Abstract
Coronary artery calcium (CAC) is a highly specific feature of coronary atherosclerosis. Based on single-center and multicenter clinical and population-based studies with short-term and long-term outcomes data (up to 15 years follow-up), CAC scoring has emerged as a widely available, consistent, and reproducible means of assessing risk of major cardiovascular outcomes, especially useful in asymptomatic people for planning primary prevention interventions such as statins and aspirin. CAC testing in asymptomatic populations is cost-effective across a broad range of baseline risk. This review summarizes evidence concerning CAC, including its pathobiology, modalities for detection, predictive role, use in prediction scoring algorithms, CAC progression, evidence that CAC changes the clinical approach to the patient and patient behavior, novel applications of CAC, future directions in scoring CAC scans, and new CAC guidelines.
Keywords: aspirin, atherosclerotic cardiovascular disease, coronary artery calcification, coronary heart disease, statins
Introduction
Coronary artery calcium (CAC), a highly specific feature of coronary atherosclerosis (1), is one of the most thoroughly studied and widely available tests in cardiovascular medicine. Following important single-center and clinical registry studies, large long-term population-based observational studies were launched in the United States (2,3), Germany (4), and the Netherlands (5) in the late 1990s and early 2000s that have produced consistent, reproducible, and convincing evidence of a strong association between CAC (6) and major cardiovascular outcomes in asymptomatic people. Clinical practice guidelines in the United States (7) and Europe (8) consider CAC scoring to be a potentially useful way of improving cardiovascular risk assessment in asymptomatic individuals and serving as a guide for initiating or deferring preventive therapies. Cost-effectiveness analyses (9–14) have concluded that CAC testing is cost-effective in asymptomatic populations. Yet, such application of CAC scoring in asymptomatic people is still sometimes regarded as experimental or unproven by many health insurance companies in the United States (15).
The purpose of this review is to summarize the evidence concerning CAC, with emphasis on asymptomatic patients, including its pathobiology, modalities for detection, predictive role, use in prediction scoring algorithms, CAC progression, evidence that CAC changes the clinical approach to the patient and patient behavior, novel applications of CAC, and future directions in scoring CAC scans.
PATHOBIOLOGY OF CORONARY ARTERY CALCIFICATION
Vascular calcification was accepted until recently as an inevitable result of aging, and the development of CAC was considered a passive process. The development of CAC is now understood to be an active pathogenic process that is not inevitable, and mechanisms that underlie vascular calcification have been identified. Ectopic bone production, a common feature of atherosclerosis, is the basis for coronary artery calcification (16). Developmental, inflammatory, and metabolic factors all influence the process. Master transcription factors, such as Msx2, Runx2, Osterix, and Sox9, have been implicated in vascular calcification, as have potent osteogenic differentiation factors, such as bone morphogenetic proteins (BMPs) 2 and 4. Matrix Gla protein is an inhibitor of BMP and is highly expressed in calcified human arteries (17). Expression of both pro- and anti-osteogenic factors in CAC highlights the extensive regulation of this process.
Inflammation, propagated by apolipoproteins and oxidized phospholipids in the artery wall, is critical to the development of both atherosclerosis and vascular calcification (18,19). Several mediators associated with oxidative stress are implicated in calcification, and oxidative stress may be a key link between inflammation and vascular calcification (20). Lipid oxidation leads to pro-osteogenic mediators, such as minimally-modified low-density lipoprotein (LDL) and oxidized phospholipids (20).
Hyperlipidemia likely has both direct and indirect effects on vascular calcification (21). Glucose can directly promote vascular cell calcification (22) and insulin can inhibit it (23). Adipose-derived factors affect calcification, with leptin promoting (24) and adiponectin inhibiting (25) vascular calcification.
MODALITIES FOR DETECTION OF CAC
Early studies of CAC used chest x-ray, fluoroscopy, or digital subtraction fluoroscopy (26), and began to show the potential value of CAC in predicting the presence of obstructive coronary artery disease (CAD) (27), as well as future coronary events (28–31). Cardiac gating, first introduced with the electron-beam computed tomography (EBCT) scanning technique, allowed detection, localization, and quantification with higher sensitivity (6). With the introduction of EBCT, more precise quantification of CAC became possible, allowing sufficient temporal resolution of the moving heart. However, EBCT scanners were inadequate for general computed tomography (CT) imaging and have been superseded by multidetector computed tomography (MDCT) scanners that are comparable to EBCT for CAC measurement (32). With gantry rotation, MDCT takes hundreds of “snapshot” images from different angles, which are used to reconstruct a complete image. Until recently, only cardiac-gated studies allowed for quantification of CAC. Due to larger numbers of detectors and faster gantry speeds in current MDCT scanners, even nongated studies can provide either semiquantitative (ordinal scores) or quantitative CAC (Agatston scoring), with high correlation to gated CT studies and cardiovascular disease (CVD) outcomes (33,34). Modern CT scans can be accomplished with 10 to 15 min of total room time at about 1 mSy of radiation, without the need for contrast agents.
EARLY STUDIES OF CAC, CORONARY PLAQUE, CLINICAL CAD
Pathological studies demonstrated a strong correlation between the presence of coronary calcium and CAD. An early focus of CAC testing was to compare results to invasive angiography, to establish the sensitivity and specificity to detect obstructive CAD. Sensitivity for obstructive CAD ranges from 88% to 100% (31,35–37). With high sensitivities for disease, a negative test has a very low probability of being associated with obstructive CAD, with negative predictive values approaching 100% (38,39).
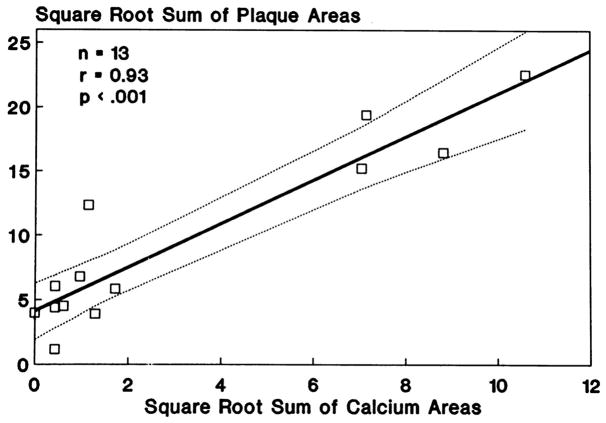
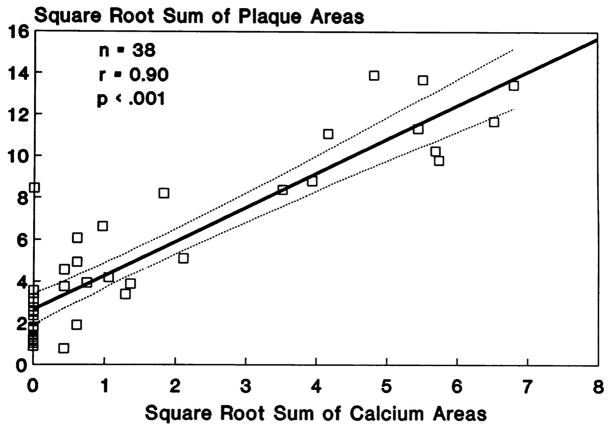
CAC quantification was found to be an excellent anatomic measure of atherosclerotic plaque burden (36,40). Sangiorgi et al. (40) showed a significant association between coronary calcification area and plaque volume, as compared with no association between coronary calcification and lumen area. Rumberger et al. (36) conducted a histopathological study of coronary arteries from autopsy hearts that were dissected, straightened, and scanned with EBCT in 3-mm contiguous increments. CAC and coronary artery plaque areas were highly correlated for the hearts as a whole, for individual coronary arteries, and for individual coronary artery segments (Figure 1). Studies of intracoronary ultrasound have also confirmed a direct association of the CAC score with the location and extent of atherosclerotic plaques in vivo (41).
