Introduction
Maternal prenatal smoking is a known risk factor for many pediatric conditions including preterm delivery, low birth weight, and childhood asthma [1,2]. Both maternal prenatal smoking and passive smoke exposure during pregnancy can damage fetal lung development which could later increase the risk of bronchiolitis, pneumonia and asthma [2–6]. Postnatal tobacco smoke exposure places infants and children at increased risk for asthma, wheezing, otitis media, cough and acute lower respiratory tract infections, including bronchiolitis especially with exposure in the first two years of life [2,7]. One systemic review reported that environmental tobacco smoke exposure specifically increases the risk of hospitalization as well as the severity of RSV-related lower respiratory tract infections in infants and children [7].
Bronchiolitis is one of the major reasons for emergency department visits and hospitalizations in younger children in the U.S. with approximately 2% to 3% of children younger than 1 year old hospitalized with bronchiolitis annually [8–10]. In 2009 bronchiolitis resulted in more than $ 1.7 billion in hospital charges [8]. Although the association between postnatal smoke exposure and childhood respiratory problems is an established risk factor for respiratory diseases, [3,7,14] the effects of prenatal maternal smoking – independent from postnatal smoke exposure – on the development of severe bronchiolitis among infants have not been studied as extensively. Some studies, which investigated maternal prenatal smoking and postnatal smoke exposure, found that prenatal maternal smoking increases the risk of severe bronchiolitis in children, whereas postnatal smoke exposure does not significantly increase the risk for bronchiolitis requiring hospitalization [15,20,21]. From a methodological standpoint, it is important to note that maternal smoking during pregnancy is likely a measure of both prenatal and postnatal tobacco smoke exposure (TSE), as it is challenging to separate in utero TSE from maternal smoking during postnatal period. Another study, a meta-analysis in 2012, looked at the effects of both maternal prenatal and postnatal TSE and reported that exposure to postnatal maternal smoking had the strongest association with the incidence of wheezing in children younger than 2 years [11]. The inconsistent results of past studies on the effects of pre-and postnatal smoke exposure warrant further research.
In the current study, we investigated the relations of both prenatal maternal smoking and postnatal smoke exposure to the risk of developing severe bronchiolitis during infancy. Given the prevalence of bronchiolitis during infancy and its burden on the healthcare system, it is imperative to establish the significance of potential risk factors including pre-and postnatal TSE. Also, since parents who smoke during pregnancy are likely to continue smoking or return to it after birth, it is important that smoking cessation interventions go on after pregnancy. We hypothesized that both maternal prenatal smoking and postnatal smoke exposure are risk factors for severe bronchiolitis during infancy.
Methods
Study Design
Using a case-control design, we analyzed electronic health record (EHR) data for children born at a single teaching hospital, and birth certificate data from the Massachusetts Department of Public Health (MDPH) for these same children. EHR data include demographics, patient diagnoses, billing codes, and patient notes from inpatient and outpatient locations. MDPH birth certificate data were collected from parents (i.e., birth certificate worksheet completed at the time of birth) and from the hospital of birth. Data include demographics, maternal exposures, maternal diseases, and details of delivery (e.g., type of delivery, complications to mother or infant, delivery interventions), and other information.
Case and Control Definitions
Children diagnosed with severe bronchiolitis during infancy (age <1 year) were identified as study cases. More specifically, children hospitalized for bronchiolitis, with an International Classification of Diseases, Ninth Revision (ICD-9) billing code for: 466.xx, respiratory syncytial virus (RSV) (ICD-9 079.6), viral pneumonia (ICD-9 480.x), asthma (ICD-9 493.xx), or wheezing (ICD-9 786.07), at age <1 year were considered to have severe bronchiolitis. Study controls were children who did not have a bronchiolitis hospitalization during the first year of life. Controls were matched 1:1 to cases by birth year.
Exposure Definitions
The primary exposures of interest were maternal cigarette smoking during pregnancy and postnatal smoke exposure. Maternal prenatal smoking status was obtained from MDPH birth certificate data; mothers were asked average number of cigarettes they smoked per day during pregnancy on the birth certificate worksheet. Postnatal smoke exposure status was gathered through chart review by a single trained reviewer (LPT) who looked for documentation of smoking by any household member during infancy; the reviewer was blinded to case-control status.
