Abstract
Tuberculosis (TB) is a zoonotic infectious disease common to humans and animals which has been caused by a rod shaped, acid fast bacterium, called Mycobacterium bovis. The rapid and sensitive detection is a great challenge for TB diagnosis. The virulent strains of Mycobacterium tuberculosis complex (MTBC) have 16 different regions of difference (RD) in their genome which encode some important antigens. The major protein of M. bovis 64 (MPT-64) is one of the main immune-stimulating antigens which are encode by RD-2 region. The aim of the present study was cloning, expression and purification of MPT-64 as a protein antigen of M. bovis in a prokaryotic system for the usage in the future diagnostic studies. In this experimental study, the mpt-64 gene with 687 bp has been proliferated from M. bovis whole genome by polymerase chain reaction (PCR) method. The PCR product has been digested by BamHI and HindIII restriction enzymes and cloned into pQE-30 plasmid. The recombinant protein has been expressed in the Escherichia coli M15 with induction by isopropyl-β-D-thiogalactopyranoside (IPTG). The expressed protein was analyzed on SDS-PAGE, and purified with Nitrilotriacetic acid (Ni-NTA) column. Finally, its biological properties were confirmed in Western blotting method using specific antibodies. Data showed the successful cloning of mpt-64 gene (as a 687 bp segment) in expression vector. The MPT-64 recombinant protein was ideally expressed and purified as a 24 kDa protein. The result of this study indicated that MPT-64 recombinant protein (24 kDa) has been successfully expressed and purified in a prokaryotic system, so this protein could be used for differential diagnosis of pathogenic and non-pathogenic Mycobacterium, in suspected BTB cases.
Key Words: Cloning, MPT-64 protein, Mycobacterium bovis, Recombinant protein
Introduction
Bovine tuberculosis (BTB) is a chronic bacterial disease and it is still common in developing countries and causes severe economic losses due to livestock deaths, chronic disease and trade restrictions. The causative agent of BTB, Mycobacterium bovis, is resistant to heat, drought and most disinfectants (Ayele et al., 2004 ▶). It belongs to the M. tuberculosis complex (MTBC) that comprises the closely related human pathogens M. tuberculosis and M. africanum (Wayne and Kubica, 1986 ▶). Unlike M. tuberculosis, M. bovis infects cattle and other animals, and so the disease can be spread to humans via contaminated milk and meat (Okada and Kobayashi, 2007 ▶).
Mycobacterium bovis mainly enters the body through breathing (Smith, 2003 ▶). In some situations, BTB may also be a serious threat to endangered species. Consequently, about 70% of the cattle bred in Latin America are held in areas with high disease prevalence and nearly 17% in areas virtually free from BTB (De Kantor and Ritacco, 2006 ▶). According to OIE (in 2010-2014) 501-1000 cases of bovine TB have been reported in Iran.
Despite the various methods of treatment, there is still death (Dye et al., 1998 ▶). The diagnosis is difficult based on the clinical symptoms in live animals, because the economic life of animals is short and disease is chronic (Smith, 2003 ▶; Baumann et al., 2006 ▶). There are several methods for detection of BTB such as molecular, serological and traditional culture methods. Unfortunately, according to common antigenic similarity of M. bovis with other mycobacteria, serological diagnosis is considered as a low sensitive method (Cho, 2007 ▶; Lange and Mori, 2010 ▶). Bacterial culture is still the most desirable option for a definitive diagnosis of the disease but bacterial growth is very slow. However, the tuberculin skin test (TST) which has been used for detection of infected animals has very low sensitivity and could not distinguish the right cases (Daniel and Janicki, 1978 ▶; Young, 1992 ▶; Coler et al., 2000 ▶; Parkash et al., 2009 ▶). The method of measuring gamma interferon has high sensitivity but is expensive. Methods of identifying antibodies have been developed in various forms however enzyme linked immunosorbent assay (ELISA) is still a reputable method (Jolley et al., 2007 ▶).
