Abstract
Introduction
Animal models have been vital for scientific discovery but have limitations, especially in infectious disease research. It is essential to develop a means to study these diseases in human models. We hypothesized that altruistic people would willingly participate in research near the end-of-life (EOL), for the benefit of science and to provide one last gift to society.
Methodology
Two surveys were administered to 377 self-reported HIV-negative and 96 HIV-positive individuals. Hypothetical questions assessed their willingness to participate in altruistic research in the last 6 months of life, which might result in a shortened lifespan or physical discomforts. The self-reported HIV-negative group was also asked about willingness to be exposed to infectious pathogens for the sake of research.
Results
Almost all responders expressed willingness to participate in research at the EOL, regardless of HIV-status. The majority of participants were willing to endure physical discomfort for the sake of research. ‘Blood draws’ was identified as the most tolerable physical discomfort (>70% in both groups). In both groups, >60% were willing to shorten their lifespans for the sake of research. A third of the self-reported HIV-negative group expressed willingness to be exposed to at least one infectious agent to participate in EOL research.
Conclusions
Our exploratory study demonstrates that people would welcome the opportunity to participate in altruistic research near the EOL. Such research could greatly impact the way infectious disease research is conducted. This study is limited however by its hypothetical nature. Further research is necessary to confirm this interest in those with terminal illness before any further clinical research effort at the EOL can be performed.
Introduction
In 1937, “Elixir Sulfanilamide”, which contained diethylene glycol, poisoned over a hundred people [1]. In 1938, the Federal Food, Drug and Cosmetic Act was passed requiring safety testing of drugs on animals before they could be marketed [2]. If all goes well, research can eventually progress to humans. As noted by Richard Klausner, former director of the National Cancer Institute, “The history of cancer research has been a history of curing cancer in the mouse. We have cured mice of cancer for decades—and it simply didn’t work in humans.” Furthermore, a 2006 news release by the U.S. Food and Drug Administration (FDA) stated “Nine out of ten experimental drugs fail in clinical studies because we cannot accurately predict how they will behave in people based on laboratory and animal studies.” This is particularly exemplified in Human Immunodeficiency Virus (HIV) research. When HIV was first isolated, mice, rats and rabbits were used for all experiments, but these animals could not be infected with HIV [3]. Eventually, in 1985 the Simian Immunodeficiency Virus (SIV) was isolated from a chimpanzee [4]. Non-human primates, like chimpanzees and rhesus macaques provide a useful animal model for the development of a wide variety of antiretroviral drug testing [5]. Even primate models have limitations and they differ from humans in achievable levels of viremia, rates of disease progression, resistance patterns, immune responses, amongst others [6]. For example, the Merck adenovirus type 5 (Ad5) trivalent HIV-1 vaccine trial (STEP trial) did not show efficacy in preventing HIV infection or even slowing disease progression [7], despite promising results in various macaque studies [8–10]. In fact, the trial had to be stopped in September 2007 when an independent panel of experts’ review revealed that the incidence of infection was lower in placebo recipients who had higher levels of Ad5 immunity compared to vaccine recipients [7]. This serves as an example of how divergent animal models can be from human reality.
To date, cancer research has been at the forefront, actively and successfully enrolling participants in Phase I clinical trials that usually harbor considerable risk to the individual [11]. Although there is always hope for tumor response and regression, that is not the primary objective of these trials. The primary objective is to understand pharmacokinetics and drug toxicity [12–15] so investigational interventions can be advanced for further testing.
Researchers have found that some individuals with terminal cancer are willing to participate in research at the End of Life (EOL), even if it had no chance of helping their underlying illness [16]. No such research has yet been conducted concerning infectious disease [17]. Many people are naturally altruistic, and perhaps would take part in a research study that may not have any benefit to them, but may benefit their friends, family or mankind. This altruism may be more acute near the EOL, because it could serve as a last meaningful contribution to society-at-large. Providing those who are terminally-ill the opportunity to participate in clinical research could revolutionize the way therapeutics move from the bench-to-clinic by offering a large supply of well-informed, eager and appropriately consented human volunteers [17].
Methods
Study design
This study was comprised of two anonymous surveys. The first was administered to individuals 18 years and older who were self-reported HIV-negative, and the second was administered to self-reported individuals living with HIV. We assessed these two study populations to evaluate EOL research attitude in both the general population and a specific population (i.e. people living with HIV).
The survey with HIV-negative respondents was administered over a period of three months. Participants were recruited online through Amazon’s Mechanical Turk (MTurk) (https://www.mturk.com/mturk/welcome) from all over the United States (non-clinical sample). This platform is commonly used to assess the attitude of the general population and has been validated for this purpose [18,19]. There was a $0.50 monetary compensation provided for participation, which is the common compensation for research studies on MTurk platform.
The survey with people living with HIV was administered from the University of California San Diego’s Owen Clinic. All of the UCSD participants were approached in person with the survey on an anonymous website managed by Qualtrics LLC (https://www.qualtrics.com/). If a participant chose to take the survey, he/she answered questions directly on an iPad, blinded to the recruiter. There was no monetary compensation provided to the HIV-positive group. This group was evaluated for insight into whether an underlying infectious condition or chronic disease would impact attitudes towards EOL research. The proposed study was designed to establish a foundational framework for the next steps in HIV clinical research at the EOL. Based on these results, similar surveys could be developed and implemented for people who are terminally ill with and without HIV.
