Abstract
Background
Sphingosine-1-phosphate (S1P) regulates endothelial barrier integrity and promotes cell survival and proliferation. We hypothesized that upregulation of S1P during ex vivo lung perfusion (EVLP) would attenuate acute lung injury and improve graft function.
Methods
C57BL/6 mice (n=4-8/group) were euthanized, followed by 1-hour of warm ischemia and 1 hour of cold preservation in a model of donation after circulatory death (DCD). Subsequently, mice underwent 1-hour of EVLP with one of four different perfusion solutions: Steen solution (Steen, control arm), Steen with added S1P (Steen+S1P), Steen plus a selective SphK2 inhibitor (Steen+SphKi), or Steen plus both additives (Steen+S1P+SphKi). During EVLP, lung compliance and pulmonary artery pressure were continuously measured. Pulmonary vascular permeability was assessed with injection of Evans Blue dye.
Results
The combination of 1-hour of warm ischemia, followed by 1-hour of cold ischemia created significant lung injury compared to lungs that were immediately harvested after circulatory death and put on EVLP. Addition of either S1P or SphKi alone did not significantly improve lung function during EVLP compared to Steen without additives. However, group Steen+S1P+SphKi resulted in significantly increased compliance (110±13.9% vs. 57.7±6.6%, p<0.0001) and decreased pulmonary vascular permeability (33.1±11.9 vs. 75.8±11.4 μg/gm tissue, p=0.04) compared to Steen alone.
Conclusions
Targeted drug therapy with a combination of S1P+SphKi during EVLP improves lung function in a murine DCD model. Elevation of circulating S1P via specific pharmacological modalities during EVLP may provide endothelial protection in marginal donor lungs leading to successful lung rehabilitation for transplantation.
Classifications: Ex Vivo Lung Perfusion, Lung Transplantation, Lung Rehabilitation, Sphingosine, Ischemia Reperfusion Injury
Introduction
The success of lung transplantation is complicated by a high rate of primary graft dysfunction due to ischemia-reperfusion injury (IRI); characterized by epithelial damage, endothelial permeability, and robust inflammation1, 2. IRI is also a risk factor for bronchiolitis obliterans, a major cause of late mortality3. Endothelial permeability leads to accumulation of pulmonary edema, which results in poor lung function and potentiates further inflammatory injury. Ex vivo lung perfusion (EVLP) has developed as a novel method to maintain donation after circulatory death (DCD) lungs in physiologically protective conditions outside the body and allows accurate evaluation of lung function, as well as providing a new platform for therapeutic treatment and repair of damaged donor lungs prior to transplantation4-7.
Sphingosine-1-phosphate (S1P) binds to a family of sphingosine receptors (S1PR 1-5) which are G-protein-coupled receptors and plays a central role in maintaining endothelial barrier integrity8, 9. It is well established that circulating S1P plays a critical role in the maintenance of vascular integrity and endothelial barrier function by promoting endothelial cell spreading and stabilizing cell-cell junctions10. Circulating S1P is synthesized largely by red blood cells via sphingosine kinase 1 (SphK1). SphK1 and SphK2 synthesize tissue S1P, but S1P lyase maintains low tissue S1P levels11. Maintenance of a S1P gradient with circulating S1P levels much higher than tissue is pivotal for vascular integrity. This gradient can be manipulated using SphK inhibitors of different selectivity, for example, SphK2 inhibitors raise blood S1P by blocking S1P clearance from the blood12 (Figure 1). Previous studies in other disease processes have shown that SphK2 inhibition attenuates kidney fibrosis13, and that activation of endothelial S1PR1 attenuates kidney IRI14. This represents a novel target for attenuation of lung IRI and requires further investigation.
Figure 1.
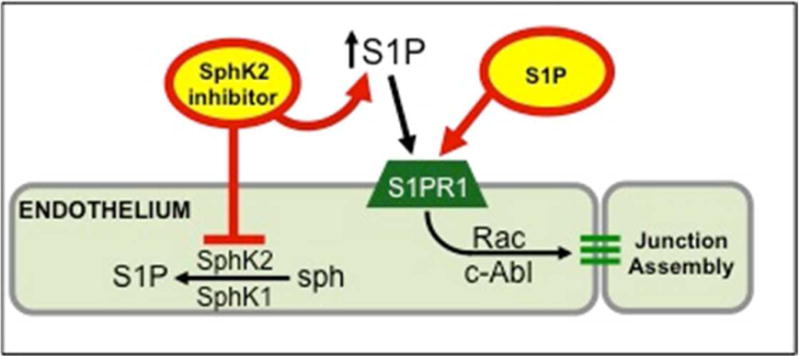
Circulating S1P is critical for maintenance of endothelial barrier by binding S1PR1 (via Rac and c-Abl signaling). Strengthening the vascular S1P gradient (circulating S1P levels that are much higher than tissue) improves endothelial barrier integrity. SphK2 inhibitors partially reduce tissue levels of S1P but elevate circulating S1P, thus increasing the S1P gradient. S1PR1 agonists and SphK2 inhibitors attenuate vascular permeability after IR.