Figure 1. Associations of CAC Area With Plaque Area (Plaque Burden) Per Patient.
(A) Calcium area with plaque area (per artery). (B) CAC is highly correlated with plaque burden, both at the level of the individual artery and in the heart as a whole. Reprinted, with permission, from Rumberger et al. (36) CAC = coronary artery calcium.
As early as 2004, single-center studies suggested a strong risk-predictive value of CAC and incremental value of CAC over traditional risk factors. The South Bay Heart Watch Study demonstrated that CAC further risk stratified individuals deemed intermediate risk by the Framingham Risk Score (42). In 2005, the St. Francis Heart Study showed a much higher risk, comparing CAC >400 versus CAC = 0, and an improvement in the area under the curve from 0.69 to 0.79 with addition of CAC to the Framingham Risk Score (43). Other studies have shown that the incremental risk predictive value of CAC extended to both younger and older individuals (44), patients with diabetes (45), smokers (46,47), and the elderly (48,49).
FINDINGS FROM MAJOR POPULATION-BASED COHORT STUDIES (Table 1)
Table 1.
Comparison of 4 Major Prospective Observational Studies of the CAC Score: Baseline Characteristics
| Study | MESA | HNR | Rotterdam | Framingham |
|---|---|---|---|---|
| Year CAC Study Started | 2000–2002 | 2000–2003 | 1997–2000 | 2002–2005 |
| Type of CT scan performed | EBCT in 3 centers and MDCT in 3 centers | EBCT | EBCT | MDCT |
| Number of participants | 6,814 | 4,487 | 2,063* | 3,238 |
| Age of participants (yrs); mean | 45–84; 62.2 ± 10.2 | 45–74; 59 ± 8 | ≥55; 71.1 ± 5.7 | Men >35; women >40; 49 ± 10.9 |
| Women | 53% | 53% | 57% | 54% |
| Systolic blood pressure (mm Hg) | 126.6 ± 21.5 | 133 ± 21 | 144 ± 21 men; 142 ± 21 women | 124.0 ± 16.7 |
| Total cholesterol (mg/dl) | 194.2 ± 35.7 | 231.2 ± 38.6 | 216.6 ± 34.8 men; 232.0 ± 34.8 women | 206.0 ± 38.2 |
| Current smoker | 12% | 23% | 18% men; 15% women | 26% |
| Previous CVD included or excluded | Clinical CVD excluded | Clinical CAD excluded† | Not excluded | Excluded from most analyses |
| Percentage with CAC >0 at baseline examination | Men: 52%– 70% Women: 35%–45%‡ |
Men: 82% Women: 55% |
91% overall (125) | Men: 40.5% Women: 20.6% |
Follow-up for atherosclerotic cardiovascular disease events was similar in all 4 studies and included hard endpoints such as myocardial infarction and cardiac death, but also, in some studies, included soft endpoints such as coronary revascularization for appropriate clinical indications.
Number with CT scans available for analysis at the baseline examination
Clinical CAD patients were excluded for this table (7% of the overall HNR study)
CAC prevalence differed in different ethnic/racial groups in MESA (50)
CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; CVD = cardiovascular disease; EBCT = electron-beam computed tomography; HNR = Heinz Nixdorf RECALL; MDCT = multidetector computed tomography; MESA = Multi-Ethnic Study of Atherosclerosis.
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective multicenter cohort sample of 6,814 men and women 45 to 84 years of age, started in 2000 (2). Approximately 38% of the recruited participants were white, 28% African-American, 22% Hispanic, and 12% Asian, predominantly of Chinese descent. MESA used EBCT scanners in 3 centers and MDCT systems in 3 centers (50). CAC distributions were similar for EBCT and MDCT (51).
The prevalence of CAC was shown to differ by ethnicity, higher in whites compared with the 3 other ethnic groups (50). Differences across ethnicities were not fully explained by risk factor differences, suggesting that other factors must account for some of the variability in CAC distributions. Most importantly, CAC convincingly predicted cardiovascular events beyond traditional risk factors, with similar strength in all 4 ethnicities. MESA introduced the presentation of estimated curves for the 50th, 75th, and 90th percentiles of calcium across age, making it possible to determine at a glance what an approximate percentile means for a particular patient (51).
The population-based Heinz Nixdorf Recall (Risk Factors, Evaluation of Coronary Calcium and Lifestyle) (HNR) Study had similar goals to the MESA Study (4). Random samples of the general population were drawn from residents’ registration offices from 3 West German cities and included men and women from 45 to 74 years of age (52). The initial examination between 2000 and 2003 involved 4,487 people who underwent EBCT scans, and results were blinded until the second examination in 2006 to 2008. The second examination included a repeat EBCT scan (53), and a third visit of the participants was organized after 10 years (2011 to 2014), in addition to yearly written and telephone contacts and plans for ongoing future follow-up (54). The mean age in the HNR study was 59 ± 8 years, and 53% of participants were women. Among 1,918 men, CAC prevalence was 82%, and in 2,148 women, CAC prevalence was 55%. CAC ≥ 400 was found in 16.3% of men and 4.4% of women. In individuals with known CAD, 100% of the men (n = 218) and 82% of the women (n = 62) had a CAC score >0, and 77.5% and 41.9%, respectively, were found to have a CAC score ≥ 400.
Based on similar designs and study protocols, the results of the MESA and HNR study cohorts were compared, including 2,220 and 3,126 participants, respectively (55). Despite differences in risk factor profiles between the 2 studies, CAC scores were very similar, as well as the age/sex distributions of CAC expressed in percentiles (51). CAC was a similar predictor of events in both studies.
The Rotterdam Study is a prospective cohort study among, initially, 7,983 persons living in the city of Rotterdam in The Netherlands (78% of 10,215 invitees) (56). All participants were at least 55 years of age. Imaging of the heart, vasculature, eyes, skeleton, and brain was completed, and biospecimens were archived. Of 3,370 eligible participants, 2,063 (61%, mean age 71 years) received an EBCT scan at first examination (57). Thus, the Rotterdam Study was an older sample than in MESA or in the HNR study. The median CAC measured 98 (25th and 75th percentiles: 10 and 430). Despite age and ethnicity differences between cohorts, findings in the Rotterdam Study for CAC and disease risk have been generally similar to those in MESA and in the HNR study (58). A strong and graded association was found between coronary calcification and myocardial infarction, and the association remained present, even in older individuals (57,58).
