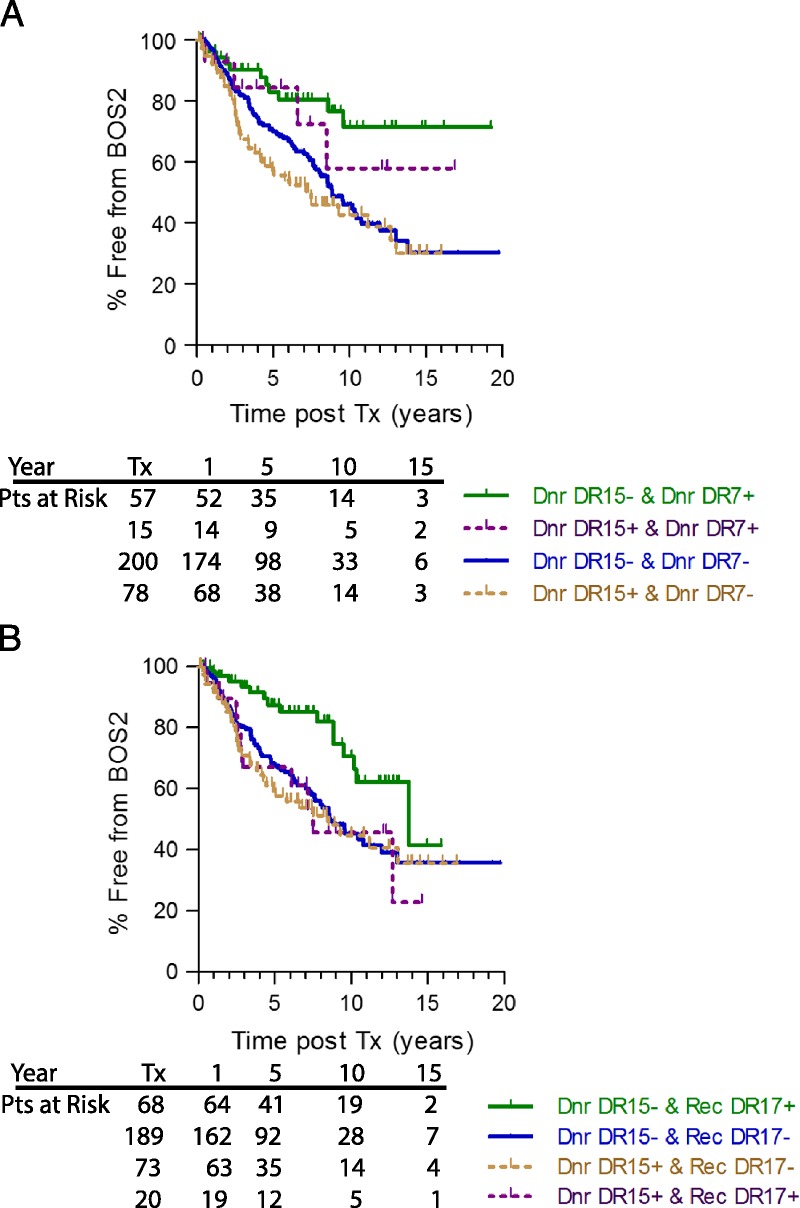
Donor DR7 protective benefit appears to be stronger than donor DR15 susceptibility risk (panel A); whereas recipient DR17 benefit from BOS ≥ 2 is only seen in patients that do not receive a donor DR15pos lung (panel B). In panel A, lung transplant recipients were divided into those that received a lung expressing both DR15 and DR7 (purple line), neither DR15, nor DR7 (blue line), DR15 without DR7 (tan line) or DR7 without DR15 (green line). In panel B, Lung transplant recipients were divided into those that were themselves DR17 and received a DR15 expressing lung (purple line) or received a lung that did not express DR15 (green line) vs those that are not DR17 and received a DR15 expressing lung (tan line) or received a lung that did not express DR15 (blue line). Numbers of subjects at risk at transplant (Tx), 1, 5, 10 and 15-years posttransplant is on the graphic.