Surveys
Both surveys gathered demographic data (e.g. age, gender-identity, race/ethnicity, marital status, household income, education, religious affiliation and current perception of health). Details regarding HIV/AIDS status, time of diagnosis, viral load and CD4+ counts were collected from the HIV-positive group. Participants were asked a series of questions regarding their willingness and desire to participate in scientific research that would not benefit them directly but could contribute to the advancement of medical research. They were asked what physical discomforts they would be willing to endure, including but not limited to nausea, vomiting, diarrhea or headaches.
The self-reported HIV-negative participants were asked if they would be willing to be exposed to and infected with pathogens like Streptococcus, Malaria, HIV or Hepatitis C near the EOL, for the sole purpose of research. Responders could select as many or as few of the options provided. All participants were asked if they would consider participating in this research if it had the potential of shortening their lifespans further, and if so, by how long.
Statistical analysis
All statistical analyses were performed using SAS 9.4. Univariate analyses were performed to compare the demographic characteristics between HIV-positive and self-reported negative groups, using Fischer’s Exact Test. We did not test group differences in attitudes about participating in research because the HIV-positive and HIV—negative groups were different in regard to demographics and other characteristics (Table 1).
Table 1. Characteristics of HIV negative and positive groups.
| Demographics n (%) |
p Value | |||
|---|---|---|---|---|
| HIV- (n = 377) |
HIV+ (n = 96) |
|||
| Gender | Male | 182 (48.3) | 84 (88.4) | < .001 |
| Female | 192 (50.9) | 6 (6.3) | ||
| Transgender/Genderqueer | 3 (0.8) | 5 (5.3) | ||
| Age | 18–24 years | 61 (16.2) | 1 (1.0) | < .001 |
| 25–44 years | 217 (57.6) | 30 (31.3) | ||
| 45–64 years | 85 (22.5) | 54 (56.3) | ||
| 65+ years | 14 (3.7) | 11 (11.5) | ||
| Income | $0 - $25,000 | 106 (30.0) | 67 (70.5) | < .001 |
| $25,001 - $50,000 | 105 (29.7) | 18 (18.9) | ||
| $50,001 - $75,000 | 75 (21.2) | 5 (5.3) | ||
| $75,001 - $100,000 | 25 (7.1) | 3 (3.2) | ||
| >$100,000 | 42 (11.9) | 2 (2.1) | ||
| Education | < HS or HS/GED | 40 (11.3) | 24 (25.3) | < .001 |
| At Least Some College | 230 (65.2) | 65 (68.4) | ||
| Masters/Advanced Degree | 83 (23.5) | 6 (6.3) | ||
| Race/ethnicity | White | 271 (77.0) | 38 (39.6) | < .001 |
| Hispanic | 23 (6.5) | 27 (28.1) | ||
| Other | 58 (16.5) | 31 (32.3) | ||
| Marital status | Single | 150 (42.3) | 55 (57.3) | < .001 |
| Married, no children | 44 (12.4) | 12 (12.5) | ||
| Married, w/ children | 84 (23.7) | 3 (3.1) | ||
| Divorced, widowed, or separated | 27 (7.6) | 12 (12.5) | ||
| Living w/partner | 50 (14.1) | 14 (14.6) | ||
| Religion | Not religious | 209 (58.9) | 45 (47.9) | < .001 |
| Catholic | 39 (11.0) | 28 (29.8) | ||
| Protestant | 50 (14.1) | 7 (7.4) | ||
| Other | 57 (16.1) | 14 (14.9) | ||
| Number of children | No children | 228 (65.5) | 74 (77.1) | 0.085 |
| 1 child | 41 (11.8) | 4 (4.2) | ||
| 2 children | 40 (11.5) | 10 (10.4) | ||
| 3+ children | 39 (11.2) | 8 (8.3) | ||
| Health status | Healthy | 318 (91.9) | 76 (79.2) | < .001 |
| Sick, not terminal | 27 (7.8) | 20 (20.8) | ||
| Terminally Ill | 1 (0.3) | 0 (0) | ||
HS = High School; GED = General Equivalency Diploma. Group differences for all variables were assessed using the Fischer’s Exact Test. Percentages are based on the number of participants who indicated a specific response divided by the number of participants who responded to the item in question.
Results
Ethics statement
The study was approved by the Institutional Review Board at the University of California San Diego. All adult participants (age ≥ 18 years) provided written informed consent. No children were included in this study.
Participant characteristics
Self-reported HIV-negative group (non-clinical sample)
Of the 377 eligible participants in the self-reported HIV-negative group, 50.9% (n = 192) were female, and 3 identified as transgender. Over half the participants (57.6%) were aged between 25 and 44 years, while 16.2% were between ages 18–24, 22.5% between 45–64 and only 3.7% were over the age of 65. Ethnically, this group identified predominantly as non-Hispanic Caucasian (77.0% n = 271). The HIV-negative group was highly educated with 65.2% (n = 230) having at least started college and another 23.5% having completed an advanced degree. Further, 40.2% (n = 142) reported an annual salary of >$50,000 and 30.0% reported an annual income between $0–25,000. With regards to marital status, half were married or in a relationship (50.1%, n = 178). Concerning religion affiliation, 58.9% (n = 209) of HIV-negative participants self-identified as “nonreligious”. From the respondents of a practicing faith, 14.1% (n = 50) were Protestant, 11.0% (n = 39) Catholic and the remaining were practicing Buddhists, Hindus, Muslims, Jews and/or Other. Concerning heath perception, 7.8% of participants reported feeling ‘sick but not terminally-ill’ (Table 1).
