Abstract
Purpose
To evaluate the frequency of tumor thrombus in the large veins draining primary pelvic osteosarcoma on early cross-sectional imaging studies and its effect on patient survival.
Materials and methods
Our retrospective study included all patients with primary pelvic osteosarcoma treated at our facility between January 2000 and May 2014, who were ≤45 years of age, and had adequate imaging studies and clinical follow up. Four radiologists evaluated for tumor in the large draining veins on initial CT, MRI and PET/CTs. A consensus evaluation by the four radiologists together with findings on operative reports, pathology reports or follow-up imaging was used as the reference standard.
Results
Thirty-nine patients with primary pelvic osteosarcoma met final inclusion criteria. Tumor thrombus was identified in the large draining veins in 10 of the 22 (45%) patients who underwent tumor resection and 10 of the 17 (59%) who did not. In the 22 patients who underwent tumor resection, tumor thrombus was significantly associated with worse overall survival (p=0.03).
Conclusions
Tumor thrombus in the large draining veins is identified in a significant proportion of initial imaging studies in patients with pelvic osteosarcoma, and is associated with worse overall survival in patients who undergo tumor resection.
Keywords: Tumor thrombus, Pelvic osteosarcoma, Large draining veins
Introduction
Tumor thrombus in the draining veins has been identified as a poor prognostic factor in various types of malignancies including osteosarcoma, renal cell carcinoma, hepatocellular carcinoma, colorectal carcinoma and gastric carcinoma [1–5]. Tumor thrombus in the large veins that drain primary and metastatic osteosarcomas has not been studied in detail; the existing information is available from few case reports and case series [6–9]. Benezech et al [1] reported peri-tumoral vascular invasion in 29% of surgically resected pediatric osteosarcomas after neo-adjuvant chemotherapy at pathologic examination. Kawai et al [10] reported intravascular extension into the large vessels in 23% of patients who underwent surgical resection for pelvic osteosarcoma. To our knowledge, no prior study has described the frequency of tumor thrombus in the large veins draining primary osteosarcoma on imaging studies and its effect on patient survival of patients. In our experience, a lack of familiarity with tumor thrombus in the veins draining the osteosarcoma and the small caliber of the draining veins in extremity osteosarcomas may lead to the under-recognition of this finding on imaging studies.
The primary purpose of this retrospective study was to evaluate the frequency of tumor thrombus in the large extra-tumoral veins draining primary pelvic osteosarcoma on imaging studies obtained within 6–12 months of initial diagnosis and prior to definitive surgery in patients who underwent surgical resection. The pelvic location was chosen over other sites as the draining veins are larger and comparison with contralateral veins is possible on most imaging studies acquired for pelvic osteosarcoma. The secondary purpose of the study was to determine the effect of tumor thrombus within the large draining veins on the overall survival of patients.
Materials and Methods
Patients
The institutional review board approval, including a waiver of informed consent for the retrospective review was obtained. The institutional tumor registry was searched for all patients with pelvic osteosarcoma who had been diagnosed between January 2000 and May 2014. Our institution started using digital imaging with Picture Archiving and Communication System in 2000.
Inclusion and Exclusion Criteria
All patients with pelvic osteosarcoma who had at least one pelvic CT or MRI study of reasonable quality within six months of diagnosis; had at least two sets of imaging studies (at least one contrast enhanced CT or MRI) on institutional PACS (including outside facility imaging studies); and adequate clinical follow up (at least 3 years for living patients and follow up until death for patients who died) were included. All patients with incomplete imaging studies, osteosarcoma secondary to prior radiation therapy to the pelvis, age at diagnosis greater than 45 years, alternative final diagnosis and alternative location of tumor, primary tumors centered on the sacrum with isolated sacral involvement, and inadequate clinical follow up were excluded. Patients with primary tumors centered on the sacrum and isolated sacral involvement can drain into veins on both sides of midline. Hence, comparison with normal contralateral veins may not be possible and unless these sacral tumors showed asymmetric involvement of one sided ilium, they were excluded from this study. Of the 132 patients with pelvic osteosarcoma, 44 met the initial inclusion/exclusion criteria and were selected for image analysis.
Imaging Studies and Analysis
One radiologist (SY) reviewed the patients’ charts, including clinical notes, pathology reports, radiology reports and imaging studies (CT/MRI/PET-CTs) and recorded data on patients’ demographics, primary tumor size on the initial study, metastasis at presentation, histologic subtype, extent of tumor thrombus in the large draining veins on initial imaging studies, findings from operative reports, pathology reports, follow up imaging, surgical resection status of the primary tumor, local recurrence and timing, and metastatic disease sites on the last available imaging studies and clinical notes until December 2016. For each patient, this radiologist selected 2–4 relevant initial imaging studies (a total of 108 studies) obtained at least one month apart for further review by three other radiologists (BA, AM and US) and consensus evaluation. The first studies included MR or CT imaging of the pelvis or both within six months of diagnosis; of these, contrast enhanced studies were available for 42 patients and only non-enhanced CTs were available for two patients. The last imaging study was a contrast enhanced MRI or CT before surgery in patients who underwent surgical resection and after the initial chemotherapy in patients who did not. At least one contrast enhanced study within the first year of diagnosis was available for all patients.
