Abstract
In this research a 100 day long treatment period was considered to unmask the probable adverse effects of long-term induced hyperthyroidism on histomorphometrical attributes of the oviduct in broiler breeder hens. A total of seventy 47-week-old Cobb 500 breeder hens were randomly allotted to two treatment groups (5 replicates of 7 hens each). Thyroxine (T4) was orally administered to the hyperthyroid group (0.3 mg/bird/d) for 100 consecutive days; whereas the control group received drinking water only throughout the trial. At 64 weeks of age, 2 birds per replicate were killed by cervical dislocation and their oviducts were removed. For histomorphometrical observations, segments were taken from five different regions. After tissue preparation and staining with haematoxylin and eosin, histological layers were evaluated using light microscopy. The assessment of histomorphometrical characteristics of oviduct showed the height of mucosal folds in the magnum, thickness of mucosal folds of the magnum and uterus, thickness of tunica muscularis in the magnum and vagina, epithelial thickness of the isthmus and vagina, and uterine tubular glands percentage were decreased in the hyperthyroid birds compared with the control counterparts. The results showed long-term induced hyperthyroidism was associated with a decrease in a number of histomorphometrical traits in different regions of the oviduct. Some studies should be done to clarify to what extent the long-term maternal hyperthyroidism might affect the egg production, fertility rate, duration of fertility, and sperm penetration rate to make a final decision on exploitation of this preventative treatment to diminish the ascites incidence in progeny chicks.
Key Words: Ascites, Breeder hen, Hyperthyroidism, Oviduct morphometry
Introduction
The oviduct in a mature hen is composed of five morphologically different portions including in-fundibulum, magnum, isthmus, uterus and vagina that each segment consists of tunica mucosa, tunica muscularis, and tunica serosa (Bakst, 1998 ▶). The epithelium of tunica mucosa has two types of cells, namely ciliated cells and non-ciliated cells (Mohammadpour et al., 2012 ▶). Some ciliated cells have secretory granules in their cytoplasm. The non-ciliated cells are known as goblet cells which are the secretory cells. The oviductal mucosa is responsible for reproductive achievement, such as gamete transport, fertilization, and early embryonic development (Yániz et al., 2000 ▶). The tunica mucosa consists of primary and secondary folds (Mohammadpour et al., 2012 ▶). The tunica muscularis is a smooth muscle consisting of two separate layers, inner circular and outer longitudinal muscles. The oviductal smooth muscle has multiple roles such as sperm transport to fertilization site and egg expulsion (Mohammadpour and Keshtmandi, 2008 ▶). The tunica serosa is consisted of a thin layer of connective tissue which is covered by mesothelium (Mohammadpour and Keshtmandi, 2008 ▶).
Infundibulum is divided to three sections which are morphologically different. The fimbriated region, which helps the passage of the ovulated ovum into the ostium; the funnel region, which is the first place that sperm meet the ovum. The chalaziferous region which has tubular glands which secrete material around the ovum (Mohammadpour and Keshtmandi, 2008 ▶; Kimaro, 2015 ▶). The longest region of the oviduct is magnum constituting 50% of the total oviductal length in domestic fowls (Kimaro et al., 2013 ▶). Shell membrane and hard shell of the egg are respectively produced by isthmus and uterus (Sharaf et al., 2013 ▶). The vagina provides a route to cloaca and produces the cuticle covering the egg before the oviposition (Sharaf et al., 2013 ▶). The hormonal factors influence the oviduct development. Several researchers reported that gonadal hormones such as estrogen affect the development of the oviduct (Yigit and Daglioglu, 2010 ▶).