The virulent strains of MTBC have 16 different regions of difference (RD) in their genome which encode some important antigens and one of the main immune-stimulating antigens is specific antigen major protein of M. bovis 64 (MPT-64) which is encoded by RD-2 region but this region is absent in Bacillus Calmette Guerin (BCG) strains and non-pathogenic or environmental mycobacteria (Fan et al., 2009 ▶; Parkash et al., 2009 ▶; Bao et al., 2013 ▶). So, the rapid and precise detection is important for TB which has been diagnosed by specific antigens. The purpose of the present work was production of MPT-64 recombinant protein from virulent strain of M. bovis for the usage in the diagnostic studies.
Materials and Methods
Materials
All the chemicals were purchased from Sigma (Sigma Aldrich) except when otherwise noted. Restriction endonucleases and T4 DNA ligase used in cloning and transformation experiments were provided from GeneAll Biotechnology Company (Seoul, Korea) Plasmid Mini kit and QIAquick Gel Extraction kit were obtained from Qiagen (GmbH, Hilden, Germany). Vector pQE30 and Escherichia coli M15 were procured from Iranian Recombinant GeneBank (Institute Pasteur, Tehran). Ordered primers were synthesized by Pishgam Company (Tehran, Iran). M. bovis AN5 standard strain was obtained from Razi Vaccine and Serum Research Institute (Tuberculin Department).
Methods
Bacterial culture and DNA extraction
The M. bovis AN5 standard strain was cultured on Loweinstein-Jensen medium supplemented with pyruvate and was incubated at 37°C for a month and grown bacteria were used for DNA extraction (Van Soolingen et al., 2001 ▶). Bacterial DNA extraction was performed according to the Cetyltrimethyl ammonium bromide/NaCl (CTAB/NaCl) method. This was done on the basis of van Solingens protocol for DNA extraction (Van Soolingen et al., 2001 ▶). The quality and quantity of purified DNA was estimated by Nano-Drop spectrophotometer at a wave length of 260/280 nm.
Primers and polymerase chain reaction
To determine the antigenic region and primer design, mpt-64 gene sequence with UniProt accession number: L0TB55 that contains 229 amino acids, was prepared [National Center for Biotechnology Information (NCBI) GeneBank]. Then, using bioinformatics software Swiss Model appropriate PCR primers were designed by software program Oligo-7 to amplify 687 bp fragment of mpt-64 gene from M. bovis genome, which encoded the MPT-64 protein. The uniqueness of the primers was confirmed by the BLAST Program. Primers were synthesized by Pishgam Company. The forward and the reverse primers sequences are as: 5´ ATC GGA TCC TCA CGG CTG TCG TTT TGC T 3´ (BamHI) and 5´ AAC GTG GGA CCA ATA CAA GCT TCT G 3´ (HindlII).
Amplification of gene was performed by PCR in volume of 30 μL and using DNA template 2 μL, 1.5 μL of each primer (10 pm), 15 μL Master Mix RED (taq 2X, 1.5 mM MgCl2) and 10 μL double distilled water (DDW).
PCR program was performed in standard condition as below: initial denaturation (94°C for 5 min, one cycle), denaturing (94°C for 45 s, 35 cycles), annealing (55°C for 30 s, 35 cycles) and extension (72°C for 45 s, 35 cycles), final amplification step (94°C for 5 min, one cycle). The 687 bp PCR products were checked by electrophoresis on 1% agarose gel. After staining with ethidium bromide solution (10 mg/ml), the gel was observed with UV transiluminator. Finally, the PCR product was purified through Qiagen mini columns as per manufacturer’s instruction.
Cloning and expression of recombinant plasmid pQE30-mpt64
For cloning of mpt-64 gene in the pQE30 vector, the PCR product was digested with restriction endonucleases HindlII and BamHI and then cloned in the pQE30 vector pre digested with the same enzymes. The ligation of mpt-64 into pQE30 was performed using T4 DNA ligase at 22°C for 1 h.
pQE30-mpt64 recombinant plasmid was transformed into E. coli M15 cells. In order to verify cloning, the transformants were screened with restriction enzyme analysis using BamHI and HindIII and PCR. Furthermore, to confirm the nucleotide sequences of PCR product, recombinant plasmid was sent to Nedayefan Co. (Iran) and the gene sequence was determined by Sanger method.
