Summary
The prevalence of systemic rheumatic diseases (SRDs) in T1DM has not been described. This study in 1,212 adults with T1DM found an age-dependent enrichment of SRDs in women with T1DM: 9.2% prevalence in women overall and 14% in women over age 50. Clinicians taking care of older women with T1DM should monitor for these SRDs.
1. Introduction
Type 1 diabetes mellitus (T1DM) is frequently associated with other endocrine and non-endocrine autoimmune diseases due to shared genetic predispositions1–3. However, we have limited insight into the epidemiology of autoimmune diseases in older adults with T1DM. Data on the prevalence of systemic rheumatic diseases (SRDs) in persons with T1DM is particularly scarce.
Systemic rheumatic diseases, including rheumatoid arthritis, scleroderma, and systemic vasculitides, are debilitating conditions that disproportionately affect women. Having an SRD in addition to T1DM may further complicate care of both conditions, and may worsen cardiovascular and metabolic bone disease risks4. Several studies have suggested a genetic link between SRDs and T1DM5–7. We now aim to better characterize the prevalence, risk factors, and ages of onset of SRDs in a cohort of patients with T1DM.
2. Methods
This observational, cross-sectional study was approved by the Washington University Human Research Protection Office. Patients with T1DM seen at the Washington University Diabetes Center from 2011 to 2018 completed a questionnaire including date of birth, gender, race, age of T1DM onset, concurrent SRD diagnoses, and age of onset of each SRD. Patient charts were reviewed for verification.
2.1 Statistical Analysis
SRD prevalence was compared in males versus females and among races using a Chi-squared test and Fishers exact test. Multivariate logistic regression modeling was used to evaluate independent effects of the presence of SRDs, gender, race, and age of T1DM onset on the prevalence of non-SRD autoimmune diseases. A two-tailed p-value of p<0.05 was considered significant. SAS version 9.4 (2012 SAS Institute Inc., Cary, NC) was used for data analysis.
3. Results
3.1 Study Population
Participants included 1,212 individuals with T1DM, mean age 46.8 ± 16.2 years, range 19–96. Participants were 51.8% female; 89.6% white, 9.0% black, and 1.4% other race/ethnicity. Median T1DM onset age was 18.0 years, mean 21.2 ± 14.4 years. 6.5% of the cohort had a SRD, and of these, 63.3% had one or more non-SRD autoimmune diseases, such as hypothyroidism, hyperthyroidism, pernicious anemia, and celiac disease. Having T1DM plus SRD significantly raised the risk of additional autoimmune diseases (adjusted OR, 2.8; 95% CI, 1.71–4.60; p<0.0001). There was no racial/ethnic predominance for any SRD in individuals with T1DM. After adjusting for gender and current age, blacks were found to be less likely than whites to develop SRD, but the relationship was not statistically significant (OR: 0.61, 95% CI: 0.30, 1.25, p=0.1758).
3.2 Prevalence of SRD by disease type and age of onset
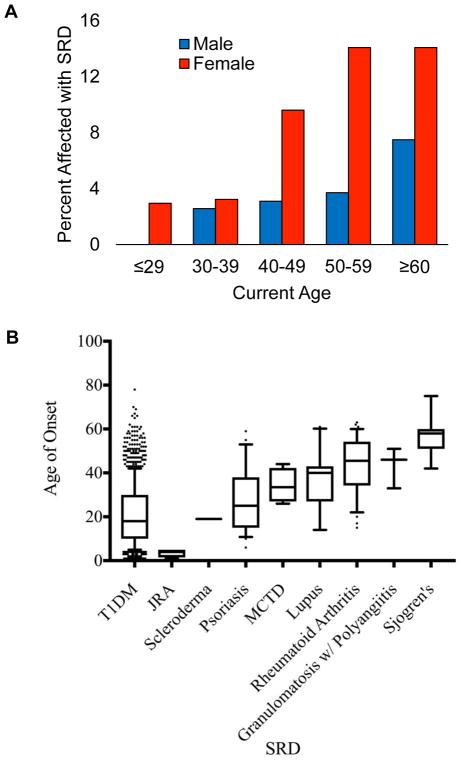
The most common SRD in this cohort was rheumatoid arthritis, comprising 4.3% of women (Table 2). Psoriasis (2.6%), systemic lupus erythematosus (1.8%), and Sjogren’s (1.0%) were less common. Figure 1B displays median ages of SRD onset. With the exception of juvenile rheumatoid arthritis, the median ages of onset for all SRDs were later than the age of T1DM onset. Scleroderma and psoriasis had relatively young median ages of onset at 19 and 25 years respectively, while rheumatoid arthritis, granulomatosis with polyangiitis, and Sjogren’s had later median ages of onset, at 45.5 years, 46 years, and 58 years respectively.
