Abstract
We report an asplenic patient who was infected with Babesia divergens–like/MO-1. The clinical course was complicated by multiorgan failure that required intubation and dialysis. The patient recovered after an exchange transfusion and antimicrobial drug therapy. Physicians should be alert for additional cases, particularly in asplenic persons.
Keywords: Babesia divergens, B. divergens–like/MO-1, parasites, protozoa, babesiosis, emerging infection, asplenic patient, tickborne disease, ticks, vector-borne infections, erythrocyte exchange transfusion, parasitemia, Michigan, United States
Babesiosis is an emerging threat in North America. In 2014, this disease was reported in 31 states in the United States (1). Protozoan intraerythrocytic parasites of the genus Babesia cause infection when transmitted by ticks or blood transfusions. Infections occur most frequently in spring or early summer, coinciding with the host-seeking activity of Ixodes scapularis nymphal ticks. Most cases occur in the northeastern upper midwestern United States. Most infections in the United States are caused by B. microti, which was first identified in 1966. Other Babesia species, including B. duncani and B. divergens–like/MO-1, have been rarely reported (2).
Clinical manifestations of babesiosis can range from asymptomatic to multiorgan failure. Severe illness is frequently seen in elderly, immunocompromised, and asplenic patients (3). We report a case of severe babesiosis caused by a B. divergens–like/MO-1 organism in an asplenic woman. This case was probably acquired in western Michigan.
The Study
A 60-year-old woman with hereditary spherocytosis status postsplenectomy and a history of pancreatic and colon cancer status post-Whipple procedure was hospitalized in 2017 because she had multiorgan failure. Fatigue, nausea, dyspnea, weakness, and chest pressure without fever had developed 5 days earlier. She was tachycardic and had jaundice but had otherwise reference (normal) vital signs.
Results of testing in the emergency department showed a leukocyte count of 20,800 cells/μL, hemoglobin 8.5 g/dL (reference 10.5 g/dL), creatinine of 5.3 mg/dL, lactate dehydrogenase 7,340 U/L, and haptoglobin <10 mg/dL, and increased levels of liver enzymes (aspartate aminotransferase 128 U/L, alanine aminotransferase 43 U/L, and total bilirubin 9.7 mg/dL). Peripheral blood smear results showed numerous intraerythrocytic parasites consistent with a Babesia sp.; parasitemia was 25%–30% (Figure, panel A).
Figure.
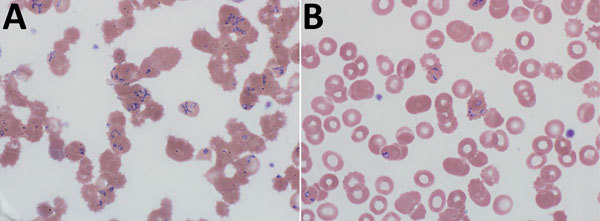
Peripheral blood films for a 60-year-old woman with probable locally acquired Babesia divergens–like infection, Michigan, USA. A) Before erythrocyte exchange transfusion. Parasitemia was 25%–30%. B) After erythrocyte exchange transfusion. Parasitemia was 3.5%. Original magnification ×1,000.
Given her multiorgan failure, the patient was transferred to a tertiary care center for exchange transfusion. At transfer, she was delirious and was admitted to the intensive care unit. She was given quinidine and clindamycin and underwent a 2-volume erythrocyte exchange transfusion. After exchange transfusion, parasitemia decreased to 3.5% (Figure, panel B). The following day she was given clindamycin (600 mg every 8 h), atovaquone (750 mg 2×/d), and azithromycin (250 mg/d) because of prolonged QTc.
Multiplex real-time PCR specific for a 204-bp region of the 18S rDNA gene (4) performed at a reference laboratory was positive for B. divergens–like/MO-1 and negative for B. microti and B. duncani. This result was confirmed by additional PCR testing at the Centers for Disease Control and Prevention (Atlanta, GA, USA). Serologic testing results were negative for Borrelia burgdorferi and Anaplasma phagocytophilum antibodies. No specific serologic analysis was performed for B. microti or B. duncani.