The plasmid pQE30-mpt64 from the correct transformant was purified from an overnight culture of recombinant E. coli DH5α cell and then transformed into the competent E. coli M15 cells and screened on LB plates containing 50 μg/ml ampicillin and 30 μg/ml kanamycin.
For the production of recombinant MPT-64 protein, the transformed E. coli M15 cells harboring the pQE30-ES plasmid was used in the expression. Several transformed E. coli were cultured in 50 ml LB medium supplemented with ampicillin (50 μg/ml) and kanamycin (30 μg/ml) at 37°C in a shaking incubator for 18 h. When OD A600 reached 0.55, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to con-centration of 0.5 and 1 mM and incubation was continued for 3 h. Sampling was performed before and after induction and was designed as T0, T1, T2, and T3. Protein expression was checked by electrophoresis in 15% SDS-polyacrylamide gel and 4% stacking gel (Sambrook and Russell, 2001 ▶). The bacterial cells were centrifuged at 13,000 rpm for 5 min at 4°C and the precipitate was suspended in 50 μL of sample-buffer and boiled for 15 min and 10 μL of each sample was loaded onto the gel and run with 120 V.
Purification
Due to Histidine sequence (6 His-tag), in N-terminal expressed protein that has been added by expression plasmid purification was carried out by Nitrilotriacetic acid (Ni-NTA) agarose resin. For this purpose, first the cells were destroyed using buffer A with pH = 8 (NaH2PO4 100 mM, Tris-HCl 10 mM, Urea 8 M). Then to 4 ml of the supernatant 1 ml Ni-NTA agarose resin was added. In the next step, resin was washed with 3 ml buffer B (same buffer A but pH = 6.3). Then protein was washed with 3 ml buffers C1, C2, C3, C4, and C5 (pH = 8). For the final separation of protein buffer E (pH = 8) and buffer E with EDTA (pH = 8) were used. Output solutions column were dissolved in sample buffer and assessed using SDS-PAGE. The protein concentration was determined with Bradford assay. In this method, three standards 10, 100, 1000 mg/ml of bovine serum albumin (BSA) in phosphate buffered saline (PBS) were prepared. 10 μL of each standard and sample containing protein was added to 200 μL Bradford reagent and OD595nm was read by spectrophotometer.
Western blot analysis
To investigate the presence and purity of recombinant protein, Western blot method was used (Sambrook and Russell, 2001 ▶). For this reason, the purified recombinant protein was electrophoresed and transferred from the acrylamide gel on nitrocellulose membrane with transfer buffer (Tris-base 2.45 g, Glysin 11.26 g, Methanol 200 ml and DDW to the final volume of 1 L) with pH = 8 at a voltage of 110 V for 2 h. The membrane was blocked with 0.5% solution of BSA at 4°C overnight and incubated for 1 h with operating dilutions of 1/500 Anti-His-Tag antibody at 37°C. Antigen-antibody complex was detected using rabbit antimouse antibodies conjugated with horseradish peroxidase (Sigma-Aldrich) in the operating dilution. The complex was incubated with the conjugate for 1 h at 37°C. After three washing steps with TBS-T (Tris-base 2.42 g, NaCL 8.78 g and with DDW volume of 1 L was reached and then 0.5 ml Tween 20) with pH = 7.4 was added. Then the membrane was stained in a solution of horseradish peroxidase substrate, 3, 3´-diaminobenzidine tetrahydrochloride (DAB).
Results
Amplification of mpt-64 gene
The concentration of genomic DNA obtained from milky colonies of M. bovis grown on Lowenstein-Jensen medium was estimated 769.8 ng/µL. This DNA, as template was used to amplify antigenic region of mpt-64 gene. After running a standard PCR protocol with designed primers from M. bovis genome, a distinct band, greater than 500 bps band appeared in which the size of the amplified gene was 687 nucleotides compared with marker (Fig. 1).
Fig. 1.