Table 2.
Systemic Rheumatic Disease Demographics
| Disease Category | Total N (%) | Male | Female | Caucasian and Other | Black |
|---|---|---|---|---|---|
|
| |||||
| Systemic Rheumatic Diseases | 79 (6.5) | 21 (3.6)** | 58 (9.2)** | 69 (6.2) | 10 (9.2) |
| Rheumatoid Arthritis | 34 (2.8) | 7 (1.2)** | 27 (4.3)** | 28 (2.5) | 6 (5.5) |
| Psoriasis | 25 (2.1) | 9 (1.5) | 16 (2.6) | 24 (2.2) | 1 (0.9) |
| MCTD | 4 (0.3) | 2 (0.3) | 2 (0.3) | 4 (0.4) | 0 (0) |
| JVRA | 4 (0.3) | 1 (0.2) | 3 (0.5) | 3 (0.3) | 1 (0.9) |
| Lupus | 12 (1.0) | 1 (0.2)** | 11 (1.8)** | 10 (0.9) | 2 (1.8) |
| Sjogren’s | 7 (0.6) | 1 (0.2) | 6 (1.0) | 6 (0.5) | 1 (0.9) |
| Granulomatosis with polyangiitis | 3 (0.2) | 0 (0) | 3 (0.5) | 2 (0.2) | 1 (0.9) |
| Scleroderma | 1 (0.1) | 0 (0) | 1 (0.2) | 1 (<0.1) | 0 (0) |
Figure 1.
(A) SRD frequency increases with age and female Gender. (B) Median ages of onset for the SRDs that occur in patients with T1DM. Boxes are 25th to 75th percentiles, whiskers are 10–90th percentiles.
3.3 SRD prevalence increases with age and gender
Prevalence of SRD in patients with T1DM increased with age. 1.4% of T1DM patients age ≤29 years had a SRD, increasing to 6.7% of T1DM patients age 40–49 years, and 10.7% age ≥60 years (Figure 1A). After adjusting for gender and race, multivariate logistic regression analysis showed that T1DM patients between the ages of 40–49, 50–59, and ≥60 years had a higher risk of having SRD when compared to patients age ≤29 years (OR:1.86, 95% CI: 1.19, 2.94, p=0.081; OR: 2.64, 95% CI: 1.70, 4.11, p<0.0001; OR: 3.27, 95% CI: 2.13, 5.02, <0.0001). A similar analysis showed no relationship between age of T1DM onset and odds of developing SRD (p=0.8724).
Women with T1DM had a higher prevalence of SRD than men (9.2% vs 3.6%, p<0.0001). The gap widens across the age spectrum, up to 14% of women age 50–59 reporting SRD versus 3.7% of men (Figure 1A). After adjusting for current age and race, multivariate logistic regression analysis showed that women were 2.71 times more likely to develop an SRD (OR: 2.71, 95% CI: 1.61, 4.55, p=0.0002). Among SRD disease types, rheumatoid arthritis and lupus were the most common, and more common in women versus men with T1DM (p<0.01). No SRD showed a male predominance (p>0.05).
4. Discussion
This study has three main findings: 1) SRD prevalence is high among persons with T1DM, 2) women with T1DM are at higher risk than men, and 3) individuals with T1DM and SRD are more likely to have additional AID than those without an SRD. Prior studies of AID prevalence in T1DM cohorts have reported low prevalence of SRD, generally under 2% (single or overall SRD)8,9. However, the mean age of these cohorts is <20 years. In contrast, our cohort with a mean age of 46 years demonstrated dramatically higher overall SRD prevalence (6.3%), particularly in women (9.2%). The most common SRD, rheumatoid arthritis, has an age-adjusted prevalence of 0.5% in the general population (0.29–0.31% males and 0.73–0.78% females)10, but the prevalence of RA is approximately 5-fold higher in our study (2.8% overall; 1.2% males and 4.3% females). This underscores the heightened risk of SRDs in T1DM, which increases with age and female gender. A potential mechanism underlying the co-existence of rheumatoid arthritis and T1DM, for example, is suggested by a recent study that showed hyperglycemia and oxidative stress from diabetes may trigger antibody binding to type II collagen11, providing a causal link between these diseases.