On initial contrast enhanced CT and T1 weighted MRIs, the radiologists evaluated for filling defects within the large draining veins (inferior vena cava (IVC); common, external, and internal iliac veins; and common femoral vein) and compared them with contralateral veins. Detection of tumor in the distal veins, particularly internal iliac vein is difficult and comparison with the contralateral veins is very helpful. On T2 weighted MR images, the radiologists evaluated for absence of flow void within the draining vein and compared with the flow voids on the contralateral side. On PET/CT increased FDG uptake corresponding to the course of the vein and on non-contrast CT/follow up imaging (including CT portion of the PET-CT) mineralization, i.e., osteoid matrix along the course of the vein were evaluated. A dilated tubular structure filled with material that showed imaging characteristics similar to primary tumor and connecting the primary tumor and the large draining veins was considered a tumor thrombus on any imaging modality. The imaging findings by consensus evaluation by all four radiologists, including the presence and location of the tumor in the draining vein, were correlated with the operative report, pathology findings and follow-up imaging. The consensus evaluation by four radiologists together with confirmation of the findings by operative report, pathology findings or follow-up imaging was used as the reference standard for the study.
Statistical Methods
Summary statistics of tumor thrombus in the large draining veins and other patient characteristics were provided using frequencies and percentages. Age and tumor size were summarized using mean, standard deviation, and range. Cox proportional hazard model was used to assess the effects of patient characteristics on overall survival and time-to-local recurrence, where events were defined as death of any cause and local recurrence, respectively. Survival curves were estimated using Kaplan-Meier method and compared using log-rank test. Fisher’s exact test was used to determine the association between metastatic disease at presentation and tumor histologic subtype with tumor thrombus. All tests were two-sided and p-values of 0.05 or less were considered statistically significant. Statistical analysis was carried out using SAS version 9.4 (SAS Institute, Cary, NC) and Spotfire S+ version 8.2 (TIBCO, Palo Alto, CA).
Results
Patient cohort
The study identified 132 patients with pelvic osteosarcoma of which 88 were excluded as detailed in Fig. 1. Incomplete imaging studies i.e., unavailability of at least one pelvic CT or MRI of reasonable quality within six months from diagnosis and at least two sets of imaging studies (of which at least one included contrast enhanced CT or MRI) on institutional PACS, was the primary reason for patient exclusion (49% of excluded patients). Forty-four patients met the study criteria and were initially included in the study for consensus evaluation of tumor thrombus in the large draining vein on imaging studies by the radiologists.
Fig. 1.
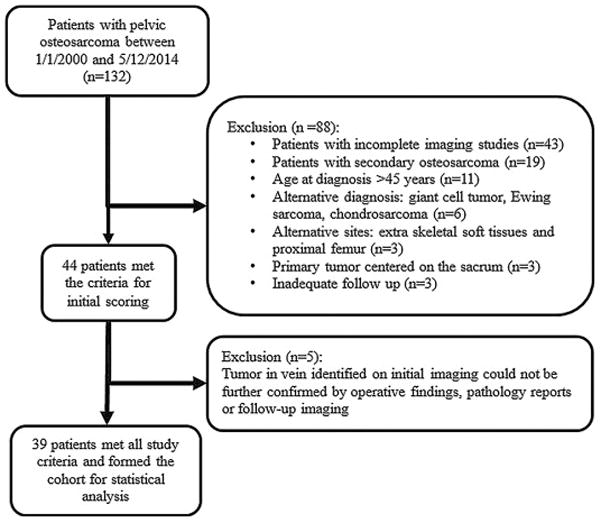
Of the 132 patients, 88 patients were initially excluded; incomplete imaging studies was the major reason for excluding patients (49%). Forty-four patients were selected for the initial imaging analysis. Five additional patients with tumor in the vein on imaging studies were excluded because of the lack of confirmation of tumor in vein by operative findings, pathology reports or follow-up imaging.