DISCUSSION
Summary: To see if the particular DR types mattered in BOS risk or protection, the type and degree of HLA-DR MM between recipient and donor at our center was studied in detail. In a study of 351 lung transplant recipients and their donors, in a population with limited ethnic heterogeneity, we have shown strong associations of 2 specific HLA-DR types in the donor with susceptibility to (DR15) or freedom from (DR7) severe BOS. We also confirmed a previous correlation of low BOS incidence in recipients with HLA-DR17.11 Thus the DR MM effect was superimposed on an underlying risk/protection matrix imparted by specific HLA-DR types expressed in the donor lung or in the recipient. An analysis of the UNOS database of 7400 lung transplant recipients in the United States found that patients with an HLA-DR15+ donor had significantly more treatments for rejection in the first year posttransplant.13 This finding, in a multicenter study, is of great interest, because it supports the underlying premise that not only recipient, but also donor HLA-DR type can significantly impact lung transplant outcome. In contrast to our findings; however, the same study found no impact of donor DR type on development of BOS 1 or long-term graft survival. There are several possible reasons for this discrepancy with the present study: (1) Gracon et al. did not distinguish severe from early BOS grade 1, which does not always progress to BOS 2 or 3; (2) the UNOS data set may not have accurately captured many BOS events, whereas all BOS events in our patient population were carefully diagnosed based on extensive medical records review; and (3) multicenter studies are notably less accurate than single-center studies at capturing HLA-class II effects on chronic autoimmune disease, due to the impact of polymorphisms within the major histocompatibility complex of different ethnic groups. In regard to the latter point, to the extent that the BOS form of CLAD is mediated by chronic autoimmune reactivity to antigens like collagen V2 and k-α1-tubulin,3 donor and recipient populations that are relatively ethnically homogeneous at a single center (Table 1) are preferable to ethnically diverse populations commingled in a multicenter HLA-DR analysis. Given the degree to which lung transplant success has been linked to control of Th17 autoimmunity, our results indicate that donor, as well as recipient, HLA-class II restricted self peptide recognition plays a key role in BOS.
In a recent elegant study by Wang et al,14 the investigators cloned a CD8 T cell and its receptor (TcR) from an HLA-A2-negative, hepatitis C virus (HCV) positive liver transplant patient. This TcR recognized a specific HCV peptide presented, not by recipient HLA class I, but only by the donor HLA-A2. Through peptide binding, structural analysis and molecular manipulation, they identified critical amino acids for TcR binding on both the HLA-A2 and HCV peptide. This result suggests that an HLA-A2 negative individual became capable of supporting the development of a T cell response to a peptide presented by a NON-SELF, that is, donor HLA, presumably to combat HCV infection. In the autoimmune-prone lung transplant setting, the unusual donor-DR dependence of the CLAD/BOS phenomenon seen in this large single-center study raises the question: can allorestricted presentation of self peptides select TcR in host CD4 T cells; for example, self peptides from antigens such as colV2,15–17 and kα1-tubulin18 involved in BOS, and differentially bound by various DR alleles?11,16,19
Opelz and collegues20 noted in a Eurotransplant database study, evidence of HLA-restricted, rather than simple HLA matching effects in lung transplant. Although this HLA-restricted effect has not yet been localized to a specific innate or adaptive T-cell type, there is considerable evidence that T cells producing IL17 are responsible for CLAD/BOS in humans2,21 as well as in mouse models.17,22 The critical IL17 producer cell appears to be CD4+ in both mouse23 and humans.16 Therefore, it is conceivable that in lung transplant recipients an allo-DR–restricted Th17 immune response to self peptide might emerge.