In conclusion, the high prevalence of SRD in older individuals with T1DM suggests that progressive loss of immune tolerance occurs with age. Clinicians taking care of older women with T1DM should monitor for SRD development. Future studies should examine disease clustering patterns and pathways underlying shared autoimmunity.
Table 1A.
Cohort Demographics
| Demographics | N (%)*(n=1212) | No SRD (n=1133) | SRD Present (n=79) | p-value |
|---|---|---|---|---|
|
| ||||
| Age (mean ± SD) | 46.8 ± 16.2 | 46.2 ± 16.3 | 54.7 ± 13.0 | <0.0001 |
|
| ||||
| Gender | ||||
| Male | 584 (48.2) | 563 (96.4) | 21 (3.6) | |
| Female | 628 (51.8) | 570 (90.8) | 58 (9.2) | <0.0001 |
|
| ||||
| Race/ethnicity | ||||
| White and Other | 1103 (89.6) | 1034 (93.7) | 69 (6.3) | |
| Black | 109 (9.0) | 99 (90.8) | 10 (9.2) | 0.2389 |
|
| ||||
| Age of T1DM Onset | 21.2 ± 14.4 | 21.1 ± 14.3 | 22.4 ± 16.6 | 0.0485 |
Acknowledgments
This work was supported by the Washington University Diabetes Research Center (DRC) through NIH grant P30 DK020579 (to J.B.M.), the Doris Duke Charitable Foundation grant #2015215 (to J.W.H.), Barnes-Jewish Foundation Grant (to G.S.T.) and NIH training grant T32DK007120 (to Y.B., J.W.H.). We thank Mary Jane Clifton, Carol Recklein, and Garrett Pagano for administrative and data collection support. We thank Dr. Philip Miller, Professor of Biostatistics at Washington University School of Medicine, for helpful discussions of data analysis. We thank the clinicians and patients in the Washington University Diabetes Center for their support and participation.
Footnotes
The authors have no known conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10(1):43–55. doi: 10.1038/nrg2489. [DOI] [PubMed] [Google Scholar]
- 2.Richard-Miceli C, Criswell LA. Emerging patterns of genetic overlap across autoimmune disorders. Genome Med. 2012;4:6. doi: 10.1186/gm305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyre S, Hinks A, Bowes J, et al. Overlapping genetic susceptibility variants between three autoimmune disorders: rheumatoid arthritis, type 1 diabetes and coeliac disease. Arthritis Res Ther. 2010;12:R175. doi: 10.1186/ar3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatol Oxf Engl. 2014;53(10):1759–1766. doi: 10.1093/rheumatology/keu162. [DOI] [PubMed] [Google Scholar]
- 5.Kiani AK, Jahngir S, John P, et al. Genetic link of type 1 diabetes susceptibility loci with rheumatoid arthritis in Pakistani patients. Immunogenetics. 2015;67(5–6):277–282. doi: 10.1007/s00251-015-0839-0. [DOI] [PubMed] [Google Scholar]
- 6.Liao KP, Gunnarsson M, Källberg H, et al. A specific association exists between type 1 diabetes and anti-CCP positive rheumatoid arthritis. Arthritis Rheum. 2009;60(3):653–660. doi: 10.1002/art.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo C-F, Grainge MJ, Valdes AM, et al. Familial Risk of Sjögren’s Syndrome and Co-aggregation of Autoimmune Diseases in Affected Families: A Nationwide Population Study. Arthritis Rheumatol Hoboken NJ. 2015;67(7):1904–1912. doi: 10.1002/art.39127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr. 2012;6(2):70–76. doi: 10.1016/j.dsx.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JW, Riddlesworth TD, DiMeglio LA, et al. Autoimmune Diseases in Children and Adults With Type 1 Diabetes From the T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2016;101(12):4931–4937. doi: 10.1210/jc.2016-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9):1551–1557. doi: 10.1007/s00296-017-3726-1. [DOI] [PubMed] [Google Scholar]
- 11.Strollo R, Rizzo P, Spoletini M, et al. HLA-dependent autoantibodies against post-translationally modified collagen type II in type 1 diabetes mellitus. Diabetologia. 2013;56(3):563–572. doi: 10.1007/s00125-012-2780-1. [DOI] [PubMed] [Google Scholar]