Ascites syndrome is a metabolic disturbance, increasing the mortality rate and decreasing the weight gain (Julian, 1993 ▶). Among different approaches to decrease ascites incidence, Akhlaghi et al. (2012 ▶) showed an association between a 4-week-long maternal hyperthyroidism and decreased cold-induced ascites incidence in their progeny chicks. Furthermore, the same treatment did not adversely affect the immune responses (Akhlaghi et al., 2013a ▶) as well as growth performance and intestinal morphology (Akhlaghi et al., 2013b ▶) in their broiler chickens. However, other plausible effects of induced hyperthyroidism on reproductive traits including the oviduct morphology in the breeder hens should be dealt with prior to making any practical recommendations. To our knowledge, the effects of induced hyperthyroidism on the morphology of oviduct in broiler breeder hens have not been reported previously. Therefore, the present study was aimed at studying the probable adverse effects of extra thyroxine (T4) on the selected oviduct characteristics of breeder hens.
Materials and Methods
All procedures in the present work were approved by the Animal Care and Welfare Committee of Department of Animal Science, College of Agriculture, Shiraz University, Shiraz, Iran. A total of seventy 47-week-old Cobb 500 breeder hens were randomly allotted to two treatment groups (5 replicates of 7 hens each), including control and hyperthyroid. Thyroxine (Iran Hormone Pharmaceutical Company, Tehran) was orally administered by gavage to the hyperthyroid group (0.3 mg/bird/d) for 100 days; whereas the control group received the drinking water only throughout the trial. The birds were maintained under the same management fed a pelleted diet (NRC, 1994; Table 1). At 64 weeks of age, two birds per replicate were killed by cervical dislocation. After removing the oviduct, total weight of oviduct was recorded. The tissues were collected immediately after slaughter; the collected samples were washed in normal saline. For histomorphometrical observations, segments of ~2.5 cm were taken from five different regions (infundibulum, isthmus, uterus, magnum, and vagina). The segments were then gently flushed with ice-cold normal saline to remove the remaining contents and fixed in 10% buffered formalin for 48 h. After fixation, the tissue blocks were dehydrated by graded series of alcohol. The segments were then embedded in paraffin, sectioned at 5 µm and processed for standard histological techniques. After tissue preparation and staining with haematoxylin and eosin (H&E), histological layers such as serosa, tunica mucosa, and muscularis were recognized using light microscopy (Olympus 01BX51) at 40 magnification and primary and secondary folds of tunica mucosa were measured using micrometry method. Olympus 01BX51 light microscope with OLYSIA software installed was used to collect the images. The density of uterine glandular tissue was determined by quantifying the glandular area in a uterine mucosal fold and relating it to the total area of the fold. Five folds were quantified in each specimen using by graticule (Berg et al., 2001 ▶).
Table 1.
Ingredients and the chemical composition of experimental diets fed to breeder hens (DM basis
| Ingredient (%) | Value |
|---|---|
| Corn grain | 36.60 |
| Wheat grain | 25.00 |
| Barley grain | 13.40 |
| Soybean meal (44%) | 15.76 |
| Oyster shell | 7.06 |
| Vitamin premix 1 | 0.10 |
| Mineral premix 2 | 0.10 |
| Sodium chloride | 0.18 |
| Sodium bicarbonate | 0.16 |
| DL-Methionine | 0.095 |
| Dicalcium phosphate | 1.48 |
| L-Thr | 0.025 |
| L-Lys | 0.04 |
| Composition | |
| ME (kcal/kg) | 2700 |
| CP (%) | 14.00 |
| Ca (%) | 2.99 |
| P (%) | 0.36 |
Supplied per kg diet: vitamin A, 14,000 IU; vitamin D3, 3000 IU; niacin, 50 mg; vitamin E, 35 mg; calcium pantothenate, 20 mg; vitamin K3, 4 mg; riboflavin, 7.0 mg; pyridoxine, 5.7 mg; vitamin B12, 25 µg, and biotin, 50 µg.