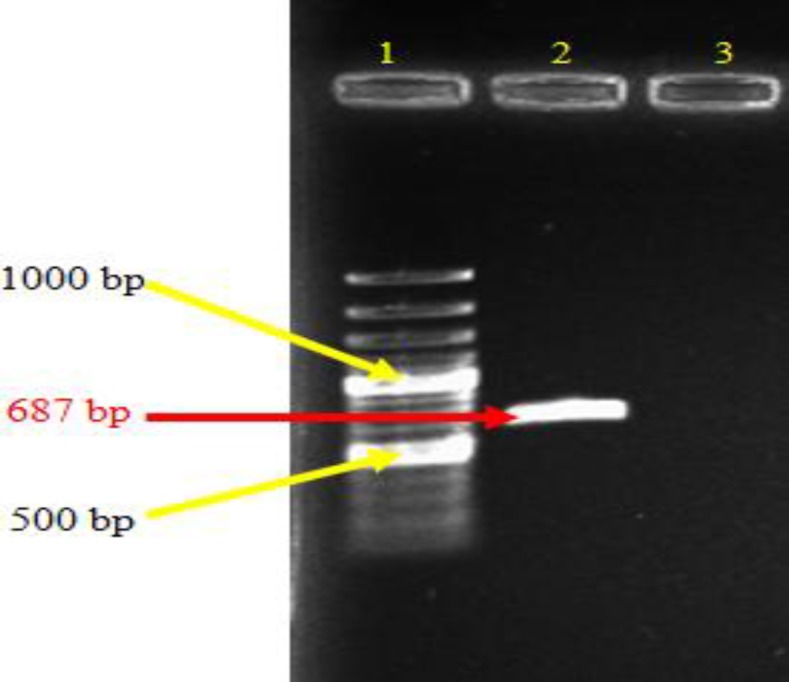
Amplifications of mpt-64 gene from M. bovis genome by PCR. Lane 1: DNA ladder 100 bp (Fermentas, #SM0248), Lane 2: PCR product and Lane 3: Negative control
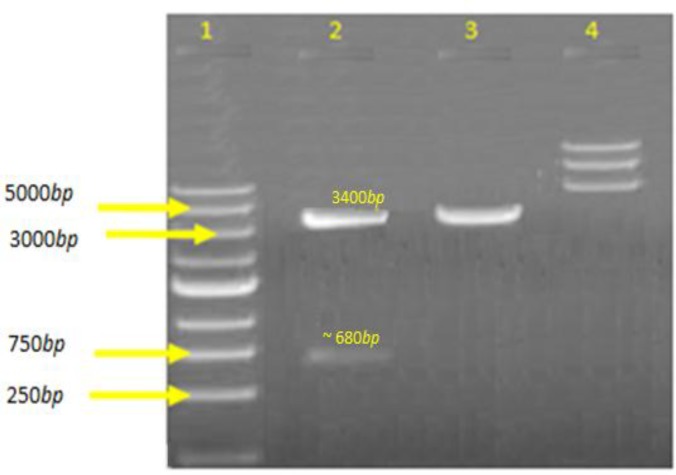
After restriction analysis of recombinant vector with HindIII and BamHI enzymes, an approximately 680 bp band of the mpt-64 gene with a ~3.4 kbp band for the remaining backbone of pQE30 vector appeared (Fig. 2). Finally, DNA sequencing confirmed that the mpt-64 gene was successfully cloned into pQE30. The result of comparison between the amplified genes sequence provided for mpt-64 gene that is registered in the NCBI GeneBank was the same with 100% homology.
Fig. 2.
Restriction analysis of recombinant plasmid pQE30-mpt64 by HindIII and BamHI. Two separated fragments were seen. Lower is ~680 bps band of the mpt-64 gene together with a part of the pQE30 backbone and upper is ~3.4 kbp band for the remaining backbone of pQE30 vector in lane 2. Lane 1: Ladder fermentas, Lane 2: Digested with BamHI/HindIII, Lane 3: Single digested, and Lane 4: Undigested
Expression of the protein MPT-64
The MPT-64 recombinant protein was produced in E. coli M15 using the His-tag cloning system and pQE30 vector. The expression of recombinant protein was induced after addition of IPTG and reached to its maximum levels after 3 h. The recombinant MPT-64 protein was confirmed with molecular weight around 24 kDa by 15% SDS-PAGE and a band was detected as recombinant protein MPT-64-(His) (Fig. 3). The highest expression was observed around 24 kDa in T3 of the pellet samples in which induction with 1 mM IPTG had more expression and no band was observed in the negative control (Fig. 3). So, the results procured from SDS-PAGE indicated that the MPT-64 recombinant protein has been successfully expressed in E. coli M15. Protein was purified with Ni-NTA method that contains two free sites for interacting with His-tag (Fig. 4). Then protein concentration in final solution was 0.5 mg/ml. The reaction of recombinant MPT-64 protein by commercial anti-MPT-64 antibody was observed as ~24 kDa band in Western blotting analysis (Fig. 5).