Covariate Definitions
From the MDPH birth certificate data, data were collected on: child sex, race, ethnicity, presence of older siblings, maternal smoking status prior to pregnancy, maternal age at delivery, mode of delivery, gestational age at birth, and breastfeeding status at discharge.
Relevant childhood conditions were identified using ICD-9 codes from the EHR. Participants who had ≥2 billing codes for atopic dermatitis (AD) or dermatitis due to food taken internally, with the first code billed during the first year of life, were considered to have a diagnosis of AD.
We also examined diagnoses of congenital heart disease (CHD; ICD-9 745.xx, 746.xx, or 747.xx). A diagnosis of CHD was made if a child had ≥3 billing codes for CHD to exclude the possibility that the child received an initial (rule-out) evaluation. Maternal history of asthma was defined as the presence of ≥3 billing codes (ICD-9 493.xx) for asthma in the mothers’ lifetime EHR. In instances where information was available in both the EHR and the MDPH birth certificate dataset, information was reviewed for concordance, with clarification by chart review, as needed.
Statistical Analyses
All statistical analyses were performed using Stata 14.1 (Stata Corp, College Station, TX). Data are presented as proportions with 95% confidence intervals (95%CIs) and medians with interquartile ranges (IQR). Unadjusted analyses of the potential risk factors for severe bronchiolitis were conducted using chi-square test, Fisher’s exact test, or Mann-Whitney test, as appropriate. All P-values were two-tailed, with P<0.05 considered statistically significant.
We performed multivariable logistic regression to investigate potential risk factors for severe bronchiolitis. Some covariates were identified a priori (such as race, maternal asthma status) and others were considered for inclusion in the model if they were found to be associated with the outcome in unadjusted analyses (P<0.20) or were thought to be clinically important. Results of the logistic regression models are reported as odds ratios (ORs) with 95%CIs.
Additionally, to investigate possible underreporting of maternal prenatal smoking, we used unique medical record numbers to link a subset of our subjects to their data available from another local study, the Massachusetts General Hospital Obstetric Maternal Study (MOMS), a prospective cohort of pregnant women enrolled during 1998-2005 (n=9,930) [16]. The details of this cohort have been described previously. We checked for exposure concordance between prenatal smoking status data provided at birth from MDPH (current study) with the data collected at the mothers’ first prenatal visit interview from the MOMS study.
Results
From EHRs, we identified 1,353 children (671 bronchiolitis cases, 682 controls) born between 1996-2011 at a single teaching hospital. Maternal and infant characteristics are shown in Table 1 and stratified by case-control status. Among severe bronchiolitis cases, 42 (6%) had a history of maternal prenatal smoking compared to 29 (4%) infant controls (P=0.10); postnatal household smoke exposure was present among 113 (17%) cases compared to 22 (3%) controls (P<0.001). Several other characteristics were found to be associated with severe bronchiolitis in unadjusted analyses, including: Hispanic ethnicity, mother’s education level less than 12 years, presence of one or more siblings in the home, younger maternal age at delivery, cesarean delivery, gestational age< 37 weeks, and AD during infancy. Breastfeeding status was not significantly related to severe bronchiolitis.
Table 1.