HIV-positive group
Of 119 individuals in the HIV-positive group, 15 surveys were excluded due to participants’ acquittal mid-administration and another 6 because the individuals reported not being HIV-infected. Of the remaining 96 participants, the majority (88.4%, n = 84) were male, 4 identified as transgender and one as genderqueer. Regarding age, over 56.3% responders (n = 54) in the HIV-positive group were between the ages of 45–64, 31.3% between the ages of 25–44 years, and only one participant was in the younger age group. Participants were ethnically diverse with 39.6% (n = 38) identifying as non-Hispanic Caucasian, 28.1% as Hispanic, and remaining as another race/ethnicity or more than one race/ethnicity. In terms of education, 68.4%, (n = 65) participants had completed college or some college, 25.3% had completed high school or GED, and 6.3% attained an advanced degree. Most participants (70.5%, n = 67) in the HIV-positive group reported an annual income between $0–25,000. There were 47.9% (n = 45) who identified as non-religious. Concerning heath perception, 20.8% of participants reported feeling ‘sick but not terminally ill’ (Table 1).
Comparing HIV-positive and self-reported HIV-negative group demographics
There was a considerably higher proportion of male respondents in the HIV-positive group (88.4% vs. 48.3%; P<0.001). This difference is consistent with previously established HIV demographics within San Diego County [20] and the United States. The HIV-positive group was older with most participants being between 45–64 (56.3% vs. 22.5%; P<0.001). The HIV-negative participants were more likely to have advanced degrees compared with the HIV-positive group (23.5% vs. 6.3%; P<0.001). Annual income was significantly lower in the HIV-positive group with 70.5% earning ≤$25,000, compared with 70.0% of HIV-negatives earning >$25,000 (P<0.001). There was no significant difference in the number of children between the two groups (P = 0.085). Notably, HIV-positive participants were more likely than HIV-negatives to report ‘feeling sick but not terminally-ill’ (20.8% vs. 7.8%; P = 0.001) when asked about health status.
Willingness to participate in research at the EOL
The overwhelming majority of HIV-negative and HIV-positive respondents reported that they would participate in various types of research studies if they were terminally ill (Table 2). Specifically, 90.8% (n = 316) of HIV-negative and 82.3% (n = 79) of HIV-positive participants demonstrated willingness to participate in EOL research; 95.1% (n = 331) of HIV-negative respondents would participate to help a friend or a relative; while 89.6% (n = 86) of HIV-positive individuals would participate to help find a cure for HIV/AIDS. Finally, 71.3% (n = 67) of HIV-positive and 41.3% (n = 145) of HIV-negative respondents would participate in research that involved receiving an experimental and likely high-risk intervention to help find a cure for HIV/AIDS, if they had less than six months to live.
Table 2. Attitudes toward research participation by HIV status.
|
Research Participation Attitudes n (%) |
||
|---|---|---|
|
HIV- (n = 377) |
HIV+ (n = 96) |
|
| Willing to participate in research if terminally ill | 316 (90.8) | 79 (82.3) |
| If terminally ill, willing to participate in research that would help | ||
| find a cure for HIV/AIDS | 86 (89.6) | |
| a friend or relative, but not self | 331 (95.1) | |
| Willing to participate in hazardous-intervention research if it might help find a cure for HIV/AIDS | 145 (41.3) | 67 (71.3) |
| Willing to endure | ||
| Blood Draws | 297 (78.8) | 81 (84.4) |
| Diarrhea | 189 (50.1) | 41 (42.7) |
| Nausea | 171 (45.4) | 39 (40.6) |
| Vomiting | 108 (28.6) | 25 (26.0) |
| Intramuscular Injection | 182 (48.3) | 38 (39.6) |
| Intravenous Injection | 210 (55.7) | 47 (49.0) |
| Headache | 220 (58.4) | 39 (40.6) |
| Fever | 138 (36.6) | 33 (34.4) |
| Willing to shorten lifespan by | ||
| < = 4 weeks | 194 (51.4) | 38 (39.6) |
| >4 weeks | 67 (17.8) | 30 (31.2) |
| Unwilling to shorten lifespan | 116 (30.8) | 28 (29.2) |
| Willing to donate organs to research after death | 299 (86.2) | 71 (75.5) |
| Willing to risk exposure to | ||
| Strep Throat | 118 (33.6) | |
| Malaria | 79 (22.5) | |
| Hepatitis C | 99 (28.2) | |
| HIV | 105 (29.9) | |
Respondents reported varying degrees of willingness to endure certain discomforts as part of a research study: 84.4% (n = 81) of HIV-positive and 78.8% (n = 297) of HIV-negative participants would endure blood draws; 42.7% (n = 41) of HIV-positive and 50.1% (n = 189) of HIV-negative participants would endure mild diarrhea; 40.6% (n = 39) of HIV-positive and 45.4% (n = 171) of HIV-negative participants would endure mild nausea; 26.0% (n = 25) of HIV-positive and 28.6% (n = 108) of HIV-negative participants would endure vomiting; 39.6% (n = 38) of HIV-positive and 48.3% (n = 182) of HIV-negative participants would endure intramuscular injections; 49.0% (n = 47) of HIV-positive and 55.7% (n = 210) of HIV-negative participants would endure intravenous injections; 40.6% (n = 39) of HIV-positive and 58.4% (n = 220) of HIV-negative participants would endure mild headaches; and 34.4% (n = 33) of HIV-positive and 36.6% (n = 138) of HIV-negative participants would endure fever (Table 2).