Although S1P or S1P analogs serve as protective agents in models of acute lung injury, little is known about the role of S1P signaling in lung transplantation and organ preservation. A study by Okazaki et al. showed that S1P treatment attenuates lung edema and leukocyte infiltration after IRI in mice15. Our laboratory has demonstrated that a selective S1PR1 agonist attenuates lung IRI and vascular permeability in mice,16 suggesting that therapeutic targeting of S1P signaling could prevent lung IRI by preserving the endothelial barrier. Thus, the purpose of the current study was to evaluate the use of activated S1P and a selective SphK2 inhibitor (SLT223307) as targeted drug therapy during EVLP in a murine model of lung ischemia and cold preservation after DCD. We hypothesize that modifying the S1P gradient (higher circulating S1P, lower levels in tissue) will attenuate lung IRI through protective effects on pulmonary endothelium resulting in improved pulmonary function and vascular permeability.
Methods
Animals and Study Groups
The study protocol was approved by the University of Virginia Animal Care and Use Committee (ACUC) and complies with the 2011 Guide for the Care and Use of Laboratory Animals as recommended by the US National Institutes of Health. Wild-type C57BL/6 mice (8-12 weeks; The Jackson Laboratory, Bar Harbor, ME) were used for all experiments. Sample sizes were based on power calculation using 85% power with mean difference of 0.45±0.25 (PA pressure – 7 animals) and 0.75±0.25 (compliance – 4 animals) between groups as demonstrated in prior studies of murine EVLP and Sphingosine pathway manipulation by our group.16, 17
Sphingosine compounds
SLT223307 (SphKi), was a gift from S. Brandon Thorpe of SphynKx Therapeutics, Charlottesville, VA. SLT223307 is approximately 10-fold selective for mouse SphK2 over mouse SphK19. The compound was purified by high-performance liquid chromatography, dissolved in 2% hydroxypropyl cyclodextrin.
Donation After Circulatory Death (DCD) Lung Procurement Procedure
A DCD mouse lung protocol was performed as we have previously described 17. Briefly, mice were euthanized with isoflurane overdose followed by cervical dislocation according to ACUC guidelines. After 1 hour of warm ischemia, the mice were intubated, a median sternotomy was performed, and a left atriotomy was made. The lungs were flushed with 3mL of 4°C Perfadex® solution (XVIVO Perfusion Inc., Englewood, CO) via infusion into the right ventricle. During the cold flush, the lungs were ventilated with room air at 120 stokes/min. Ice slush was then added to the thoracic cavity and mice were stored at 4°C for 1 hour.
Ex Vivo Lung Perfusion
Mice underwent 1 hour of EVLP with Steen solution™ (Steen, n=8; XVIVO Perfusion Inc., Englewood, CO), Steen with physiologically active S1P (Steen+S1P, n=4), Steen plus drug therapy (Steen+ SphKi, n=4) or Steen and both additives (Steen+S1P+SphKi, n=8). S1P was added to Steen at a concentration of 0.5 mM. SLT223307 was added to Steen at a concentration of 3.0 mM and was selected for this experiment based on prior studies suggesting selective SphK2 inhibition reduces intracellular production of S1P, which drives up the S1P gradient12.
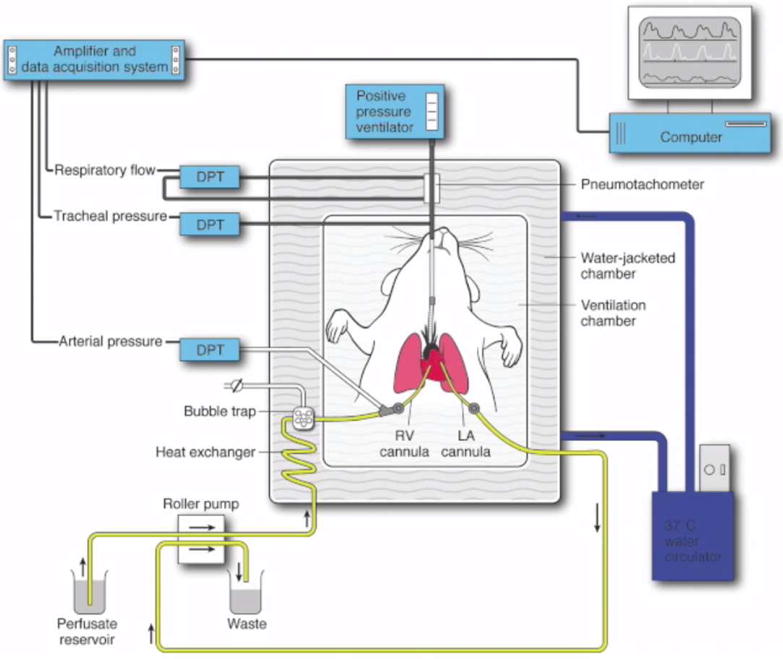
Mouse lung EVLP was performed as previously described by our laboratory (Video) 17. Briefly, mice were ventilated via a tracheostomy. The pulmonary artery (PA) was cannulated to allow inflow of the lung perfusion solution (Figure 2). Lungs were perfused using an isolated, murine lung apparatus (Hugo Sachs Elektronik, March-Huggstetten, Germany)18. Depending on groups, the lungs were perfused with each solution at a rate of 60 μl/g body weight/minute. All EVLP perfusates were supplemented with heparin (10,000 IU), cefazolin (500 mg), and methylprednisolone (500 mg) per 1500 mL of solution, and warmed to 37°C over the first 10 minutes of EVLP.
Video.