Characteristics of mother-infant dyad, by inpatient bronchiolitis status (n=1,353)
| Inpatient bronchiolitis (n=671) |
No inpatient bronchiolitis (n=682) |
p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Maternal prenatal smoking | 0.10 | ||||
| No | 628 | 94 | 651 | 96 | |
| Yes | 42 | 6 | 29 | 4 | |
| Postnatal smoke exposure | <0.001 | ||||
| No | 437 | 65 | 369 | 54 | |
| Yes | 113 | 17 | 22 | 3 | |
| Unknown | 121 | 18 | 291 | 43 | |
| Infant’s age at diagnosis in days, median (IQR) | 671 | 108 (47-205) | – | – | – |
| Sex | 0.06 | ||||
| Male | 400 | 60 | 372 | 55 | |
| Female | 271 | 40 | 310 | 45 | |
| Race | <0.001 | ||||
| White | 272 | 41 | 358 | 52 | |
| Black | 33 | 5 | 33 | 5 | |
| Other | 288 | 43 | 243 | 36 | |
| Unknown | 78 | 12 | 48 | 7 | |
| Ethnicity | <0.001 | ||||
| Hispanic | 280 | 42 | 194 | 28 | |
| Non-Hispanic | 320 | 48 | 448 | 66 | |
| Unknown | 71 | 11 | 40 | 6 | |
| Mother’s education | <0.001 | ||||
| < 12 years | 204 | 30 | 115 | 17 | |
| ≥ 12 years | 467 | 70 | 567 | 83 | |
| Older sibling present in home | <0.001 | ||||
| No sibling | 222 | 33 | 316 | 46 | |
| 1 sibling | 234 | 35 | 222 | 33 | |
| > 1 sibling | 213 | 32 | 144 | 21 | |
| Congenital heart disease | <0.001 | ||||
| No | 558 | 83 | 513 | 75 | |
| Yes | 19 | 3 | 7 | 1 | |
| Unknown | 94 | 14 | 162 | 24 | |
| Maternal asthma status | 0.22 | ||||
| No | 512 | 76 | 509 | 75 | |
| Yes | 51 | 8 | 42 | 6 | |
| Unknown | 108 | 16 | 131 | 19 | |
| Mother’s age at delivery in years, median (IQR) | 671 | 29 (23-33) | 682 | 31 (25-35) | <0.001 |
| Method of Delivery | 0.001 | ||||
| Vaginal | 455 | 68 | 468 | 69 | |
| Primary c section | 105 | 16 | 145 | 21 | |
| Repeat c section | 102 | 15 | 63 | 9 | |
| Unknown | 9 | 1 | 6 | 1 | |
| Gestational age | <0.001 | ||||
| ≥ 37 | 535 | 80 | 601 | 88 | |
| 32 - 36 | 91 | 14 | 66 | 10 | |
| < 32 | 41 | 6 | 13 | 2 | |
| Breastfeeding at discharge | 0.13 | ||||
| Yes | 591 | 88 | 619 | 91 | |
| No | 79 | 12 | 63 | 9 | |
| Atopic dermatitis during infancy | <0.001 | ||||
| No | 520 | 78 | 482 | 71 | |
| Yes | 57 | 8 | 38 | 6 | |
| Unknown | 94 | 14 | 162 | 24 | |
Percentage totals may not equal 100% because of rounding.
Abbreviations: IQR, Inter quartile range
Figure 1 highlights that postnatal smoke exposure was associated with significantly higher risk of severe bronchiolitis. There was no significant increase in the risk of bronchiolitis when infants were only exposed to prenatal smoke, but not postnatal smoke (adjusted OR 0.71, P=0.55). The ORs were highest among infants exposed to both prenatal and postnatal smoke (adjusted OR 10.1, 95%CI 2.3-44.3, P<0.01) and the risk was still significantly increased among those only exposed to postnatal smoke (adjusted OR=3.80, 95%CI 2.2-6.5, P<0.001). The overlap between the two 95%CI suggests that most of the increased risk comes from postnatal TSE and that the two significant results are likely similar.
Figure 1. Risk of severe bronchiolitis with prenatal smoke exposure and postnatal smoke exposure among participants with complete data (n=940).
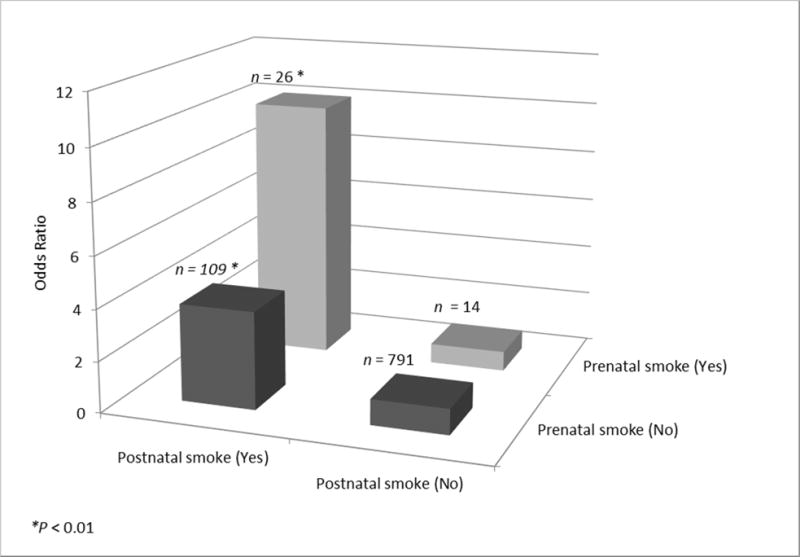
Association of combined exposures for prenatal and postnatal smoke and the risk of severe bronchiolitis (*P < 0.01).