Fig. 3.
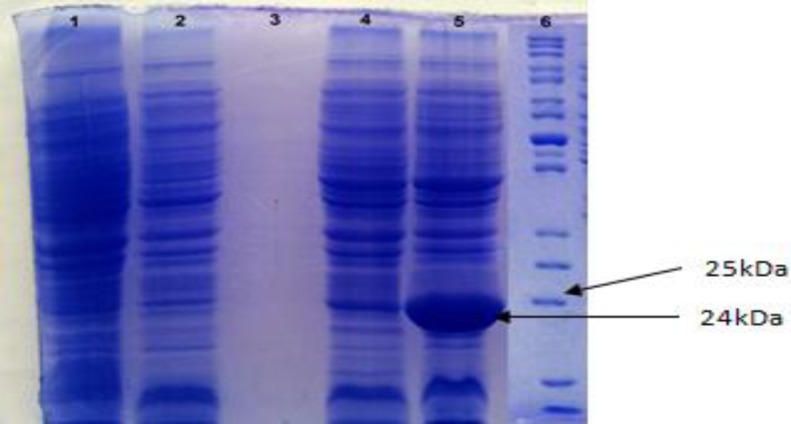
Expression of the MPT-64 recombinant protein. Distinct band around 24 kDa was observed in Lane 5, after 3 h induction with IPTG 1 mM. Lane 1: Before induction with IPTG (T0), Lane 2: After 1 h induction with IPTG (T1), Lane 3: Supernatant, after 3 h induction with IPTG, Lane 4: After 2 h induction with IPTG (T2), Lane 5: After 3 h induction with IPTG (T3), and Lane 6: Protein ladder (Fermentas, #SM0661
Fig. 4.
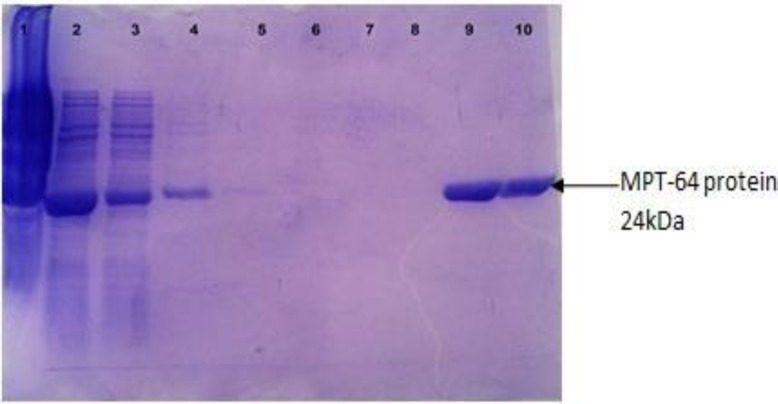
Purification of the protein MPT-64 with Ni-NTA. Lanes 1, 2, 3: Before purification, Lane 4: Washing with B buffer, Lane 5: Washing with C1 buffer, Lane 6: Washing with C2 and C3 buffers, Lane 7: Washing with C4 and C5 buffers, Lane 8: Washing with wash buffer, Lane 9: After purification with Elution buffer, and Lane 10: After purification with Elution and EDTA buffer
Fig. 5.