The patient required mechanical ventilation, pressor support, and renal replacement therapy. Serial peripheral blood smears showed the following consecutive parasitemia values over a 13-day period: 25%–30%, 3.5%, 2%, 1.8%, 0.5%, <0.1%, and 0%. Her hemoglobin and platelet levels returned to reference ranges during this period. Seven days after admission, she was extubated and renal function eventually improved. Antimicrobial drugs were continued after discharge for 4 weeks. At follow-up on day 29 postpresentation after her initial emergency department visit, her clinical status continued to improve, and repeat peripheral blood smears were negative for Babesia spp.
The patient gardened at her home in Berrien County, Michigan, USA, and walked along the Lake Michigan shoreline. She did not report any known tick bites, but the region is known to be endemic for Ixodes scapularis ticks. She had traveled to Kansas City, Missouri, USA, 2.5 weeks before symptom onset, but stayed in urban nonpark areas and did not have contact with animals. She did not have blood transfusions during the year before her illness. Given this history, the most likely source of disease acquisition was in Michigan.
Conclusions
We report a case of severe B. divergens–like/MO-1 infection in the upper midwestern United States. The patient probably contracted the disease from a tick in southwestern Michigan. She did not have any blood transfusions within the previous year, and the longest reported period between transfusion-transmitted babesiosis and a recipient diagnosis is 384 days (5).
Although B. microti is the predominant cause of babesiosis in the United States, 5 cases caused by B. divergens–like organisms have been reported. Reports include residents of Missouri (1992 and 2010), Kentucky (2001), Washington (2002), and Arkansas (2017) (4,6–8). All case-patients were asplenic and had high levels of parasitemia. Three of these 5 patients died. Neither of the survivors received an exchange transfusion. Of the patients who died, 1 received an exchange transfusion, 1 did not, and the status for the third patient was unknown. B. divergens–like/MO-1 parasites have also been identified in cottontail rabbits and Ixodes spp. ticks on Nantucket Island, Massachusetts, USA (9).
In Europe, B. divergens is the most frequent cause of human babesiosis (≈40 reported cases), although the seroprevalence might be higher; 13% of patients with Lyme disease were seropositive in Sweden (10,11). Further analysis of cases of B. divergens–like/MO-1 infection in the United States showed that this infection is distinct from that of B. divergens in Europe on the basis of sequence analysis, lack of infectiousness to cattle, and distinct morphologic differences when grown in vitro (12). A case of B. divergens–like infection was reported in a patient on the Canary Islands in 1994 (11). Sequence analysis showed similarities to B. divergens but neither the organism nor its vector was present on the islands. Two possible B. divergens–like species were also identified in China in 2011 (11).
The distribution and number of babesiosis infections in the United States is increasing. In 2014, babesiosis was reported in 31 states, compared with 27 in 2013. The number of reported cases increased from 1,126 in 2011 to nearly 1,744 in 2014. Seroprevalance data from disease-endemic regions ranged from 6% to 16% for B. microti, which suggests the reported disease prevalence is underestimated (2). In 2016, there were 2 confirmed cases of B. microti infection in residents of Michigan, both of whom lived near the Wisconsin border. However, B. microti was not detected in the tick vector.
Although microscopic features are similar for all human-infecting Babesia species, B. divergens and B. divergens–like organisms are more likely to have tetrad forms (Maltese cross forms) and accole forms on peripheral blood smears than B. microti. Octad forms are rarely seen.
Treatment recommendations for Babesia infections are made on the basis of data for B. microti because clinical information regarding B. divergens–like infections is limited to case reports and treatment recommendations are available elsewhere (3). A recent study supports treating persons who are immunosuppressed for >6 weeks, including persons with negative blood smears for 2 weeks before discontinuation of therapy (13). In case-patients with parasitemia >10% or evidence of end-organ dysfunction, an emergent automated erythrocyte exchange transfusion is indicated. A 2-volume erythrocyte exchange should lead to a 90% reduction of parasite load (14,15). This procedure not only removes parasite-infected erythrocytes but also removes vasoactive factors, including thromboplastic substances and cytokines, which contribute to development of disseminated intravascular coagulation and renal failure (14).