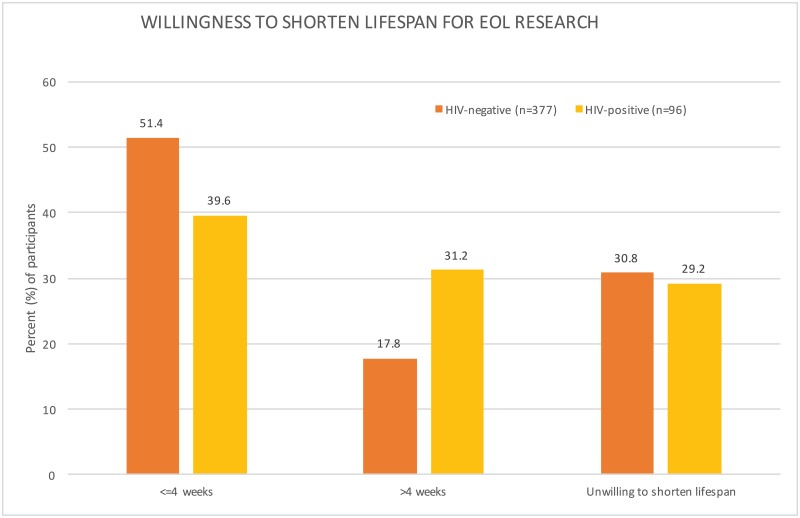
Interestingly, a majority of respondents were willing to shorten their lives by participating in research that would help advance HIV cure research and to donate their organs upon death. Specifically, 39.6% (n = 38) of HIV-positive and 51.4% (n = 194) of HIV-negative respondents would be willing to decrease their lifespans by up to 4 weeks and 31.2% (n = 30) of HIV-positive and 17.8% (n = 67) of HIV-negative respondents would decrease their lives by more than 4 weeks (Fig 1). Furthermore, 75.5% (n = 71) of HIV-positive and 86.2% (n = 299) of HIV-negative participants reported that they would be willing to donate parts of their body to help advance science.
Fig 1. Willingness to shorten lifespan for end of life research.
Our surveys found that 69.2% (n = 68) of HIV-positive and 70.8% (n = 261) of HIV-negative participants were willing to shorten their lifespans for the sake of end of life research. A higher proportion (31.2%) of the HIV-positive group was willing to donate >4 weeks of their lives. About a third of respondents in both groups stated that they were unwilling to shorten their lifespans for the sake of research.
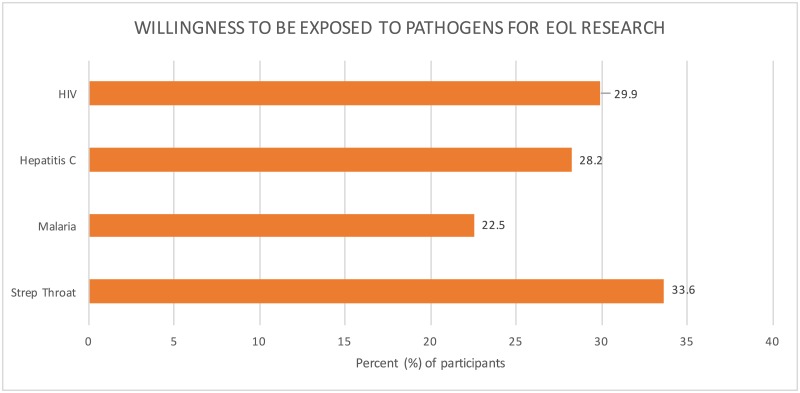
Finally, many HIV-negative respondents reported that they would be willing to risk exposure to an infectious agent for the sake of research. Specifically, 33.6% (n = 118) would risk exposure to strep throat, 22.5% (n = 79) to malaria, 28.2% to Hepatitis C (n = 99), and 29.9% (n = 105) to HIV to help advance infectious disease research (Fig 2).
Fig 2. Willingness to be exposed to infectious pathogens for end of life research.
Our surveys found that around one-third of the HIV-negative group was willing to be exposed to and infected by an infectious pathogen for the sake of research. Participants were most willing to be exposed to streptococcus (33.6%. n = 118) infections, followed by HIV (29.9%, n = 105).
Discussion
Biomedical research is available at the EOL, but mostly for the purposes of alleviating suffering, gaining more time, or trying last ditch interventions to cure illnesses [21,22]. Such studies are mostly conducted in the setting of oncology [23]; these studies are crucial and should not be replaced by other research efforts. There are people, however who do not qualify for, want, or have the opportunity for research in this setting. Instead, when faced with their approaching mortality, such individuals may be willing to participate in research that offers no hope for their condition but that provides one final gift to society. To explore this, we conducted two surveys aimed at understanding hypothetical willingness to participate in clinical research in the last few months of life, aimed at both the general population and separately at the population living with HIV, as a representative of a special population with an incurable chronic infectious disease.