Supplied per kg diet: Fe (FeSO4·H2O), 85 mg; Mn (MnSO4·H2O), 90 mg; Zn (ZnO), 67.3 mg; Cu (CuSO4·5H2O), 11.1 mg, and Se (Na2SeO3), 0.19 mg
Statistical analysis
The experiment was carried out as a completely randomized design. The data were tested for normality and subjected to the PROC GLM (SAS, 2003 ▶). The means were compared by the least squares procedure and the level of significance was set at P≤0.05.
Results
The results of this study showed the oviductal weight was not different between the control (96.3 ± 6.98 g) and hyperthyroid hens (93.2 ± 9.01 g). Table 2 presents the morphometric values for the different traits of infundibulum and magnum, where higher primary folds (4075.00 ± 215.05 µm) and thicker secondary folds (970.00 ± 106.38 µm) in the magnum were found for the control group. The effect of hyperthyroidism on the thickness of tunica muscularis was significant for magnum (P<0.05). Hyperthyroidism was associated with a decreased thickness of muscularis in the magnum. The thickness of tunica serosa in the magnum was decreased in the hyperthyroid hens (P<0.05); whereas, the height of secondary folds, the thickness of primary folds, and the thickness of epithelium in the infundibulum and magnum did not differ significantly (P>0.05). Histology of magnum in Cobb breeder hens is illustrated in Figs. 1A-B. The thickness of primary folds and serosa in the uterus of the control birds were greater than that of the hyperthyroid counterparts (P<0.05; Table 3). Moreover, the epithelial thickness of isthmus in the hyperthyroid group was smaller than that of the control group, although other traits of isthmus and uterus were not affected by T4 administration (Table 3). The effect of induced hyperthyroidism on the thickness of tunica muscularis in the vagina was significant (P<0.05), where the higher thickness of vaginal tunica muscularis was recorded for the control group (522.91 ± 15.01 µm). The effect of treatment on the tunica serosa and the epithelial thickness were significant in vagina (P<0.05; Tables 4); however, the height of primary and secondary folds and the thickness of primary and secondary folds in vagina were not affected by long-term hyperthyroidism. In this study a higher mean value of epithelial thickness was seen for vagina in the control group (29.50 ± 1.97 µm); whereas the lower record was observed for epithelial thickness in the infundibulum of hyperthyroid hens (15.62 ± 3.82 µm). The effect of long-term induced hyperthyroidism on the uterine glandular density was significant (P<0.05). Uterine glandular density was 86.00 ± 5.5% for the control groups which was reduced to 46.40 ± 5.53% in the hyperthyroid group. Histology of uterus and the uterus glands in Cobb breeder hens have shown in Figs. 2A-B.
Table 2.
Mean (±SE) morphometric values for different traits of infundibulum and magnum in breeder hens 1
| Trait (μm) | Infundibulum |
P-value | Magnum |
P-value | ||
|---|---|---|---|---|---|---|
| Control | Hyperthyroid | Control | Hyperthyroid | |||
| Height of primary folds | 2012.5±371.85 | 1581.2±371.85 | NS | 4075.0±215.05a | 3200.0±215.05b | 0.0206 |
| Height of secondary folds | 500.0±146.19 | 506.2±146.19 | NS | 1560.0±206.62 | 1465.0± 06.62 | NS |
| Thickness of primary folds | 590.0±45.46 | 550.0±58.68 | NS | 1425.0±119.29 | 1162.5±133.37 | NS |
| Thickness of secondary folds | 305.0±69.14 | 341.6±89.26 | NS | 970.0±106.38a | 462.5±118.94b | 0.0155 |
| Thickness of tunica muscularis | 43.6±3.76 | 35.5±3.76 | NS | 69.1±1.89a | 57.5±1.89b | 0.0024 |
| Thickness of serosa | 168.7±13.26 | 125.0±13.26 | NS | 220.0±19.73a | 137.5±22.05b | 0.0270 |
| Thickness of epithelium | 22.0±3.42 | 15.6±3.82 | NS | 22.5±3.45 | 23.75±3.86 | NS |
Within rows and for each region, values with different superscripts differ significantly (P≤0.05).