How might a particular HLA-DR specificity on the donor lung influence the susceptibility to BOS? One possibility is a strictly “HLA-allo” effect, that is, direct allorecognition of DR15 always induces a stronger alloimmune response than does HLA-DR7. Although theoretically possible, there is no evidence from mixed lymphocyte reaction studies to support this idea. In fact the donor HLA-DR7 data (Figure 3B) suggests that it supercedes DR matching effects, implying active protection of the graft from BOS. Another possibility is that of an “HLA-restriction” effect. In the case of HLA-DR15, we have shown that there are several colV(α1) peptides that have very high binding, and expression of HLA-DR15 was associated with elevated pretransplant colV response.11 Additionally, HLA-DR15 frequency is increased in some,24,25 but not all populations26 of patients with idiopathic pulmonary fibrosis, a disease we have found to be associated with colV autoimmunity.10 Importantly, we have observed that 2 patients who were HLA-DR15neg, but received a lung expressing HLA-DR15 can exhibit T cell responses to DR15-restricted peptides 2 to 7 years posttransplant.11,16
One possible avenue for allorestricted presentation of self antigen is via the “semi-direct”, exosome mediated, allorecognition pathway.27 Recent evidence suggests that lungs secrete exosomes or extracellular vesicles containing donor HLA as well as self antigens colV and kα1-tubulin.28 Additionally, Nayak et al.29 has shown that CD163/169/206 triple+, non–T cells in BAL from lung transplant patients constitute HLA-DRpos tissue-resident alveolar macrophages. These cells, which patrol the surface of the airway, turn over very little from bone marrow and were found to remain largely donor-type at 3 years posttransplant. Furthermore these resident donor cells were recently found to act as an ongoing reservoir producing extracellular vesicles carrying donor HLA (class I or class II), and self antigens that trigger autoimmunity in BOS.30 Extracellular vesicle-based mechanisms may help explain increased risk31 or protection32 of a woman from developing rheumatoid arthritis based on persisting fetal microchimerism in her body after parturition.
The protective influence of donor DR7 is still a mystery. We have preliminary data that coexpression of HLA-DR7 and DR15 in a lung transplant recipient prevents the immune response to a colV(α1) peptide normally presented by DR15, whereas permitting responses to other colV(α1) peptides. In our patient population, it was notable that such coexpression of DR7 overcame the increased BOS risk of a having a DR15pos donor (Figure 5A), something that recipient expression of the otherwise protective DR17 allele did not (Figure 5B). Evidence for active suppression of BOS was shown in a mouse lung transplant model where induced T regulatory cells were found to suppress the IL-17 production and reduce the degree of BOS and cytokine production.33
In a different autoimmune disease, Goodpasture syndrome,34 HLA-DR15, and HLA-DR7 also had opposite influences on susceptibility; with a higher frequency of HLA-DR15 and a much lower frequency of DR7 in patients with the disease. Peptide analysis by Phelps et al35 showed that disease protection by DR7 was not due to lack of binding of the pathogenic colIV(α3) peptides to DR7, as the known peptides actually bound with a higher affinity to DR7 than to DR15. They postulate that autoantigenic epitopes were “stolen” by DR7 and presented harmlessly preventing DR15-associated Goodpasture disease.35 In another disease, psoriasis, HLA-DR7 appears to present peptides that stimulate IFNγ production by T cells, whereas HLA-DR15 does not.36 In this case, HLA-DR7 appears to be the pathologic genotype, whereas DR15 is a protective one.
ColV is only one type of antigen that might be important in the immune responses in lung transplant recipients; microbiota37,38 may be drastically altered by the presence of different DR alleles39 or environmental antigens.40 Interplay of donor-MHC and the lung microenvironment, in the case of both types of antigens, could mean that the profound donor and recipient DR associations found in this single-center study might not be universal, but rather specific to the strongly Caucasian/Northern European bias in the ethnicity of our lung donor and recipient populations. The particular DR-type/BOS associations seen in a given lung transplant center are likely to reflect their ethnic diversity, as different human populations show different DR-based autoimmune disease associations.41
The mechanistic basis of the donor DR-BOS associations are yet to be fully elucidated, but are likely to involve acquisition by the host of new MHC class II immune response, gene products from the transplant donor, possibly by the same exosome-dependent process that can lead to early acute rejection.42 Because lung transplants that survive the first year become particularly susceptible to autoimmune damage, these donor class II-based, acquired susceptibilities and protections are likely to involve reshaping of the autoimmune CD4 TcR repertoire of the host, as has been shown for a donor class I reshaping the host viral-specific CD8 T cell immune repertoire.14
Footnotes
This work was supported by NIH grants RO1AI119140 and PPG AI084853.
The authors declare no conflicts of interest.
L.D.H., W.J., J.M., K.C.M., W.J.B. participated in research design. L.D.H., W.J.B., G.L., K.C.M. participated in the writing of the article. L.D.H., W.J., G.L. participated in the performance of the research. L.D.H. and G.L. participated in data analysis.