Tumor thrombus in the large draining veins was identified in 25 of 44 (57%) patients by consensus scoring by all four radiologists. The presence of tumor in the draining veins was confirmed by findings from operative reports, pathology reports, or both (n=7) or follow-up imaging (n=13) in 20 of the 25 patients with tumor thrombus on imaging (Figs. 2 and 3). Four patients who did not undergo surgical resection died early in the course of their disease before undergoing surgery, and therefore they did not have operative or pathology reports and did not have follow up imaging studies for confirmation of tumor in vein. In addition, no operative or pathology reports were available for one patient who underwent surgery at an outside facility. These 5 patients with tumor thrombus in large draining veins on imaging per final consensus score who lacked confirmation by either operative findings or pathology reports or follow up imaging were excluded from the subsequent statistical analysis. Thirty-nine patients formed the cohort for statistical analysis.
Fig. 2.
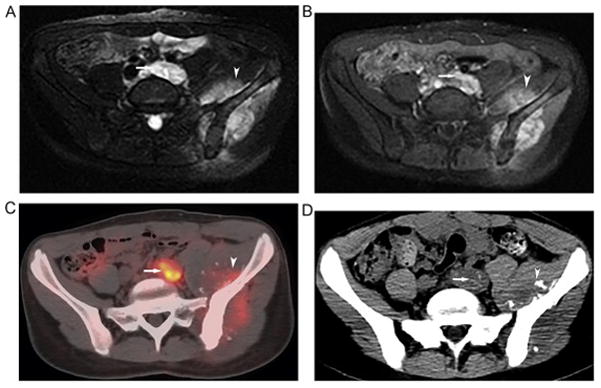
14-year old girl with left pelvic osteosarcoma. Axial T2 (A) and post-contrast T1-weighted (B) MRI illustrates left pelvic osteosarcoma (arrowhead) and tumor thrombus within the left common iliac vein (arrow). The primary tumor (arrowhead) and the venous tumor thrombus (arrow) were FDG avid on baseline axial fused PET/CT (C) confirming the malignant nature of the thrombus. Follow-up non contrast CT 4 months later (D) shows faint mineralization of the tumor thrombus within the persistently dilated left common iliac vein (arrow) similar to matrix mineralization within the primary tumor (arrow head) also confirming the malignant nature of the thrombus.
Fig. 3.
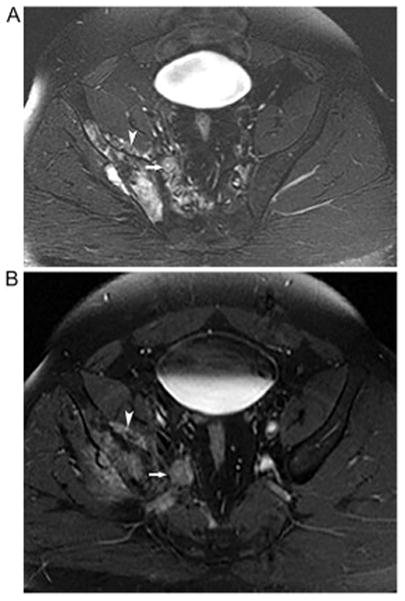
34-year old man with right pelvic osteosarcoma. Axial T2 (A) and post-contrast T1-weighted (B) MRI illustrates right pelvic osteosarcoma (arrowhead) and tumor within the right internal iliac vein (arrow). This patient underwent surgical resection of the tumor and the tumor thrombus in the large draining veins is confirmed by surgical pathology.
Clinical Characteristics
The clinical characteristics including patient demographics, disease stage and tumor size at diagnosis, osteosarcoma subtype, surgical resection, and patient life status of the 39 patients are detailed in Table 1. Age at diagnosis ranged between 11 and 44 years (median: 19 years) and female to male ratio was 23:16. The primary tumor involved the ilium in 33 patients (with extension into ischiopubic region in 7 patients; lumbosacral region in 14 patients; and ischiopubic region and sacrum in 3 patients), ischiopubic region in 3 patients and pubis in 3 patients. A chondroblastic component was identified in 25 (64%) patients including 20 with chondroblastic subtype and five patients with mixed subtype. Twenty two of 39 patients underwent surgical resection of the primary tumor including three patients who underwent surgical resection at an outside facility. Of these, 15 patients underwent internal hemipelvectomy and 7 patients underwent external hemipelvectomy. The intent of surgery was curative in 21 patients and palliative in one patient. All patients received neoadjuvant chemotherapy prior to surgical resection and two patients received radiation therapy initially for local control (including one patient who underwent palliative resection for pain, and another patient with extensive local disease treated with chemotherapy and radiation therapy as the patient and parents refused surgery initially but eventually agreed to undergo external hemipelvectomy for recurrence about 2 years after diagnosis). Surgical margins were positive in 6 patients; four out of these 6 patients received radiation therapy after surgery. One patient with positive margins refused any further treatment after one cycle of adjuvant chemotherapy. The remaining patient with positive margin underwent palliative resection with no further therapy due to poor wound healing and pulmonary metastatic disease and died 2.5 months after surgical resection. Seventeen of 39 (44%) patients did not undergo surgical resection of the primary tumor secondary to extensive metastatic disease in lung and/or bone (n=10) or locally advanced primary tumor (n=7). Metastatic disease at presentation was identified in 14 of 17 (82%) patients who did not undergo surgical resection and five of 22 (23%) patients who underwent surgical resection, indicating advanced disease stage at presentation in the majority of patients who did not undergo surgical resection.