Thyroxine was orally administered to the hyperthyroid group (0.3 mg/bird/day) for 100 days and the control group received the drinking water only during 47 to 64 weeks of age. The tissues were collected after slaughter for histological observations at 64 weeks of age
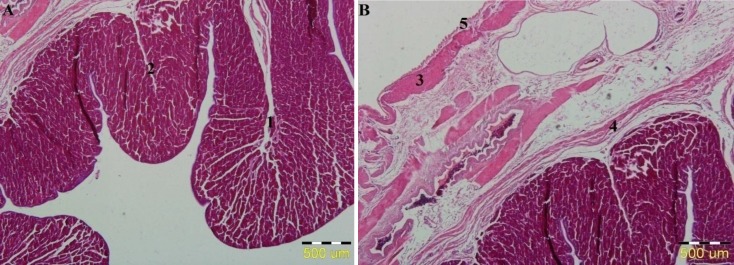
Fig. 1.
Light micrograph of magnum (A): primary fold (1), and secondary fold (2). (B): Tunica muscularis: outer longitudinal muscle (3), inner circular muscle (4), and tunica serosa (5) (H&E, ×100). Thyroxine was orally administered to the hyperthyroid group (0.3 mg/bird) for 100 successive days and the control group received the drinking water only
Table 3.
Mean (±SE) morphometric values for different traits of isthmus and uterus in breeder hens 1
| Trait (μm) | Isthmus |
P-value | Uterus |
P-value | ||
|---|---|---|---|---|---|---|
| Control | Hyperthyroid | Control | Hyperthyroid | |||
| Height of primary folds | 2950.0±295.93 | 3070.0±295.93 | NS | 2325.0±226.28 | 2765.0±226.28 | NS |
| Height of secondary folds | 1335.0±102.80 | 1215.0±102.80 | NS | 620.0±137.81 | 695.0±137.81 | NS |
| Thickness of primary folds | 825.0±49.37 | 750.0±49.37 | NS | 706.2±81.25a | 393.7±81.25b | 0.034 |
| Thickness of secondary folds | 480.0±35.79 | 460.0±35.79 | NS | 415.0±74.70 | 233.3±96.44 | NS |
| Thickness of tunica muscularis | 67.1±1.02 | 66.1±1.02 | NS | 109.0±13.29 | 99.6±13.29 | NS |
| Thickness of serosa | 145.0±22.81 | 103.3±22.81 | NS | 135.0±9.01a | 100.0±9.01b | 0.0252 |
| Thickness of epithelium | 25.0±1.64a | 16.0±1.64b | 0.0047 | 26.0±3.33 | 18.7±3.72 | NS |
Within rows and for each region, values with different superscripts differ significantly (P≤0.05).
Thyroxine was orally administered to the hyperthyroid group (0.3 mg/bird) for 100 days and the control group received the drinking water only during 47 to 64 weeks of age. The tissues were collected after slaughter for histological observations at 64 weeks of age
Table 4.
Mean (±SE) morphometric values for different traits of vagina in breeder hens 1
| Parameter (μm) | Control | Hyperthyroid | P-value |
|---|---|---|---|
| Height of primary folds | 2295.0 ± 164.19 | 2437.5 ± 183.57 | NS |
| Height of secondary folds | 805.0 ± 96.12 | 841.6 ± 124.08 | NS |
| Thickness of primary folds | 275.0 ± 18.39 | 212.5 ± 18.39 | NS |
| Thickness of secondary folds | 225.0 ± 26.39 | 181.2 ± 26.39 | NS |
| Thickness of tunica muscularis | 522.9 ± 15.01a | 458.3 ± 16.78b | 0.0241 |
| Thickness of serosa | 134.0 ± 8.80a | 97.0 ± 8.80b | 0.0178 |
| Thickness of epithelium | 29.5 ± 1.97a | 20.0 ± 1.97b | 0.0092 |
Within rows and for each region, values with different superscripts differ significantly (P≤0.05).