Murine EVLP apparatus with a set of murine lungs being perfused with Steen solution on the Ex Vivo circuit with pressure measurements.
Figure 2.
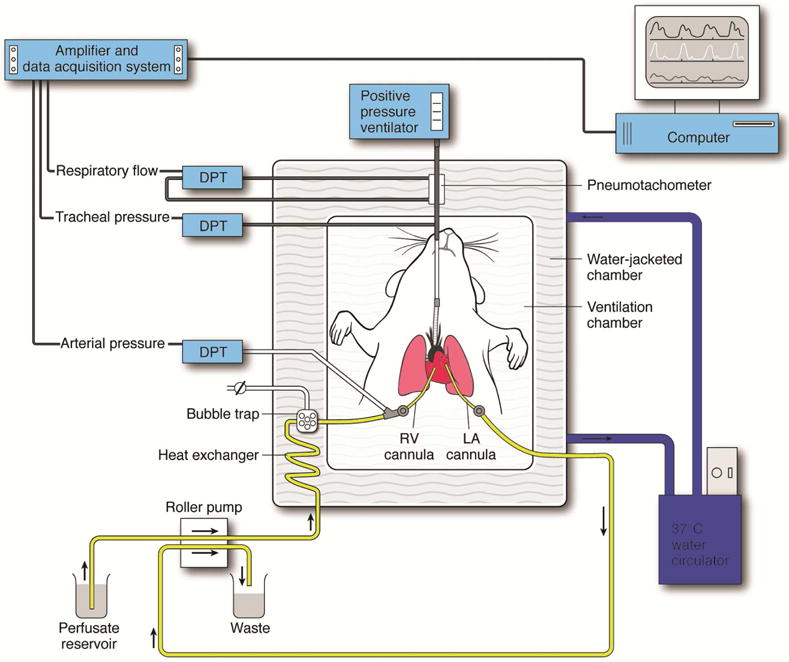
Schematic demonstration of the murine EVLP model. An isolated, buffer-perfused mouse lung system was used where mice underwent endotracheal intubation, followed by right ventricular (RV) cannulation for inflow, and left atrial (LA) cannulation for outflow of perfusate. Several differential pressure transducers (DPT) and a pneumotachometer were used to measure arterial pressure, tracheal pressure, and respiratory flow via the PULMODYN data acquisition software.
Evans Blue Vascular Permeability assay
Evans blue dye (Sigma-Aldrich Co., St. Louis, MO, USA) was flushed through the lungs (n=4/group, groups Steen and Steen+S1P+SphKi)) during the last 5 minutes of EVLP to evaluate pulmonary vascular permeability. After EVLP, lungs were harvested and homogenized in phosphate buffer saline. Lung homogenates were analyzed by measuring absorbance values measured at 740 nm and 620 nm. The following formula was used to calculate the concentration of Evans Blue dye in whole lung lysate: A620 (corrected) = A620 − (1.426 × A740 + 0.030) to correct for heme pigments. The average of each group is demonstrated as concentration of Evans Blue dye per weight of left lung.
Statistical Analysis
The primary outcomes were change in lung compliance and pulmonary artery pressure from beginning to the end of EVLP. Secondary outcomes included pulmonary vascular permeability as measured by Evans Blue dye test. Assumptions of normality and equal variance were evaluated with normal probability plots and variance ratios prior to comparing groups. Given their normal distribution, when only two groups were compared (Figures 3 and 6) a student t-test was used to determine statistical significance. A two-way ANOVA with repeated measures and Bonferroni’s correction for multiple comparisons was used to compare raw compliance and PA pressure (Figures 4A and 5A). Finally, given variability within the model the percent change was compared by group (Figures 4B and 5B) using one-way ANOVA with Bonferroni’s correction for multiple comparisons. Prism 7 (GraphPad Software Inc., La Jolla, CA) was used to perform statistical calculations and all data were reported as mean ± standard deviation, and p<0.05 was used for statistical significance.
Figure 3.
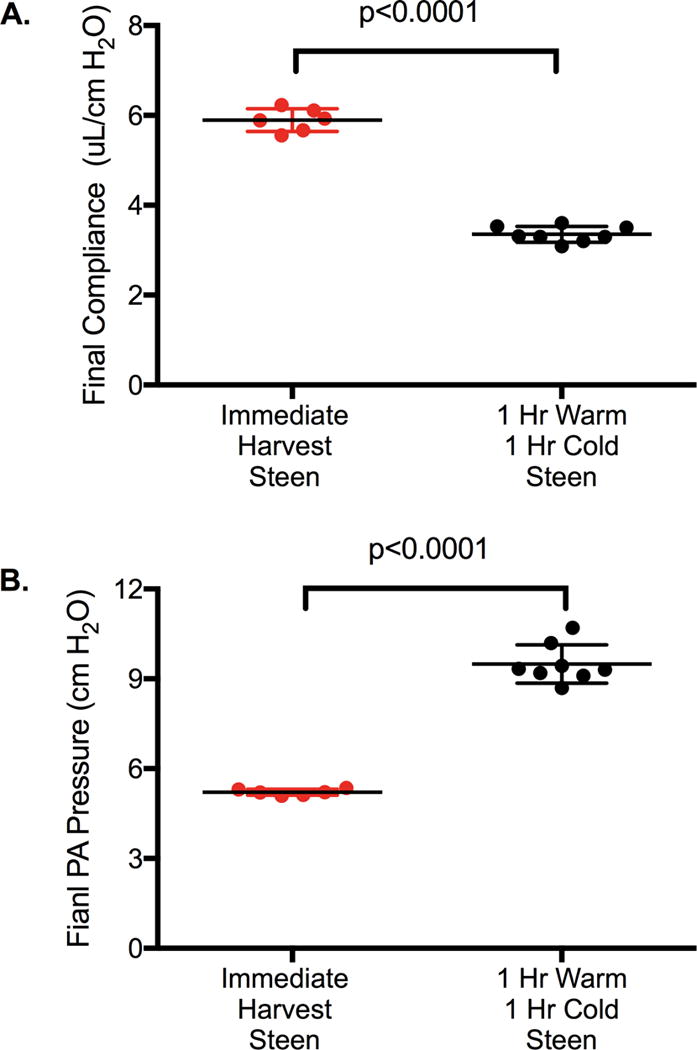
A. Dynamic Lung compliance and B. Pulmonary Artery Pressure were significantly worse in lungs undergoing 1-hr warm and 1-hr cold ischemia prior to EVLP with Steen solution compared to immediate harvest and EVLP with Steen solution. Results are presented as mean +/−SD; n=6-8 mice/group; p-value calculated by students t-test.