Table 1.
Clinical characteristics of 39 patients with pelvic osteosarcoma and data on tumor thrombus in the large draining veins on imaging by final consensus
| Characteristic | Result (n=39) |
|---|---|
|
| |
| Age at Diagnosis, years | |
| Range | 11–45 |
| Median | 19 |
|
| |
| Female: male ratio | 23:16 |
|
| |
| Race | |
| Caucasian | 24 |
| Hispanic | 5 |
| African | 5 |
| Asian | 5 |
|
| |
| Tumor size at diagnosis or on first available scan within 6 months of diagnosis, cm | |
| Range | 4–18 |
| Median | 12.3 |
|
| |
| Subtype of osteosarcoma, n | |
| Chondroblastic | 20 |
| Osteoblastic | 8 |
| Mixed | 5 |
| Small cell | 1 |
| Not otherwise specified | 5 |
|
| |
| Patients who underwent surgical resection of the primary tumor, n | 22 |
| Patients with metastatic disease at presentation, n (%) | 5 (23) |
| Tumor thrombus in the large draining veins on imaging, n (%) | 10 (45) |
|
| |
| Patients who did not undergo surgical resection of the primary tumor, n | 17 |
| Patients with metastatic disease at presentation, n (%) | 14 (82) |
| Tumor thrombus in the large draining veins on imaging, n (%) | 10 (59) |
Imaging Findings
Of the 39 patients, tumor thrombus in large draining veins was identified on imaging studies in 10 of the 22 (45%) patients who underwent surgical resection and 10 of the 17 (59%) patients who did not. The tumor thrombus reached the right atrium (n=1), IVC (n=4), common iliac vein (n=8), internal iliac vein (n=6), and common femoral vein (n=1). Of the 10 patients with tumor thrombus in the large draining veins on imaging who underwent surgical resection, tumor thrombus was identified and resected with clear margins during surgery in 6 patients. Operative report was not available in one patient who underwent external hemipelvectomy at an outside facility, but no residual tumor thrombus was seen on follow up imaging in this patient. Residual tumor thrombus was identified in the common iliac vein in the remaining 3 patients on follow up imaging: two patients had positive surgical margins and received adjuvant radiation therapy, and one patient is the patient described above who underwent resection with positive margins for palliation after prior radiation therapy, and had dense adhesions along the iliac vessels.
Metastatic disease at presentation was seen in 12 of the 20 (60%) patients with tumor thrombus in the large draining veins on imaging studies and 7 of the 19 (37%) patients without tumor thrombus. However, Fisher’s exact test did not show a statistically significant higher incidence of metastatic disease at presentation in patients with tumor thrombus on imaging (p= 0.2) (Table 2). The last available imaging studies did not show local recurrence or metastatic disease in 9 patients. The sites of disease in the remaining 30 patients included the pelvis (local recurrence, n=8), lungs (n=25), bones (n=14), pleura (n=5), hilar and mediastinal lymph nodes (n=5), and other (n=6). A chondroblastic component was identified in 25 of the 39 (64%) patients. Of these 25 patients, 17 (68%) showed tumor thrombus on imaging studies. Fisher’s exact test confirmed the association between the chondroblastic subtype and a significantly high proportion of tumor thrombus in draining veins (p=0.008) (Table 2).
Table 2.
Summary of the association analysis between metastatic disease at presentation and histologic subtype with tumor thrombus in the large draining veins on imaging in 39 patients with pelvic osteosarcoma
| Characteristic | Tumor thrombus in the large draining veins | P value | |
|---|---|---|---|
|
| |||
| No, n (%) | Yes, n (%) | ||
| Metastatic disease at presentation | |||
| No | 12 (60) | 8 (40) | 0.20 |
| Yes | 7 (37) | 12 (63) | |
| Subtype | |||
| Chondroblastic | 8 (32) | 17 (68) | 0.008 |
| Other | 11 (79) | 3 (21) | |
P-values by Fisher’s exact test.