Correspondence: William J. Burlingham, PhD, G4/701 CSC, 600 Highland Avenue, Madison, WI 53792-7375. (burlingham@surgery.wisc.edu).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
In a retrospective study of outcomes in 351 lung transplant recipients, the authors demonstrate that some donor HLA class II types are associated with susceptibility (especially HLA-DR15) or protection from the development of severe bronchiolitis obliterans syndrome. Supplemental digital content is available in the text.
REFERENCES
- 1.Mares DC, Heidler KM, Smith GN. Type V collagen modulates alloantigen-induced pathology and immunology in the lung Am J Respir Cell Mol Biol 2000. 2362–70 [DOI] [PubMed] [Google Scholar]
- 2.Burlingham WJ, Love RB, Jankowska-Gan E. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants J Clin Invest 2007. 1173498–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goers TA, Ramachandran S, Aloush A. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection J Immunol 2008. 1804487–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer KC, Raghu G, Verleden GM. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome Eur Respir J 2014. 441479–1503 [DOI] [PubMed] [Google Scholar]
- 5.Verleden GM, Raghu G, Meyer KC. A new classification system for chronic lung allograft dysfunction J Heart Lung Transplant 2014. 33127–133 [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Waddell TK, Wagnetz U. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction J Heart Lung Transplant 2011. 30735–742 [DOI] [PubMed] [Google Scholar]
- 7.Verleden SE, Todd JL, Sato M. Impact of CLAD phenotype on survival after lung retransplantation: a multicenter study Am J Transplant 2015. 152223–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zazueta OE, Preston SE, Moniodis A. The presence of pretransplant HLA antibodies does not impact the development of chronic lung allograft dysfunction or CLAD-related death Transplantation 2017. 1012207–2212 [DOI] [PubMed] [Google Scholar]
- 9.Royer PJ, Olivera-Botello G, Koutsokera A. Chronic lung allograft dysfunction: a systematic review of mechanisms Transplantation 2016. 1001803–1814 [DOI] [PubMed] [Google Scholar]
- 10.Bobadilla JL, Love RB, Jankowska-Gan E. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation Am J Respir Crit Care Med 2008. 177660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller MR, Haynes LD, Jankowska-Gan E. Epitope analysis of the collagen type V-specific T cell response in lung transplantation reveals an HLA-DRB1*15 bias in both recipient and donor. PLoS One. 2013;8:e79601. doi: 10.1371/journal.pone.0079601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estenne M, Maurer JR, Boehler A. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria J Heart Lung Transplant 2002. 21297–310 [DOI] [PubMed] [Google Scholar]
- 13.Gracon AS, Liang TW, Rothhaar K. Human leukocyte antigen-DR13 and DR15 are associated with short-term lung transplant outcomes J Surg Res 2016. 20382–90 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Singh NK, Spear TT. How an alloreactive T-cell receptor achieves peptide and MHC specificity Proc Natl Acad Sci U S A 2017. 114E4792–E4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkes DS, Heidler KM, Yasufuku K. Cell-mediated immunity to collagen V in lung transplant recipients: correlation with collagen V release into BAL fluid. J Heart Lung Transplant. 2001;20:167. doi: 10.1016/s1053-2498(00)00308-9. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan JA, Jankowska-Gan E, Hegde S. Th17 responses to collagen type V, kα1-tubulin, and vimentin are present early in human development and persist throughout life Am J Transplant 2017. 17944–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan L, Benson HL, Vittal R. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation Am J Transplant 2011. 11911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiriveedhi V, Gautam B, Sarma NJ. Pre-transplant antibodies to Kα1 tubulin and collagen-V in lung transplantation: clinical correlations J Heart Lung Transplant 2013. 32807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park AC, Huang G, Jankowska-Gan E. Mucosal administration of collagen V ameliorates the atherosclerotic plaque burden by inducing interleukin 35-dependent tolerance J Biol Chem 2016. 2913359–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opelz G, Susal C, Ruhenstroth A. Impact of HLA compatibility on lung transplant survival and evidence for an HLA restriction phenomenon: a collaborative transplant study report Transplantation 2010. 90912–917 [DOI] [PubMed] [Google Scholar]
