Abstract
Objective
To gain an understanding of the effectiveness of golimumab in a ‘real-world’ setting.
Design
Retrospective cohort study using prospectively maintained clinical records.
Setting
Two UK tertiary IBD centres.
Patients
Patients with ulcerative colitis (UC) were given golimumab at Guy’s & St Thomas and King’s College Hospitals between September 2014 and December 2016.
Intervention
Golimumab, a subcutaneously administered antitumour necrosis factor agent.
Main outcome measures
Clinical disease activity was assessed at baseline and at the first clinical review following induction therapy using the Simple Clinical Colitis Activity Index (SCCAI). Response was defined as an SCCAI reduction of 3 points or more. Remission was defined as an SCCAI of less than 3.
Results
Fifty-seven patients with UC completed golimumab induction therapy. Paired preinduction and postinduction SCCAI values were available for 31 patients and fell significantly from 7 (2–19) to 3 (0–11) (p<0.001). To these 31, an additional 13 patients who did not have paired SCCAI data but stopped treatment due to documented ‘non-response’ in the opinion of their supervising clinician, were added. Among this combined cohort, 23/44 (52%) had a clinical response, 15/44 (34%) achieved remission and 13/44 (30%) achieved corticosteroid-free remission.
Faecal calprotectin and CRP fell (FC: pre-induction: 1096 (15-4800) μg/g, post-induction: 114 (11-4800) μg/g, p = 0.011; n = 20; CRP: pre-induction: 4 (1-59) mg/L, post-induction: 2 (1-34) mg/L, p = 0.01 for n = 43). Post-induction endoscopy was carried out in 23 patients and a mucosal healing (Mayo 0 or 1) rate of 35% was observed.
Conclusions
Our experience mirrors previously reported real-world cohorts and demonstrates similar outcomes to those observed in randomised controlled trials. These data demonstrate a meaningful reduction in clinical, biochemical and endoscopic disease activity as well as a steroid-sparing effect in patients with previously refractory disease.
Keywords: ulcerative colitis, inflammatory bowel disease, Golimumab, Simponi
Introduction
Ulcerative colitis (UC) is a chronic relapsing and remitting inflammatory bowel disease (IBD), with a characteristic leucocytic infiltration of the colonic mucosa. Bloody diarrhoea is the most common presentation with a peak onset during the third and fourth decades of life.1 The National Institute for Health and Care Excellence (NICE) estimates it affects approximately 146 000 people in the UK. In combination with Crohn’s disease, IBD care costs the National Health Service (NHS) in excess of £700 million per year.2 The condition also has a significant impact on quality of life with 73% of patients with UC reporting interference in their leisure activities, two-thirds describing a negative impact on their work, and over a quarter having to alter their work to accommodate their disease.3
Before the advent of biological therapies, options for UC treatment primarily consisted of the stepwise use of mesalazine, corticosteroids and immunomodulators. Although a large proportion of patients respond to mesalazine treatment alone,4 5 a significant minority require additional agents. However, corticosteroids are inappropriate for long-term use and thiopurine intolerance is relatively common.6 In addition, despite optimisation, some patients have disease that remains refractory to immunomodulation.7 There is now good evidence from large-scale, randomised controlled trials (RCTs) demonstrating the efficacy of antitumour necrosis factor (anti-TNF) agents in this subgroup of patients.8 Following a NICE multiple technology appraisal (TA329) in 2015, all three anti-TNF agents (infliximab, adalimumab and golimumab) were granted approval for use in moderate-to-severe UC. These approvals have led to significant changes in UC treatment paradigms in the UK with associated patient benefit.9
Golimumab is a transgenic, fully human monoclonal immunoglobulin G1 antibody that is synthesised from TNF-immunised transgenic mice expressing human immunoglobulin G.10 11 It is administered subcutaneously and is licensed for the treatment of UC, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. NICE approval for UC was granted based on the efficacy demonstrated during an integrated phase 2 and 3 trial program (PURSUIT, Program of Ulcerative colitis Research Studies Utilizing an Investigational Treatment).12 13 During the induction phase (PURSUIT-SC), golimumab-treated patients achieved a response (defined as a decrease in Mayo score by at least 3 points and by 30% or more, with a bleeding subscore of 0 or 1, or decrease ≥1) significantly more frequently than those on placebo, thereby meeting the induction primary endpoint. Two different induction regimens were investigated and after 6 weeks of treatment, just over 50% of golimumab-treated patients had responded, compared with 30% in the placebo group (p<0.0001).12 NICE approval allows treatment at 200 mg at week 0, 100 mg at week 2 and weight-based dosing at week 6 (100 mg for patients weighing 80 kg and over or 50 mg for those under 80 kg).