Prenatal smoke (No) | Postnatal smoke (No): Odds Ratio (reference group) = 1.00
Prenatal smoke (No) | Postnatal smoke (Yes): Odds Ratio (95% CI) = 3.80 (2.22-6.53);
Prenatal smoke (Yes) | Postnatal smoke (No): Odds Ratio (95% CI) = 0.77 (0.25-2.31);
Prenatal smoke (Yes) | Postnatal smoke (Yes): Odds Ratio (95% CI) = 10.07 (2.29-44.32).
In the unadjusted logistic regression model for the association between maternal prenatal smoking and severe bronchiolitis, maternal prenatal smoking was not a statistically significant risk factor for severe bronchiolitis (unadjusted OR 1.50, 95%CI 0.92-2.44). In the multivariable logistic regression models adjusted for several infant and maternal characteristics, the association between prenatal smoke exposure and severe bronchiolitis was non-significant both without adjustment for postnatal smoke exposure (adjusted OR 1.17, 95%CI 0.69-1.98) and with adjustment for postnatal smoke exposure (adjusted OR 1.02, 95%CI 0.56-1.84). By contrast, postnatal smoke exposure was associated with a >300% increased odds for severe bronchiolitis in the full multivariable model (adjusted OR 4.19, 95%CI 2.51-6.98) (Table 2). Other independent risk factors included Hispanic ethnicity, presence of 1 or more siblings in the home, mother’s younger age at delivery, repeat cesarean section, gestational age<37 weeks, and AD during infancy.
Table 2.
Multivariable logistic regression model for prenatal and postnatal tobacco smoke exposure and risk of severe bronchiolitis during infancy (n=1,353)
| Characteristics | OR (95% CI)* | p-value |
|---|---|---|
| Maternal prenatal smoking | 1.02 (0.56-1.84) | 0.96 |
| Post-natal smoke exposure | ||
| No | Reference | |
| Yes | 4.19 (2.51-6.98) | <0.001 |
| Unknown | 0.38 (0.28-0.50) | <0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.21 (0.95-1.54) | 0.12 |
| Race | ||
| White | Reference | |
| Black | 1.15 (0.65-2.01) | 0.63 |
| Other | 0.85 (0.61-1.19) | 0.34 |
| Unknown | 0.87 (0.33-2.33) | 0.78 |
| Ethnicity | ||
| Non-Hispanic | Reference | |
| Hispanic | 1.52 (1.06-2.17) | 0.02 |
| Unknown | 1.88 (0.67-5.29) | 0.23 |
| Older sibling present in home | ||
| No sibling | Reference | |
| 1 sibling | 1.57 (1.16-2.12) | 0.003 |
| > 1 sibling | 2.16 (1.54-3.03) | <0.001 |
| Mother’s age at delivery | 0.98 (0.95-0.99) | 0.03 |
| Maternal asthma status | ||
| No | Reference | |
| Yes | 0.95 (0.58-1.55) | 0.83 |
| Unknown | 0.75 (0.55-1.03) | 0.1 |
| Method of Delivery | ||
| Vaginal | Reference | |
| Primary c section | 0.77 (0.55-1.08) | 0.14 |
| Repeat c section | 1.58 (1.07-2.35) | 0.02 |
| Unknown | 0.94 (0.28-3.14) | 0.93 |
| Gestational age | ||
| ≥ 37 | Reference | |
| 32 - 36 | 1.95 (1.33-2.85) | 0.001 |
| < 32 | 4.38 (2.18-8.81) | <0.001 |
| Atopic dermatitis during infancy | ||
| No | Reference | |
| Yes | 1.71 (1.08-2.73) | 0.02 |
| Unknown | 0.83 (0.59-1.16) | 0.28 |
Abbreviations: OR, odds ratio; CI, confidence interval
Mutually adjusted Odds Ratios with 95% Confidence Intervals.