Patient Survival
Of the 22 patients who underwent tumor resection, 12 (55%) died of disease and 10 (45%) are alive at the last follow-up. All 17 patients who did not undergo resection of the primary tumor died. The median follow-up for the surviving patients was 7 years (range, 3.6 to 12 years). The 5-year overall survival from the time of surgical resection of the primary tumor is 45% (95% CI, 28%–72%). All 17 patients who did not undergo surgical resection of the primary tumor died within 5 years from diagnosis. Of these 17 patients, one patient with locally advanced disease died of cardiac failure secondary to anthracycline-induced cardiotoxicity and another patient with locally advanced disease died of sepsis and multiorgan failure from treatment complications.
Among the 22 patients who underwent surgical resection of the primary tumor, those with tumor thrombus in the large draining veins had statistically significant worse overall survival than those without tumor thrombus (p=0.021 by log-rank test) (Fig. 4). In the 17 patients who did not undergo surgical resection, there was no statistically significant difference in overall survival by tumor thrombus (p=0.372 by log-rank test). Local recurrence was seen in eight patients who underwent surgical resection. Tumor thrombus in the large draining veins on imaging was seen in four of these 8 patients with local recurrence. A Cox proportional hazard analysis was used to assess the effect of tumor thrombus in the large draining veins, sex, metastatic disease at presentation, percent tumor necrosis at surgery (≥90% necrosis or <90%), tumor subtype (chondroblastic versus other), age (1 year increase), and tumor size (1 unit increase) on overall survival and time to local recurrence in the 22 patients who underwent surgical resection (Table 3). Patients with tumor thrombus in the large draining veins had significantly lower overall survival than those without tumor thrombus (p=0.03). There was possible association between overall survival and tumor size (p=0.07), and metastatic disease at presentation (p=0.08). No variables were associated with statistically significant changes in time to local recurrence.
Fig. 4.
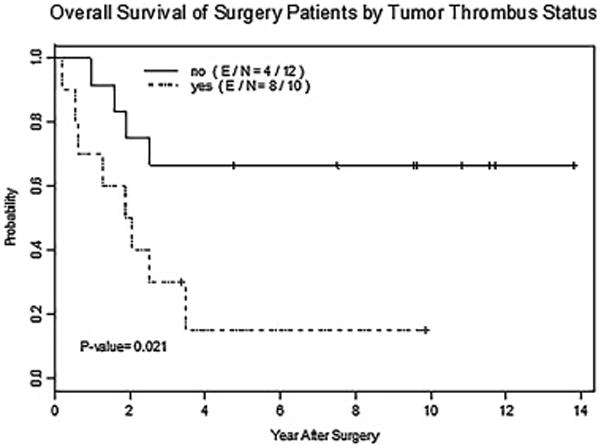
Overall survival curves of patients who underwent surgical resection of tumor without (no) and with (yes) tumor thrombus in the large draining veins. P-value by log-rank test. E = number of deaths, N = total number of patients.
Table 3.
Summary of univariate Cox proportional hazard model results correlating clinical factors with survival endpoints after surgery in 22 patients with pelvic osteosarcoma
| Endpoint factor | Hazard ratio | 95% LCL | 95% UCL | P value | No. events | No. censored |
|---|---|---|---|---|---|---|
| OS | ||||||
| Tumor thrombus in the large draining veins (yes vs. no) | 3.8 | 1.1 | 12.9 | 0.03 | 12 | 10 |
| Sex (female vs. male) | 1.9 | 0.6 | 6.2 | 0.31 | 12 | 10 |
| Metastasis (no vs. yes) | 0.3 | 0.1 | 1.1 | 0.08 | 12 | 10 |
| Percent of tumor necrosis (≥90% vs. <90%) | 4.2 | 0.5 | 32.6 | 0.17 | 12 | 10 |
| Subtype (chondroblastic vs. other) | 2.4 | 0.7 | 9 | 0.19 | 12 | 10 |
| Age (1 year increase) | 1 | 1 | 1.1 | 0.39 | 12 | 10 |
| Tumor size (1 unit increase) | 1.2 | 1 | 1.6 | 0.07 | 12 | 10 |
| TTR | ||||||
| Tumor thrombus in the large draining veins (yes vs. no) | 1.8 | 0.5 | 7.4 | 0.39 | 8 | 14 |
| Sex (female vs. male) | 3 | 0.6 | 14.7 | 0.18 | 8 | 14 |
| Metastasis (no vs. yes) | 0.5 | 0.1 | 2.6 | 0.42 | 8 | 14 |
| Percent tumor necrosis (≥90% vs. or <90%) | 2.8 | 0.3 | 22.7 | 0.34 | 8 | 14 |
| Subtype (chondroblastic vs. other) | 6.3 | 0.8 | 51 | 0.09 | 8 | 14 |
| Age (1 year increase) | 1 | 0.9 | 1.1 | 0.70 | 8 | 14 |
| Tumor size (1 unit increase) | 1.2 | 0.9 | 1.6 | 0.15 | 8 | 14 |
LCL, lower confidence limit; UCL, upper confidence limit; OS, overall survival; TTR, time to local recurrence. Hazard ratio higher than 1 indicates worse prognosis.