During the maintenance phase of the clinical trials program (PURSUIT-M), patients were randomised to either 50 or 100 mg every 4 weeks. The response observed during induction was demonstrated to be durable, with 47% and 50% of patients, respectively, achieving a sustained response, compared with 31% in the placebo group (p=0.010 and p<0.001, respectively) thus, meeting the maintenance primary endpoint.13 On the basis of weight-based differences in response rates seen in PURSUIT-M, the European Medicines Agency (and subsequently NICE) approved the use of 4-weekly maintenance therapy at a dose of 100 mg for patients weighing 80 kg and over or 50 mg for those under 80 kg. It should be noted that this differs from the US Food and Drug Administration approval, which allows 100 mg, 4-weekly for all patients, regardless of weight. An additional point of interest regarding PURSUIT-M was the stringent definition of maintained response; long-term continuous efficacy was evaluated over the course of 15 prospective assessments over 54 weeks, without loss of response permitted at any time-point. This compares with only three assessments undertaken as part of the ACT-114 maintenance trial and two in the ACT-214 and ULTRA15 maintenance trials (the landmark RCTs of infliximab and adalimumab in UC, respectively).
Although the data generated by large-scale, RCTs represent high-quality evidence,16 there is growing appreciation of the importance of observational, real-life data in IBD.17 Effectiveness relates to how well a treatment works in clinical practice, which is different from efficacy, which relates to how well it works in clinical trials.18 Using the cohorts from Guy’s & St Thomas (GSTT) and King’s College Hospitals (KCH), we aimed to contribute to the growing body of existing observational data from Europe19–23 and the US,24 25 the majority of which is available only in abstract (or letter26–28) form.
Methods
We performed a retrospective cohort study by reviewing prospectively maintained clinical records for all patients starting golimumab at GSTT or KCH between September 2014 and January 2017. We screened the records of 58 patients, who received at least one injection of golimumab for UC; one patient, who received only the first dose before needing an emergency colectomy for disease-progression, was excluded. Records of the remaining 57 patients were reviewed. Demographic information and disease-related data are presented in table 1. Data regarding dose-escalation during maintenance therapy from 50 mg to 100 mg were also collected.
Table 1.
Baseline characteristics
| Characteristic | n=57 |
| Gender, male:female | 38:19 |
| Median age at time of starting golimumab (range), years | 35 (20–72) |
| Median disease duration (range), years | 5 (1–52) |
| Median duration of golimumab treatment (range), months | 7 (2–28) |
| Concomitant immunomodulator | |
| Thiopurine | 41 (72%) |
| Methotrexate | 2 (4%) |
| None | 14 (24%) |
| Previous antitumour necrosis factor experience | |
| Naïve | 40 (70%) |
| Exposed | 17 (30%) |
| Prior infliximab | 10 |
| Prior adalimumab | 7 |
| Prior infliximab and adalimumab | 0 |
| Disease extent | |
| Proctitis | 6 (11%) |
| Left-sided | 20 (35%) |
| Extensive | 31 (54%) |
Our primary outcome was the clinical effectiveness of golimumab. This was evaluated using the Simple Clinical Colitis Activity Index29 (SCCAI). Where available, we compared paired evaluations, taken prior to treatment initiation and again at the first clinical review after completing the 6-week induction regimen (figure 1). Rather than being fixed, these time points varied from patient to patient with postinduction clinical assessments (SCCAI) being carried out at a median of 12 weeks from treatment initiation. Postinduction C reactive protein (CRP) and faecal calprotectin (FC) measurements were made at medians of 8 and 10 weeks, respectively. Clinical response was defined as a reduction ≥3 in SCCAI and clinical remission was defined as a SCCAI <3.30
Figure 1.
Study design and evaluations. CRP, C reactive protein; FC, faecal calprotectin; GOL, golimumab; SCCAI, Simple Clinical Colitis Index.
Secondary outcomes included the effect of golimumab on biochemical markers of disease activity, endoscopic outcomes, rates of corticosteroid use and the need for colectomy. A CRP ≥5 mg/L or FC >150 µg/g were considered biochemical evidence of disease activity.31 Endoscopic Mayo scores of 0 or 1 were considered to represent mucosal healing.32 Dose–response and dose–remission analyses were carried out using maintenance dose (either 50 mg or 100 mg) and body weight (kg) at treatment initiation, to calculate each patient’s dose on a mg/kg basis. Rates of corticosteroid usage at each time point were also collected and colectomy was included if it occurred while on golimumab therapy.
Continuous data are summarised as medians and ranges (in brackets). Paired SCCAI, CRP and FC values were compared using Wilcoxon signed-rank test. Categorical variables were compared using the Fisher’s exact test (GraphPad Prism V.7.0a). Unless stated, p values are non-significant. All data below/above the limit of quantification were substituted with the value of the lower/upper limit of quantification, that is, CRP 1 mg/L for levels of <1 mg/L and FC 4800 µg/g for levels>4800 µg/g.
Results
Clinical disease activity
Paired preinduction and postinduction SCCAI values were available for 31 patients. Prior to starting golimumab (preinduction), the median SCCAI was 7 (range 2–19). The corresponding postinduction score fell to 3 (0–11, p<0.0001) (figure 2) at a median time of 12 (6–36) weeks from initiation of treatment.
Figure 2.
Change in Simple Clinical Colitis Index (SCCAI) for patients with paired preinduction and postinduction data available (n=31, *p<0.0001). Median shown in orange. Postinduction scores assessed at a median of 12 weeks following treatment initiation.