Due to similarities between the 3 cohort studies, meta-analyses including all 3 studies have examined subcohorts of interest. A meta-analysis in low-risk women (59) found that CAC >0 was present in approximately one-third and was associated with an increased risk of atherosclerotic cardiovascular disease (ASCVD) and modest improvement in prognostic accuracy compared with traditional risk factors. A meta-analysis was conducted in the elderly subjects (mean age 70 years) (58) from among 4,778 participants from 3 U.S. cohorts, including MESA, Framingham, and the Cardiovascular Health Study. Over 11 years of follow-up, 405 coronary heart disease (CHD) and 228 stroke events occurred. CAC score (vs. age) had a greater association with incident CHD and modestly improved prediction of incident stroke. Findings were similar in the Rotterdam and HNR cohorts.
The Framingham Heart Study (FHS) added a CAC measurement by MDCT to the examinations of the Framingham Offspring and Third Generation cohorts in 2005. The FHS is limited to white men and women, but distributions of CAC >0 and CAC >100 were very similar to those previously reported from MESA. A novel analysis from the CAC data evaluated whether information on the distribution of CAC and coronary dominance, as detected by MDCT, was incremental to the traditional Agatston score in predicting incident CHD. During a median follow-up of 7 years, the number of coronary arteries with CAC and the presence of CAC in the proximal dominant coronary artery were significantly associated with major CHD events after multivariable adjustment for Framingham risk score and categories of Agatston score. This analysis suggested that additional information from MDCT can augment the traditional Agatston score for risk prediction (60).
The CARDIA (Coronary Artery Risk Development in Young Adults) study measured CAC during follow-up and is the first prospective cohort to include data on CAC among subjects from 32 to 46 years of age. CARDIA showed that CAC >0 is not uncommon in this age group, particularly when a risk factor is present (61,62). Over an approximately 10-year follow-up, CAC strongly predicted risk beyond traditional risk factors in these young individuals (63).
The Jackson Heart Study measured CAC during follow-up. Among African Americans, CAC predicted risk beyond the traditional risk factors and has been shown to better identify individuals most likely to benefit from preventive therapies (64,65).
CAC was measured during follow-up of the Women’s Health Initiative, with CAC showing incremental predictive value over risk factors in post-menopausal women (66). CAC is currently being measured within the ARIC (Atherosclerosis Risk in Communities) study, which is expected to provide important insight into its risk predictive capability in adults >75 years of age.
USING CAC IN RISK SCORES FOR CLINICAL DECISION-MAKING
For more than 40 years, clinical decisions in preventive cardiology have been based on risk assessment equations (67). Clinical practice guidelines, including those from the United States (7), Europe (8,68), and Canada (69), have universally recommended risk factor equations that use office-based measurements of blood lipids, blood pressure, age, smoking history, and presence or absence of diabetes as mainstays of clinical risk assessment. Although it is widely recognized that CAC scoring can improve upon these clinical risk assessments, guidelines have not recommended using risk scores that require CAC testing (Table 2). To date, there is only one risk score that has incorporated CAC into the model and validated the score in external population samples (70). After 10 years of follow-up, McClelland et al. (70) used MESA data to derive and validate a risk score to estimate 10-year CHD risk using CAC plus traditional risk factors. External validation was conducted in the HNR study and the Dallas Heart Study (DHS). Inclusion of CAC results in the MESA risk score offered significant improvements in risk prediction. External validation in HNR demonstrated very good discrimination and calibration. The MESA risk score is available online (71) and via smartphone application, and can be used when communicating risk to patients and when determining risk-based treatment strategies.
Table 2.
Summary of 4 Major Guidelines and Expert Consensus Documents on Use of CAC for Risk Assessment in Asymptomatic Patients
| Guideline/Statement | Summary of CT Recommendations |
|---|---|
| 2013 ACC/AHA Risk Assessment Guideline | If, after quantitative risk assessment using traditional risk factors, a risk-based treatment decision is uncertain, CAC score may be considered to inform treatment decision-making. Class IIb, Level of Evidence: B (7). |
| 2016 European Guidelines on CVD Prevention | CAC scoring may be considered as a risk modifier in CV risk assessment. Class IIb, Level of Evidence: B (8). |
| 2017 Expert Consensus from the Society of Cardiovascular Computed Tomography | It is appropriate to perform CAC testing in the context of shared decision making for asymptomatic individuals without clinical ASCVD who are 40–75 years of age in the 5%–20% ten-year ASCVD risk group and selectively in the <5% ASCVD risk group, such as those with a family history of premature CAD (91). |
| 2018 U.S. Preventive Services Task Force Draft Guideline on Non-Traditional Risk Factors | In asymptomatic adults, the current evidence is insufficient to assess the balance of benefits and harms of adding CAC score to traditional risk assessment for CVD prevention. Class I (123). |
ASCVD = atherosclerotic cardiovascular disease; CT = computed tomography CVD = cardiovascular disease. Other abbreviations as in Table 1.
CAC scoring was also tested in a cardiovascular event prediction model created by machine learning techniques in the MESA dataset (72). The CAC score was the most important predictor of CHD and all ASCVD combined outcomes, improving on more than 700 other baseline variables.
COST-EFFECTIVENESS OF CAC IN PREVENTIVE CARDIOLOGY
Cost-effectiveness of CAC testing in the primary prevention context has been evaluated by several independent groups using generally similar assumptions and simulations (9–14). The datasets for 6 separate cost-effectiveness studies have been based on results from the Rotterdam Study, MESA, and the FHS. Critical factors that drive conclusions in all of the cost-effectiveness studies are the cost of statins and the rating of discomfort or side effects from statins, as well as the general desire to avoid lifelong preventive therapy (“disutility”). With relatively small differences in these inputs into the models, conclusions have varied from “treat all” with statins above a fairly low-risk threshold (10,12) without performing CAC to “perform CAC in all” in selected risk categories and treat with statins if CAC >0 (11,14). In a recent analysis by Hong et al. (14), outcomes were similar between using CT scans for CAC measurement or the 2013 ACC/AHA guidelines (7,73) to guide long-term statin therapy among individuals at intermediate cardiovascular risk. However, fewer patients would be treated using a CAC screening strategy. Hong et al. (14) therefore proposed that cost-effectiveness analyses should not be the only criterion for clinical decision making, but a shared decision-making model is most important for clinicians, patients, and policymakers. Their analysis and previous cost-effectiveness analyses are consistent with the concept that CAC testing represents a reasonable option to risk stratify as well as facilitate shared decision-making without any significant downstream adverse outcomes, loss of quality of life, and/or increased costs (14).
CAC PROGRESSION
As interscan variability of CAC score results is low (~10%), quantitative estimates of CAC progression are possible. MESA reported results of CAC progression in 5,756 participants with an average of 2.4 years between 2 CT scans (74). CAC scores increased by about 20% to 25% per year, and about 20% of individuals with CAC = 0 progressed to CAC >0 within 4 to 5 years. As CAC progression is most strongly predicted by baseline CAC, the distribution of CAC is always heavily right-skewed, underscoring the exponential nature of CAC change over time, which was confirmed by the HNR study (75).
In the HNR study, with CT scans spaced 5 years apart, CAC incidence was identified in a cohort of men and women who had CAC = 0 at the first examination. The probability of incident CAC at 5 years among those with no CAC initially steadily increased with age, from 23% in men 45 to 49 years of age to 67% in the 70 to 74 years of age category. In women, new onset of CAC was seen in 15% (age 45 to 49 years) and 43% (age 70 to 74 years), respectively. Findings were similar after adjusting for traditional risk factors (76).