Discussion
Tumor thrombus in the draining veins is a well-known poor prognostic indicator in renal cell carcinoma, hepatocellular carcinoma, and colorectal carcinoma [2–4, 11]. Hoehn et al [2] described a 46% incidence of macroscopic venous tumor invasion in the renal veins or IVC in patients with surgically resected renal cell carcinomas; this finding was associated with a significantly lower overall survival rate. Kudo et al [11] reported 16% and 6% rates of portal venous and hepatic venous involvement in patients who underwent surgical resection of hepatocellular carcinoma. A multivariate analysis by Ikai et al [3] indicated that portal venous invasion and hepatic venous invasion were two among multiple independent prognostic factors in patients with resected hepatocellular carcinoma. Krasna et al [4] reported a 38% rate of vascular invasion in patients who underwent surgical resection for colorectal carcinoma, which was shown to be associated with significantly worse prognosis.
Our study showed a high rate of tumor in the veins draining the primary tumor in patients with pelvic osteosarcoma. The frequency of tumor thrombus in the large draining veins was 45% on initial and pre-operative imaging studies in surgically resected patients in our study, which is higher than 29% peri-tumoral vascular involvement described by Benezech et al [1] in surgically resected osteosarcomas from all locations in a pediatric population. We believe that this result was related to the pelvic location of the primary tumor in our study; in general, these patients tend to present with advanced disease and have poor prognosis more often than the patients with osteosarcomas in other locations [12]. A univariate analysis showed a statistically significant difference in the overall survival of our 22 patients who underwent surgical resection by tumor thrombus in the large draining veins and possible associations between overall survival and tumor size (p=0.07) and overall survival and metastatic disease at presentation (p=0.08). In a study of 1702 patients with newly diagnosed high-grade osteosarcomas of the extremities and trunk, Bielack et al reported that tumor site and size, metastatic disease at presentation, response to chemotherapy (percent tumor necrosis), and surgical remission were independent prognostic factors in patients with high grade osteosarcoma of the extremities and trunk [13]. Our inability to demonstrate a statistically significant association between overall survival and metastatic disease at presentation may be related to the small number of patients with metastatic disease at presentation among patients who underwent surgical resection (five of 22 patients; Table 1).
We believe that, as in renal cell carcinoma, hepatocellular carcinoma, and colorectal cancer, tumor thrombus in the draining veins is an important prognostic factor in the overall survival of patients with osteosarcoma. Future studies in a larger cohort are needed to assess the prognostic significance of tumor thrombus in the large draining veins compared to other known factors (e.g., tumor site and size, metastatic disease at presentation, and response to chemotherapy) [13]. In our study, tumor thrombus in the large draining veins on imaging studies appeared to be associated with a higher rate of metastatic disease at presentation compared to those without tumor thrombus; however, this association was not statistically significant (Table 2). This could be related to small number of patients in our series. While the presence of detectable tumor in the veins draining the primary tumor may be associated with a higher incidence of metastatic disease, the absence of detectable thrombus does not exclude the possibility of metastatic disease. We showed a statistically significant association between tumor thrombus in the large draining veins and chondroblastic subtype (Table 2). The chondroblastic subtype accounted for 64% of our patients, which is similar to the 68% reported [14] in 25 patients with osteosarcoma of the pelvis and 58% reported [10] in 40 patients with osteosarcoma of the pelvis and sacrum. In contrast, the chondroblastic subtype accounted only for 15% in 2114 patients with osteosarcomas at all sites [15]. Tumor thrombus in the large draining veins was predominantly associated with the chondroblastic subtype in our study, but it was also seen in other subtypes. We identified 10 case reports describing 11 patients with tumor thrombus in the draining veins in pelvic osteosarcoma since the year 2000 [7, 8, 16–23]. Of these, four patients had the chondroblastic subtype, one patient had the osteoblastic subtype, and the subtype was not specified in the other six patients.
Accurate detection of tumor thrombus in the large draining veins on the pre-operative imaging studies may potentially impact surgical margins. Shao et al have reported a fatal tumor embolism into bilateral pulmonary arteries shortly after surgical resection in a child with osteosarcoma [24]. Although, fatal tumor embolism has been described in a few case reports, it is crucial for the multidisciplinary team to be aware of the possibility of tumor embolism into the pulmonary arteries during the peri-operative period to enable early diagnosis. Since the inception of this study, we have identified tumor in the draining veins from osteosarcomas at other locations, particularly in the humerus, and from other sarcomas. In light of our findings, we recommend evaluation of the draining veins on the routine baseline and follow-up imaging studies of patients with osteosarcomas.