This study provided important observations. First, both HIV-positive and self-reported HIV-negative individuals expressed considerable willingness to participate in infectious disease clinical research at the EOL, even if this research had no relevance to their terminal condition (both >80%). Second, there was a high rate of willingness to donate their body parts for research purposes after death (both >75%). Third, respondents in both groups were hypothetically willing to participate in such research, even if it involved physical discomforts (>70% were willing to endure blood draws and >60% were willing to endure intravenous injections), or even if it reduced their lifespans (both > 50%). Interestingly, HIV-positive participants were more likely to be willing to sacrifice >4 weeks of life to research, compared to the self-reported HIV-negative group. Similarly, another recent study found that HIV-infected people who perceived themselves as ‘not very healthy/not at all healthy’ were significantly more likely to be willing to participate to HIV cure research compared to otherwise healthy chronically HIV-infected individuals, suggesting that people might be more likely to participate when they become (or perceive themselves as) sick [24]. Finally, a high percentage of HIV-negative individuals expressed a willingness to be infected by disease pathogens in the setting of EOL research (i.e. >30% of respondents would allow exposure to HIV, HCV, malaria and/or strep throat). While this last point was provocative, it demonstrates a generally positive attitude and willingness to endure some level of discomfort for altruistic reasons. All of these factors were influenced by various socio-demographic factors, but there were no clear indicators that certain groups would not uniformly participate in the hypothetical infectious disease clinical research at the EOL.
In particular, HIV cure-related research with individuals who are terminally ill could represents a new scientific area, which has the potential to significantly advance the field. For example, by collecting tissue samples from multiple organs at the time of death, we will be able to understand how the virus distributes within the human body and how these tissue reservoirs correlate with each other and with blood reservoirs before death. It also provides an opportunity to understand ethical issues associated with this type of research. As with any clinical research, scientists are expected to obey basic ethical principles, such as beneficence (doing good), non-maleficence (minimizing or preventing harm), autonomy (ensuring informed consent free of coercion), and justice (ensuring the fair distribution of risks and benefits), as articulated by Beauchamp and Childress [25][26]. Additional ethical principles include social value and scientific validity of research, independent review of research, and respect for potential and enrolled participants [27][28].
Currently, there are very few opportunities for terminally-ill people to participate in clinical research, due to various cultural taboos and ethical concerns, such as vulnerability, coercion and exploitation [29]. Vulnerability refers to “increased potential that one’s interests cannot be protected” [30]. Authors have argued against categorizing people at the EOL as being inherently vulnerable [30], and favor describing special protective measures that should be in place given the reality of each clinical study protocol [31]. Coercion is a relational concept that refers to a “credible and irresistible force exerted by one person that negatively limits the option of another person” [30]. Coercion undermines autonomy and can be avoided by clearly demarcating clinical research from medical care at the EOL [32]. Exploitation is an “unfair distribution of the benefits and burdens from a transaction” and must be addressed at the level of the institutional review board overseeing the clinical research [30].
Some may argue that it is unethical to engage people at the EOL in clinical trials [2,33]. However, others feel, along with the principle of representational and distributive justice, that it would be unethical and unjust to withhold this opportunity from them, as long as they have the capability for consent [34]. Informed consent is determined by three fundamental conditions: intentionality, clear understanding and lack of controlling influence [35]. When associated with HIV cure-related research, informed consent must be robust and clearly state that participants will not be cured from HIV from participating in research, but are contributing to creating generalizable knowledge that will benefit future patients and society [28][36][37][38].
Recruiting people for cure studies has been a subject of significant controversy for years [12,15,39,40]. Critics charge that a “conflict of interest” will always exist between the researcher-physician wanting to make scientific strides and the patient-participant hoping for a miraculous treatment or cure [12,15,39,40]. The main critique, which is one of great import, is that this conflict will diminish a participant’s ability to give true informed consent. Prior studies have in fact shown that despite informed consent, participants in these clinical trials hold on to hope for therapeutic benefit, a phenomenon coined “therapeutic optimism” in the literature [41–43]. Other scholars disagree with this claim, citing that hope does not, in itself, compromise informed consent [44]. We would argue that EOL studies are actually if anything devoid of the risk for “therapeutic optimism”, as they do not offer any hope of clinical improvement. They do allow interested parties however the choice of participating in a study that could improve the life of another, and in that, provide a Last Gift to the society. In this sense, participants must understand that they enter into a ‘gifting relationship’ with the research team, future patients and the HIV cure research community [16].
Denying individuals the opportunity to participate in research at the EOL based on a general assumption of vulnerability is simplistic. It undermines their autonomy and right to self-determination and does not take the diversity of the population into account [34]. A recent review concerning EOL research and its impact on participants, demonstrated that the ethical concerns regarding research participation at EOL are often unjustified [34]. Of course, these studies require careful design and execution that incorporates sensitivity to participants’ needs, concerns and preferences, as well as observance of all legal requirements [45][46][47]. The research that an individual engages in should be tailored ethically and specifically to that participant’s illness and expectations [45]. Interestingly, health professionals have been found to be the most likely to raise concerns regarding involving participants at the EOL in research, while patients themselves were generally willing to contribute to research and reported this engagement as a positive experience [34].