Postinduction response and remission rates
In addition to the 31 patients in our cohort with paired preinduction and postinduction SCCAI data, 13 patients discontinued treatment due to non-response, judged by their supervising physician but with no documented paired SCCAI scores. These groups were combined thus, increasing the cohort included in the following response and remission analysis to a total of 44 (see online supplementary figure 1). Among this cohort, 23/44 (52%) had a clinical response to golimumab and 15/44 (34%) achieved remission. The rate of corticosteroid-free remission was 13/44 (30%) (figure 3).
Figure 3.
Rates of response, remission and corticosteroid-free remission (n=44). Response defined as SCCAI reduction ≥3. Remission defined as SCCAI <3. Postinduction scores assessed at a median of 12 weeks following treatment initiation. SCCAI, Simple Clinical Colitis Index.
flgastro-2017-100895supp001.pdf (26KB, pdf)
Clinical response rates were significantly higher among patients who were anti-TNF-naïve (20/31, 65%) than anti-TNF-exposed (3/13, 23%)(p=0.020). Corresponding remission rates were 13/31 (42%) and 2/13 (15%), respectively (p=0.16) (figure 4).
Figure 4.
Rates of response, remission and corticosteroid-free remission when dividing patients by prior anti-TNF exposure (n=44, *p=0.020). Response defined as SCCAI reduction ≥3. Remission defined as SCCAI <3. Postinduction scores assessed at a median of 12 weeks following treatment initiation. SCCAI, Simple Clinical Colitis Index; TNF, tumour necrosis factor.
Response rates were 5/13 (38%) in patients on golimumab monotherapy and 18/31 (58%) in patients on combination therapy with an immunomodulator while remission rates were 5/13 (38%) for monotherapy and 10/31 (32%) for combination therapy. This did not reach statistical significance and neither did other comparisons included in our univariate analysis (table 2), although these findings should be considered in terms of the limited patient numbers.
Table 2.
Univariate analysis comparing responder and non-responder groups
| Responders (n=23) |
Non-responders (n=21) |
p Value | |
| Age, years (median (range)) | 34 (18–81) | 38 (20–62) | 0.60 |
| Gender, f:m (n (%)) | 1.6:1 | 2:1 | 0.76 |
| Duration of disease, years (median (range)) |
5 (0.3–18) | 5 (1.5–30) | 0.66 |
| Concomitant immunomodulator (n (%)) |
5 (22%) | 8 (38%) | 0.33 |
| Prior antitumour necrosis factor exposure (n (%)) | 3 (13%) | 10 (48%) | 0.020* |
| Maintenance dose 50 mg (n (%)) | 13 (57%) | 12 (57%) | >0.99 |
| Maintenance dose, mg/kg (median (range)) |
0.94 (0.63–1.92) | 0.79 (0.64–1.17) | 0.046* |
| Corticosteroids at initiation (n (%)) | 12 (52%) | 8 (38%) | 0.38 |
* p<0.05
Weight-based dose–response analysis
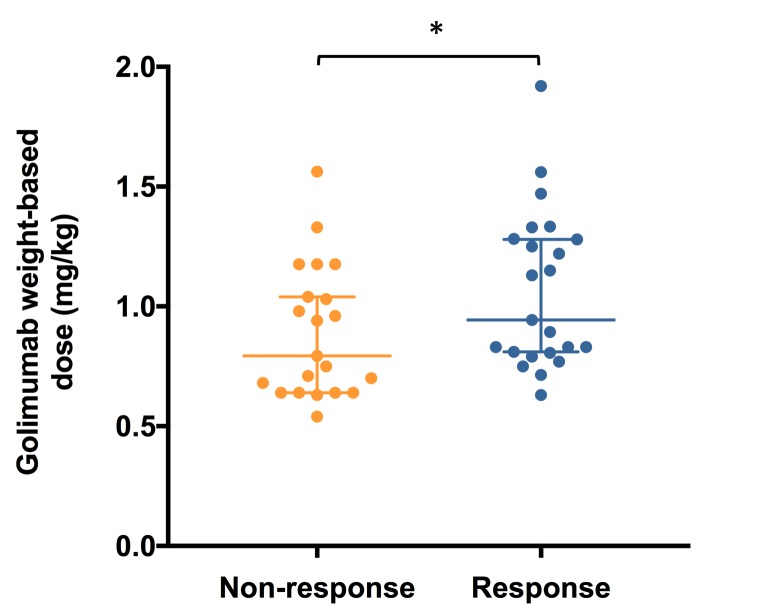
When each patient’s individual maintenance dose was calculated on a mg/kg basis, there appeared to be a dose–response relationship. The median dose among responders was significantly higher (0.94 mg/kg (0.63–1.92 mg/kg, n=23)) than that observed among non-responders (0.79 mg/kg (0.64–1.17 mg/kg, n=21) (p=0.046)) (figure 5). However, although the pattern was similar, this significant difference was not evident when comparing those who achieved remission with those who did not (remission: 0.94 mg/kg (0.63–1.33, n=15) versus non-remission: 0.79 mg/kg (0.54–1.92, n=29) (p=0.13)).
Figure 5.