Thyroxine was orally administered to the hyperthyroid group (0.3 mg/bird) for 100 days and the control group received the drinking water only during 47 to 64 weeks of age. The tissues were collected after slaughter for histological observations at 64 weeks of age
Fig. 2.
Light micrograph of uterine glands in control (A) and hyperthyroid (B) hens. Arrow: uterine glands (H&E, ×40). Thyroxine was orally administered to the hyperthyroid group (0.3 mg/bird) for 100 successive days and the control group received the drinking water only
Discussion
In an effort by Akhlaghi et al. (2012 ▶) a 4-week-long maternal administration of T4 in the drinking water caused ascites incidence to diminish in cold-induced progeny chicks, which lead to current study to determine how long-term hyperthyroidism for 100 days, recommended for reducing ascites in the progeny, can affect on breeder hens reproductive traits such as histomorphometrical changes of different regions of the oviduct. It seemed that this research is the first study to determine these effects in breeder hens.
The infundibulum has secretory function and secretes the egg coat, the chalazae to set the embryo in proper position. The neck of the infundibulum is the reservoir for the spermatozoa (Mohammadpour and Keshtmandi, 2008 ▶). Therefore, infundibulum function can be affected by any various changes influencing on fertility; however, in this experiment, none of the morphometrical traits in the infundibulum was different in hyperthyroid state.
The present study showed the height and the thickness of the mucosal folds in the magnum decreased in hyperthyroid hens. The magnum epithelial cells have an essential role in egg formation, if any deformities occur in these cells, the quality of egg white will be affected adversely (Kimaro et al., 2013 ▶; Saemi et al., 2018 ▶). Palmiter and Gutman (1972 ▶), reported the formation of ovalbumin, conalbumin, ovomucoid and lysozyme take place in chick oviduct magnum. The firmness of albumen is an important factor that affects on egg quality (Kimaro et al., 2013 ▶). Besides, Silversides and Scott (2001 ▶) indicated albumen height determined Haugh units, which is an indicator of egg quality. In this study long-term hyperthyroidism decreased height and the thickness of the mucosal folds in the magnum and these morphological changes might adversely affect the egg quality. Although in this research the effect of long-term hyperthyroidism on egg quality was not evaluated.
The results of this morphometric study showed the decrease in the epithelial thickness of isthmus, the thickness of primary folds and the serosa in the uterus, and the uterine tubular glands percentage in the hyperthyroid hens. It is well known that the shell membrane and egg shell are produced by the isthmus and shell gland (uterus), respectively (Sharaf et al., 2013 ▶; Qi et al., 2016 ▶). Both alkaline phosphatase and carbonic anhydrase activities in isthmus and uterus are thought to affect on egg shell calcification mechanism (Rodríguez-Navarro et al., 2015 ▶). The association was found between the egg production and egg shell thickness of the hens with the alkaline phosphatase activity in their blood plasma (Gutowska et al., 1943 ▶). Therefore, any malformation in the isthmus and uterus structure can affect on egg production and egg shell thickness adversely. Although in this experiment the effect of thyroxine administration on egg production and egg shell thickness were not assessed.
Overall, in this experiment the long-term induced hyperthyroidism caused a decrease in the height of the mucosal folds in the magnum, the thickness of mucosal folds of the magnum and the uterus, the thickness of tunica muscularis in the vagina, the epithelial thickness of the isthmus and the vagina, and uterine tubular glands percentage. These structural histomorphometrical changes in oviduct can be ascribed to thyroid hormones by affecting on steroid hormones. The effect of gonadal steroids on the development of the oviduct associated with thyroid hormones was investigated by Klandorf et al. (1992 ▶) who injected thyroid hormone (intra-muscularly) to broiler breeder pullets for assessing the histological changes of the shell gland. They observed the height of the epithelial cells and the uterine tubular glands were reduced in hyperthyroidism state. Also, they reported thyroid hormones decreased the concentration of plasma estradiol. Estrogen has an essential role for differentiation and development of the epithelial cells and tubular glands in the oviduct (Kohler et al., 1969 ▶; Yigit and Daglioglu, 2010 ▶). As mentioned before, the influence of thyroid hormones on clearance of gonadal steroids, the diminishing of some histomorphometrical traits in hyperthyroid breeder hens can be ascribed to reduced concentration of plasma estradiol, however the estradiol concentration was not determined in this study.