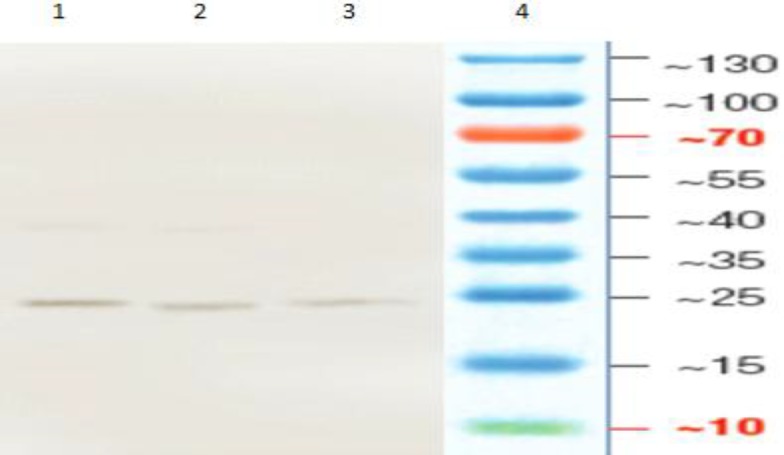
Western blotting to confirm the recombinant protein MPT-64 with different concentrations of purified protein. Lane 1: Pure protein, Lane 2: 1/2 and lane 3: 1/4, and Land 4: Protein ladder
Discussion
Despite all the efforts to control BTB, this disease causes a significant economic burden to the agricultural industries and for human health. Eradication programs by tuberculin testing and slaughter of positive animals have been successful in many countries. However, a tuberculin test is limited in its specificity and sensitivity and bacterial culture is long. Moreover, molecular technics like PCR can also detect M. bovis directly in clinical samples. Hence, production of synthetic peptides can help us for rapid differential diagnosis of pathogenic and non-pathogenic Mycobacterium and increasing the sensitivities and specificities of the serological assays in suspected BTB as a diagnostic tool in control TB.
Regions of different (RD) encoded proteins have been absent in non-pathogenic strains of mycobacteria and could be considered candidate antigens in serodiagnosis of TB. Some of these antigens are encoded by RD located in the genome of M. tuberculosis, M. africanum, M. bovis but are absent in all the Bacillus Galmette Guerin substrains and many of the environmental Mycobacteria (Parkash et al., 2009 ▶). One of the specific antigens of M. bovis is MPT-64 that encoded by RD-2 and is absent only in some BCG substrains and non-pathogenic Mycobacteria (Li et al., 1993 ▶) and reported as a highly immunogenic (Berther et al., 1998). In this study, recombinant MPT-64 protein, containing antigenic region of mpt-64 gene was produced in E. coli M15 with molecular weight around 24 kDa. In a previous study all isolated protein molecules were able to induce delayed hypersensitivity skin reaction in sensitized guinea pigs and proliferation of T-cells in vitro (Zavaran Hoseini et al., 1997 ▶). Regarding the fact that any TB control strategy is dependent mainly on sensitive and specific diagnosis methods, it is suggested that the molecules isolated in this study may be useful in conceiving future studies for development of diagnostic tests for TB infection (Zavaran Hoseini et al., 1997 ▶).
Unfortunately, the studies for BTB detection with serologic methods in Iran are few. In a forward study, the ESAT-6 of M. tuberculosis H37Rv have been cloned and expressed in prokaryotic system (Zavaran Hoseini et al., 2009 ▶). In another report, ESAT-6 and CFP-10 antigens were performed and suggested for the development of diagnostic kit for M. tuberculosis in future (Pourakbari et al., 2013 ▶). However, some researchers focused on ESAT-6 recombinant proteins for serodiagnosis of TB infection (Tebianian et al., 2015 ▶). In a study conducted with Aghazadeh et al. (2016 ▶) it has been demonstrated that ELISA with the use of the ESAT-6 antigens is simple and sensitive and can be used to analyze large numbers of samples for the serodiagnosis of BTB. In another study, the, CFP-10 and ESAT-6/CFP-10 recombinant proteins were used in the diagnosis and detection of TB by ELISPOT assays and showed sensitivity of 93%, 90%, and 100%, respectively. These data were significantly higher than the values obtained in conventional TST (Mostafavi-Pour et al., 2011 ▶). In a study, Esat-6 recombinant protein was used as a skin marker diagnosis of TB infection in guinea pigs (Tebianian et al., 2015 ▶). The recombinant proteins CFP-10, ESAT-6, Mb0143, MPB83, PE5, PE13, TB10.4, TB15.3 and a chimera of ESAT-6/MPB70/MPB83 (fusion protein) were tested as ELISA antigens for the diagnosis of BTB (Souza et al., 2012 ▶). The proteins EsxI, Mb0143, PE5, and PE13 are antigens tested for the first time in skin test on cattle. The results showed that the recombinant proteins have potential to be assessed as antigens in skin tests in cattle (Melo et al., 2015 ▶). Expression of M. tuberculosis proteins MPT63 and MPT83 was suggested as a fusion to the development of improved diagnostic tools or vaccines against human and/or cattle tuberculosis (Redchuk et al., 2012 ▶).