Figure 6.
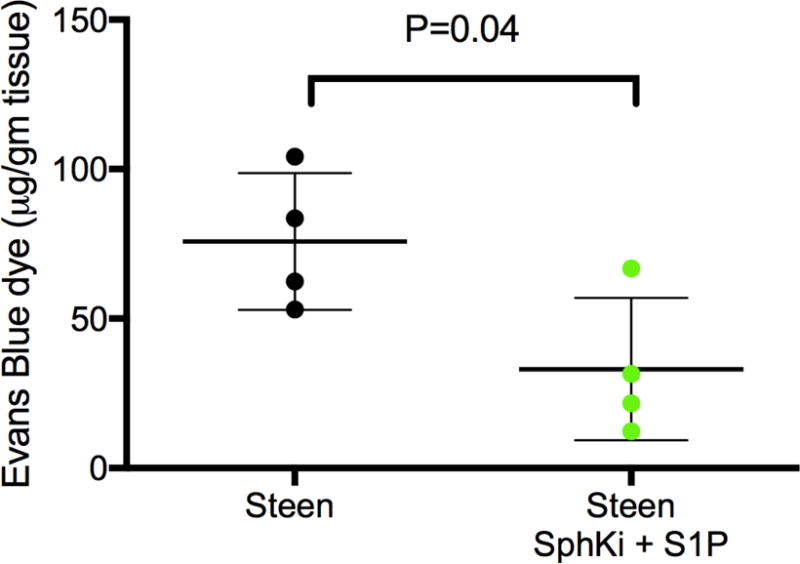
Evans blue vascular permeability assay is a more sensitive measure of pulmonary vascular permeability and was utilized to detect a difference between Steen groups. Mouse lungs treated with EVLP using Steen and a combination of SphKi and S1P demonstrated a significant decrease in lung edema compared to Steen alone. Results are presented as mean +/− SD; n=4 mice/group; p-value calculated by students t-test.
Figure 4.
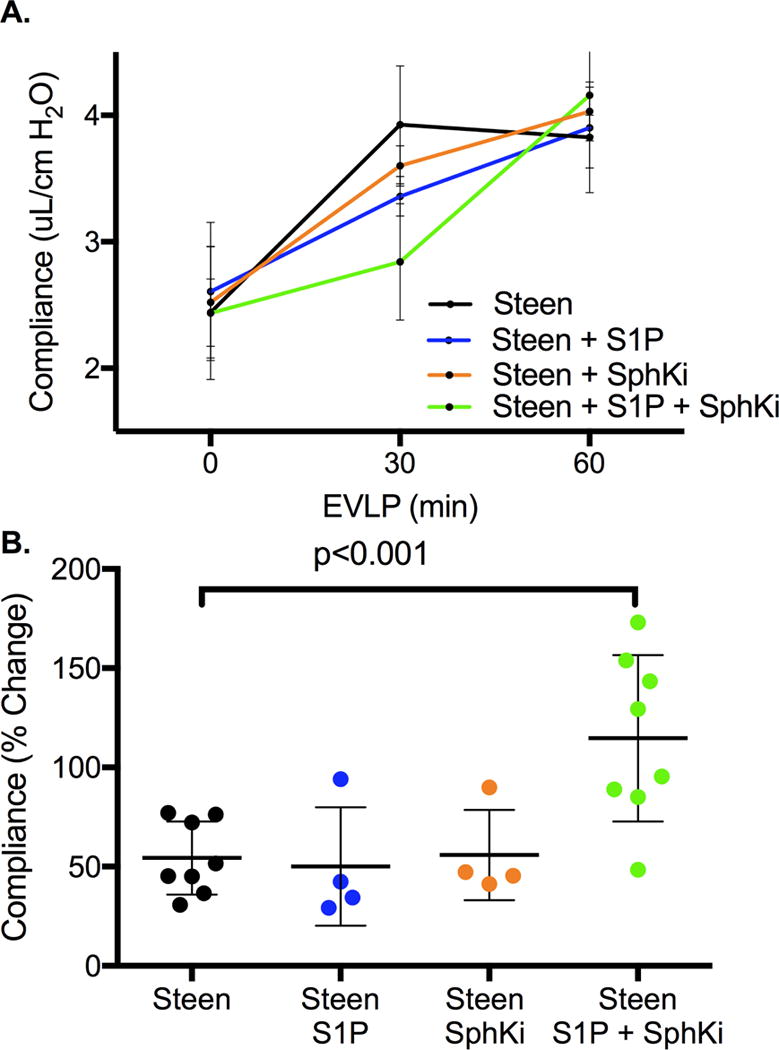
Sequential changes in lung function during EVLP. A: Changes in Dynamic Lung Compliance over the 1-hr perfusion period of EVLP demonstrates improvement over time for all groups (p<0.0001) with maximal protection after treatment with Steen+S1P+Sph Ki compared to Steen alone (p=0.01). Results are presented as mean +/− SD; n=4-8 mice/group; p-value calculated by two-way ANOVA with repeated measures and Bonferroni’s correction for multiple comparisons. B: Percent change in dynamic compliance from time 0 to 60 minutes of EVLP shows a significant increase after treatment with Steen+SphKi and Steen+S1P+SphKi groups compared to Steen alone. n=4-8 mice/group; p-value calculated by one-way ANOVA with Bonferroni’s correction for multiple comparison.