Another argument against research of this nature could be that terminally ill individuals have traditionally been hard to recruit and retain in studies aimed to better their current condition [30,32,48–50]. However, and as our study shows, with 95.1% of HIV-negative respondents willing to participate in EOL research to help a friend or relative, and 89.6% of those with HIV to help find a cure for HIV/AIDS, altruism may be a better motivator than self-gain for those approaching the EOL [51]. Altruism could outweigh the discomfort or uneasiness that participating in a clinical trial may bring [52]. Another instance is a mother with muscular dystrophy dying of cancer who wants to participate in a clinical trial on muscular dystrophy to possibly improve the quality of life for her son, who also has the disease. Similarly, a person living with HIV might be willing to participate to HIV-research at the EOL to help his/her infected partner or peers. This is analogous to the early HIV epidemic when thousands of gay men and other affected community members enrolled in research at enormous self-sacrifice, with little or no personal benefit [53]. Those same altruistic individuals have often been denied (for various reasons) the opportunity to contribute to HIV research in recent decades.
There will be various obstacles that this type of research will encounter, but we argue that these barriers can be overcome by understanding and addressing the wants and needs of the participant population in due course.
This study has a number of limitations. First and foremost, the survey information presented in this study was not collected from people at their EOL, but rather from two convenience groups recruited through MTurk and our local HIV clinic in San Diego. People may feel very differently about research participation when they actually have a terminal illness with only six months left to live and our results may not be generalizable across larger populations. Self-report might not be predictive of future behavior. However, websites (as MTurk) are widely used in behavioral research and offer a useful platform that provides convenient access to a large set of people who are willing to undertake tasks at a relatively low cost [54]. We also previously performed unstructured interviews of 12 people receiving hospice services. All of these individuals expressed a desire to be able to ‘give back’ in some way, especially at this time near the EOL [17]. Further, the sample size was relatively small which might have limited our ability to perform stratified analysis. Another key limitation is that the differences between recruitment methods, groups and sample sizes precluded statistical comparisons between people living with HIV and people not living with HIV and the paper mostly focused on descriptive data. Lastly, generalizability of findings from research at EOL might not be directly applicable to the general population (e.g. efficacy of strategies to cure HIV might be very different at the EOL compared to otherwise healthy HIV-infected people).
We call for a broad, frank, and pragmatic discussion about research near the EOL, which may represent a new, innovative paradigm in how we conduct research with human participants (“less to lose” versus “otherwise healthy volunteers”). In this dialogue, we envision that cultural, ethical and legal challenges can be resolved, and that research safeguards can be developed. Research in this setting offers a valuable alternative to animal testing which is more generalizable to the human condition, and allows those who are dying one more chance to give to future generations. We must tackle the cultural, ethical and infrastructure barriers that prevent people from participating in clinical research at the EOL to help overcome many of the important health challenges of our day. To address this issues, we propose the following plan of action: 1) Perform a similar survey among people who are at the EOL; 2) Consider all ethical aspects for research at the EOL (in particular the role of informed consent, autonomy, altruism and vulnerability in the decision making process); 3) Recognize the role of family members and other stakeholders in decisions to participate in research at the EOL; 4) Consider all practical aspects of research at the EOL so that it can be done effectively and ethically; 5) Take into account values regarding quantity versus quality of life at each step of this process.
Acknowledgments
We are grateful to all the study participants and to Christy Anderson and Florin Vaida for their invaluable help and input with the statistical analysis.
Data Availability
All data are presented in the paper.
Funding Statement
This work was supported primarily by a grant from the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research, P30-AI027763 (CNIHR), California HIV Reserch Program Ideal award to Sara Gianella, by the department of Veterans Affairs, the James B. Pendleton Charitable Trust and additional grants from the National Institutes of Health: AI100665, MH100974, MH097520, DA034978, AI007384, AI027763, AI106039, AI43638, AI074621, AI036214, MH101012, UL1TR000100, CARE U19 AI096113 and AI068636-09. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hajar R. Animal testing and medicine. Heart Views. 2011. January;12(1):42 10.4103/1995-705X.81548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Act of June 25, 1938 (Federal Food, Drug, and Cosmetic Act), Public Law 75–717, 52 STAT 1040, which prohibited the movement in interstate commerce of adulterated and misbranded food, drugs, devices, and cosmetics. 1938 p. 75–717.