Limitations
A major limitation of this study is the variation in imaging techniques in the studies performed over a decade and at multiple institutions. However, the reasonable image quality of these studies allowed fairly confident identification of tumor thrombus in the large draining veins in most cases. Detection of tumor in the internal iliac vein is difficult, which could be due to slow blood flow and that the existing imaging techniques are not tailored to detect tumor in these distal veins. The fact that not all imaging studies followed a single structured protocol and were not tailored to the detection of tumor thrombus might have resulted in under diagnosis. This study emphasizes the need to develop imaging techniques to detect tumor thrombus in the small veins; perhaps PET/MRI may play a potential role in delineating tumor thrombi in small veins. Since our center is a tertiary cancer care facility, we understand that there is a selection bias towards advanced cancer stage that is inherent to patients referred to our center.
Conclusions
Tumor thrombus in the large veins draining primary pelvic osteosarcoma was seen in a significant proportion (45%) of initial cross-sectional imaging studies of patients with pelvic osteosarcoma who underwent surgical resection and in a higher percentage in patients who could not undergo surgical resection. In our study, among patients who underwent surgical resection of the primary tumor those with tumor thrombus showed statistically significant lower overall survival than those without. We also showed that the chondroblastic subtype is statistically significantly associated with tumor thrombus in the large draining veins. In view of the frequency of tumor thrombus in the large draining veins in pelvic osteosarcoma and its potential impact on surgical margins and patient prognosis, radiologists should routinely evaluate the veins that drain primary pelvic osteosarcomas for tumor thrombus on imaging studies.
Highlights.
Tumor thrombus is frequently seen in the larger veins draining primary pelvic osteosarcoma on routine baseline and follow up staging studies.
Accurate detection of tumor thrombus in the large draining veins on the pre-operative imaging studies may potentially impact surgical margins.
In our study, among patients who underwent surgical resection of the primary tumor those with tumor thrombus showed statistically significant lower overall survival than those without.
Acknowledgments
Funding: The study was supported in part by the NIH/NCI under award number P30 CA016672. The funding source supports MD Anderson core facilities.
Institutional review board statement: The study was approved by the University of Texas, MD Anderson Cancer Center Institutional Review Board
The authors thank Ms. Ann Sutton in the Department of Scientific Publications at MD Anderson for editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benezech S, Chabaud S, Chambon F, Dijoud F, Chotel F, Marec-Berard P. Prognostic Value of Vascular Invasion in Pediatric Osteosarcomas. Pathol Oncol Res. 2016;22:847–852. doi: 10.1007/s12253-016-0074-5. [DOI] [PubMed] [Google Scholar]
- 2.Hoehn W, Hermanek P. Invasion of veins in renal cell carcinoma - frequency, correlation and prognosis. Eur Urol. 1983;9:276–280. doi: 10.1159/000474103. [DOI] [PubMed] [Google Scholar]
- 3.Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K, Yamaoka Y. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi: 10.1002/cncr.20426. [DOI] [PubMed] [Google Scholar]
- 4.Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018–1023. doi: 10.1002/1097-0142(19880301)61:5<1018::aid-cncr2820610527>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Kim TU, Kim S, Lee NK, Kim HJ, Han GJ, Lee JW, Baek HJ, Jeon TY, Kim HS, Park DY. Prognostic Value of Computed Tomography-Detected Extramural Venous Invasion to Predict Disease-Free Survival in Patients With Gastric Cancer. J Comput Assist Tomogr. 2016;7:7. doi: 10.1097/RCT.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 6.NP, JSM, JJK, SJD, TPC, IRV, HB Osteosarcoma tumor thrombus: A case report with a review of the literature. Texas Hear Inst J. 2013;40:75–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Lin WC, Lin CH, Chao YH, Lin HC, Chen PY, Wu HP, Wu KH. Simultaneous pulmonary and inferior vena cava thromboembolism secondary to pelvic osteosarcoma. J Pediatr Hematol Oncol. 2013;35:e320–2. doi: 10.1097/MPH.0b013e3182707a1a. [DOI] [PubMed] [Google Scholar]