Figure 5.
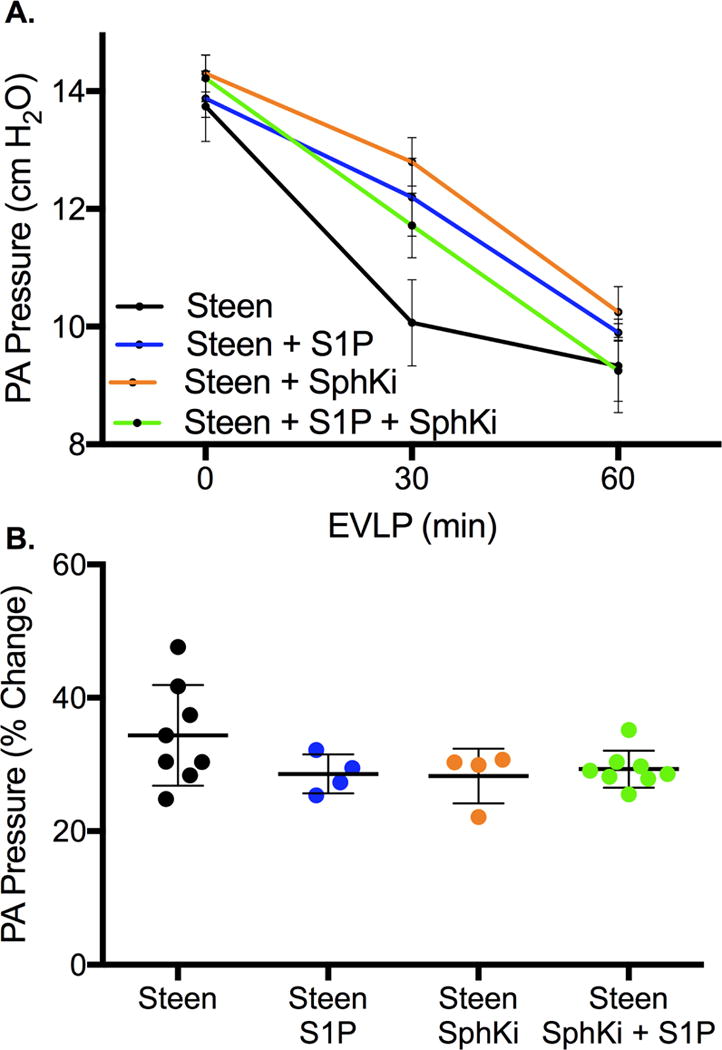
A: Change in pulmonary artery pressure over the 1-hr perfusion period of EVLP demonstrates improvement over time for all groups (p<0.0001) with no difference between groups (p=0.24). Results are presented as mean +/− SD; n=4-8 mice/group; p-value calculated by two-way ANOVA with repeated measures and Bonferroni’s correction for multiple comparisons. B: Percent change in pulmonary artery pressure from time 0 to 60 minutes of EVLP. All p>0.05 compared to Steen control. n=4-8 mice/group; p-value calculated by one-way ANOVA with Bonferroni’s correction for multiple comparison.
Results
EVLP with Steen rehabilitates DCD lungs in a murine lung injury model
All lungs were subjected to 1 hour of EVLP according to the protocol. The model of 1 hour warm followed by 1 hour cold prior to EVLP resulted in significant lung injury compared to lungs immediately harvested after circulatory death and placed on EVLP as measured by lung compliance (3.35±0.3 vs 5.85±0.2 μL/cm H2O, Injured + Steen and Normal + Steen respectively, p<0.0001, 95% CI −2.8 to −2.3, Figure 3A) and PA pressure (9.50±0.6 vs 5.20±0.1 cm H2O Injured + Steen and Normal + Steen respectively, p<0.0001, 95% CI 3.7 to 4.9, Figure 3B).