Overall, in this research the long-term T4 treatment reduced some histomorphometrical traits in breeder hens’ oviduct. The effects of structural malformations in oviduct of breeder hens at long-term hyperthyroidism state on fertility, duration of fertility, sperm penetration rate, egg quality, hatchability, and chick quality remain to be elucidated to make commercial recommendations for diminishing the incidence of ascites in progeny chicks.
Thyroid hormones affect on oviduct development and histomorphometrical and histological changes. In this research some histomorphometrical traits were reduced by long-term induced hyperthyroidism and might affect adversely on reproductive traits. Providing hyper-thyroidism as an application for reducing ascites in the chicks produced from hyperthyroid breeder hens might have plausible effects on another reproductive performance, therefore further studies are needed to recommend this treatment.
Acknowledgements
The authors wish to express their appreciation to the Research Council of University and the academic members of the Animal Science Department for their technical assistance.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Akhlaghi, A , Zamiri, MJ , Jafari Ahangari, Y , Atashi, H , Ansari Pirsaraei, Z , Deldar, H , Eghbalian, AN , Akhlaghi, AA , Navidshad, B , Yussefi Kelarikolaei, K , Hashemi, SR Oral exposure of broiler breeder hens to extra thyroxine modulates early adaptive immune responses in progeny chicks. Poult. Sci. 2013a;92:1040–1049. doi: 10.3382/ps.2012-02545. [DOI] [PubMed] [Google Scholar]
- Akhlaghi, A , Zamiri, MJ , Jafari Ahangari, Y , Mollasalehi, MR , Shojaie, H , Atashi, H , Navidshad, B , Akhlaghi, AA , Dadpasand, M Growth performance and intestinal morphology in broiler chickens produced from hyperthyroid breeder hens. Anim. Prod. Sci. 2013b;53:1046–1051. [Google Scholar]
- Akhlaghi, A , Zamiri, MJ , Jafari Ahangari, Y , Nejati Javaremi, A , Rahimi Mianji, G , Mollasalehi, MR , Shojaie, H , Akhlaghi, AA , Deldar, H , Atashi, H , Ansari Pirsaraei, Z , Zhandi, M Maternal hyper-thyroidism is associated with a decreased incidence of cold-induced ascites in broiler chickens. Poult. Sci. 2012;91:1165–1172. doi: 10.3382/ps.2011-02021. [DOI] [PubMed] [Google Scholar]
- Bakst, M Structure of the avian oviduct with emphasis on sperm storage in poultry. J. Exp. Zool. 1998;282:618–626. [PubMed] [Google Scholar]
- Berg, C , Holm, L , Brandt, I , Brunstrom, B Anatomical and histological changes in the oviducts of Japanese quail, Coturnixcoturnix japonica, after embryonic exposure to ethynyloestradiol. Reproduction. 2001;121:155–165. doi: 10.1530/rep.0.1210155. [DOI] [PubMed] [Google Scholar]
- Gutowska, MS , Parkhurst, RT , Parrott, EM , Verburg, RM Alkaline phosphatase and egg formation. Poult. Sci. 1943;22:195–204. [Google Scholar]
- Julian, RJ Ascites in poultry. Avian Pathol. 1993;22:419–454. doi: 10.1080/03079459308418934. [DOI] [PubMed] [Google Scholar]
- Kimaro, WH Effect of carbendazim® in the in-fundibulum of the Japanese quail: morphometrical and ultrastructural studies. Toxicol. Environ. Health Sci. 2015;7:58–64. [Google Scholar]