According to the results, MPT-64 recombinant protein due to expression plasmid pQE30 could be successfully expressed to relatively high amounts in E. coli M15. So, this antigen could be used for differential diagnosis of pathogenic and non-pathogenic Mycobacterium, in suspected BTB for development of diagnostic Kit.
Acknowledgements
We are thankful to all staff at the Tuberculin and R&D Departments of RVSRI, in particular, Dr. M. Taghizadeh.
References
- Aghazadeh, R , Tebianian, M , Mahdavi, M Evaluation of specific Anti-ESAT-6 antibody in diagnosis of bovine tuberculosis. J. Knowledge Health. 2016;72:78 –10. (in Persian) [Google Scholar]
- Ayele, WY , Neill, SD , Zinsstag, J , Weiss, MG , Pavlik, I Bovine tuberculosis: an old disease but a new threat to Africa. Int. J. Tuberc. Lung. Dis. 2004;8:924–937. [PubMed] [Google Scholar]
- Bao, L , Fan, Q , Lu, M , Xia, ZY Mycobacterium tuberculosis MPT-64 stimulates the activation of murine macrophage modulated by IFN-γ. Eur. Rev. Med. Pharmacol. Sci. 2013;17:3296–3305. [PubMed] [Google Scholar]
- Baumann, S , Eddine, A , Kaufmann, SH Progress in tuberculosis vaccine development. Curr. Opin. Immunol. 2006;18:438–448. doi: 10.1016/j.coi.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Cho, SN Current issues on molecular and immunological diagnosis of tuberculosis. Yonsei Med. J. 2007;48:347–359. doi: 10.3349/ymj.2007.48.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler, RN , Skeiky, YA , Ovendale, PJ Cloning of a Mycobacterium tuberculosis gene encoding a purifed protein derivative protein that elicits strong tuberculosis-specific delayed-type hypersensitivity. J. Infect Dis. 2000;182:224–233. doi: 10.1086/315677. [DOI] [PubMed] [Google Scholar]
- Daniel, TM , Janicki, BW Mycobacterial antigens: a review of their isolation chemistry and immunological properties. Microbiology. 1978; 42:84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kantor, IN , Ritacco, V An update on bovine tuberculosis programmes in Latin American and Caribbean countries. Vet. Microbiol. 2006;112:111–118. doi: 10.1016/j.vetmic.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Dye, C , Garnett, GP , Sleeman, K , Williams, BG Prospects for worldwide tuberculosis control under the World Health Organization (WHO) DOTS strategy. Lancet. 1998;352:1886–1891. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- Fan, X , Wang, C , Shi, C An improved whole-blood gamma interferon assay based on the CFP21-MPT64 fusion protein. Clin. Vaccine Immunol. 2009;16:686–691. doi: 10.1128/CVI.00486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley, ME , Nasir, MS , Surujballi, OP , Romanowska, A , Renteria, TB , De La Mora, A Fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in bovine. Vet. Microbiol. 2007;120:113–121. doi: 10.1016/j.vetmic.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Lange, C , Mori, T Advances in the diagnosis of tuberculosis. Respirology. 2010;15:220–240. doi: 10.1111/j.1440-1843.2009.01692.x. [DOI] [PubMed] [Google Scholar]
- Li, H , Ulstrup, JC , Jonassen, TO , Melby, K , Nagai, S , Harboe, M Evidence for absence of the mpb-64 gene in some substrains of Mycobacterium bovis BCG. Infect. Immun. 1993;61:1730–1734. doi: 10.1128/iai.61.5.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo, E , Souza, I , Ramos, C , Osório, A , Verbisck, N , Araújo, F Evaluation of the use of recombinant proteins of Mycobacterium bovis as antigens in intradermal tests for diagnosis of bovine tuberculosis. Arch. Med. Vet. 2015;47:273–280. [Google Scholar]