Sphingosine Gradient Targeted Manipulation Enhances EVLP Lung Rehabilitation
Each group demonstrated a significant improvement in dynamic lung compliance over the 1-hour perfusion period with a statistically significant group effect (time effect p<0.0001, group effect p=0.01, Figure 4A). After adjusting for multiple comparisons, the Steen+S1P+SphKi was the only group to demonstrate superior improvement in dynamic compliance compared to Steen alone (mean difference 0.34±0.10, p=0.01, 95% CI 0.07 to 0.60). Similarly, each group demonstrated a significant reduction in PA pressure over the 1-hour perfusion period with no difference between groups (time effect p<0.0001, group effect p=0.24, Figure 5A). After adjusting for multiple comparisons, there was no difference in PA pressure between groups (all p>0.05).
Addition of S1P to Steen solution did not significantly improvement in percent change in lung compliance (50.1±29.9% vs 57.7±19.9%, Steen+S1P and Steen respectively, p=0.59, 95% CI −22.9 to 38.1, Figure 4B) or percent change in PA pressure (28.6±2.9% vs 34.4±7.5%, Steen+S1P and Steen respectively, p=0.18, 95% CI −3.1 to 14.7, Figure 5B) during EVLP compared to Steen solution alone. Addition of SphKi to Steen solution alone also did not improve percent change in lung compliance (55.9±22.8% vs 57.7±19.9%, Steen+SphKi and Steen respectively, p=0.89, 95% CI −29.2, 25.6, Figure 4B) or percent change in PA pressure (28.3±4.1% vs 34.4±7.5%, Steen+SphKi and Steen respectively, p=0.17, 95% CI −3.0 to 15.3, Figure 5B) during EVLP compared to Steen solution alone. However, addition of both S1P and SphKi resulted in a significant improvement in percent change in lung compliance (110.0±13.9% vs. 57.7±7.5%, Steen+S1P+SphKi and Steen respectively, p<0.0001, 95% CI −92.4 to −28.3, Figure 4B) with a trend toward lower percent change in PA pressure (29.3±2.7% vs 34.39±7.5%, p=0.09, Steen+S1P+SphKi and Steen respectively, 95% CI −11.2 to 1.0, Figure 5B) compared to Steen solution alone.
Sphingosine Gradient Targeted Manipulation Protects Vascular Endothelium
To evaluate pulmonary vascular permeability in the Steen groups, the Evans blue dye test was utilized. The Steen treatment group demonstrated significant reduction in vascular permeability compared to control (33.1±23.8 vs. 75.8±22.8 μg/gm tissue, Steen+S1P+SphKi and Steen respectively, p=0.04, 95% CI −83.1 to −2.4, Figure 6).
Discussion
Our study demonstrates that targeted drug therapy with S1P and SphKi during EVLP with Steen solution provides attenuation of lung injury in murine DCD lungs. These effects are confirmed through documentation of improved lung function as measured by dynamic lung compliance and PA pressure. Furthermore, microvascular permeability is significantly attenuated in the lungs treated with S1P and SKi addition to Steen compared to control lungs undergoing EVLP with Steen alone.
Our DCD model of mouse EVLP provides reproducible lung injury confirmed by both decrease in lung compliance and increase in PA pressures (Figure 3). Despite this injury we see a significant improvement in lung function during 1 hour of EVLP for all of the groups perfused with Steen solution. Several studies have demonstrated EVLP with Steen solution provides improvement in lung function in murine, porcine and human lungs7, 19-22. Given this incremental improvement, we sought to further rehabilitate lungs through targeted manipulation of the S1P gradient, which is known to effect endothelial permeability. With the growing body of literature supporting this hypothesis, we first added physiologically active S1P directly to the perfusate with no detectable difference in lung function. We next targeted SphKi to decrease tissue levels of S1P, therefore driving up the gradient. Next, we sought to evaluate the theoretic synergistic effect of increasing intravascular S1P with addition of physiologically active S1P while simultaneously driving down tissue S1P levels with a use of an SphK2 selective inhibitor. Lungs undergoing EVLP with both compounds added to Steen solution demonstrated significant improvement in lung function above and beyond each compound individually.
The improvement of lung function with treatment during EVLP, demonstrated by decreased vascular permeability in this study, suggests that targeting pulmonary vascular endothelium could be a vital target for S1P-mediated attenuation of lung injury. Lung IRI is characterized by increased endothelial permeability, activation of inflammatory cells, and development of pulmonary edema23-25. A majority of research into targeted drug therapy has focused on decreasing activation of inflammatory cells26-29. The current study sought to apply a novel protective strategy during EVLP though targeted manipulation of pulmonary vascular permeability. Furthermore, with the rise of EVLP for donor lung evaluation, several groups have touted this technology as a therapeutic intervention that may be used to delivery therapy to rehabilitate lungs for transplantation4, 30, 31. While this approach has been adopted for targeted therapy to reduce activation of inflammatory cells and treat donor infection, we sought to target the endothelial barrier during EVLP as a means to ameliorate lung IRI32, 33.
Given the complex mechanism by which the S1P gradient augments endothelial binding, we used Evan’s blue permeability assay to demonstrate reduced vascular permeability in the Steen+S1P+SphKi group compared to Steen alone. These effects may reduce migration of inflammatory cells and we demonstrate a reduction in pulmonary edema. Through these mechanisms the treatment groups exhibit superior lung function with improved compliance and reduced pulmonary artery pressure. This is further evidenced by the more gradual improvement in lung compliance for group Steen+S1P+SphKi, gaining most of the benefit in the last half of the perfusion time. A growing body of literature supports this mechanism of action though augmentation of the S1P gradient in both renal and pulmonary physiology34-37. Additional studies are needed to expand our understanding of this complex gradient and characterize optimal concentrations as well as improved therapeutic approaches. However, these data demonstrate that increasing the S1P gradient (i.e. increased circulating S1P with decreased intracellular S1P) impacts endothelial binding and ameliorates lung IRI in this murine model of EVLP therapy.