Weight-based dose–response analysis demonstrating the individual dose received on a mg/kg basis (median and 95% CI), for responders and non-responders (n=44, *p=0.046).
The effect of dose escalation
A total of 7/25 patients on 50 mg 4 weekly underwent dose escalation to 100 mg. In all cases of dose escalation, the decision was made in view of a clinically suboptimal response as judged by the supervising clinician having taken into account symptoms and objective measures of disease activity. In 3/7 cases, dose escalation proved clinically ineffective and treatment was stopped (however, it should be noted that in one patient FC fell from 2608 to 880 µg/g and in another from 1000 to 368 µg/g). In 2/7 cases, clinical remission was achieved (both SCCAI 0) with both continuing treatment (total durations of 12 and 27 months). In 1/7 cases, there was clinical and biochemical improvement (SCCAI fell from 19 to 5 and FC fell from 1560 to 171 µg/g) and the patient continues on treatment (total duration 12 months). The final patient recently required dose-escalation and has not yet been reassessed (total duration of treatment 4 months)
Biochemical disease activity
Paired preinduction and postinduction serum CRP data were available for 43 patients. Baseline CRP fell from a median of 4 (1–59) mg/L to 2 (1–34) mg/L following induction therapy (p=0.010) at a median of 8 (6–24) weeks after treatment initiation (see online supplementary figure 2). Eighteen of the 43 patients (42%) had an elevated CRP (≥5 mg/L) at baseline. Of the 18 patients with an elevated preinduction CRP, a fall was observed in 16/18 (89%) and normalisation (<5 mg/L) in 11/18 (61%).
flgastro-2017-100895supp002.jpg (186.5KB, jpg)
Paired preinduction and postinduction FC data were available for 20 patients. The median FC fell significantly from a median of 1096 (15–4800) µg/g to 114 (11–4800) µg/g (p=0.011) at a median of 10 (6–36) weeks after treatment initiation (see online supplementary figure 3). A total of 18/20 patients (90%) had an elevated (≥150 µg/g) FC at baseline in whom a fall was observed in 15/18 (83%) and normalisation (<150 µg/g) in 9/18 (50%).
flgastro-2017-100895supp003.jpg (217.1KB, jpg)
Endoscopic outcomes
A total of 23/57 (40%) patients underwent postinduction endoscopies with endoscopic Mayo scoring (Mayo 0: 2 (9%), Mayo 1: 6 (26%), Mayo 2: 6 (26%), Mayo 3: 9 (39%)) with a mucosal healing (Mayo 0 or 1) rate of 8/23 (35%). Of the eight patients who achieved mucosal healing, six were in clinical remission and steroid-free, while two remained mildly symptomatic.
Corticosteroid usage, treatment discontinuation and surgery
At baseline, 27/57 (43%) patients were receiving corticosteroid treatment, which fell to 9/57 (16%) at postinduction clinical follow-up (at a median of 12 weeks from golimumab initiation); that is, 18 of the 27 (67%) patients on corticosteroids at baseline were successful withdrawn from them while on golimumab.
A total of 22/57 (39%) patients discontinued golimumab treatment for the following reasons: 16 primary non-response, 4 secondary loss of response, 1 due to drug-induced rash and 1 patient was withdrawn from the GO-COLITIS clinical trial due to breach of trial protocol (figure 6). Four of the patients who failed to respond to golimumab underwent colectomy, resulting in an overall surgery rate of 4/57 (7%).
Figure 6.
Patients remaining on golimumab. Kaplan–Meier analysis illustrating the rate of golimumab continuance.
The median duration of treatment of the entire cohort (including patients continuing on treatment as well as those who discontinued) was 7 (2-28) months. Of the 36 patients who had started treatment more than 12 months ago, 18 (50%) remained on treatment at 1 year.
Discussion
The results of our study closely resemble those seen in large scale, randomised, placebo-controlled trials12 13 as well as previously reported ‘real-world’ cohorts.19–28 Patients in our cohort had a similar duration of disease and rates of corticosteroid usage to those seen in the PURSUIT trial. However, as previous anti-TNF exposure was an exclusion criterion in PURSUIT, our cohort had a higher rate of prior anti-TNF experience (30%). This finding partly reflects previous patterns of management in the UK, in which anti-TNF agents were not approved for use as maintenance therapy until mid-2015. This resulted in some patients effectively receiving episodic infliximab rescue therapy with a maximum of three induction infusions given during periods of increased disease activity. Of relevance in this context, PURSUIT-M was the first randomised withdrawal study of an anti‐TNF in UC and clarified that induction only is insufficient to maintain a long-term response in the majority of patients.13 Despite the difference in prior anti-TNF exposure, our observed change in SCCAI (−4) and response rate at median of 12 weeks (52%) was broadly similar to the change in Mayo score (−2) and response rate at week 6 (51%) seen in PURSUIT-SC.12 Consistent with other cohorts across multiple biologic drugs, we observed a significantly greater response rate among patients who were anti-TNF naïve compared with anti-TNF experienced. This finding was not replicated in terms of remission, perhaps due to the relatively smaller numbers of patients achieving this outcome. Unfortunately, we did not have the outcomes of all previous anti-TNF exposure as our hospitals are tertiary referral centres and many of those previously treated with anti-TNF agents were treated elsewhere. However, we do appreciate the importance of this aspect of the history when making decisions regarding subsequent biologic choices; prior anti-TNF discontinuation for primary non-response is likely to confer a less favourable outcome with subsequent anti-TNF agents than discontinuation for other reasons (secondary non-response, intolerance or in view of complete remission).