To evaluate potential underreporting of maternal prenatal smoking, we compared the prenatal smoking status used in the current study (provided by the MDPH and ascertained at birth) with data collected from the overlapping MOMS study (a prospective cohort of 9,930 pregnant women enrolled during 1998-2005 with prenatal smoking data collected at the mothers’ first prenatal visit interview) [16]. Among our analytical cohort of 1,353 infants, 196 (14%) also had prenatal smoking data available from the MOMS cohort. Comparing the two data sources, (Figure 2), there were 55 discrepant responses including: 39 (20%) mothers who reported that they stopped smoking at the first prenatal visit interview, but later reported as never-smokers at child’s birth. Another 13 (7%) mothers who reported being current smokers at the first prenatal visit interview (MOMS database), but approximately 6 months later, at the child’s birth, reported being never-smokers. However, most of the responses were consistent; 141 (72%) of the 196 mothers reported the same prenatal smoking status in both the MDPH birth certificate and MOMS data source (MDPH database).
Figure 2. Comparison of prenatal smoking status responses: first pre-natal visit interview versus response approximately 6 months later at birth (n=196).
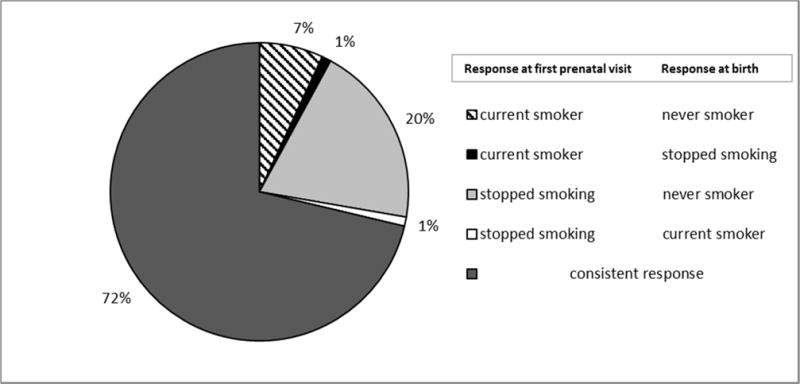
Percent change in response from first prenatal visit to response recorded at birth.
Discussion
In this case-control study, we found no significant association between maternal prenatal smoking and severe bronchiolitis in infancy in both unadjusted and adjusted multivariable analyses after controlling for potential confounders. By contrast, postnatal smoke exposure was associated with significantly increased odds of developing severe bronchiolitis. Other factors found to be associated with the risk of severe bronchiolitis in our study were Hispanic ethnicity, presence of 1 or more siblings in the home, mother’s younger age at delivery, repeat cesarean section, gestational age<37 weeks, and AD during infancy.
The adverse health effects of postnatal smoke exposure on infants are well-recognized. For example, prior studies have established that children of households with TSE are at higher risk for lower respiratory tract infections, such as bronchiolitis, and are more likely to require hospitalization for such illnesses especially for those younger than 2 years old [13, 17, 18]. To date, however, limited studies have distinguished between the risks associated with maternal prenatal smoking and postnatal smoke exposure in developing bronchiolitis; [2,11,15,20,21,22,25] the results of these studies have been discordant. It is also important to note that women who smoke during pregnancy are likely to continue smoking or return to it after delivery and so smoking cessation programs should advance after pregnancy to reduce postnatal TSE by preventing parents from going back to smoking.
Most earlier studies report a significant association between maternal prenatal smoking and severe bronchiolitis [15,20,21,22]. Studies have also demonstrated an increased risk of ICU admission and endotracheal intubation among children hospitalized with bronchiolitis who were born to mothers who smoked during pregnancy [23,24]. One of these studies was a large retrospective cohort study of 101,245 mother-infant dyads enrolled in the Tennessee Medicaid program which reported that infants of mothers who smoke during pregnancy had higher risks of developing bronchiolitis than infants who were not exposed to prenatal maternal smoking [19]. However, they did not have information on the smoking status of the other household members. Therefore, they did not differentiate between maternal prenatal smoking and postnatal TSE; maternal smoking during pregnancy therefore represented some combination of both prenatal and postnatal exposures [19]. This might explain the difference in the results with our study that it was the postnatal smoke exposure that caused the harm.