In the future EVLP will be used to deliver multi-targeted therapy to rehabilitate lungs for transplantation resulting in an expanded donor pool and reduced waitlist mortality. Manipulation of the Sphingosine gradient is the first EVLP supplement demonstrated to target endothelial permeability to ameliorate lung injury. In combination with cellular targets in the inflammatory pathway it will be possible to attenuate the effects of IRI in DCD lungs. The additions of anti-infectious and anti-inflammatory compounds will allow for the use of almost all lungs from donors that are currently rejected as demonstrated by the Toronto group and others.33, 38, 39 Several other groups have demonstrated the importance of reactive oxygen and nitrogen species and suggested a role for scavengers in the ideal perfusion solution.40 Furthermore, these rehabilitative approaches may yield the ability to use lobes of previously rejected lung if the entire organ is unable to be recovered as demonstrated by Miyoshi et al.41
The limitations of this study include the murine model, which is technically unsuitable for longer durations of EVLP or transplantation to evaluate effects after reperfusion. However, given the large body of literature indicating excellent outcomes after lung transplantation of lungs demonstrating significant improvements in lung compliance during EVLP, our results support the conclusion. The SphKi was selected from a variety of options based on binding affinity and prior in vitro studies9, 12, 42.
In conclusion, this study emphasizes the importance of targeted drug therapy with S1P and SphKi to protect endothelial barrier function during EVLP to enhance lung function in a murine model of marginal lung rehabilitation. Upregulation of S1P via specific pharmacological modalities on EVLP in marginal donor lungs may provide endothelial protection leading to attenuation of lung injury. As a result, this may provide successful lung rehabilitation for transplantation to increase the donor pool and improve survival in patients with end stage lung disease.
Perspective.
Sphingosine-1-phosphate (S1P), a sphingolipid metabolite formed via phosphorylation of sphingosine by sphingosine kinase, regulates endothelial barrier integrity and immune cell activity in lung ischemia-reperfusion injury. Upregulation of S1P during EVLP allows for rehabilitation of marginal lungs in a murine model and if implemented clinically may increase the number of lung transplants performed.
Central Message.
Upregulation of sphingosine-1-phosphate during EVLP in marginal donor lungs provides endothelial protection leading to attenuation of lung injury in a murine model.
Central Image.
S1P and SphKi provide synergistic benefit on EVLP with improved dynamic lung compliance
Acknowledgments
Funding
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL119218 (ILK), R01GM121075 (KRL), T32HL007849 (ILK), and UM1HL088925 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- S1P
Sphingosine-1-phosphate
- SphK
Sphingosine Kinase
- EVLP
Ex Vivo Lung Perfusion
- SphKi
Sphingosine Kinase Inhibitor
- Steen
Steen Solution™
- PA
Pulmonary Artery
- IRI
Ischemia Reperfusion Injury
- DCD
Donation after Cardiac Death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report–2010. J Heart Lung Transplant. 2010;29:1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 3.Ailawadi G, Lau CL, Smith PW, et al. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg. 2009;137:688–694. doi: 10.1016/j.jtcvs.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Mehaffey JH, Charles EJ, Sharma AK, et al. Airway pressure release ventilation during ex vivo lung perfusion attenuates injury. J Thorac Cardiovasc Surg. 2017;153:197–204. doi: 10.1016/j.jtcvs.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehaffey JH, Hawkins RB, Charles EJ, Tribble CG. Revisiting successful transplantation with marginal lungs: Fourteen years later, a new era of extended criteria. J Thorac Cardiovasc Surg. 2016;152:e135–e136. doi: 10.1016/j.jtcvs.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles EJ, Huerter ME, Wagner CE, et al. Donation After Circulatory Death Lungs Transplantable Up to Six Hours After Ex Vivo Lung Perfusion. Ann Thorac Surg. 2016;102:1845–1853. doi: 10.1016/j.athoracsur.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Im DS, Heise CE, Nguyen T, O’Dowd BF, Lynch KR. Identification of a molecular target of psychosine and its role in globoid cell formation. J Cell Biol. 2001;153:429–434. doi: 10.1083/jcb.153.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch KR, Macdonald TL. Sphingosine 1-phosphate chemical biology. Biochim Biophys Acta. 2008;1781:508–512. doi: 10.1016/j.bbalip.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkerson BA, Argraves KM. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim Biophys Acta. 2014;1841:1403–1412. doi: 10.1016/j.bbalip.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharel Y, Mathews TP, Gellett AM, et al. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem J. 2011;440:345–353. doi: 10.1042/BJ20110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharel Y, Morris EA, Congdon MD, et al. Sphingosine Kinase 2 Inhibition and Blood Sphingosine 1-Phosphate Levels. J Pharmacol Exp Ther. 2015;355:23–31. doi: 10.1124/jpet.115.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajwa A, Huang L, Kurmaeva E, et al. Sphingosine Kinase 2 Deficiency Attenuates Kidney Fibrosis via IFN-gamma. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry HM, Huang L, Ye H, et al. Endothelial Sphingosine 1Phosphate Receptor1 Mediates Protection and Recovery from Acute Kidney Injury. J Am Soc Nephrol. 