Our results corroborate a previously reported (abstract) observational study by Taxonera et al in which 60% of patients treated with golimumab were anti-TNF experienced.23 Like us, they conducted a retrospective, multicentre cohort study and described a significant difference in initial response rates based on prior anti-TNF experience. They also reported a numeric difference in longer-term maintained response after 10 months of golimumab, which was observed in 60% of patients who were anti-TNF naïve but only 39% of those with prior anti-TNF exposure (p=0.15).
As with most biological agents in IBD, golimumab displays an exposure efficacy relationship. This was originally described in PURSUIT-SC with patients in the highest serum golimumab concentration quartiles having greater improvement in Mayo scores and greater rates of clinical response and clinical remission when compared with those in the lower quartiles.12 This pattern was also seen during the maintenance phase (PURSUIT-M) with greater proportions of patients in the higher serum golimumab concentration quartiles achieving clinical response through to week 54 or clinical remission at both weeks 30 and 54, when compared with those in the lower serum concentration quartiles.13 In a recent publication, Adedokun and colleagues reported a study of the pharmacokinetics and pharmacodynamics of golimumab using samples taken as part of the PURSUIT trials. As part of these analyses, the authors found serum golimumab concentrations to be dose-proportional and that a positive correlation existed between concentrations and efficacy outcomes (clinical response, clinical remission and mucosal healing) during induction and maintenance therapy. They then went further by using receiver-operating-characteristics (ROC) curve analysis to define serum golimumab concentrations that may serve as potential targets for treatment optimisation; proposing thresholds of 2.5 µg/mL at week 6 and 1.4 µg/mL during steady-state maintenance therapy.33 Prior to this, similar findings were also reported by a group from Leuven as part of an observational study of 21 patients being treated with golimumab in a clinical setting. Median golimumab concentrations were significantly higher in partial clinical responders than in non-responders at week 2 (10.0 vs 7.4 µg/mL, p=0.035) and week 6 (5.1 vs 2.1 µg/mL, p=0.037). Their ROC curve analysis revealed a cut-off of 2.6 µg/mL at week 6 (90% specificity, 56% sensitivity, area under the curve 0.79 (95% CI), p=0.034) for the association with a partial clinical response at week 14.19 Although a commercial assay is not currently available in the UK, and our study did not include therapeutic drug monitoring, there was, nonetheless evidence of a dose-response relationship. With the knowledge that golimumab concentrations are dose proportional, this would appear to offer further observational evidence of an exposure–response relationship. Other than this difference and prior anti-TNF experience, our univariate analysis comparing responders and non-responders identified no other factors associated with response (table 2).
This is further supported by the observed benefit derived from dose-escalation in our study. We observed a benefit in 50% of patients who underwent dose escalation. This suggests that the drug is inadequately dosed in some cases of suboptimal response. Although it should be noted that dose-escalation is not within license or NICE guidance, the 50 and 100 mg maintenance doses are priced equally in the UK suggesting that dose escalation is a cost-effective option in patients with a suboptimal response to 50 mg maintenance dosing. Of course, this is not a particularly novel approach in the use of biologics for IBD; evidence-based dose escalation strategies already exist for infliximab,34 adalimumab35 and vedolizumab36 37 and seem likely also to be useful for ustekinumab.38 However, empirical dose escalation can incur undue costs39–41 and perhaps a more rational approach to dose optimisation would be to use therapeutic drug monitoring to quantify serum golimumab concentrations as well as to identifying the presence or absence of antidrug antibodies.42 Several studies are currently planned or underway to further clarify therapeutic thresholds and to validate commercially available golimumab assays.