- 3.Dezzutti CS. Animal and human mucosal tissue models to study HIV biomedical interventions: can we predict success? J Int AIDS Soc. 2015;18:20301 10.7448/IAS.18.1.20301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel M, Letvin N, King N, Kannagi M, Sehgal P, Hunt R, et al. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science (80-). 1985;228(4704):1201–4. [DOI] [PubMed] [Google Scholar]
- 5.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012. December 16;10(12):852–67. 10.1038/nrmicro2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Policicchio BB, Pandrea I, Apetrei C. Animal Models for HIV Cure Research. Front Immunol. 2016. January 28;7:12 10.3389/fimmu.2016.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald DW, Janes H, Robertson M, Coombs R, Frank I, Gilbert P, et al. An Ad5-Vectored HIV-1 Vaccine Elicits Cell-mediated Immunity but does not Affect Disease Progression in HIV-1–infected Male Subjects: Results From a Randomized Placebo-Controlled Trial (The Step Study). J Infect Dis. 2011. March 15;203(6):765–72. 10.1093/infdis/jiq114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001. April 6;292(5514):69–74. [DOI] [PubMed] [Google Scholar]
- 9.Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000. October 20;290(5491):486–92. [DOI] [PubMed] [Google Scholar]
- 10.Shiver JW, Fu T-M, Chen L, Casimiro DR, Davies M-E, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002. January 17;415(6869):331–5. 10.1038/415331a [DOI] [PubMed] [Google Scholar]
- 11.Roberts TG, Goulart BH, Squitieri L, Stallings SC, Halpern EF, Chabner BA, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004. November 3;292(17):2130–40. 10.1001/jama.292.17.2130 [DOI] [PubMed] [Google Scholar]
- 12.Joffe S, Miller FG. Rethinking risk-benefit assessment for phase I cancer trials. J Clin Oncol. 2006. July 1;24(19):2987–90. 10.1200/JCO.2005.04.9296 [DOI] [PubMed] [Google Scholar]
- 13.Freireich EJ. Ethical considerations in cancer chemotherapy. Annu Rev Pharmacol Toxicol. 1979. April;19(1):547–57. [DOI] [PubMed] [Google Scholar]
- 14.Pratt CB. The conduct of phase I-II clinical trials in children with cancer. Med Pediatr Oncol. 1991;19(4):304–9. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal M, Emanuel EJ. Ethics of phase 1 oncology studies: reexamining the arguments and data. JAMA. 2003. August 27;290(8):1075–82. 10.1001/jama.290.8.1075 [DOI] [PubMed] [Google Scholar]
- 16.Quinn GP, Murphy D, Pratt C, Muñoz-Antonia T, Guerra L, Schabath MB, et al. Altruism in terminal cancer patients and rapid tissue donation program: does the theory apply? Med Heal Care Philos. 2013. November 29;16(4):857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianella S, Taylor J, Brown TR, Kaytes A, Achim CL, Moore DJ, et al. Can research at the end of life be a useful tool to advance HIV cure? AIDS. 2017. January 2;31(1):1–4. 10.1097/QAD.0000000000001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprouse J. A validation of Amazon Mechanical Turk for the collection of acceptability judgments in linguistic theory. Behav Res Methods. 2011. March;43(1):155–67. 10.3758/s13428-010-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crump MJC, McDonnell J V., Gureckis TM. Evaluating Amazon’s Mechanical Turk as a Tool for Experimental Behavioral Research. Gilbert S, editor. PLoS One. 2013. March 13;8(3):e57410 10.1371/journal.pone.0057410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.County of San Diego, Health and Human Services Agency (HHSA). Monthly STD Report, Vol 1, Issue 1, published Jan 31. 2014.
- 21.Sanghavi DM. How Dying Patients Get Access to Experimental Drugs—NYTimes.com [Internet]. [cited 2017 Nov 2]. http://www.nytimes.com/2013/11/03/magazine/how-dying-patients-get-access-to-experimental-drugs.html?pagewanted=all
- 22.Enzinger AC, Zhang B, Weeks JC, Prigerson HG. Clinical trial participation as part of end-of-life cancer care: associations with medical care and quality of life near death. J Pain Symptom Manage. 2014. June;47(6):1078–90. 10.1016/j.jpainsymman.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber JS, Levit LA, Adamson PC, Bruinooge S, Burris HA, Carducci MA, et al. American Society of Clinical Oncology policy statement update: the critical role of phase I trials in cancer research and treatment. J Clin Oncol. 2015. January 20;33(3):278–84. 10.1200/JCO.2014.58.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubé K, Evans D, Sylla L, Taylor J, Weiner BJ, Skinner A, et al. Willingness to participate and take risks in HIV cure research: survey results from 400 people living with HIV in the US. J virus Erad. 2017. January 1;3(1):40–50.e21. [PMC free article] [PubMed] [Google Scholar]
- 25.Holm S. Principles of Biomedical Ethics, 5th edn: Beauchamp TL, Childress JF. Oxford University Press, 2001, pound19.95, pp 454. ISBN 0-19-514332-9. J Med Ethics. 2002;28(5):332-NaN-332. [Google Scholar]
- 26.Beauchamp T, Childress J. Principles of Biomedical Ethics. Third Oxford: Oxford University Press; 1989. 470 pages. [Google Scholar]
- 27.Emanuel EJ, Wendler D, Grady C. What Makes Clinical Research Ethical? JAMA J Am Med Assoc. 2013;283(20):2701–11. [DOI] [PubMed] [Google Scholar]