2016;27:3383–3393. doi: 10.1681/ASN.2015080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki M, Kreisel F, Richardson SB, et al. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant. 2007;7:751–758. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 16.Stone ML, Sharma AK, Zhao Y, et al. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1245–1252. doi: 10.1152/ajplung.00302.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone ML, Sharma AK, Mas VR, et al. Ex Vivo Perfusion With Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs After Circulatory Death. Transplantation. 2015;99:2494–2503. doi: 10.1097/TP.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma AK, Linden J, Kron IL, Laubach VE. Protection from pulmonary ischemia-reperfusion injury by adenosine A2A receptor activation. Respir Res. 2009;10:58. doi: 10.1186/1465-9921-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles EJ, Huerter ME, Wagner CE, et al. Donation After Circulatory Death Lungs Transplantable Up to Six Hours After Ex Vivo Lung Perfusion. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes LM, Mariani AW, Medeiros IL, et al. Alternative solution for ex vivo lung perfusion, experimental study on donated human lungs non-accepted for transplantation. Acta Cir Bras. 2015;30:359–365. doi: 10.1590/S0102-865020150050000008. [DOI] [PubMed] [Google Scholar]
- 21.Lindstedt S, Hlebowicz J, Koul B, et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg. 2011;12:162–165. doi: 10.1510/icvts.2010.244830. [DOI] [PubMed] [Google Scholar]
- 22.Popov AF, Sabashnikov A, Patil NP, et al. Ex vivo lung perfusion - state of the art in lung donor pool expansion. Med Sci Monit Basic Res. 2015;21:9–14. doi: 10.12659/MSMBR.893674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Kron IL. Pulmonary macrophages are involved in reperfusion injury after lung transplantation. Ann Thorac Surg. 2001;71:1134–1138. doi: 10.1016/s0003-4975(01)02407-9. discussion 1138-1139. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AK, Mulloy DP, Le LT, Laubach VE. NADPH oxidase mediates synergistic effects of IL-17 and TNF-alpha on CXCL1 expression by epithelial cells after lung ischemia-reperfusion. Am J Physiol Lung Cell Mol Physiol. 2014;306:L69–79. doi: 10.1152/ajplung.00205.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. discussion 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma AK, Laubach VE, Ramos SI, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reece TB, Ellman PI, Maxey TS, et al. Adenosine A2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1137–1143. doi: 10.1016/j.jtcvs.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 28.Gazoni LM, Walters DM, Unger EB, Linden J, Kron IL, Laubach VE. Activation of A1, A2A, or A3 adenosine receptors attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;140:440–446. doi: 10.1016/j.jtcvs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaPar DJ, Laubach VE, Emaminia A, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulloy DP, Stone ML, Crosby IK, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg. 2012;144:1208–1215. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung JC, Cypel M, Waddell TK, van Raemdonck D, Keshavjee S. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques–non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19:261–274. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Wagner CE, Pope NH, Charles EJ, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2015 doi: 10.1016/j.jtcvs.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima D, Cypel M, Bonato R, et al. Ex Vivo Perfusion Treatment of Infection in Human Donor Lungs. Am J Transplant. 2016;16:1229–1237. doi: 10.1111/ajt.13562. [DOI] [PubMed] [Google Scholar]
- 34.Hla T. Plugging vascular leak by sphingosine kinase from bone marrow progenitor cells. Circ Res. 2009;105:614–616. doi: 10.1161/CIRCRESAHA.109.207068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krenn K, Klepetko W, Taghavi S, Paulus P, Aharinejad S. Vascular endothelial growth factor increases pulmonary vascular permeability in cystic fibrosis patients undergoing lung transplantation. Eur J Cardiothorac Surg. 2007;32:35–41. doi: 10.1016/j.ejcts.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 37.Jo SK, Bajwa A, Ye H, et al. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 2009;75:167–175. doi: 10.1038/ki.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens A, Ordies S, Vanaudenaerde BM, et al. Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem Cell Res Ther. 2017;8:159. doi: 10.1186/s13287-017-0603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am J Transplant. 2015;15:2404–2412. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma AK, LaPar DJ, Stone ML, et al. NOX2 Activation of Natural Killer T Cells Is Blocked by the Adenosine A2A Receptor to Inhibit Lung Ischemia-Reperfusion Injury. Am J Respir Crit Care Med. 2016;193:988–999. doi: 10.1164/rccm.201506-1253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi K, Oto T, Konishi Y, et al. Use of Extended-Criteria Lungs on a Lobe-by-Lobe Basis Through Ex Vivo Lung Perfusion Assessment. Ann Thorac Surg. 2015;99:1819–1821. doi: 10.1016/j.athoracsur.2014.06.115. [DOI] [PubMed] [Google Scholar]
- 42.Kharel Y, Mathews TP, Kennedy AJ, Houck JD, Macdonald TL, Lynch KR. A rapid assay for assessment of sphingosine kinase inhibitors and substrates. Anal Biochem. 2011;411:230–235. doi: 10.1016/j.ab.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