Our study has several limitations. Most notable are its retrospective design and the subjective nature of the clinical disease activity scores. In addition, other than rates of surgery, we did not have systematically collected data regarding adverse events. Our cohort also included some patients without paired SCCAI, FC or CRP measurements made before and after induction. Although paired CRP data were available for the majority of patients, we had paired FC data on only a limited number of patients. Even with these limited data, FC seemed to outperform CRP in terms of indicating disease activity (elevated in 90%, compared with 42% with an elevated CRP at baseline), although both appeared responsive to the change in disease activity induced by golimumab (an elevated CRP fell in 89% and an elevated FC fell in 83%). Additionally, only nine patients had all three assessments (SCCAI, CRP and FC) documented before and after golimumab induction treatment and of these only four had all three carried out at the same interval. This highlights the realities of studying ‘real-life’ cohorts where assessments are performed at variable time points compared with protocol-driven assessments in clinical trials. Our endoscopic data may also be subject to a negative selection bias based on the premise that most clinicians are more likely to repeat endoscopy in patients with a suboptimal response. Despite this, the proportion we observed to have mucosal healing (Mayo 0 or 1) was similar to that in the PURSUIT-SC (35% vs 42%, respectively), as was the proportion with complete mucosal normalisation (Mayo 0) (9% vs 8.3%, respectively). In an attempt to compensate for missing treatment outcome data, we analysed treatment persistence (as proxy marker of response) and found that half of our patients remained on golimumab after a year. This finding is not dissimilar to that observed by Bressler et al in their retrospective study of 136 golimumab-treated patients with UC, in which a 1 year persistence rate of 63% was described.25
Despite the above limitations, we believe the results generated in our study are relevant, reliable and generalisable. Indeed, this type of observational effectiveness research is becoming increasingly recognised as significant and necessary.17 This recognition is based on the growing understanding that patients included in RCTs are not necessarily representative of patients encountered in clinical practice43 and that the intensity of follow-up during trial protocols may be partly responsible for the high placebo effect observed.44 Nonetheless, readers should be aware of the inherent methodological deficiencies associated with effectiveness research. Studies, such as this one, are commonly subject to a number of possible biases and confounders (eg, selection, attrition and misclassification) and in addition are less likely to have rigorous study designs and statistical methods.17 To remedy some of these shortcomings, the following publications have attempted to define optimal methods with which to carry out observational research: Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)45 and Good Research for Comparative Effectiveness (GRACE).46
Our own previously reported experience of vedolizumab for UC demonstrated similar effectiveness to those described here for golimumab (response rates of 55% and 52%, respectively and remission rates of 39% and 34%, respectively).47 With NICE-approval of vedolizumab coming at a similar time to the approval of anti-TNF agents, clinicians now have a broader range of treatments for UC than ever before. Although certain factors may make vedolizumab preferable,48 the choice of mechanism for first-line biological treatment in UC remains a current ‘hot-topic’ for debate. The overall effectiveness of golimumab presented here should be considered in the context of the relatively high rate of anti-TNF naïvety (70%) in our cohort. It is likely that this would be lower in a group with a higher rate of prior anti-TNF exposure. A direct head-to-head trial of the comparative effectiveness of anti-TNF (infliximab) versus selective leucocyte adhesion molecule inhibition (etrolizumab, which has a similar mechanism of action as vedolizumab) is ongoing and may be informative in this regard.49 However, in the absence of RCT evidence, our practice is to discuss each case in a multidisciplinary setting, where established IBD pathways50 devised in cooperation with our local Clinical Commissioning Group guide decisions. The appropriate choice and management of biological drugs is often a matter of nuance that incorporates multiple factors, best addressed by physicians, clinical nurse specialists and pharmacists with a specialist interest in IBD. In addition, practical factors such as patient preference for route of administration and the management of pressures on infusion suite capacity should be considered. Our pathway makes the following statement regarding biological choice for chronic active UC: “At present subcut anti-TNF is first line in this group, dictated by informed patient choice and unless clinically inappropriate; As experience increases, vedolizumab may become first line’. In reality, our use of subcutaneous therapy was largely driven by the need to manage our infusion capacity. It should be appreciated that this is a rapidly moving field and that the next wave of biological (eg, ustekinumab and etrolizumab) and small molecule (eg, tofacitinib and ozanimod) drugs will add further complexity.
Conclusion
Our early experience with golimumab in two tertiary IBD centres mirrors the effectiveness observed in other real-world cohorts as well as the efficacy demonstrated by the PURSUIT trial program. Our data demonstrate a clear benefit in terms of symptom control and improvement of objective markers of disease activity as well as a steroid-sparing effect. It also offers further evidence of the dose–response relationship associated with the use of golimumab.
Significance of this study.
What is already known about this subject?
The PURSUIT trial program (an integrated phase 2 and 3, randomised controlled trial) demonstrated the efficacy of golimumab for both the induction and maintenance of remission in moderate-to-severe ulcerative colitis (UC).
An exposure–response relationship was observed in a posthoc analysis of samples collected as part of the PURSUIT trial program.
What this study adds?
Our data demonstrate the effectiveness of golimumab in controlling symptoms and improving objective markers of disease activity in a ‘real-world’ cohort of patients with UC.
Our findings support a dose–response relationship when golimumab is used in clinical practice.
How might it impact on clinical practice in the foreseeable future?
Clinicians should consider golimumab in patients with UC who are failing conventional therapies as well as in those who are steroid-dependent.
In cases of suboptimal response to golimumab 50 mg, 4-weekly maintenance therapy, clinicians may consider dose escalation to 100 mg, 4-weekly administration. It should be understood that this regimen is unlicensed in patients weighing less than 80 kg.
Footnotes
Contributors: MAS, PP, BHH and PMI were responsible for the original concept and planning of the study. MAS, PP, ELJ, JD-B, AD, RP, AOA and LM were responsible for data collection and analysis. MAS drafted the manuscript, which GC- F, PD, IK, NP, SHCA, JS, BHH and PMI critically reviewed and revised.