In a prospective cohort study of 2,210 Italian children in 2015, the investigators found that both active maternal prenatal smoking and passive prenatal TSE were significant risk factors for severe bronchiolitis; yet, they only found marginally higher, non-significant rates of hospitalization with bronchiolitis among infants who were exposed to postnatal smoke exposure [15]. However, they reported that the risk of bronchiolitis hospitalization for infants of mothers who smoked during pregnancy is dose-dependent and only found significantly increased risk when mothers were smoking more than 15 cigarettes per day (HR 3.5, 95% 1.5-8.1). For mothers smoking less than 15 cigarettes per day, there was no significant change in risk of bronchiolitis requiring hospitalization (HR 1.3, 95% 0.7-2.4) [15]. They concluded that reducing the number of cigarettes mothers smoke during pregnancy can decrease the risk for hospitalization in infants [15]. In our cohort, most smoking mothers smoked less than 10 cigarettes per day. Among the mothers who smoked during pregnancy in our study, 63% smoked less than 10 cigarettes a day. We evaluated the association of the number of cigarettes smoked per day by mothers with the risk of severe bronchiolitis in adjusted and unadjusted analysis and they were not significant. The possibility of lower number of cigarettes smoked by mothers in our study could explain the difference in our results.
There are studies that found similar results to ours – i.e., they also identified postnatal TSE as a major risk factor for severe bronchiolitis [2,11,25]. This postnatal risk highlights the importance of smoking cessation programs even after birth to address this issue, as suggested by prior studies [27]. In one meta-analysis on the effects of prenatal maternal smoking and postnatal TSE, authors found that exposure to postnatal maternal smoking had the strongest association with risk of wheeze in children younger than 2 years (OR 1.70, 95% 1.24-2.35) [11]. Another supportive study is a prospective cohort study of 1,236 pregnant women and their children in Singapore, which looked at TSE and respiratory morbidity of these children. The authors reported that TSE significantly increased the risk of total number of wheezing episodes and hospitalizations due to respiratory disease (adjusted RR 1.71, 95% 1.38-2.11 and adjusted RR 1.89, 95% 1.02-3.5). Only 12.5% of the mothers smoked regularly prior to pregnancy and 2.3% continued to do so during their pregnancy. The authors did not examine the independent effects of smoking during pregnancy versus postnatally because of insufficient power to detect the differences [26]. An American study of 206 infants with severe bronchiolitis focused on tobacco smoke exposure and the severity of bronchiolitis (measured by low oxygen saturations), and reported that prenatal TSE was not significantly associated with lower oxygen saturation among infants hospitalized with bronchiolitis [25]. Similar to our results, they also reported that the severity of bronchiolitis between infants who were only exposed to prenatal tobacco smoke was not significantly different than those infants who were never exposed to tobacco smoke [25].
Postnatal smoke exposure increases susceptibility to lower respiratory tract infections including bronchiolitis by directly affecting the lungs. TSE impairs the protective mechanisms of the airways such as muco-ciliary clearance and weakens both cell mediated and humoral immunity in infants [2,15]. Prenatal smoke exposure is associated with reduced lung function and may impair in utero airway development [2,25]. Fetal breathing movements are necessary for fetal lung growth and maturation, and animal studies show that nicotine from prenatal smoke exposure reduces such movements resulting in hypoplasia of fetal lungs. Also, cotinine, an inflammatory marker that increases in response to TSE, causes placental vascular damage that can lead to placental insufficiency. Reduced blood flow through the placenta to the fetus can impair the development of lungs and airways [2]. Although prenatal smoke exposure is likely to interfere with lung growth and function, postnatal smoke exposure suppresses the immune response and increases the risk of respiratory infections. Moreover, parents who smoke are more likely to develop respiratory infections themselves and can transmit the infection to their children, adding to the harms of postnatal tobacco smoke exposure [7].