CAC progression has been associated with higher risk for myocardial infarction and all-cause mortality (77–79). Various studies have used differing algorithms in order to quantify the degree of CAC progression, which may have influenced the different outcomes between studies (80,81). Lehmann et al. (82), in the HNR study, reported a method to differentiate rapid and slow CAC progression compared to an expected and calculated norm. Based on additional HNR data, the prediction of coronary and cardiovascular events was compared for 10 published algorithms (54). Analysis of CAC progression did not add any benefit to risk prediction models that included the most recent CT scan and the most recent follow-up risk factors. The best coronary disease prognosis was found for participants with “double zero,” meaning CAC = 0 both at baseline and at the CT scan 5 years later. This pair was associated with a 10-year risk of only 1.4%, followed by incident CAC after 5 years with a 10-year risk estimate of 1.8%. Therefore, a repeat scan after 5 years seems to be of additional value, except for those who already have a double zero CAC scan or have already been classified as high-risk individuals due to a CAC ≥ 400 (54).
CAC AND PREVENTIVE THERAPIES
Although there is now ample evidence that CAC improves statistical risk reclassification (83,84), that is, modifying risk estimates in individuals free of events into lower-risk categories and those with events into higher-risk categories (net reclassification index), there is also evidence that CAC might directly guide risk-based selection of appropriate preventive therapies (85). MESA investigators studied 950 individuals who met inclusion criteria for the JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) clinical trial (86). Of these “eligible” individuals, 47% had CAC = 0, whereas 25% had CAC >100. Using observed absolute event rates from MESA, coupled with the relative risk reduction observed with rosuvastatin in the JUPITER trial, the 5-year Number Needed to Treat (NNT5) to prevent one cardiovascular event varied from 124 for individuals with CAC = 0, to just 19 for those with CAC >100. Similar analyses demonstrating that CAC might identify those expected to derive both the most and the least net benefit from statin therapy have been performed for patients meeting criteria for any one of the statin clinical trials (87), among elderly patients (88), or for all patients with dyslipidemia (89).
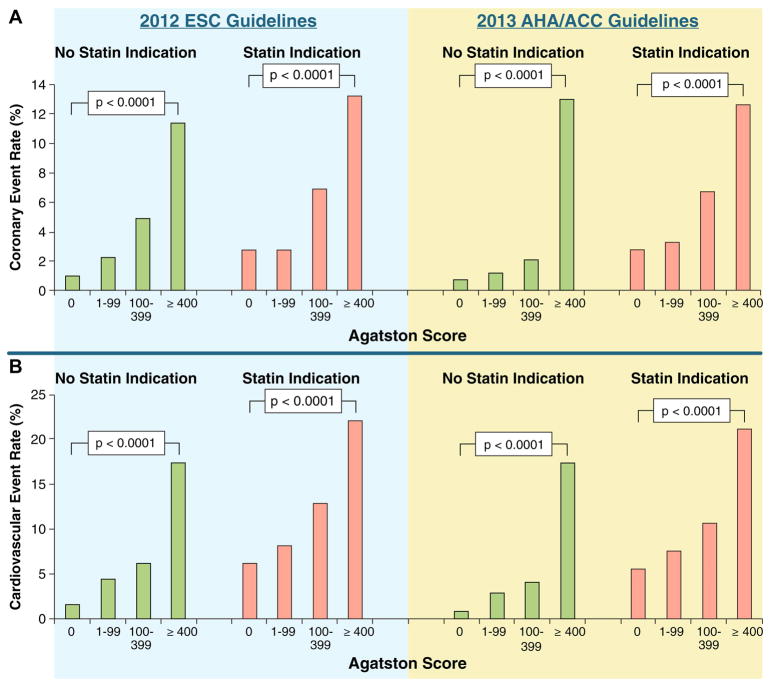
Nasir et al. (90) conducted an analysis of the potential impact of CAC on statin allocation in the context of the 2013 ACC/AHA Cholesterol Treatment guidelines. In MESA participants with a 10-year ASCVD risk of between 5% and 7.5% using the Pooled Cohort Equation (7,90), a finding of CAC = 0 was associated with observed ASCVD event rates below the guideline-recommended treatment threshold of 7.5% (actual event rate: ~1.5%), whereas any CAC >0 was associated with event rates above the accepted threshold for statin benefit. Likewise, in participants with 10-year ASCVD risk between 7.5% and 20%, CAC = 0 was associated with event rates below the guideline-based threshold of statin benefit (~4.5%), whereas any CAC >0 was associated with events consistent with net benefit from statin therapy (~10.5%). In this analysis, CAC had no role in middle-aged adults with a 10-year ASCVD risk >20%. This study directly informed the recommendation by the Society of Cardiovascular Computed Tomography (SCCT) to consider CAC testing, within the context of shared decision-making, in intermediate-risk patients between 40 and 75 years of age with a 10-year ASCVD risk between 5% and 20% (91). Mahabadi et al. (92) conducted a similar analysis relating to the indication for statin therapy using ESC and ACC/AHA guidelines. The CAC score consistently stratified risk of ASCVD events across both statin-recommended and non–statin-recommended groups (Figure 2). Thus, CAC scoring may help to match intensified risk factor modification to atherosclerotic plaque burden as well as actual risk, while avoiding statin therapy in patients with low CAC scores and low 10-year event rates (sometimes called derisking) (92,93).
Figure 2. Cardiovascular Event Rate for HNR Study Participants.
The cardiovascular event rates for participants in the HNR study with and without statin indication according to ESC and ACC/AHA guidelines are shown, stratified by CAC. Group. There figure shows a distinct increase in cardiovascular event rates with increasing CAC score, irrespective of statin indication according to ESC and ACC/AHA guidelines. Reprinted, with permission, from Mahabadi et al. (92). ACC/AHA = American College of Cardiology/American Heart Association; CAC = coronary artery calcium; ESC = European Society for Cardiology; HNR = Heinz Nixdorf RECALL.
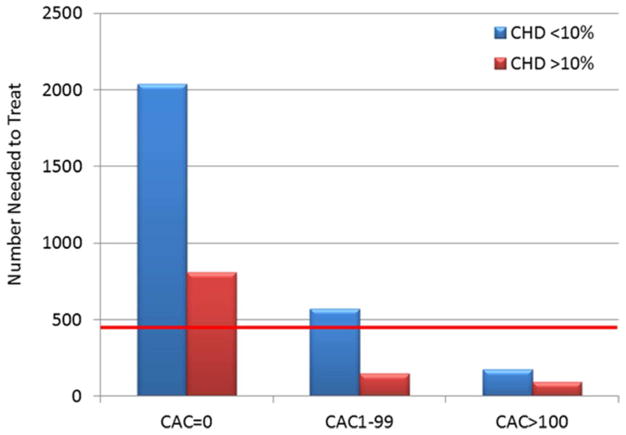
CAC may have also value in the decision to recommend prophylactic daily aspirin. Miedema et al. (94) studied the potential net benefit of aspirin in 4,229 individuals free of diabetes in MESA. This analysis found that there would be a predicted net harm with aspirin therapy when CAC = 0 (number of bleeds exceeding number of ASCVD events prevented), and net benefit of aspirin therapy, regardless of risk factors, in those with CAC >100 (Figure 3). These data also helped inform recent SCCT guidelines recommending consideration of aspirin therapy for all patients with CAC >100 (91).