In summary, babesiosis is a potentially fatal disease caused by protozoan parasites of the genus Babesia. B. divergens–like/MO-1 infections are rare in the United States, and many patients who are infected had major illness and high mortality rates. Physicians should be alert for additional cases, particularly in asplenic persons. Further epidemiologic investigations of ticks in the upper midwestern United States for B. divergens–like organisms are warranted.
Acknowledgment
We thank the Centers for Disease Control and Prevention for confirming the diagnosis of B. divergens–like/MO-1 infection in this study.
Biography
Dr. Herc is a senior staff physician in the Division of Infectious Diseases, Henry Ford Health Systems, Henry Ford Hospital, Detroit, MI. Her research interests are antibiotic resistance and stewardship and global health.
Footnotes
Suggested citation for this article: Herc E, Pritt B, Huizenga T, Douce R, Hysell M, Newton D, et al. Probable locally acquired Babesia divergens–like infection in woman, Michigan, USA. Emerg Infect Dis. 2018 Aug [date cited]. https://doi.org/10.3201/eid2408.180309
References
- 1.Centers for Disease Control and Prevention. Surveillance for babesiosis—United States, 2014. Annual summary [cited 2018 May 19]. https://www.cdc.gov/parasites/babesiosis/resources/babesiosis_surveillance_summary_2016.pdf
- 2.Ord RL, Lobo CA. Human babesiosis: pathogens, prevalence, diagnosis and treatment. Curr Clin Microbiol Rep. 2015;2:173–81. 10.1007/s40588-015-0025-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 4.Burgess MJ, Rosenbaum ER, Pritt BS, Haselow DT, Ferren KM, Alzghoul BN, et al. Possible transfusion-transmitted Babesia divergens–like/MO-1 infection in an Arkansas patient. Clin Infect Dis. 2017;64:1622–5. 10.1093/cid/cix216 [DOI] [PubMed] [Google Scholar]
- 5.Tonnetti L, Eder AF, Dy B, Kennedy J, Pisciotto P, Benjamin RJ, et al. Transfusion-transmitted Babesia microti identified through hemovigilance. Transfusion. 2009;49:2557–63. 10.1111/j.1537-2995.2009.02317.x [DOI] [PubMed] [Google Scholar]
- 6.Herwaldt B, Persing DH, Précigout EA, Goff WL, Mathiesen DA, Taylor PW, et al. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med. 1996;124:643–50. 10.7326/0003-4819-124-7-199604010-00004 [DOI] [PubMed] [Google Scholar]
- 7.Beattie JF, Michelson ML, Holman PJ. Acute babesiosis caused by Babesia divergens in a resident of Kentucky. N Engl J Med. 2002;347:697–8. 10.1056/NEJM200208293470921 [DOI] [PubMed] [Google Scholar]
- 8.Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, et al. Babesia divergens-like infection, Washington State. Emerg Infect Dis. 2004;10:622–9. 10.3201/eid1004.030377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goethert HK, Telford SR III. Enzootic transmission of Babesia divergens among cottontail rabbits on Nantucket Island, Massachusetts. Am J Trop Med Hyg. 2003;69:455–60. [PubMed] [Google Scholar]
- 10.Uhnoo I, Cars O, Christensson D, Nyström-Rosander C. First documented case of human babesiosis in Sweden. Scand J Infect Dis. 1992;24:541–7. 10.3109/00365549209052642 [DOI] [PubMed] [Google Scholar]
- 11.Yabsley MJ, Shock BC. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2012;2:18–31. 10.1016/j.ijppaw.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman PJ, Spencer AM, Telford SR III, Goethert HK, Allen AJ, Knowles DP, et al. Comparative infectivity of Babesia divergens and a zoonotic Babesia divergens-like parasite in cattle. Am J Trop Med Hyg. 2005;73:865–70. [PubMed] [Google Scholar]
- 13.Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–6. 10.1086/525852 [DOI] [PubMed] [Google Scholar]
- 14.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the use of therapeutic apheresis in clinical practice evidence-based approach from the writing committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 2016;31:149–62. [DOI] [PubMed] [Google Scholar]
- 15.Spaete J, Patrozou E, Rich JD, Sweeney JD. Red cell exchange transfusion for babesiosis in Rhode Island. J Clin Apher. 2009;24:97–105. 10.1002/jca.20197 [DOI] [PubMed] [Google Scholar]