One possible explanation for our null prenatal smoke findings could be maternal underreporting of prenatal smoking. For maternal prenatal smoking status, we relied on the MDPH birth data, which were obtained through parental report after delivery. Mothers may have been reluctant to report their true smoking status throughout pregnancy after they have given birth. This could explain the discrepancy between our two data sources, the MDPH and MOMS, among a subset of the participants. Although 72% of the responses were consistent in both datasets, we found that 27% of the matched participants changed their smoking status from previous smokers and current smokers to never smokers. This might have undermined the effects of prenatal maternal smoking and the risk of severe bronchiolitis in our cohort. Prior studies have also reported that the estimated number of pregnant smokers are likely underestimated and at least 20% of pregnant smokers misreport their smoking status during childbearing [28].
Considering the findings of several prior studies mentioned earlier in discussion which reported an association between prenatal maternal smoking and risk of bronchiolitis, together with some degree of underreporting of smoking status in expecting mothers, it is probable that prenatal maternal smoking has an effect (albeit smaller than postnatal smoke exposure) on the development of infant bronchiolitis. It remains important to focus on smoking cessation during pregnancy to avoid this smaller risk, along with the many well-established harms of prenatal maternal smoking, such as preterm delivery and low birth weight [2].
One limitation in our study is that we did not verify self-reported smoking status with objective biomedical testing such as salivary or blood cotinine levels. However, data suggest good agreement between self-reported smoking status of the parents and biomedical markers in the household environment and children’s urine sample [29]. Another limitation is that the absence of an association between prenatal smoke exposure and severe bronchiolitis was due to low statistical power (38%) to detect a small difference in the overall smoking prevalence.
Conclusion
Bronchiolitis is one of the most common reasons for ED visits and hospitalizations among infants, and TSE is a strong risk factor for this condition. TSE is a modifiable risk factor; therefore, it is important to identify its effects and constitute proper interventions to help patients with smoking cessation, especially during pregnancy and early parenthood. Our results show that while maternal prenatal smoking was not significantly associated with severe bronchiolitis during infancy, postnatal smoke exposure was a significant risk factor for severe bronchiolitis. It is still important to focus on smoking cessation during pregnancy due to the established harms of prenatal maternal smoking, such as preterm delivery and low birth weight [2]. Moreover, since women are more likely to quit smoking during pregnancy than any other time, counseling should be provided to expectant mothers who smoke [15]. However, interventions should extend beyond pregnancy to reduce postnatal TSE by preventing parents from going back to smoking. Healthcare professionals play an important role in promoting smoking cessation and raising awareness about the harms of passive smoke exposure on infants during prenatal and well-child care visits. Our data can be used by physicians and other health care providers who are taking care of pregnant women and infants during counseling to new parents about the risks of postnatal smoke exposure.
Highlights.
Postnatal smoke exposure increases the risk of severe bronchiolitis in infancy.
Other risk factors; Hispanic ethnicity, preterm birth and younger maternal age.
Maternal prenatal smoking did not significantly increase infant bronchiolitis.
Acknowledgments
Financial Disclosure: This work was supported by grant T32 HL-116275 from the National Institutes of Health (Bethesda, MD).
Abbreviations
- TSE
tobacco smoke exposure
- AD
atopic dermatitis
- CHD
congenital heart disease
- MDPH
Massachusetts Department of Public Health
- MOMS
Massachusetts General Hospital Obstetric Maternal Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ko T-J, Tsai L-Y, Chu L-C, Yeh S-J, Leung C, Chen C-Y, et al. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatrics & Neonatology. 2014;55(1):20–7. doi: 10.1016/j.pedneo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Hofhuis W, De Jongste J, Merkus P. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Archives of disease in childhood. 2003;88(12):1086–90. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vardavas C, Hohmann C, Patelarou E, Martinez D, Henderson A, Granell R, et al. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. European Respiratory Journal. 2016;48(1):115–24. doi: 10.1183/13993003.01016-2015. [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Rosenthal DG, Sherman S, Zelikoff J, Gordon T, Weitzman M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Current problems in pediatric and adolescent health care. 2014;44(8):219–41. doi: 10.1016/j.cppeds.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. American Journal of respiratory and critical care medicine. 1995;152(3):977–83. doi: 10.1164/ajrccm.152.3.7663813. [DOI] [PubMed] [Google Scholar]