Figure 3. Estimated Risk/Benefit of Aspirin in Primary Prevention by CAC Score in MESA Participants.
The estimated risk/benefit of aspirin in primary prevention by CAC score in MESA participants is shown. Coronary heart disease (CHD) risk was calculated using the Framingham Risk Score. The red line represents the estimated 5-year number needed to harm based on a 0.23% increase in major bleeding over 5 years. The 5-year number needed to treat estimations are based on an 18% relative reduction in CHD events. In patients with CAC = 0, aspirin was not estimated to be beneficial, regardless of estimated CHD risk. Conversely, when CAC >100, aspirin was estimated to provide net benefit, regardless of CHD risk estimate. Reprinted, with permission, from Miedema et al. (94). CAC = coronary artery calcium; MESA = Multi-Ethnic Study of Atherosclerosis.
With more formal incorporation of absolute risk assessment in future cholesterol and blood pressure guidelines, CAC may take on a greater role in the selection of cholesterol and blood pressure therapeutic targets. For example, individuals with CAC >100 have event rates closer to stable secondary prevention (89), and could benefit from LDL cholesterol goals of <70 mg/dl (95). McEvoy et al. (96) demonstrated that the 10-year NNT of pursuing aggressive blood pressure targets varies considerably by baseline CAC status (NNT10 of 99 for CAC = 0 vs. NNT10 of 24 for CAC >100).
CAC AND CLINICIAN-PATIENT BEHAVIOR
Risk reclassification by any test, including CAC, will only result in clinical benefit if there is an impact on patient or physician behavior. The EISNER study randomized 2,137 volunteers to CAC scanning versus no CAC scan and followed individuals for the 4-year change in risk factors and 10-year estimated risk score (97). In the primary analysis, those randomized to CAC scanning experienced a net favorable change in blood pressure, LDL cholesterol, and waist circumference, along with a lower Framingham Risk Score at the 4-year follow-up. Medical costs in the CAC scanning group were similar to those in the no-scanning group, with decreased costs in those with CAC = 0 balanced by increased spending in those with CAC ≥400 (i.e., closer association between risk and medical expenditure in the CAC scanning arm of the trial) (98).
Observational studies suggested an impact of high CAC score on initiation and continuation of preventive medications (99), and more definitive evidence has been provided by a recent meta-analysis. In a pooled analysis of 6 studies including 11,256 participants, with a mean follow-up time ranging from 1.6 to 6 years, Gupta et al. (100) demonstrated significantly higher odds of aspirin initiation, lipid-lowering medication initiation and continuation, antihypertensive medication initiation, increased exercise, and dietary change in individuals with CAC >0 compared to those with CAC = 0. Findings persisted after adjustment for demographic factors as well as cardiovascular risk factors.
CAC USING NONGATED CHEST CT
Several studies have confirmed a role for identifying CAC on nongated chest CT (34,101). Although a formal quantitative CAC score cannot be obtained from a nongated study, experienced readers can provide a qualitative CAC assessment (none, mild, moderate, severe) that correlates closely with traditional CAC score groups (0, 1 to 100, 101 to 400, >400) (102,103). The role of CAC in nongated chest CT takes on importance with the increasing acceptance of lung cancer screening in those between 55 and 80 years of age who have a 30-pack-year smoking history and who have smoked within the last 15 years. Leigh et al. (104) demonstrated that CAC predicted ASCVD risk in all smokers and in those eligible for lung cancer screening, although the improvement over the risk factor score alone was modest. Recent guidelines from the SCCT/Society of Thoracic Radiology provide a Class I indication for evaluation and reporting of at least qualitative CAC scoring on all noncontrast chest CT examinations (91).
CAC IN SINGLE-PHOTON EMISSION COMPUTED TOMOGRAPHY AND POSITION EMISSION TOMOGRAPHY PERFUSION IMAGING
A limitation of routine stress testing is the reliance on functional data (i.e., evidence of ischemia), with an inability to quantify the anatomic atherosclerosis burden. However, CAC scoring can be added to single-photon emission computed tomography and positron emission tomography myocardial perfusion imaging using hybrid scanners (105). CAC scores obtained from the attenuation scans obtained at the time of perfusion imaging are highly predictive of risk, including in patients for whom there is no evidence of myocardial ischemia (106,107). CAC testing at the time of stress testing can improve assessment of pre-test risk (38), increase interpreter certainty (108), and lead to more risk-based preventive medical decision-making compared with stress testing alone (109).
FUTURE DIRECTIONS–IMPROVING THE CAC SCORE
When a formal approach to CAC scoring was introduced in 1990 (6), little was known about the relationship between calcification, total atherosclerotic plaque, and ASCVD risk. However since then, the understanding of why CAC scoring predicts risk has matured, with increasing attention paid to potential ways to improve the Agatston score. The Agatston score is limited in its assumption that scores should be upweighted with higher calcium density, its failure to capture information about the regional distribution of calcified plaque, and its fixed scanning parameters (120 kV, 3-mm slice thickness) (110). Following evidence demonstrating that low attenuation of a plaque is a high-risk feature, studies have suggested that the Agatston score predicts risk better if it is inversely weighted for calcium density (111). In addition, studies have shown that the regional distribution of CAC (in particular the total number of coronary arteries with CAC) adds prognostic information to the Agatston score, with higher risk in those with more diffuse plaque distributions (112,113).
CAC scoring can be accomplished with little ionizing radiation with simple modifications to the scanning protocol. For example, radiation could be reduced to well below 1 mSv of radiation with use of a lower-energy photon, although CAC scores must then be recalibrated (114). In addition, emerging microcalcification may be detected using thinner slices, allowing detection of higher-risk cases among those with CAC = 0 (115).
The need for a new CAC score is a matter of current debate (116). A new CAC score may potentially incorporate extracoronary calcification, as evidence mounts that aortic valve calcification, aortic calcification, and mitral annular calcification add risk predictive value, particularly from stroke and other cardiovascular outcomes (117).
FUTURE DIRECTIONS–CAC AND NON-CVD RISK
CAC provides a summary measure of atherosclerotic disease, reflecting the cumulative lifetime effect of both measurable (i.e., risk factors) and unmeasurable (i.e., all genetic and environmental factors) risk determinants directly on vulnerable tissue (118). Given its role as a “risk integrator,” there is increasing interest in the role of CAC in predicting non-CVD outcomes. CAC has been shown to predict incident cancer, chronic kidney disease, chronic obstructive pulmonary disease, and hip fracture independent of age, sex, and risk factors (119). CAC has also been shown to be an independent predictor of dementia (120). Ongoing work seeks to clarify the role of CAC in predicting risk of ASCVD versus cancer across the lifespan (121). CAC also appears valuable in identifying long-term “healthy agers” –those surviving into old age with CAC = 0 (122).
CAC AND GUIDELINES (Table 2)
In 2010, the ACC/AHA guidelines on risk prediction in the asymptomatic patient assigned CAC a Class IIA recommendation for intermediate-risk patients, a Class IIB recommendation in low-intermediate–risk patients and advised against CAC testing in very low-risk patients, as defined by the Framingham Risk Score. In 2013, the ACC/AHA guidelines on risk assessment gave CAC a Class IIB recommendation for patients in whom risk or the decision to treat with statins is unclear. Recent 2017 guidelines from the SCCT recommended consideration of CAC testing (equivalent of a Class II recommendation), in the context of shared decision-making, for individuals with a 10-year ASCVD risk of 5% to 20%, or in those with <5% ten-year risk but with another strong indication, such as a family history of premature CAD (91). Other guidelines (8,123) have provided recommendations similar to the 2013 ACC/AHA risk assessment guideline; see Table 2 for summary of recommendations.