- 21.Vanaudenaerde BM, De Vleeschauwer SI, Vos R. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation Am J Transplant 2008. 81911–1920 [DOI] [PubMed] [Google Scholar]
- 22.Lemaitre PH, Vokaer B, Charbonnier LM. IL-17A mediates early post-transplant lesions after heterotopic trachea allotransplantation in Mice. PLoS One. 2013;8:e70236. doi: 10.1371/journal.pone.0070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Gupta PK, Suzuki H. CD4 T cells but not Th17 cells are required for mouse lung transplant obliterative bronchiolitis Am J Transplant 2015. 151793–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fingerlin TE, Zhang W, Yang IV. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet. 2016;17:74. doi: 10.1186/s12863-016-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue J, Gochuico BR, Alawad AS. The HLA class II Allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One. 2011;6:e14715. doi: 10.1371/journal.pone.0014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HP, Zou J, Xie P. Association of HLA and cytokine gene polymorphisms with idiopathic pulmonary fibrosis Kaohsiung J Med Sci 2015. 31613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera OB, Golshayan D, Tibbott R. A novel pathway of alloantigen presentation by dendritic cells J Immunol 2004. 1734828–4837 [DOI] [PubMed] [Google Scholar]
- 28.Gunasekaran M, Xu Z, Nayak DK. Donor-derived exosomes with lung self-antigens in human lung allograft rejection Am J Transplant 2017. 17474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayak DK, Zhou F, Xu M. Long-term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor-specific immune responses Am J Transplant 2016. 162300–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nayak DK, Zhou F, Xu M, et al. Zbtb7a induction in alveolar macrophages is implicated in anti-HLA-mediated lung allograft rejection. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Z, Aydelotte T, Gadi VK. Acquisition of the rheumatoid arthritis HLA shared epitope through microchimerism Arthritis Rheum 2011. 63640–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guthrie KA, Dugowson CE, Voigt LF. Does pregnancy provide vaccine-like protection against rheumatoid arthritis? Arthritis Rheum 2010. 621842–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Zhou X, Gaowa S. The critical role of induced CD4+ FoxP3+ regulatory cells in suppression of interleukin-17 production and attenuation of mouse orthotopic lung allograft rejection Transplantation 2015. 991356–1364 [DOI] [PubMed] [Google Scholar]
- 34.Phelps RG, Rees AJ. The HLA complex in Goodpasture’s disease: a model for analyzing susceptibility to autoimmunity Kidney Int 1999. 561638–1653 [DOI] [PubMed] [Google Scholar]
- 35.Phelps RG, Jones V, Turner AN. Properties of HLA class II molecules divergently associated with Goodpasture’s disease Int Immunol 2000. 121135–1143 [DOI] [PubMed] [Google Scholar]
- 36.Baker BS, Ovigne JM, Fischetti VA. Reduced IFN-gamma responses associated with HLA-DR15 presentation of streptococcal cell wall proteins to dermal Th-1 cells in psoriasis J Clin Immunol 2003. 23407–414 [DOI] [PubMed] [Google Scholar]
- 37.Becker J, Poroyko V, Bhorade S. The lung microbiome after lung transplantation Expert Rev Respir Med 2014. 8221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernasconi E, Pattaroni C, Koutsokera A. Airway microbiota determines innate cell inflammatory or tissue remodeling profiles in lung transplantation Am J Respir Crit Care Med 2016. 1941252–1263 [DOI] [PubMed] [Google Scholar]
- 39.Gomez A, Luckey D, Yeoman CJ. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One. 2012;7:e36095. doi: 10.1371/journal.pone.0036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhinder S, Chen H, Sato M. Air pollution and the development of posttransplant chronic lung allograft dysfunction Am J Transplant 2014. 142749–2757 [DOI] [PubMed] [Google Scholar]
- 41.Taneja V, Giphart MJ, Verduijn W. Polymorphism of HLA-DRB,-DQA1, and -DQB1 in rheumatoid arthritis in Asian Indians: association with DRB1*0405 and DRB1*1001 Hum Immunol 1996. 4635–41 [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, Rojas-Canales DM, Divito SJ. Donor dendritic cell-derived exosomes promote allograft-targeting immune response J Clin Invest 2016. 1262805–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]