- 6.Health UDo. Services H The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. p. 709. [PubMed] [Google Scholar]
- 7.DiFranza JR, Masaquel A, Barrett AM, Colosia AD, Mahadevia PJ. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. BMC pediatrics. 2012;12(1):81. doi: 10.1186/1471-2431-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meissner HC. Viral bronchiolitis in children. New England Journal of Medicine. 2016;374(1):62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 9.Akenroye AT, Baskin MN, Samnaliev M, Stack AM. Impact of a bronchiolitis guideline on ED resource use and cost: a segmented time-series analysis. Pediatrics. 2014;133(1):e227–34. doi: 10.1542/peds.2013-1991. [DOI] [PubMed] [Google Scholar]
- 10.Lieberthal AS, Bauchner H, Hall CB, Johnson DW, Kotagal U, Light MJ, et al. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 11.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 12.Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. European journal of pediatrics. 2009;168(8):897–905. doi: 10.1007/s00431-009-0967-3. [DOI] [PubMed] [Google Scholar]
- 13.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113(Supplement 3):1007–15. [PubMed] [Google Scholar]
- 14.Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax. 1997;52(12):1081–94. doi: 10.1136/thx.52.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanari M, Vandini S, Adorni F, Prinelli F, Di Santo S, Silvestri M, et al. Prenatal tobacco smoke exposure increases hospitalizations for bronchiolitis in infants. Respiratory research. 2015;16(1):152. doi: 10.1186/s12931-015-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstetrics & Gynecology. 2001;98(5):757–62. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 17.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respiratory research. 2011;12(1):5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gürkan F, Kıral A, Dağlı E, Karakoç F. The effect of passive smoking on the development of respiratory syncytial virus bronchiolitis. European journal of epidemiology. 2000;16(5):465–8. doi: 10.1023/a:1007658411953. [DOI] [PubMed] [Google Scholar]
- 19.Carroll KN, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Wu P, et al. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007;119(6):1104–12. doi: 10.1542/peds.2006-2837. [DOI] [PubMed] [Google Scholar]
- 20.Cano FJ, Zabaleta CC, de la Torre MdN, Yep CG, Melendi CJ, Sánchez BM. Pre and postnatal tobacco exposure and bronchiolitis. Anales de pediatria (Barcelona, Spain: 2003) 2003 doi: 10.1016/s1695-4033(03)78014-x. [DOI] [PubMed] [Google Scholar]
- 21.Fuentes-Leonarte V, Estarlich M, Ballester F, Murcia M, Esplugues A, Aurrekoetxea JJ, et al. Pre-and postnatal exposure to tobacco smoke and respiratory outcomes during the first year. Indoor air. 2015;25(1):4–12. doi: 10.1111/ina.12128. [DOI] [PubMed] [Google Scholar]
- 22.Gilliland FD, Li Y-F, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. American journal of respiratory and critical care medicine. 2001;163(2):429–36. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson MD, Mansbach JM, Mowad E, Dunn M, Clark S, Piedra PA, et al. Prenatal versus postnatal tobacco smoke exposure and intensive care use in children hospitalized with bronchiolitis. Academic pediatrics. 2016;16(5):446–52. doi: 10.1016/j.acap.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansbach JM, Piedra PA, Stevenson MD, Sullivan AF, Forgey TF, Clark S, et al. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics. 2012;130(3):e492–e500. doi: 10.1542/peds.2012-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115(1):e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 26.Snodgrass AM, Tan PT, Soh SE, Goh A, Shek LP, Van Bever HP, Gluckman PD, Godfrey KM, Chong YS, Saw SM, Kwek K, Teoh OH, The GUSTO Study Group Tobacco Smoke Exposure and Respiratory Morbidity in Young Children. Tobacco Control. 2016;25(e2):e75–e82. doi: 10.1136/tobaccocontrol-2015-052383. [DOI] [PubMed] [Google Scholar]
- 27.Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 28.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batscheider A, Zakrzewska S, Heinrich J, Teuner CM, Menn P, Bauer CP, Hoffmann U, Koletzko S, Lehmann I, Herbarth O, von Berg A. Exposure to second-hand smoke and direct healthcare costs in children–results from two German birth cohorts, GINIplus and LISAplus. BMC health services research. 2012 Oct 2;12(1):344. doi: 10.1186/1472-6963-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]