CONCLUSIONS
Coronary artery calcification has emerged as the most predictive single cardiovascular risk marker in asymptomatic individuals, capable of adding predictive information beyond the traditional cardiovascular risk factors. CAC scoring appears to be useful for making decisions about preventive statin and/or aspirin use. In most studies, CAC testing has been shown to be cost-effective compared to alternative approaches when factoring in patient preferences about taking preventive medications. In the Central Illustration, we suggest a clinical approach, modified from the ACC/AHA lipid treatment guideline (73) and from Nasir et al. (90,124), incorporating a broader use of CAC testing in selected individuals.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. The Heinz Nixdorf Recall Study acknowledges the role of the German Ministry of Education and Science, Bonn, Germany for their role as an international advisory board, quality control, and event committee.
ABBREVIATIONS AND ACRONYMS
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CT
computed tomography
- CVD
cardiovascular disease
- EBCT
electron-beam computed tomography
- MDCT
multidetector computed tomography
Footnotes
Disclosures: Dr. Budoff has received funding from NIH and General Electric Company. Dr. Blaha has reported membership on Advisory Boards for MedImmune, Akcea, Novartis, Amgen, Sanofi, and Regeneron. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The Heinz Nixdorf Recall Study was funded by The Heinz Nixdorf Foundation; the German Aero-space Center [Deutsches Zentrum für Luft- und Raumfahrt (DLR)], Bonn, Germany; and the German Research Council Assessment. Dr. Blaha has reported grant funding from Aetna Foundation and the Amgen Foundation. The MESA Study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–41. 1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmermund A, Möhlenkamp S, Stang A, et al. Heinz Nixdorf RECALL Study Investigative Group. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Am Heart J. 2002;144:212–8. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 5.Oei HH, Vliegenthart R, Hak AE, et al. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39:1745–51. doi: 10.1016/s0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 6.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Published correction appears in: J Am Coll Cardiol 2014;63:3026] J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Authors/Task Force Members. Piepoli MF, Hoes AW, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur J Prev Cardiol. 2016;23:NP1–NP96. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 9.van Kempen BJ, Spronk S, Koller MT, et al. Comparative effectiveness and cost-effectiveness of computed tomography screening for coronary artery calcium in asymptomatic individuals. J Am Coll Cardiol. 2011;58:1690–701. doi: 10.1016/j.jacc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 10.Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7:276–84. doi: 10.1161/CIRCOUTCOMES.113.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts ET, Horne A, Martin SS, et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the Multi-Ethnic Study of Atherosclerosis (MESA) PloS One. 2015;10:e0116377. doi: 10.1371/journal.pone.0116377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galper BZ, Wang YC, Einstein AJ. Strategies for primary prevention of coronary heart disease based on risk stratification by the ACC/AHA lipid guidelines, ATP III guidelines, coronary calcium scoring, and C-reactive protein, and a global treat-all strategy: a comparative--effectiveness modeling study. PloS One. 2015;10:e0138092. doi: 10.1371/journal.pone.0138092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kempen BJ, Ferket BS, Steyerberg EW, Max W, Myriam Hunink MG, Fleischmann KE. Comparing the cost-effectiveness of four novel risk markers for screening asymptomatic individuals to prevent cardiovascular disease (CVD) in the US population. Int J Cardiol. 2016;203:422–31. doi: 10.1016/j.ijcard.2015.10.171. [DOI] [PubMed] [Google Scholar]
- 14.Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines: a cost-effectiveness analysis. J Am Coll Cardiol Img. 2017;10:938–52. doi: 10.1016/j.jcmg.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed May 24, 2018];Blue Cross Blue Shield CT Providers by State. 2014 Available at: http://www.calciumscan.com/wp-content/uploads/2014/07/Blue-Cross-Blue-Shield-CTA-Providers.pdf.
- 16.Tintut Y, Alfonso Z, Saini T, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–10. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 17.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Hong S, Qian J, Zheng Y, Yang J, Yi Q. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood. 2010;116:210–7. doi: 10.1182/blood-2009-11-255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFκB ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–6. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 20.Bear M, Butcher M, Shaughnessy SG. Oxidized low-density lipoprotein acts synergistically with β-glycerophosphate to induce osteoblast differentiation in primary cultures of vascular smooth muscle cells. J Cell Biochem. 2008;105:185–93. doi: 10.1002/jcb.21812. [DOI] [PubMed] [Google Scholar]
- 21.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 22.Chen NX, Duan D, O’Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21:3435–42. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, Sorribas V, Sharma G, Levi M, Draznin B. Insulin attenuates vascular smooth muscle calcification but increases vascular smooth muscle cell phosphate transport. Atherosclerosis. 2007;195:e65–75. doi: 10.1016/j.atherosclerosis.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–60. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 25.Luo XH, Zhao LL, Yuan LQ, Wang M, Xie H, Liao EY. Development of arterial calcification in adiponectin-deficient mice: adiponectin regulates arterial calcification. J Bone Miner Res. 2009;24:1461–8. doi: 10.1359/jbmr.090227. [DOI] [PubMed] [Google Scholar]
- 26.Detrano R, Markovic D, Simpfendorfer C, et al. Digital subtraction fluoroscopy: a new method of detecting coronary calcifications with improved sensitivity for the prediction of coronary disease. Circulation. 1985;71:725–32. doi: 10.1161/01.cir.71.4.725. [DOI] [PubMed] [Google Scholar]
- 27.Hamby RI, Tabrah F, Wisoff BG, Hartstein ML. Coronary artery calcification: clinical implications and angiographic correlates. Am Heart J. 1974;87:565–70. doi: 10.1016/0002-8703(74)90493-1. [DOI] [PubMed] [Google Scholar]
- 28.Bartel AG, Chen JT, Peter RH, Behar VS, Kong Y, Lester RG. The significance of coronary calcification detected by fluoroscopy. A report of 360 patients. Circulation. 1974;49:1247–53. doi: 10.1161/01.cir.49.6.1247. [DOI] [PubMed] [Google Scholar]
- 29.Rifkin RD, Parisi AF, Folland E. Coronary calcification in the diagnosis of coronary artery disease. Am J Cardiol. 1979;44:141–7. doi: 10.1016/0002-9149(79)90263-7. [DOI] [PubMed] [Google Scholar]
- 30.Detrano R, Salcedo EE, Hobbs RE, Yiannikas J. Cardiac cinefluoroscopy as an inexpensive aid in the diagnosis of coronary artery disease. Am J Cardiol. 1986;57:1041–6. doi: 10.1016/0002-9149(86)90671-5. [DOI] [PubMed] [Google Scholar]
- 31.Margolis JR, Chen JT, Kong Y, Peter RH, Behar VS, Kisslo JA. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. 1980;137:609–16. doi: 10.1148/radiology.137.3.7444045. [DOI] [PubMed] [Google Scholar]
- 32.Mao SS, Pal RS, McKay CR, et al. Comparison of coronary artery calcium scores between electron beam computed tomography and 64-multidetector computed tomographic scanner. J Comput Assist Tomogr. 2009;33:175–8. doi: 10.1097/RCT.0b013e31817579ee. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Nasir K, Kinney GL, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5:113–8. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease [Published correction appears in Radiology 2011;259:617] Radiology. 2010;257:541–8. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 35.Simons DB, Schwartz RS, Edwards WD, Sheedy PF, Breen JF, Rumberger JA. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol. 1992;20:1118–26. doi: 10.1016/0735-1097(92)90367-v. [DOI] [PubMed] [Google Scholar]