- 8.Mavrogenis AF, Angelini A, Sakellariou VI, Skarpidi E, Ruggieri P, Papagelopoulos PJ. Osteosarcoma invasion of the inferior vena cava and right atrium. J Surg Orthop Adv. 2012;21:107–112. doi: 10.3113/jsoa.2012.0107. [DOI] [PubMed] [Google Scholar]
- 9.Yedururi S, Morani AC, Gladish GW, Vallabhaneni S, Anderson PM, Hughes D, Wang WL, Daw NC. Cardiovascular involvement by osteosarcoma: an analysis of 20 patients. Pediatr Radiol. 2016;46:21–33. doi: 10.1007/s00247-015-3449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai A, Huvos AG, Meyers PA, Healey JH. Osteosarcoma of the pelvis. Oncologic results of 40 patients. Clin Orthop Relat Res. 1998;1998:196–207. [PubMed] [Google Scholar]
- 11.Kudo M, Izumi N, Ichida T, Ku Y, Kokudo N, Sakamoto M, Takayama T, Nakashima O, Matsui O, Matsuyama Y. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2016;46:372–90. doi: 10.1111/hepr.12697. [DOI] [PubMed] [Google Scholar]
- 12.Isakoff MS, Barkauskas DA, Ebb D, Morris C, Letson GD. Poor survival for osteosarcoma of the pelvis: A report from the children’s oncology group. Clin Orthop Relat Res. 2012;470:2007–2013. doi: 10.1007/s11999-012-2284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Wemer M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 14.Fahey VGRM, Spanier SS. Osteosarcoma of the Pelvis. A clinical and histopathological study of 25 patients. J Bone Jt Surg Am. 1992;74:321–330. [PubMed] [Google Scholar]
- 15.Pakos EE, Nearchou AD, Grimer RJ, Koumoullis HD, Abudu A, Bramer JAM, Jeys LM, Franchi A, Scoccianti G, Campanacci D, Capanna R, Aparicio J, Tabone MD, Holzer G, Abdolvahab F, Funovics P, Dominkus M, Ilhan I, Berrak SG, Patino-Garcia A, Sierrasesumaga L, San-Julian M, Garraus M, Petrilli AS, Filho RJG, Macedo CRPD, de Alves MTS, Seiwerth S, Nagarajan R, Cripe TP, Ioannidis JPA. Prognostic factors and outcomes for osteosarcoma: An international collaboration. Eur J Cancer. 2009;45:2367–2375. doi: 10.1016/j.ejca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Arkader A, Morris CD. Inferior vena cava extension of pelvic osteogenic sarcoma. Cancer Imaging. 2008;8:6–7. doi: 10.1102/1470-7330.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jani JC, Massad M, Kpodonu J, Alagiozian-angelova V, Guzman G. High-Grade Pelvic Osteosarcoma With Intravascular Extension to the Right Side of the Heart A Case Report and Review of the Literature. Arch Pathol Lab Med. 2005;129:241–243. doi: 10.5858/2005-129-241-HPOWIE. [DOI] [PubMed] [Google Scholar]
- 18.Chrouser KL, Sim FH, Lieber MM. Intravascular extension of an osteosarcoma of the pubic bone into periprostatic venous plexus. Urology. 2005;65 doi: 10.1016/j.urology.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Yin WH, Young MS, Lan GY, Chung MT, Wei J. Osteogenic sarcoma metastatic to the heart via infradiaphragmatic venous extension - A case report. Acta Cardiol Sin. 2004;20:52–55. [Google Scholar]
- 20.Shah AP, Parmar S, Regan RO. Right Atrial and Ventricular Thrombus Infiltrated with Osteoblastic Osteosarcoma. J Cardiovasc Pharmacol Ther. 2003;8(4):307–31. 307–311. doi: 10.1177/107424840300800408. [DOI] [PubMed] [Google Scholar]
- 21.Newkirk L, Vater Y, Oxorn D, Mulligan M, Conrad E. Intraoperative TEE for the management of pulmonary tumour embolism during chondroblastic osteosarcoma resection. Can J Anesth Can D’anesthésie. 2003;50:886–890. doi: 10.1007/BF03018733. [DOI] [PubMed] [Google Scholar]
- 22.Garcia ND, Morasch MD, Sam AD, II, Satcher RL, Blum MG, Fullerton DA. Inferior Vena Cava Thrombus Removal Using Hypothermic Circulatory Arrest in Two Patients with Osteosarcoma. Ann Vasc Surg. 2003;17:686–689. doi: 10.1007/s10016-003-0077-z. [DOI] [PubMed] [Google Scholar]
- 23.Mitsunaga MM, Bateni C, Bindra J. Radiology Case Reports Venous tumor thrombus from a pelvic osteosarcoma. Radiol Case Reports. 2013;8:18–20. doi: 10.2484/rcr.v8i3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao L, Willard MJ, Lowe LH, Singh V. Fatal pulmonary tumor embolism in a child with chondroblastic osteosarcoma. Pediatr Dev Pathol. 2008;11:156–159. doi: 10.2350/07-02-0241.1. [DOI] [PubMed] [Google Scholar]