Competing interests: MAS advisory fees: hospira lecture fees: hospira, takeda, MSD, Janssen, falk; ELJ : Lecture fees: takeda; JD-Bell : Lecture fees: takeda, MSD; NP : advisory fees: abbvie, allergan, debiopharm International, ferring, vifor pharma lecture fees: allergan, falk pharma; PMI : advisory fees: abbvie, warner chilcott, takeda, MSD, vifor pharma, pharmacosmos, topivert, genentech, hospira, samsung bioepis lecture fees: abbvie, warner chilcott, ferring, falk pharma, takeda, MSD, Johnson and Johnson, Shire financial support for research: MSD, takeda.
Ethics approval: The Health Research Authority does not consider post-marketing surveillance, research and therefore, suggests that NHS REC approval was not necessary.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no unpublished data associated with the study.
Presented at: BSG 2017, Manchester, UK
References
- 1. Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–17. 10.1053/j.gastro.2004.01.063 [DOI] [PubMed] [Google Scholar]
- 2. Bassi A, Dodd S, Williamson P, et al. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut 2004;53:1471–8. 10.1136/gut.2004.041616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis 2007;1:10–20. 10.1016/j.crohns.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 4. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2006;19:CD000544. [DOI] [PubMed] [Google Scholar]
- 5. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2006;19:CD000543. [DOI] [PubMed] [Google Scholar]
- 6. Goel RM, Blaker P, Mentzer A, et al. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis 2015;6:138–46. 10.1177/2040622315579063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louis E, Irving P, Beaugerie L. Use of azathioprine in IBD: modern aspects of an old drug. Gut 2014;63:1695–9. 10.1136/gutjnl-2013-306711 [DOI] [PubMed] [Google Scholar]
- 8. Samaan MA, Bagi P, Vande Casteele N, et al. An update on anti-TNF agents in ulcerative colitis. Gastroenterol Clin North Am 2014;43:479–94. 10.1016/j.gtc.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 9. Samaan MA, Irving PM. The impact of updated NICE guidelines on biologic treatment of ulcerative colitis: reflections on past practices, the changing present and implications for the future. Expert Opin Biol Ther 2016;16:975–7. 10.1080/14712598.2016.1189529 [DOI] [PubMed] [Google Scholar]
- 10. Hutas G. Golimumab, a fully human monoclonal antibody against TNFalpha. Curr Opin Mol Ther 2008;10:393–406. [PubMed] [Google Scholar]
- 11. Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol 2005;23:1117–25. 10.1038/nbt1135 [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95. 10.1053/j.gastro.2013.05.048 [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109. 10.1053/j.gastro.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 14. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. 10.1056/NEJMoa050516 [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65. 10.1053/j.gastro.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 16. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305–10. 10.1097/PRS.0b013e318219c171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salleron J, Danese S, D’Agay L, et al. Effectiveness research in inflammatory bowel disease: a necessity and a methodological challenge. J Crohns Colitis 2016;10:1096–102. 10.1093/ecco-jcc/jjw068 [DOI] [PubMed] [Google Scholar]
- 18. Guidance for Industry Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. U.S. Dept. of health and human services, food and drug administration, center for drug evaluation and research: center for biologics evaluation and research: Rockville MD, 1998. [Google Scholar]
- 19. Detrez I, Dreesen E, Van Stappen T, et al. Variability in golimumab exposure: a ’real-life' observational study in active ulcerative colitis. J Crohns Colitis 2016;10:575–81. 10.1093/ecco-jcc/jjv241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bosca-Watts MM, Cortes X, Iborra M, et al. Short-term effectiveness of golimumab for ulcerative colitis: observational multicenter study. World J Gastroenterol 2016;22:10432–9. 10.3748/wjg.v22.i47.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castro-Laria L, Argüelles-Arias F, García-Sánchez V, et al. Initial experience with golimumab in clinical practice for ulcerative colitis. Rev Esp Enferm Dig 2016;108:129–32. doi:10.17235/reed.2016.4068/2015 [DOI] [PubMed] [Google Scholar]
- 22. Varvara D, Costantino G, Privitera AC, et al. Efficacy and safety of golimumab in patients with ulcerative colitis: a prospective multicentre study. 11th Congress of ECCO. Amsterdam, The Netherlands, 2016. [Google Scholar]
- 23. Taxonera C, Bertoletti F, Rodríguez C, et al. Real-Life experience with golimumab in ulcerative colitis patients according to prior anti-TNF use. Gastroenterology 2016;150:S979 10.1016/S0016-5085(16)33318-2 [DOI] [Google Scholar]
- 24. Hamed K, Kathleen S, Ashwin A, et al. Efficacy and safety of golimumab in ulcerative colitis: experience from two large boston academic hospitals. Inflammatory Bowel Diseases 2014;20:S54. [Google Scholar]
- 25. Bressler B, Williamson MA, Camacho F, et al. Real world use and effectiveness of golimumab for ulcerative colitis in Canada. Gastroenterology 2016;150:S811 10.1016/S0016-5085(16)32744-5 [DOI] [Google Scholar]