- 28.Lo B, Grady C. Ethical Considerations in HIV Cure Research: Points to Consider. Curr Opin HIV AIDS. 2013. May;8(3):243–9. 10.1097/COH.0b013e32835ea1c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grande GE, Todd CJ. Why are trials in palliative care so difficult? Palliat Med. 2000. January;14(1):69–74. 10.1191/026921600677940614 [DOI] [PubMed] [Google Scholar]
- 30.Agrawal M. Voluntariness in clinical research at the end of life. J Pain Symptom Manage. 2003. April;25(4):S25–32. [DOI] [PubMed] [Google Scholar]
- 31.Henry B, Scales D. Ethical Challenges in Conducting Research on Dying Patients and Those at Risk of Dying. Account Res. 2012;19:1–12. 10.1080/08989621.2011.622173 [DOI] [PubMed] [Google Scholar]
- 32.Mackin ML, Herr K, Bergen-Jackson K, Fine P, Forcucci C, Sanders S. Research participation by older adults at end of life: barriers and solutions. Res Gerontol Nurs. 2009. July 1;2(3):162–71. 10.3928/19404921-20090421-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allmark P, Tod AM. Ethical challenges in conducting clinical research in lung cancer. Transl lung cancer Res. 2016. June;5(3):219–26. 10.21037/tlcr.2016.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gysels MH, Evans C, Higginson IJ. Patient, caregiver, health professional and researcher views and experiences of participating in research at the end of life: a critical interpretive synthesis of the literature. BMC Med Res Methodol. 2012. August 17;12(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faden R, Beauchamp T. A History and Theory of Informed Consent. New York: Oxford University Press; 1986. [Google Scholar]
- 36.Sugarman J. HIV Cure Research. Expanding the Ethical Considerations. Ann Intern Med. 2013;159:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson GE . The Ethics of HIV “Cure” Research: What Can We Learn from Consent Forms? AIDS Res Hum Retroviruses. 2014;31(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubé K, Henderson GE, Margolis DM. Framing Expectations in Early HIV Cure Research. Trends Microbiol. 2014. October;22(10):547–9. 10.1016/j.tim.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassel JB, Del Fabbro E, Arkenau T, Higginson IJ, Hurst S, Jansen LA, et al. Phase I Cancer Trials and Palliative Care: Antagonism, Irrelevance, or Synergy? J Pain Symptom Manage. 2016. September;52(3):437–45. 10.1016/j.jpainsymman.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 40.Pentz RD, White M, Harvey RD, Farmer ZL, Liu Y, Lewis C, et al. Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer. 2012. September 15;118(18):4571–8. 10.1002/cncr.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horng S, Grady C. Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, and therapeutic optimism. IRB. 2003;25(1):11–6. [PubMed] [Google Scholar]
- 42.Jansen LA. The problem with optimism in clinical trials. Vol. 28, IRB Ethics and Human Research. 2006. p. 13–9. [PubMed] [Google Scholar]
- 43.Jansen LA. Two concepts of therapeutic optimism. J Med Ethics. 2011. September 1;37(9):563–6. 10.1136/jme.2010.038943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller FG, Joffe S. Benefit in phase 1 oncology trials: therapeutic misconception or reasonable treatment option? Clin Trials. 2008. December 1;5(6):617–23. 10.1177/1740774508097576 [DOI] [PubMed] [Google Scholar]
- 45.Bolt EE, Pasman HRW, Willems D, Onwuteaka-Philipsen BD. Appropriate and inappropriate care in the last phase of life: an explorative study among patients and relatives. BMC Health Serv Res. 2016. December 15;16(1):655 10.1186/s12913-016-1879-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fried T, Bradley E, Twole V, Allore H. Understanding the Treatment Preferences of Seriously Ill Patients. N Engl J Med. 2002;346(14):1061–6. 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 47.Steinhauser K, Christakis N, Clipp E, Mcintyre L. Factors Considered Important at the End of Life by Patients, Family, Physicians, and Other Care Providers. JAMA. 2000;284(19):2476–82. [DOI] [PubMed] [Google Scholar]
- 48.Stiel S, Heckel M, Bussmann S, Weber M, Ostgathe C. End-of-life care research with bereaved informal caregivers—analysis of recruitment strategy and participation rate from a multi-centre validation study. BMC Palliat Care. 2015. May 2;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casarett D, Ferrell B, Kirschling J, Levetown M, Merriman MP, Ramey M, et al. NHPCO Task Force Statement on the Ethics of Hospice Participation in Research. J Palliat Med. 2001. December;4(4):441–9. 10.1089/109662101753381566 [DOI] [PubMed] [Google Scholar]
- 50.Rinck GC, van den Bos GA, Kleijnen J, de Haes HJ, Schadé E, Veenhof CH. Methodologic issues in effectiveness research on palliative cancer care: a systematic review. J Clin Oncol. 1997. April;15(4):1697–707. 10.1200/JCO.1997.15.4.1697 [DOI] [PubMed] [Google Scholar]
- 51.Doukas DJ, Hardwig J. Patient informed choice for altruism. Camb Q Healthc Ethics. 2014. October 17;23(4):397–402. 10.1017/S0963180114000073 [DOI] [PubMed] [Google Scholar]
- 52.de Waal FBM. Putting the Altruism Back into Altruism: The Evolution of Empathy. Annu Rev Psychol. 2008. January;59(1):279–300. [DOI] [PubMed] [Google Scholar]
- 53.Shilts R. And the Band Played On: Politics, People and the AIDS Epidemic. 1987. [Google Scholar]
- 54.Estellés-Arolas E, González-Ladrón-De-Guevara F. Towards an integrated crowdsourcing definition. Artic J Inf Sci. 2012;38:189–200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the paper.