- 36.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 37.Rumberger JA, Schwartz RS, Simons DB, Sheedy PF, III, Edwards WD, Fitzpatrick LA. Relation of coronary calcium determined by electron beam computed tomography and lumen narrowing determined by autopsy. Am J Cardiol. 1994;73:1169–73. doi: 10.1016/0002-9149(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 38.Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation. 2002;105:1791–6. doi: 10.1161/01.cir.0000014483.43921.8c. [DOI] [PubMed] [Google Scholar]
- 39.Shavelle DM, Budoff MJ, LaMont DH, Shavelle RM, Kennedy JM, Brundage BH. Exercise testing and electron beam computed tomography in the evaluation of coronary artery disease. J Am Coll Cardiol. 2000;36:32–8. doi: 10.1016/s0735-1097(00)00696-3. [DOI] [PubMed] [Google Scholar]
- 40.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–33. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 41.Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol. 1997;30:57–64. doi: 10.1016/s0735-1097(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 42.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals [Published correction appears in JAMA 2004;291:563] JAMA. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 43.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 44.LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–9. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 45.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 46.McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. J Am Coll Cardiol Img. 2012;5:1037–45. doi: 10.1016/j.jcmg.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw LJ, Raggi P, Callister TQ, Berman DS. Prognostic value of coronary artery calcium screening in asymptomatic smokers and non-smokers. Eur Heart J. 2006;27:968–75. doi: 10.1093/eurheartj/ehi750. [DOI] [PubMed] [Google Scholar]
- 48.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–62. doi: 10.1093/eurheartj/ehs230. [DOI] [PubMed] [Google Scholar]
- 50.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 51.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–7. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 52.Stang A, Moebus S, Dragano N, et al. Heinz Nixdorf Recall Study Investigation Group. Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf Recall Study: identifiability of phone numbers as the major determinant of response. Eur J Epidemiol. 2005;20:489–96. doi: 10.1007/s10654-005-5529-z. [DOI] [PubMed] [Google Scholar]
- 53.Erbel R, Möhlenkamp S, Moebus S, et al. Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann N, Erbel R, Mahabadi AA, et al. Heinz Nixdorf Recall Study Investigators. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR Study (Heinz Nixdorf Recall) Circulation. 2018;137:665–79. doi: 10.1161/CIRCULATIONAHA.116.027034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erbel R, Delaney JA, Lehmann N, et al. Multi-Ethnic Study of Atherosclerosis; Investigator Group of the Heinz Nixdorf Recall Study. Signs of subclinical coronary atherosclerosis in relation to risk factor distribution in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR) Eur Heart J. 2008;29:2782–91. doi: 10.1093/eurheartj/ehn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofman A, Brusselle GG, Darwish Murad S, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol. 2015;30:661–708. doi: 10.1007/s10654-015-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–7. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 58.Yano Y, O’Donnell CJ, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2:986–94. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kavousi M, Desai CS, Ayers C, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA. 2016;316:2126–34. doi: 10.1001/jama.2016.17020. [DOI] [PubMed] [Google Scholar]
- 60.Ferencik M, Pencina KM, Liu T, et al. Coronary artery calcium distribution is an independent predictor of incident major coronary heart disease events: results from the Framingham Heart Study. Circ Cardiovasc Imaging. 2017;10:e006592. doi: 10.1161/CIRCIMAGING.117.006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okwuosa TM, Greenland P, Ning H, Liu K, Lloyd-Jones DM. Yield of screening for coronary artery calcium in early middle-age adults based on the 10-year Framingham Risk Score: the CARDIA study. J Am Coll Cardiol Img. 2012;5:923–30. doi: 10.1016/j.jcmg.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozanski A, Berman DS. Use of coronary artery calcium scanning to screen for coronary atherosclerosis among early middle-age adults. J Am Coll Cardiol Img. 2012;5:931–4. doi: 10.1016/j.jcmg.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Carr JJ, Jacobs DR, Jr, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–9. doi: 10.1001/jamacardio.2016.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah RV, Spahillari A, Mwasongwe S, et al. Subclinical atherosclerosis, statin eligibility, and outcomes in African American individuals: the Jackson Heart Study. JAMA Cardiol. 2017;2:644–52. doi: 10.1001/jamacardio.2017.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sung JH, Yeboah J, Lee JE, et al. Diagnostic value of coronary artery calcium score for cardiovascular disease in African Americans: the Jackson Heart Study. Br J Med Med Res. 2016;11 doi: 10.9734/BJMMR/2016/21449. BJMMR/2016/21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manson JE, Allison MA, Carr JJ, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 2010;17:683–91. doi: 10.1097/gme.0b013e3181d683b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson PW, Castelli WP, Kannel WB. Coronary risk prediction in adults (the Framingham Heart Study) Am J Cardiol. 1987;59:91G–94G. doi: 10.1016/0002-9149(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 68.JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3) Heart. 2014;100(Suppl 2):ii1–ii67. doi: 10.1136/heartjnl-2014-305693. [DOI] [PubMed] [Google Scholar]
- 69.Anderson TJ, Gregoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–82. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 70.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study) J Am Coll Cardiol. 2015;66:1643–53. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MESA. MESA 10-Year CHD Risk with Coronary Artery Calcification. Collaborative Health Studies Coordinating Center; 2018. [Accessed May 23, 2018]. Available at: https://www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. [Google Scholar]
- 72.Ambale-Venkatesh B, Yang X, Wu CO, et al. Cardiovascular event prediction by machine learning: the Multi-Ethnic Study of Atherosclerosis. Circ Res. 2017;121:1092–101. doi: 10.1161/CIRCRESAHA.117.311312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Published corrections appear in: J Am Coll Cardiol 2015;6:2812 and J Am Coll Cardiol 2014;63:3024–5] J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 75.Erbel R, Lehmann N, Churzidse S, et al. Heinz Nixdorf Recall Study Investigators. Progression of coronary artery calcification seems to be inevitable, but predictable - results of the Heinz Nixdorf Recall (HNR) study. Eur Heart J. 2014;35:2960–71. doi: 10.1093/eurheartj/ehu288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehmann N, Möhlenkamp S, Mahabadi AA, et al. Effect of smoking and other traditional risk factors on the onset of coronary artery calcification: results of the Heinz Nixdorf recall study. Atherosclerosis. 2014;232:339–45. doi: 10.1016/j.atherosclerosis.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 77.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–7. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 78.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–9. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. J Am Coll Cardiol Img. 2010;3:1229–36. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 80.McEvoy JW, Blaha MJ, Defilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–22. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 81.Paixao AR, Chakravorty R, Khera A, et al. Disagreement between different definitions of coronary artery calcium progression. J Am Coll Cardiol Img. 2015;8:743–4. doi: 10.1016/j.jcmg.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Lehmann N, Erbel R, Mahabadi AA, et al. Accelerated progression of coronary artery calcification in hypertension but also prehypertension. J Hypertens. 2016;34:2233–42. doi: 10.1097/HJH.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 83.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Möhlenkamp S, Lehmann N, Moebus S, et al. Heinz Nixdorf Recall Study Investigators. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57:1455–64. doi: 10.1016/j.jacc.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 85.Kianoush S, Mirbolouk M, Makam RC, Nasir K, Blaha MJ. Coronary artery calcium scoring in current clinical practice: how to define its value? Curr Treat Options Cardiovasc Med. 2017;19:85. doi: 10.1007/s11936-017-0582-y. [DOI] [PubMed] [Google Scholar]