- 26. Renna S, Orlando E, Macaluso FS, et al. Letter: a prospective real life comparison of the efficacy of adalimumab vs. golimumab in moderate to severe ulcerative colitis. Aliment Pharmacol Ther 2016;44:310–1. 10.1111/apt.13692 [DOI] [PubMed] [Google Scholar]
- 27. Tursi A, Allegretta L, Della Valle N, et al. Effectiveness of golimumab in inducing remission and clinical response in outpatient ulcerative colitis. Clin Res Hepatol Gastroenterol 2016;40:e61–3. 10.1016/j.clinre.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 28. Tursi A, Della Valle N, Penna A, et al. Letter: effectiveness of golimumab to induce remission in outpatient ulcerative colitis in Italy. Aliment Pharmacol Ther 2016;43:657–8. 10.1111/apt.13509 [DOI] [PubMed] [Google Scholar]
- 29. Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. 10.1136/gut.43.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54:782–8. 10.1136/gut.2004.056358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19. 10.1038/ajg.2015.120 [DOI] [PubMed] [Google Scholar]
- 32. Samaan MA, Mosli MH, Sandborn WJ, et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis 2014;20:1465–71. 10.1097/MIB.0000000000000046 [DOI] [PubMed] [Google Scholar]
- 33. Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis 2017;11:35–46. 10.1093/ecco-jcc/jjw133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taxonera C, Barreiro-de Acosta M, Calvo M, et al. Infliximab dose escalation as an effective strategy for managing secondary loss of response in ulcerative colitis. Dig Dis Sci 2015;60:3075–84. 10.1007/s10620-015-3735-4 [DOI] [PubMed] [Google Scholar]
- 35. Wolf D, D’Haens G, Sandborn WJ, et al. Escalation to weekly dosing recaptures response in adalimumab-treated patients with moderately to severely active ulcerative colitis. Aliment Pharmacol Ther 2014;40:486–97. 10.1111/apt.12863 [DOI] [PubMed] [Google Scholar]
- 36. Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe crohn’s disease: results from the us victory consortium. Am J Gastroenterol 2016;111:1147–55. 10.1038/ajg.2016.236 [DOI] [PubMed] [Google Scholar]
- 37. Bruce S, Marla D, Severine V, et al. Effects of Increased vedolizumab dosing frequency on clinical remission and response in ulcerative colitis and crohnʼs disease. Inflamm Bowel Dis 2014;20:S67 10.1097/01.MIB.0000456837.14633.30 [DOI] [Google Scholar]
- 38. Battat R, Kopylov U, Bessissow T, et al. Association of ustekinumab trough concentrations with clinical, biochemical and endoscopic outcomes. Gastroenterology 2016;150:S144–5. 10.1016/S0016-5085(16)30587-X [DOI] [PubMed] [Google Scholar]
- 39. Black CM, Yu E, McCann E, et al. Dose escalation and healthcare resource use among ulcerative colitis patients treated with adalimumab in english hospitals: an analysis of real-world data. PLoS One 2016;11:e0149692 10.1371/journal.pone.0149692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. 10.1136/gutjnl-2013-305279 [DOI] [PubMed] [Google Scholar]
- 41. Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66. 10.1016/j.cgh.2012.12.035 [DOI] [PubMed] [Google Scholar]
- 42. Vande Casteele N, Khanna R. Therapeutic Drug Monitoring of Golimumab in the Treatment of Ulcerative Colitis. Pharm Res 2017;34:1556–1563. [DOI] [PubMed] [Google Scholar]
- 43. Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–7. quiz e78 10.1016/j.cgh.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 44. Jairath V, Zou G, Parker CE, et al. Systematic review and meta-analysis: placebo rates in induction and maintenance trials of ulcerative colitis. J Crohns Colitis 2016;10:607–18. 10.1093/ecco-jcc/jjw004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 46. Dreyer NA, Schneeweiss S, McNeil BJ, et al. GRACE principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care 2010;16:467–71. [PubMed] [Google Scholar]
- 47. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2017;8:196–202. 10.1136/flgastro-2016-100720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dart RJ, Samaan MA, Powell N, et al. Vedolizumab: toward a personalized therapy paradigm for people with ulcerative colitis. Clin Exp Gastroenterol 2017;10:57–66. 10.2147/CEG.S110547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. A study comparing the efficacy and safety of etrolizumab to infliximab in patients with moderate to severe ulcerative colitis who are naive to tnf inhibitors. https://clinicaltrials.gov/ct2/show/NCT02136069
- 50. South East London area prescribing committee. Primary & Secondary Care Inflammatory Bowel Disease Pathway August 2017. A partnership between NHS organisations in South East London: Bexley, Bromley, Greenwich, Lambeth, Lewisham and Southwark Clinical Commissioning Groups (CCGs) and GSTFT/KCH /SLAM/Oxleas NHS Foundation Trusts/Lewisham & Greenwich NHS Trust http://www.lambethccg.nhs.uk/news-and-publications/meeting-papers/south-east-london-area-prescribing-committee/Documents/Clinical_guidelines_and_pathways/IBD_pathways_August_2017.pdf (accessed: Aug 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2017-100895supp001.pdf (26KB, pdf)
flgastro-2017-100895supp002.jpg (186.5KB, jpg)
flgastro-2017-100895supp003.jpg (217.1KB, jpg)