- Mostafavi-Pour, Z , Hemmati, M , Seghatoleslam, A , Rasti, M , Ebadat, M , Mosavari, N , Habibagahi, M , Taheri, M , Sardarian, A Expression and purification of recombinant Mycobacterium tuberculosis diagnosis antigens, ESAT-6, CFP-10, and ESAT-6/CFP-10, and their potential use in the diagnosis and detection of tuberculosis. Iran. Red Crescent Med. J. 2011;13:558–565. [PMC free article] [PubMed] [Google Scholar]
- Okada, M , Kobayashi, K Recent progress in mycobacteriology. Kekkaku. 2009;82:783–799. [PubMed] [Google Scholar]
- Parkash, O , Singh, B , Pai, M Regions of differences encoded antigens as targets for immuno-diagnosis of tuberculosis in humans. Scandinavian J. Immunol. 2009;70:345–357. doi: 10.1111/j.1365-3083.2009.02312.x. [DOI] [PubMed] [Google Scholar]
- Pourakbari, B , Mahmoudi, S , Mamishi, S , Ghazi, M , Hosseinpour Sadeghi, R Cloning, expression and purification of Mycobacterium tuberculosis ESAT-6 and CFP-10 antigens. Iran. J. Microbiol. 2013;5:374–378. [PMC free article] [PubMed] [Google Scholar]
- Redchuk, T , Korotkevich, N , Gorbatiuk, O , Gilchuk, P , Kaberniuk, A , Oliynyk, O , Kolibo, D , Komisarenko, S Expression of Mycobacterium tuberculosis proteins MPT63 and MPT83 as a fusion: purification, refolding and immunological characterization. J. Appl. Biomed. 2012;10:169–176. [Google Scholar]
- Sambrook, J , Russell, DW . 3rd Edn. New York: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning: a laboratory manual; pp. 5–40. [Google Scholar]
- Smith, I Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, II , Melo, ES , Ramos, CA , Farias, TA , Osório, AL , Jorge, KS , Vidal, CE , Silva, AS , Silva, MR , Pellegrin, AO , Araújo, FR Screening of recombinant proteins as antigens in indirect ELISA for diagnosis of bovine tuberculosis. Springerplus. 2012;1:77. doi: 10.1186/2193-1801-1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebianian, M , Moradi, J , Mosavari, N , Ebrahimi, M , Arefpajohi, R Evaluation of Mycobacterium tuberculosis early secreted antigenic target 6 recombinant protein as a diagnostic marker in skin test. Osong Public Health Res. Perspect. 2015;6:e34–e38. doi: 10.1016/j.phrp.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soolingen, D , De Hass, EWP , Kremer, K Restriction fragment length polymorphism (RFLP) typing of Mycobacteria. Methods Mol. Med. 2001;54:165–203. doi: 10.1385/1-59259-147-7:165. [DOI] [PubMed] [Google Scholar]
- Wayne, LG , Kubica, GP . The Mycobacteria. In: Sneath, PHA , Holt, JG , editors. Bergeys manual of systematic bacteriology. Baltimore: Williams & Wilkins Co; 1986. pp. 1435–1457. [Google Scholar]
- Young, DB Heat-shock proteins: immunity and autoimmunity. Curr. Opin. Immunol. 1992;4:396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- Zavaran Hoseini, A , Eslami, MB , Jalali, M Purification antigen isolated from Mycobacterium tuberculosis (H37Rv strain) and their effects on cell-mediated immune responses guinea pigs. M.J.IRI. 1997;10:291–297. [Google Scholar]
- Zavaran Hoseini, A , Tebianian, M , Ebrahimi, SM , Rezaei Mokaram, A , Taghizadeh, M , Asli, E Cloning and expression of Mycobacterium tuberculosis ESAT-6 in prokaryotic system. Arch. Razi Inst. 2009;64:1–7. [Google Scholar]