- 86.Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–92. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mortensen MB, Falk E, Li D, et al. Statin trials, cardiovascular events, and coronary artery calcification: implications for a trial-based approach to statin therapy in MESA. J Am Coll Cardiol Img. 2018;11:221–30. doi: 10.1016/j.jcmg.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mortensen MB, Fuster V, Muntendam P, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage Study. J Am Coll Cardiol. 2016;68:881–91. doi: 10.1016/j.jacc.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 89.Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the Multi-Ethnic Study of Atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015;66:1657–68. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 91.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–68. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 92.Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. J Am Coll Cardiol Img. 2017;10:143–53. [Google Scholar]
- 93.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2016;133:849–58. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–60. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blaha MJ. Personalizing treatment: between primary and secondary prevention. Am J Cardiol. 2016;118:4A–12A. doi: 10.1016/j.amjcard.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 96.McEvoy JW, Martin SS, Dardari ZA, et al. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135:153–65. doi: 10.1161/CIRCULATIONAHA.116.025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaw LJ, Min JK, Budoff M, et al. Induced cardiovascular procedural costs and resource consumption patterns after coronary artery calcium screening: results from the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) study. J Am Coll Cardiol. 2009;54:1258–67. doi: 10.1016/j.jacc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 99.Nasir K, McClelland RL, Blumenthal RS, et al. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: the Multi-Ethnic Study of Atherosclerosis (MESA) Circ Cardiovasc Qual Outcomes. 2010;3:228–35. doi: 10.1161/CIRCOUTCOMES.109.893396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies: a systematic review and meta-analysis. J Am Coll Cardiol Img. 2017;10:833–42. doi: 10.1016/j.jcmg.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie X, Zhao Y, de Bock GH, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–21. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 102.Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11:8–15. doi: 10.1016/j.jcct.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Blair KJ, Allison MA, Morgan C, et al. Comparison of ordinal versus Agatston coronary calcification scoring for cardiovascular disease mortality in community-living individuals. Int J Cardiovasc Imaging. 2014;30:813–8. doi: 10.1007/s10554-014-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leigh AMJ, Garg P, Carr J, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers: MESA. J Am Coll Cardiol Img. 2018 Feb 9; doi: 10.1016/j.jcmg.2017.12.017. E-pub ahead of print. [DOI]
- 105.Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 106.Chang SM, Nabi F, Xu J, et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54:1872–82. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 107.Engbers EM, Timmer JR, Ottervanger JP, Mouden M, Knollema S, Jager PL. Prognostic value of coronary artery calcium scoring in addition to single-photon emission computed tomographic myocardial perfusion imaging in symptomatic patients. Circ Cardiovasc Imaging. 2016;9:e003966. doi: 10.1161/CIRCIMAGING.115.003966. [DOI] [PubMed] [Google Scholar]
- 108.Uretsky S, Cohen R, Argulian E, et al. Combining stress-only myocardial perfusion imaging with coronary calcium scanning as a new paradigm for initial patient work-up: an exploratory analysis. J Nucl Cardiol. 2015;22:89–97. doi: 10.1007/s12350-014-9958-5. [DOI] [PubMed] [Google Scholar]
- 109.Bybee KA, Lee J, Markiewicz R, et al. Diagnostic and clinical benefit of combined coronary calcium and perfusion assessment in patients undergoing PET/CT myocardial perfusion stress imaging. J Nucl Cardiol. 2010;17:188–96. doi: 10.1007/s12350-009-9159-9. [DOI] [PubMed] [Google Scholar]
- 110.Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239:109–17. doi: 10.1016/j.atherosclerosis.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 111.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–8. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol Img. 2016;9:1407–16. doi: 10.1016/j.jcmg.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tota-Maharaj R, Joshi PH, Budoff MJ, et al. Usefulness of regional distribution of coronary artery calcium to improve the prediction of all-cause mortality. Am J Cardiol. 2015;115:1229–34. doi: 10.1016/j.amjcard.2015.01.555. [DOI] [PubMed] [Google Scholar]
- 114.Baron KB, Choi AD, Chen MY. Low radiation dose calcium scoring: evidence and techniques. Curr Cardiovasc Imaging Rep. 2016;9:12. doi: 10.1007/s12410-016-9373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Urabe Y, Yamamoto H, Kitagawa T, et al. Identifying small coronary calcification in non-contrast 0. 5-mm slice reconstruction to diagnose coronary artery disease in patients with a conventional zero coronary artery calcium score. J Atheroscler Thromb. 2016;23:1324–33. doi: 10.5551/jat.35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: is it time for a change in methodology? J Am Coll Cardiol Img. 2017;10:923–37. doi: 10.1016/j.jcmg.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 117.Tison GH, Guo M, Blaha MJ, et al. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9:406–14. doi: 10.1016/j.jcct.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: Clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging. 2014;7:398–408. doi: 10.1161/CIRCIMAGING.113.000341. discussion 408. [DOI] [PubMed] [Google Scholar]
- 119.Handy CE, Desai CS, Dardari ZA, et al. The association of coronary artery calcium with noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol Img. 2016;9:568–76. doi: 10.1016/j.jcmg.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujiyoshi A, Jacobs DR, Jr, Fitzpatrick AL, et al. Coronary artery calcium and risk of dementia in MESA (Multi-Ethnic Study of Atherosclerosis) Circ Cardiovasc Imaging. 2017;10:e005349. doi: 10.1161/CIRCIMAGING.116.005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blaha MJ, Whelton SP, Al Rifai M, et al. Rationale and design of the Coronary Artery Calcium Consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11:54–61. doi: 10.1016/j.jcct.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. J Am Coll Cardiol Img. 2015;8:1393–400. doi: 10.1016/j.jcmg.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 123.U.S. Preventive Services Taks Force. [Accessed May 23, 2018];Draft Recommendation Statement: Cardiovascular Disease: Risk Assessment With Nontraditional Risk Factors. 2018 Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/coronary-heart-disease-screening-using-non-traditional-risk-assessment.
- 124.Pender A, Lloyd-Jones DM, Stone NJ, Greenland P. Refining statin prescribing in lower-risk individuals: informing risk/benefit decisions. J Am Coll Cardiol. 2016;68:1690–7. doi: 10.1016/j.jacc.2016.07.753. [DOI] [PubMed] [Google Scholar]
- 125.O’Donnell CJ, Kavousi M, Smith AV, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation. 2011;124:2855–64. doi: 10.1161/CIRCULATIONAHA.110.974899. [DOI] [PMC free article] [PubMed] [Google Scholar]