- Kimaro, WH , Madekurozwa, MC , Groenewald, HB Histomorphometrical and ultrastructural study of the effects of carbendazim on the magnum of the Japanese quail (Coturnixcoturnix japonica) Onderstepoort J. Vet. 2013;80:1–17. doi: 10.4102/ojvr.v80i1.579. [DOI] [PubMed] [Google Scholar]
- Klandorf, HB , lauwiekel, R , Qin, X , Russell, RW Characterization of a sustained-release estrogen implant on oviduct development and plasma Ca concentrations in broiler breeder chicks: modulation by feed restriction and thyroid state. Gen. Comp. Endocr. 1992;86:469–482. doi: 10.1016/0016-6480(92)90072-r. [DOI] [PubMed] [Google Scholar]
- Kohler, PO , Grimley, PM , O’Malley, BW Estrogen-induced cytodifferentiation of the ovalbumin-secreting glands of the chick oviduct. J. Cell Biol. 1969;40:8–27. doi: 10.1083/jcb.40.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour, AA , Keshtmandi, M Histo-morphometrical study of infundibulum and magnum in turkey and pigeon. World J. Zool. 2008;3:47–50. [Google Scholar]
- Mohammadpour, AA , Zamanimoghadam, A , Heidari, M Comparative histomorphometrical study of genital tract in adult laying hen and duck. Vet. Res. Forum. 2012;3:27–30. [PMC free article] [PubMed] [Google Scholar]
- NRC. Nutrient requirements for poultry. New York: National Research Council; 1994. [Google Scholar]
- Palmiter, RD , Gutman, GA Fluorescent antibody localization of ovalbumin, conalbumin, ovomucoid and lysozyme in chick oviduct magnum. J. Biol. Chem. 1972;247:6459–6461. [PubMed] [Google Scholar]
- Qi, X , Tan, D , Wu, C , Tang, C , Li, T , Han, X , Wang, J , Liu, C , Li, R , Wang, J Deterioration of eggshell quality in laying hens experimentally infected with H9N2 avian influenza virus. Vet. Res. 2016;47:35. doi: 10.1186/s13567-016-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro, AB , Marie, P , Nys, Y , Hincke, MT , Gautron, J Amorphous calcium carbonate controls avian eggshell mineralization: a new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015;190:291–303. doi: 10.1016/j.jsb.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Saemi, F , Shahneh, AZ , Zhandi, M , Akhlaghi, A , Khaksar, Z , Dadpasand, M Long-term effects of thyroxine-induced hyperthyroidism on the histological attributes of the oviduct in broiler breeder hens. Comp. Clin. Path. 2018;27:605–609. [Google Scholar]
- SAS. User’s Guide: Statistics. Version 9.1 Edn. Cary, NC: Inst., Inc; 2003. [Google Scholar]
- Sharaf, A , Eid, W , Abuel-Atta, AA Age-related morphology of the ostrich oviduct (isthmus, uterus and vagina) Bulg. J. Vet. Med. 2013;16:145–158. [Google Scholar]
- Silversides, FG , Scott, TA Effect of storage and layer age on quality of eggs from two lines of hens. Poult. Sci. 2001;80:1240–1245. doi: 10.1093/ps/80.8.1240. [DOI] [PubMed] [Google Scholar]
- Yániz, JL , Lopez-Gatius, F , Santolaria, P , Mullins, KJ Study of the functional anatomy of bovine oviductal mucosa. Anat. Rec. 2000;260:268–278. doi: 10.1002/1097-0185(20001101)260:3<268::AID-AR60>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yigit, F , Daglioglu, S Histological changes in the uterus of the hens after embryonic exposure to bisphenol A and diethylstilbestrol. Protoplasma. 2010;247:57–63. doi: 10.1007/s00709-010-0140-x. [DOI] [PubMed] [Google Scholar]